Abstract
Intranasal application of vesicular stomatitis virus (VSV) produces a well-characterized model of viral encephalitis in mice. Within one day post-infection (PI), VSV travels to the olfactory bulb and, over the course of 7 days, it infects regions and tracts extending into the brainstem followed by clearance and recovery in most mice by PI day 14 (PI 14). Infectious diseases are commonly accompanied by excessive sleepiness; thus, sleep is considered a component of the acute phase response to infection. In this project, we studied the relationship between sleep and VSV infection using C57BL/6 (B6) and BALB/c mice. Mice were implanted with transmitters for recording EEG, activity and temperature by telemetry. After uninterrupted baseline recordings were collected for 2 days, each animal was infected intranasally with a single low dose of VSV (5 × 104 PFU). Sleep was recorded for 15 consecutive days and analyzed on PI 0, 1, 3, 5, 7, 10, and 14. Compared to baseline, amounts of non-rapid eye movement sleep (NREM) were increased in B6 mice during the dark period of PI 1–5, whereas rapid eye movement sleep (REM) was significantly reduced during the light periods of PI 0–14. In contrast, BALB/c mice showed significantly fewer changes in NREM and REM. These data demonstrate sleep architecture is differentially altered in these mouse strains and suggests that, in B6 mice, VSV can alter sleep before virus progresses into brain regions that control sleep.
1. Introduction
Vesicular stomatitis virus (VSV) is a member of the Vesiculovirus genus in the Rhabdoviridae family and it is the prototypic virus of this family that includes rabies virus. The genome of the virus is a single molecule of negative-sense RNA that encodes five major proteins: glycoprotein (G), matrix protein (M), nucleoprotein (N), large protein (L) and phosphoprotein (Kang and Prevec 1969). It is a “simple” virus that has been well characterized biochemically and immunologically.
Administered intranasally, VSV initially infects and replicates in olfactory receptor neurons and then invades the olfactory bulb (OB) via the olfactory tract and, over the course of 7 days, infects regions and tracts extending into the brainstem. Reiss and colleagues as well as our own more recent studies have extensively characterized this virus encephalitis model induced by a neurotropic viral pathogen. In the VSV encephalitis model, neutrophils are the initial inflammatory cell seen in the OB around 24 hours post-infection (Chen, Restivo et al. 2001; Blank, Nijholt et al.), a time when VSV antigens are first detected in this brain region (Huneycutt, Plakhov et al. 1994). Around days 6–8 PI, a robust mixed cellular infiltrate dominated by neutrophils, T cells, macrophages and to a lesser extent dendritic cells accumulates in the brain parenchyma (Bi, Barna et al. 1995; Ciavarra, Stephens et al. 2006; Steel, Stephens et al. 2008; Steel, Hahto et al. 2009). Resident glia cells (astrocytes, microglia) are not quiescent but undergo a rapid and impressive expansion (astrogliosis and microgliosis) following VSV neuroinvasion (Christian, Barna et al. 1996). This acute inflammatory response is temporally associated with peak viral titers at this time (Forger, Bronson et al. 1991; Reiss, Plakhov et al. 1998). While intranasal instillation of VSV can result in hind limb paralysis and death (Miyoshi, Harter et al. 1971; Rabinowitz, Dal Canto et al. 1976; Forger, Bronson et al. 1991), mice that survive completely clear VSV from the CNS, an event dependent on T cell-mediated immunity (Forger, Bronson et al. 1991; Andersson, Christensen et al. 1994; Soilu-Hanninen, Roytta et al. 1997; Christensen, Andreasen et al. 2001; Bullard, Hu et al. 2005). Because mice often survive, VSV has become a valuable model for the neurological complications that can persist after transient viral infections of the brain (van den Pol, Dalton et al. 2002).
VSV does not produce global infection of the CNS; however, it does course through brain regions that are involved in the regulation of sleep. Major brain structures that show VSV antigen include the septal region, amygdala, bed nucleus of the stria terminalis, hypothalamus, thalamus and hippocampus. More caudally, VSV strongly impacts the dorsal pons and infects the serotonergic dorsal raphe nucleus (Mohammed, Maehlen et al. 1992; Huneycutt, Bi et al. 1993; Huneycutt, Plakhov et al. 1994) and noradrenergic locus coeruleus (Lundh, Love et al. 1988; Huneycutt, Bi et al. 1993; Huneycutt, Plakhov et al. 1994), two nuclei implicated in the regulation of REM (Steriade and McCarley 1990) as well as a variety of behaviors.
Infectious diseases are commonly accompanied by alterations in sleep, fatigue and fever. For example, intranasal inoculation with the influenza virus in mice results in increases in non-rapid eye movement sleep (NREM) and decreases in rapid eye movement sleep (REM), though body temperature is decreased (Fang, Sanborn et al. 1995; Toth, Rehg et al. 1995; Fang, Tooley et al. 1996; Toth and Williams 1999; Toth and Opp 2001). Genetics play a role in the susceptibility and resistance to infectious agents (Cooke and Hill 2001; Hill 2001; De Maio, Torres et al. 2005; Tuite and Gros 2006; Trammell and Toth 2008; Trammell, Liberati et al. 2012); e.g., the increases in NREM with influenza appear to be strain dependent because C57BL/6 (B6) mice display increases whereas BALB/c mice do not (Toth, Rehg et al. 1995).
To date, no one has examined the effects of VSV on sleep. The well-characterized path and temporal course of VSV as it travels through the brain suggest that it may be a good model for examining the relationship between viral infection and sleep. In this study, we examined the effects of VSV on sleep from the time of initial infection through virus clearance (acute and recovery phases of infection). We also compared the effects of VSV on B6 and BALB/c mice, two strains that show differences in sleep responses to influenza (Toth, Rehg et al. 1995).
2. Methods
2.1. Subjects
Six male B6 mice and 8 male BALB/c mice weighing 20 to 25 g (ages 9 to 10 weeks) were obtained from the Jackson Laboratory, Bar Harbor, Maine. All mice were maintained in a sanitized colony room and were individually housed in sterile cages with bedding. Sterile food and water were given ad libitum. The colony room was kept on a 12:12 light-dark cycle and ambient temperature was maintained at 24°C ± 0.5°C. Throughout the experimental procedures, measures were taken to avoid unnecessary pain and discomfort of the animals. When animals became moribund or severely hypothermic (temperature < 26 °C), and/or non-ambulatory/unresponsive, they were euthanized. A total of 3 mice were euthanized (2 BALB/c on PI 6 and 1 B6 on PI 10). All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by at Eastern Virginia Medical School’s Animal Care and Use Committee.
2.2. Surgery
After at least 1 week of acclimation to the colony room, the mice were implanted intraperitoneally with telemetry transmitters (ETA10-F20, DataSciences, St. Paul, MN) for recording EEG, activity and temperature in order to monitor sleep and wakefulness as previously described (Tang and Sanford 2002). EEG leads from the transmitter body were led subcutaneously to the head, and the free ends were placed into holes (one in the left anterior quadrant and one in the right posterior quadrant near Bregma) drilled in the dorsal skull to allow recording cortical EEG. Aseptic techniques were used for all surgical procedures. Prior to surgery, the animals received potassium penicillin (100 IU/g weight), dexamethasone (0.005 mg/g weight), and gentamicin (0.005 mg/g weight) subcutaneously. All surgery was conducted under isofluorane (as inhalant: 5% induction; 2% maintenance) anesthesia. Ibuprofen (30 mg/kg, oral) was continuously available in each animal’s drinking water, for 24 to 48 h preoperatively and for a minimum of 72 h postoperatively, to alleviate potential postoperative pain. The animals were given at least 3 weeks for post-surgery recovery and kept undisturbed, except for weekly bedding changes, until used in the study.
2.3. Virus Infection
Wild-type VSV (Indiana strain) provided by Dr. Philip Marcus, University of Connecticut, was grown in confluent monolayers of Vero cells as previously described (Marvaldi, Lucas-Lenard et al. 1977; Sekellick and Marcus 1979). Virus inoculation was carried out near the beginning of the light period on PI day 0. Mice were lightly anesthetized in a closed container saturated with isofluorane, and then intranasally infected with a single low dose of VSV (5 × 104 PFU) administered equally to both nostrils in a total volume of 10 μl as previously described (Steel, #145). Mice were generally ambulant within a few minutes after the procedure and returned to home cage for recording.
2.4. Recording and Determination of Behavioral State
Recording was started by activating the transmitters with a magnetic switch and placing the mice, in their home cages, on a telemetry receiver (Model RPC-1, Data Sciences, St. Paul, MN). All recordings were performed in the colony room. Signals (EEG, body temperature, and activity) from the transmitter were processed by DataSciences (St. Paul, MN)) software at 250 Hz and saved to the hard disk for subsequent offline data analyses and for visual determination of sleep and wakefulness in 10-s epochs using the SleepSign™ (Kissei America, Inc., Irvine, CA) scoring program. This program also processed the EEG data. The EEG data were subjected to online spectral analysis every 2 s by fast Fourier Transformation (FFT) with a Hanning window treatment. SleepSign uses finite impulse response filtering with 4 digital filters: a low pass filter, a high pass filter, a band pass filter and a band stop filter to reject artifact. Behavioral state was visually scored by trained observers in 10 s epochs as either wakefulness (active wakefulness (AW), or quiet wakefulness (QW; not reported)), NREM, or REM based on EEG and gross whole-body activity as previously described (Tang and Sanford 2002).
Prior to VSV inoculation, uninterrupted recordings were collected for 2 days and used as baseline values. Recording was continuous during the course of infection.
2.5. Data Analyses
The following sleep parameters were evaluated: total NREM sleep, number of NREM sleep episodes, total REM sleep, number of REM episodes. An episode was defined as a number of continuous 10 s epochs spent in each behavioral state, and then assessed using the SleepSign Program. In addition, delta wave amplitude (DWA, 0.5–4 Hz) during NREM and theta wave amplitude (TWA, 5–9 Hz) during REM were normalized as percentage of baseline prior to analysis. Sleep was analyzed in 12-h blocks (light and dark periods) for baseline and on PI 0, 1, 3, 5, 7, 10, and 14. Data were analyzed using SigmaStat V12 (Systat Software, San Jose, CA).
To directly compare the two strains for potential differences in responses to VSV infection, data from each animal were normalized by subtracting baseline values from post-VSV infection values. Time effect, strain differences, and interaction between time and strain were examined using ANOVA procedures. Following ANOVA all pairwise post hoc comparisons were conducted using Tukey test when appropriate. In instances where the equal variance test failed, data were analyzed by a Kruskal-Wallis ANOVA on ranks followed by Dunn’s method for comparisons to control (baseline). Differences were considered significant at p < 0.05. Error bars in each figure are ± SEM.
The non-surviving mice were included in the pre-infection analysis (baseline strain comparison), but were excluded from the post-infection analysis. The effect of VSV on sleep in these mice is described separately.
3. Results
3.1. Baseline strain comparisons
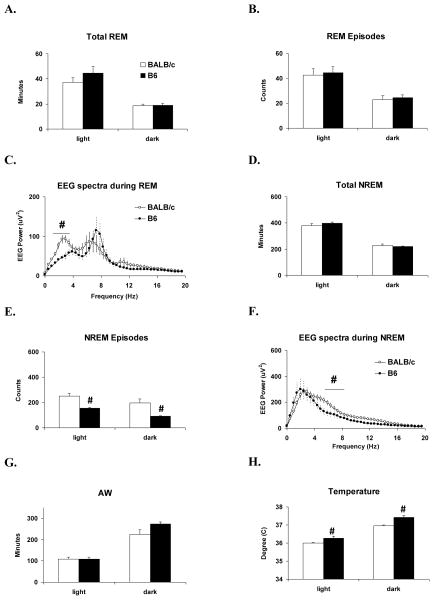
B6 and BALB/c mice did not significantly differ in baseline amounts of total REM (Fig. 1A) or number of REM episodes (Fig. 1B). There was no strain difference in theta band EEG activity during REM. However, the analysis for delta band activity was significant (F (1, 24) = 16.9, p < 0.001)); EEG power was significantly greater in the delta band in BALB/c mice (Fig. 1C).
Figure 1.
Comparisons between BALB/c (open bars & dots) and B6 (filled bars & dots) mice for baseline amounts of sleep, activity and temperature monitored for 24-h before VSV inoculation. Values (mean ± SEM) are plotted for 12-h light and dark periods. (A) Total time spent in REM. (B) REM episode counts. (C) 24 h EEG spectra during REM. (D) Total time spent in NREM. (E) NREM episode counts. (F) 24 h EEG spectra during NREM. (G) Total time spent in AW. (H) Body temperature. # denotes significant differences between strains (p < 0.05).
B6 and BALB/c mice also did not differ in baseline amounts of total NREM (Fig. 1D); however, the number of NREM episodes (Fig. 1E) significantly differed between strains during light (Kruskal Wallis; H (1) = 6.0, p = 0.013) and dark periods (F (1, 12) = 8.6, p = 0.013). BALB/c mice showed greater numbers of NREM episodes in both light and dark periods. There was no strain difference in NREM delta. However, EEG power was significantly greater (F (1, 24) = 7.4, p = 0.012) in the theta band during NREM in BALB/c mice (Fig. 1F).
Amounts of AW did not significantly differ between strains (Fig. 1G). However, body temperature significantly differed between strains during light (F (1, 12) = 5.9, p = 0.032) and dark periods (F (1, 12) = 18.4, p = 0.001). B6 mice maintained a higher body temperature during both light and dark periods (Fig. 1H).
3.2. Effects of VSV on REM
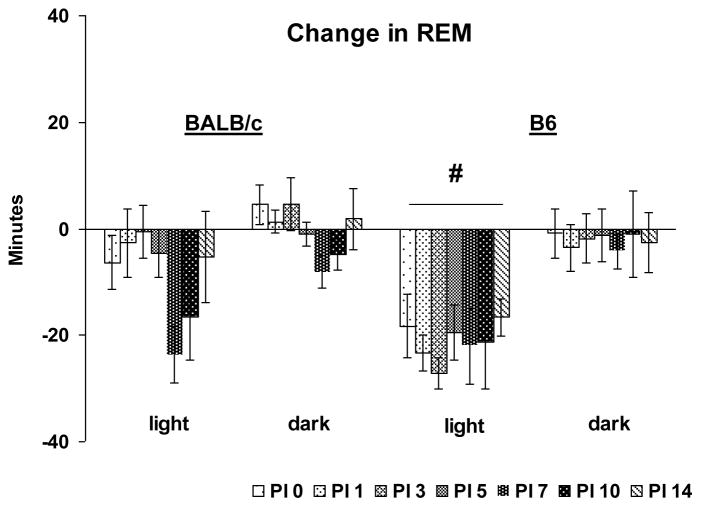
One of the most prominent effects of VSV was a significant reduction in REM that was most apparent in B6 mice. A direct strain comparison revealed a significant group main effect for light period REM amount (F (1, 63) = 14.9, p < 0.001) indicating a greater overall reduction in REM in B6 mice compared to BALB/c mice (Fig. 2). No other comparisons were significant.
Figure 2.
Direct strain comparison of VSV-induced changes in total REM between BALB/c and B6 mice. Data from each animal were normalized by subtracting baseline values from post-VSV infection values and averaged by strains. PI days 0, 1, 3, 5, 7, 10 and 14 (shaded bars) are presented. Values (mean ± SEM) are plotted for 12-h light and dark periods. # denotes significant differences between strains (p < 0.05).
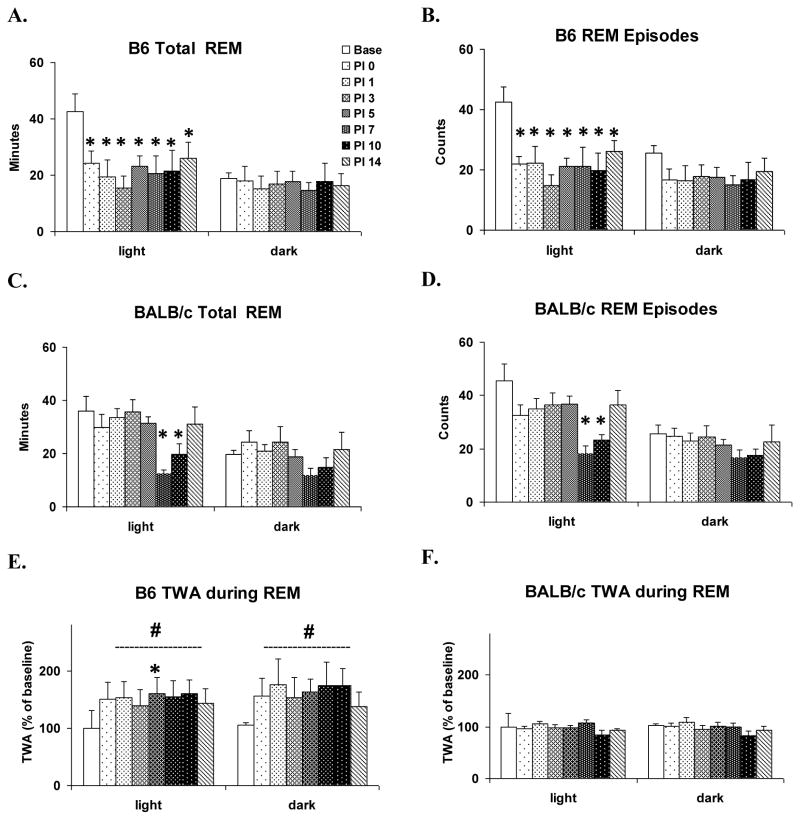
As indicated in the direct strain comparison, changes in REM were more pronounced in B6 mice. ANOVAs conducted across days for B6 mice revealed significant time effects for light period REM sleep amounts (Fig. 3A) (F (7, 28) = 4.7, p <0.002) and number of REM sleep episodes (Fig. 3B) (F (7, 28) = 5.3, p < 0.001). Compared to baseline, amount of REM sleep and number of REM sleep episodes were significantly reduced during the light periods of PI 0–14. There were no significant differences in dark period REM sleep across days.
Figure 3.
Within strain comparisons of the effects of VSV for selected REM parameters. Values (mean ± SEM) are plotted during 12-h light and dark periods for baseline (Base, open bar) and PI days 0, 1, 3, 5, 7, 10 and 14 (shaded bars). (A) Total time spent in REM of B6 mice. (B) REM episode counts of B6 mice. (C) Total time spent in REM of BALB/c mice. (D) REM episode counts of BALB/c mice. (E) Theta-wave amplitude (TWA) during REM of B6 mice. (F) TWA during REM of BALB/c mice. TWA is plotted as % of baseline. * denotes p < 0.05 relative to baseline values at the comparable time point. # denotes significant differences between strains (p < 0.05).
In BALB/c mice, the ANOVAs for comparisons across days were also significant for light period REM sleep amounts (Fig. 3C) (F (7, 35) = 6.5, p < 0.001) and number of REM sleep episodes (Fig. 3D) (F (7, 35) = 6.7, p < 0.001). However, changes in REM sleep occurred later in BALB/c; compared to baseline, amount and number of REM sleep episodes were reduced only in the light period of PI 7 and PI 10. Dark period REM sleep did not significantly differ for any of the parameters we examined, either across days or conditions.
We also compared TWA during REM, corrected for baseline differences, across strains and across PI days within strains. Between strain comparisons revealed significant main effects for group during both the light period (F (1, 72) = 25.8, p < 0.001) and dark period (F (1, 72) = 29.7, p < 0.001). This was due to an overall increase in TWA in B6 mice (Fig 3E) compared to BALB/c mice (Fig. 3F). Comparisons within strains revealed a significant increase in theta wave amplitude during light period on PI 5 compared to baseline in B6 mice (Chi-square (7) = 14.7, p < 0.05). There were no other significant differences in the comparison either between or within strains.
3.3. Effects of VSV on NREM
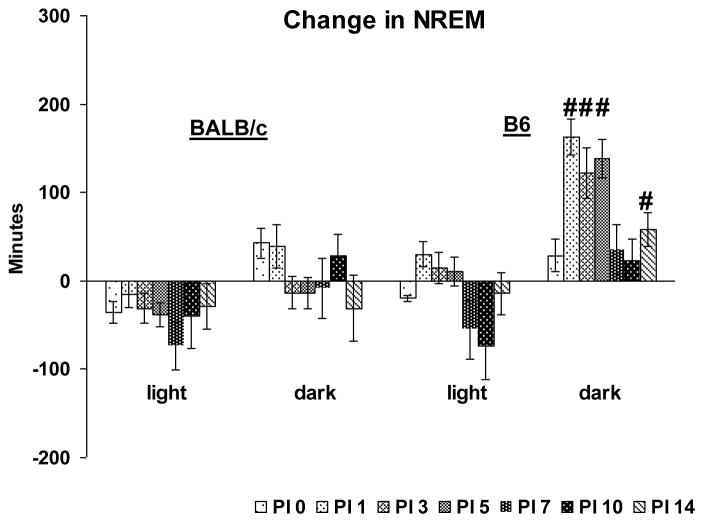
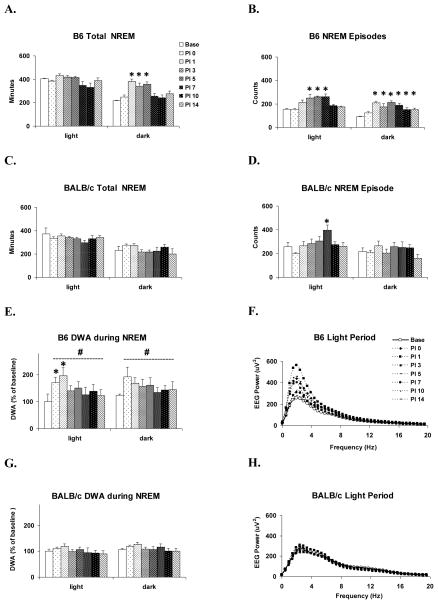
The direct strain comparison of NREM amounts after VSV infection revealed a significant main effect for strain (F (1, 63) = 31.4, p < 0.001) and a significant strain x PI day interaction (F (13, 63) = 3.6, p = 0.004) during the dark period (Fig. 4). Post-hoc comparisons within PI days revealed that NREM amounts in B6 mice was significantly increased on PI 1, 3, 5 and 14 compared to BALB/c.
Figure 4.
Direct strain comparison of VSV-induced changes in total NREM between BALB/c and B6 mice. Data from each animal were normalized by subtracting baseline values from post-VSV infection values and averaged by strains. PI days 0, 1, 3, 5, 7, 10 and 14 (shaded bars) are presented. Values (mean ± SEM) are plotted for 12-h light and dark periods. # denotes significant differences between strains (p < 0.05).
The within strain analysis found that B6 mice showed significant differences in dark period NREM amounts (F (7, 28) = 8.3, p < 0.001) as well as episode numbers (F (7, 28) = 11.0, p < 0.001) across recording days. Compared to baseline, dark period NREM was significantly increased on PI 1, 3 and 5 (Fig. 5A) and there were increases in the number of dark period NREM episodes on PI 1 through PI 14 (Fig. 5B). The analysis did not find any significant alteration in light period total NREM sleep across recording days. However, there was a significant alteration in NREM episode number across days (F (7, 28) = 7.1, p < 0.001). The number of NREM episodes was increased during the light periods of PI 3, 5 and 7 compared to baseline.
Figure 5.
Within strain comparisons of the effects of VSV on selected NREM parameters. Values (mean ± SEM) are plotted for 12-h light and dark periods. (A) Total time spent in NREM of B6 mice. (B) NREM episode counts of B6 mice. (C) Total time spent in NREM of BALB/c mice. (D) NREM episode counts of BALB/c mice. (E) Delta-wave amplitude (DWA) during NREM of B6 mice. (F) EEG spectra of B6 during light period NREM. (G) DWA during NREM of BALB/c mice. (H) EEG spectra of BALB/c during light period NREM. DWA is plotted as % of baseline. * denotes p < 0.05 relative to baseline values at the comparable time point. # denotes significant differences between strains (p < 0.05).
In contrast to the findings for B6 mice, the within strain analysis for BALB/c mice did not reveal any significant alterations for total NREM in either the light or dark period (Fig. 5C). The ANOVA was significant for number of NREM episodes (F (7, 35) = 5.824, p < .001) in the light period (Fig. 5D). This was primarily due to a significant increase in the number of NREM episodes on PI 7 compared to baseline.
We compared DWA during NREM, corrected for baseline differences, across strains and across PI days within strains after VSV infection. The between strain comparisons revealed significant main effects for group during both the light period (F (1, 72) = 29.2, p < 0.001) and dark period (F (1, 72) = 25.3, p < 0.001). This was due to an overall increase in DWA in B6 mice (Fig 5E) compared to BALB/c mice (Fig. 5G).
DWA of the B6 mice (Figs. 5E–F) showed significant changes in the light period across days (F (7, 28) = 5.1, p < 0.001). Compared to baseline, DWA was increased during the light period on PI 0 and 1. The analysis for the dark period did not reach significance. DWA in BALB/c mice (Figs. 5G–H) did not differ across days.
3.4. Sleep after VSV Infection in Non-surviving Mice
Three mice became severely hypothermic (temperature < 26 °C) and/or non-ambulatory/unresponsive, and were euthanized before completing the study (2 BALB/c mice on PI 6 and 1 B6 mouse on PI 10). Data for these mice were used in the strain comparisons of baseline recordings, but were not used in the subsequent analysis after VSV infection. While it was not feasible to conduct a statistical analysis of data from these mice, it is worth noting that these 3 non-survivors did not show any notable differences in post-infection NREM in comparison to the survivors. However, the REM response to VSV infection in two of the non-survivors did appear to deviate from that of the survivors. The non-surviving B6 mouse did not appear to show the prominent reduction in REM exhibited by the survivors and one of the BALB/c non-survivors showed an apparent increase in dark period REM from PI 0 onward. Outside these observations, we did not observe any post-infection changes that could distinguish the surviving and non-surviving mice of either strain.
3.5. Effects of VSV on Active Wakefulness
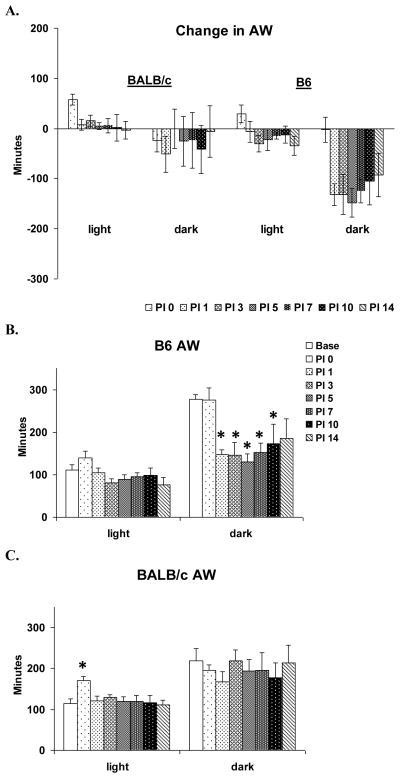
The direct strain comparison of AW across days found no significant difference between BALB/c and B6 in the effect of VSV infection on AW (Fig. 6A). This was likely due to relatively large individual variation found in the BALB/c mice during the dark period.
Figure 6.
Effects of VSV infection on active wakefulness (AW) during baseline (Base, open bar) and PI days 0, 1, 3, 5, 7, 10 and 14 (shaded bars). Values (mean ± SEM) are plotted for 12-h light and dark periods. (A) Direct strain comparison of VSV-induced changes in AW between BALB/c and B. Data from each animal were normalized by subtracting baseline values from post-VSV infection values and averaged by strains. (B) Total time spent in AW of B6 mice. (C) Total time spent in AW of BALB/c mice. * denotes p < 0.05 relative to the baseline values at the comparable time point.
The within strain analysis for B6 mice revealed a significant day effect for dark period AW (F (7, 28) = 4.2, p< 0.004). Compared to baseline, AW amounts were significantly decreased on PI 1–10 (Fig. 6B); however, the apparent decrease in AW on PI 14 did not reach significance (p = 0.06). Light period amounts of AW were not significantly altered.
BALB/c showed a significant day effect (F (7, 28) = 2.7, p = 0.023) for light period AW (Fig. 6C). This primarily resulted from a significant increase in AW immediately after VSV infection on PI 0 that was associated with the intranasal inoculation procedure.
3.6. Effects of VSV on Core Body Temperature
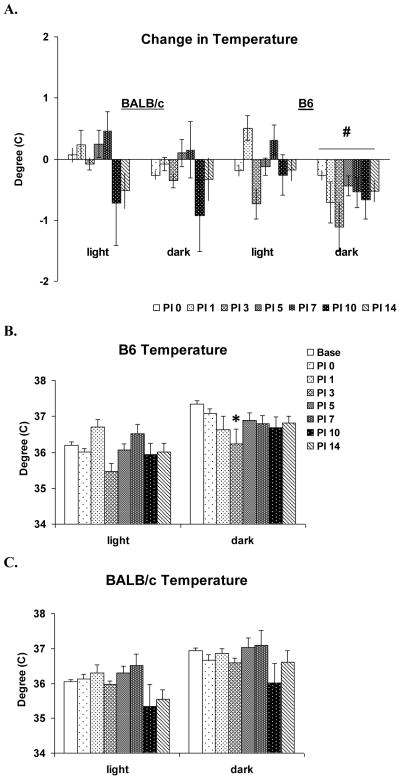
The direct strain comparison revealed significant differences between B6 and BALB/c mice in dark period body temperature (F (1, 63) = 4.79, p = 0.032) (Fig 7A). This was likely due to the more consistent decrease, relative to baseline, in body temperature across days in the B6 mice. The analysis for light period body temperature did not reveal any significant differences between B6 and BALB/c mice.
Figure 7.
Effects of VSV infection on body temperature during baseline (Base, open bar) and PI days 0, 1, 3, 5, 7, 10 and 14 (shaded bars). Values (mean ± SEM) are plotted for 12-h light and dark periods. (A) Direct strain comparison of VSV-induced changes in temperature between BALB/c and B6 mice. Data from each animal were normalized by subtracting baseline values from post-VSV infection values and averaged for strains. Baseline value (C°): BALB/c: 36.1 ± 0.05 (light period), 36.9 ± 0.07 (dark period), B6: 36.2 ± 0.09 (light period), 37.3 ± 0.09 (dark period). (B) Body temperature of B6 mice. (C) Body temperature of BALB/c mice. * denotes p < 0.05 relative to the baseline values at the comparable time point. # denotes significant differences between strains (p < 0.05).
The within strain analysis for B6 mice showed VSV-induced across-day effects in body temperature both during light (F (7, 28) = 5.2, p < 0.001) and dark F (7, 28) = 2.9, p = 0.021) periods (Fig. 7B). However, the post hoc comparisons revealed only a significant decrease in dark period temperature on PI 3 compared to baseline.
The within strain analysis for BALB/c mice showed a significant effect for temperature during the light period (Chi-square (7) = 16.0, p = 0.025); however, post hoc comparisons with the conservative Tukey test did not reveal any differences across days. The analysis for the dark period was not significant.
4. Discussion
We found that intranasal inoculation with a single low dose of VSV produced significant alterations in sleep, and that the magnitudes of impact of VSV on REM, NREM and body temperature varied with strain. In B6 mice, VSV can begin impacting REM before the virus progresses beyond the olfactory bulb and can persist even after viral clearance. By comparison, reductions in REM in BALB/c mice were observed only on PI 7 and 10. Moreover, alterations in NREM in B6 began on PI 1 and persisted through PI 7, whereas amounts of NREM in BALB/c mice were relatively stable until PI 7, when mice showed more fragmented NREM sleep in the light period. Core body temperature was altered in mice of both strains, but the changes were greater in B6 mice.
While there have been no direct comparisons of VSV infection in B6 and BALB//c mice, separate studies using a higher intranasal dosage of VSV (2 × 105 PFU) than used in this study found similar patterns of morbidity and mortality, kinetics of immunohistochemically detectable VSV protein, anatomic distribution of VSV and cellular distribution of staining in both strains (Bi, Barna et al. 1995; Christian, Barna et al. 1996). Indeed, based on several measures, B6 and BALB/c mice showed no difference in susceptibility, resistance or recovery to VSV challenge at this higher dosage (Christian, Barna et al. 1996). This conclusion is consistent with our results which had similar levels of morbidity in the two strains, though we had an admittedly small sample. Thus, in contrast to a variety of other measures of immune system function and infection outcome (Bi, Barna et al. 1995; Christian, Barna et al. 1996), the current study demonstrates that sleep is a sensitive marker of strain differences in the impact of VSV.
Our finding of strain differences in the sleep response to VSV infection have similarities to those reported for the influenza virus in that B6 mice show increases in NREM sleep that lasts for 4 days or more whereas BALB/c mice do not (Toth, Rehg et al. 1995). However, the time course of the increase in NREM in B6 mice was different for influenza and VSV. With influenza, increases in NREM occurred within 24 h of inoculation (Toth, Rehg et al. 1995) whereas with VSV, increases in NREM began in the dark period of the second day. With both influenza and VSV, BALB/c mice showed less marked changes in NREM sleep. The only significant alterations in NREM in BALB/c mice after VSV infection was more fragmented NREM sleep in the light period of PI 7. DWA, which reflects sleep pressure or sleep intensity (Pappenheimer, Koski et al. 1975; Borbely 1982), also appears to be differentially altered by VSV and influenza. With influenza, both B6 and BALB/c mice showed significant reductions in DWA during NREM (Toth, Rehg et al. 1995). With VSV, B6 mice showed enhanced NREM DWA on PI 0 and 1 compared to baseline, whereas BALB/c mice showed no significant alterations in NREM DWA across any of the days we examined.
We saw significant strain differences in REM sleep. Reductions in REM amounts in B6 mice began on the day of infection and persisted throughout the recording period. Unfortunately, at this point, we do not know whether the reductions in REM are permanent, but they did continue beyond the period where VSV is cleared from the CNS. By comparison, reductions in REM in BALB/c mice were observed only on PI 7 and 10, after which they returned to baseline levels. The decrease in REM in B6 mice also occurred prior to changes in NREM sleep whereas with influenza increases in NREM sleep precede decreases in REM (Fang, Sanborn et al. 1995; Fang, Tooley et al. 1996). Interestingly, there is some suggestion that rabies, which belongs to the same family as VSV, can produce an early decrease of REM sleep (Gourmelon, Briet et al. 1986; Gourmelon, Briet et al. 1991); however data from the onset of infection were not provided.
The early decrease in REM sleep in B6 mice also is interesting in that it was associated with an increase in TWA on some recording days whereas there was no apparent change in TWA in BALB/c mice over the entire recording period. TWA in the EEG is continuous throughout REM in adult rodents and has been associated with the circadian component of sleep regulation (Yasenkov and Deboer 2011) as well as with behaviors in wakefulness (Brown 1964; Vanderwolf 1988; Franken 1998). However, the possible significance of the increase in B6 mice in this study is not clear.
Following VSV infection, B6 mice, and BALB/c mice to a lesser degree, showed sporadic decreases in body temperature leading to a loss of the circadian temperature rhythm. However, neither strain showed the persistent and prolonged hypothermia reported after inoculation with influenza where both strains showed similar decreases in core temperature that began within 24 h after inoculation and persisted in both the light and dark periods for at least 4 days (Toth, Rehg et al. 1995).
It has been suggested that the decrease in body temperature observed with influenza in mice may in turn decrease hypothalamic temperature which may play a role in decreased REM (van den Pol, Dalton et al. 2002). However, with VSV, we saw early and persisting decreases in REM in B6 mice without any obvious relationship to changes in temperature. While one cannot make direct comparisons between the effects of influenza and VSV, it is interesting to note that with both viruses, alterations in core body temperature did not follow those in sleep. The potential for the separation of sleep and thermoregulatory responses has been previously noted (reviewed in (Krueger and Takahashi 1997)).
The reason for the pronounced strain difference in the sleep responses to VSV is not clear though there are a number of genetic differences between B6 and BALB/c mice which are likely to be important in responding to VSV infection. These mouse strains differ in alleles for the interferon (IFN) gene (Huygen and Palfliet 1984), which results in reduced IFN production by BALB/c mice (Toth 1996). For example, examination of IFN-gamma production in response to tuberculin in BCG (Bacillus Calmette-Guerin)-vaccinated mice revealed that B6 mice produced about ten times more IFN-gamma than did BALB/c mice (Huygen and Palfliet 1984). Although an anti-VSV effect of innate IFN-gamma has not been found in the periphery (Muller, Steinhoff et al. 1994), intravenously transferred IL-12, a strong activator of IFN-gamma production, has shown protective effects against VSV infection in the CNS (Bi, Quandt et al. 1995), thereby suggesting the involvement of IFN-gamma during VSV infection in the CNS (Bi, Barna et al. 1995). IFN also has somnogenic effects (Toth 1996) suggesting that the VSV-induced increase in NREM in B6 may be influenced by the production of IFN.
The immune response to CNS infection in B6 mice is often primarily Th1 mediated whereas that in BALB/c mice often shows a Th2-dominated immune response (Christian, Barna et al. 1996). Th1 and Th2 responses are distinguished based on cytokine profiles and the Th1 pathway promotes cellular immunity whereas the Th2 pathway promotes humoral immunity (Schwarz, Chiang et al. 2001). These pathways have also been associated with differences in sleep. There also have been suggestions of higher levels of sleep difficulties being associated with the shift towards the Th2 pathway in chronic fatigue syndrome patients (Torres-Harding, Sorenson et al. 2008).
Compared to B6 mice, BALB/c mice have reduced benzodiazepine receptors only in the amygdala (Hode, Ratomponirina et al. 2000). The amygdala strongly responds to peripheral immune challenge and plays a central role in mediating sickness behavior (Dantzer, O’Connor et al. 2008). Compared to injections of saline, intraperitoneal administration of lipopolysaccharide (LPS) and staphylococcal enterotoxin B (SEB) in adult rats induced increases in amygdaloid neuronal activity 90 to 120 minutes post-injection and increased c-Fos expression in both basal and central nuclei of the amygdala (Engler, Doenlen et al. 2011; Prager, Hadamitzky et al. 2012). Both also produced an increase in anxiety-related behavior in the elevated plus-maze test as indicted by reduced time in the open arms and increased head dips. LPS also enhanced IL-1beta, IL-6 and TNF-alpha (which are involved in the regulation of sleep (Opp 2005; Imeri and Opp 2009)) mRNA expression in the amygdala at 60 to 200 minutes after treatment (Engler, Doenlen et al. 2011; Prager, Hadamitzky et al. 2012). In line with these findings, we found that intranasal application into B6 mice of a dosage of VSV identical to that used in this study induced mRNA expression of lymphotoxin (homologus to TNF-beta) in the amygdala at 180 minutes post-infection (unpublished data). Lymphotoxin, produced in the Th1 pathway, also has been implicated in regulating sleep (Kapas and Krueger 1992; Deboer, Fontana et al. 2002). Together, these responses indicate that the amygdala can rapidly register and alter behavior in response to an immune challenge, and that the responses can involve cytokines that can alter sleep. The amygdala also plays a significant role in regulating sleep, in particular REM sleep, in response to stressful challenges (Liu, Yang et al. 2009; Liu, Wellman et al. 2011), though, to our knowledge, its potential role in regulating sleep during sickness has not been examined. However, given the role of the benzodiazepines in regulating sleep (Mendelson 1984; Borbely, Mattmann et al. 1985; Tan, Uchida et al. 1998) and the immune system (Miyawaki, Sogawa et al. 2001; Casellas, Galiegue et al. 2002; Wilms, Claasen et al. 2003), differences in benzodiazepine receptors in the amygdala have the potential to be involved in the influence of infection on sleep.
There also are strain differences in the corticotropin system (Anisman, Prakash et al. 2007) and the noradrenergic LC (Touret, Valatx et al. 1982), both of which play roles in the stress response and in the regulation of sleep and arousal. Given the bidirectional influences of the immune and stress systems (Silverman, Pearce et al. 2005), these differences could play roles in immune-stress interactions and their impact on sleep. While these linkages are speculative, they do suggest that differences in the sleep responses of B6 and BALB/c mice may involve innate neurobiological differences in the CNS as well as in their immune systems (Toth, Rehg et al. 1995).
There is a general conception that sleep plays a beneficial role during sickness. One study reported that rabbits subjected to microbial challenges (Escherichia coli, Staphylococcus aureus, Candida albicans) had a greater probability for survival if they exhibited robust increases in NREM and slow wave EEG activity during NREM compared to animals that showed extended periods of NREM suppression (Toth, Tolley et al. 1993). However, a clear association between sleep and recovery from infection has not been established (for discussion see (Majde and Krueger 2005; Imeri and Opp 2009)). As with most previous studies, we saw no clear association between sleep and recovery from the virus as both B6 and BALB/c mice showed significantly different sleep responses to VSV, but virtually equivalent sickness and recovery based on the parameters we examined.
With intranasal inoculation, VSV has a well characterized temporal course of infection and sickness. VSV also has a relatively well constrained regional pattern of infection in the brain and it traverses through several regions (Huneycutt, Plakhov et al. 1994) that have demonstrated roles in regulating sleep and arousal (e.g., septal region, the amygdala, hypothalamus, thalamus and hippocampus, LC and DRN). These factors make it an excellent model for studying immune function and sleep, as one can follow the time course of changes in sleep prior to and after the virus enters the brain, during sickness and after it is cleared from the CNS.
VSV is neurotropic; thus, one cannot rule out the possibility that later changes in sleep involve damage to sleep regulatory regions. However, there are suggestions that VSV may have a selective affinity for some neuron types. For instance, infant rats that survived VSV infection showed a loss of neurons in the medial and dorsal raphe and a permanent reduction in serotonin (Mohammed, Magnusson et al. 1990; Mohammed, Maehlen et al. 1992). By comparison, indices of dopamine, noradrenaline, GABA and acetylcholine showed no marked changes in adulthood (Mohammed, Magnusson et al. 1990; Mohammed, Maehlen et al. 1992). These factors have led to the suggestion that VSV may also be an excellent model for studying how transient viral infections of the brain can lead to permanent neurological or mental dysfunction (van den Pol, Dalton et al. 2002).
In conclusion, intranasal inoculation with VSV produced significant alterations in sleep, activity and temperature that varied with strain. In B6 mice, changes in sleep began very soon after infection and persisted after virus is presumably cleared from the brain whereas changes in sleep in BALB/c mice were more limited. Thus, VSV should provide an excellent model for examining the mechanisms linking sickness behavior and sleep.
Highlights.
Vesicular stomatitis virus produces early and persisting decreases in rapid eye movement sleep and increases in non-REM sleep during encephalitis that vary with mouse strain.
Footnotes
Conflict of Interest Statement
All authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson EC, Christensen JP, et al. Changes in cell adhesion molecule expression on T cells associated with systemic virus infection. J Immunol. 1994;152(3):1237–1245. [PubMed] [Google Scholar]
- Anisman H, Prakash P, et al. Corticotropin releasing hormone receptor alterations elicited by acute and chronic unpredictable stressor challenges in stressor-susceptible and resilient strains of mice. Behav Brain Res. 2007;181(2):180–190. doi: 10.1016/j.bbr.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Bi Z, Barna M, et al. Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. Journal of virology. 1995;69(10):6466–6472. doi: 10.1128/jvi.69.10.6466-6472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Z, Quandt P, et al. IL-12 promotes enhanced recovery from vesicular stomatitis virus infection of the central nervous system. J Immunol. 1995;155(12):5684–5689. [PubMed] [Google Scholar]
- Blank T, Nijholt I, et al. Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J Neurosci. 2003;23(2):700–707. doi: 10.1523/JNEUROSCI.23-02-00700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Human neurobiology. 1982;1(3):195–204. [PubMed] [Google Scholar]
- Borbely AA, Mattmann P, et al. Effect of benzodiazepine hypnotics on all-night sleep EEG spectra. Human neurobiology. 1985;4(3):189–194. [PubMed] [Google Scholar]
- Brown BB, Shryne JE. EEG theta activity and fast activity sleep in cats as related behavioral traits. Neuropsychologia. 1964;2:311–326. [Google Scholar]
- Bullard DC, Hu X, et al. Critical Requirement of CD11b (Mac-1) on T Cells and Accessory Cells for Development of Experimental Autoimmune Encephalomyelitis. J Immunol. 2005;175(10):6327–6333. doi: 10.4049/jimmunol.175.10.6327. [DOI] [PubMed] [Google Scholar]
- Casellas P, Galiegue S, et al. Peripheral benzodiazepine receptors and mitochondrial function. Neurochemistry international. 2002;40(6):475–486. doi: 10.1016/s0197-0186(01)00118-8. [DOI] [PubMed] [Google Scholar]
- Chen N, Restivo A, et al. Leukotrienes play protective roles early during experimental VSV encephalitis. Journal of neuroimmunology. 2001;120(1–2):94–102. doi: 10.1016/s0165-5728(01)00415-5. [DOI] [PubMed] [Google Scholar]
- Christensen JE, Andreasen SO, et al. CD11b expression as a marker to distinguish between recently activated effector CD8(+) T cells and memory cells. Int Immunol. 2001;13(4):593–600. doi: 10.1093/intimm/13.4.593. [DOI] [PubMed] [Google Scholar]
- Christian AY, Barna M, et al. Host immune response to vesicular stomatitis virus infection of the central nervous system in C57BL/6 mice. Viral immunology. 1996;9(3):195–205. doi: 10.1089/vim.1996.9.195. [DOI] [PubMed] [Google Scholar]
- Ciavarra RP, Stephens A, et al. Evaluation of immunological paradigms in a virus model: are dendritic cells critical for antiviral immunity and viral clearance? J Immunol. 2006;177(1):492–500. doi: 10.4049/jimmunol.177.1.492. [DOI] [PubMed] [Google Scholar]
- Cooke GS, Hill AV. Genetics of susceptibility to human infectious disease. Nature reviews Genetics. 2001;2(12):967–977. doi: 10.1038/35103577. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews Neuroscience. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maio A, Torres MB, et al. Genetic determinants influencing the response to injury, inflammation, and sepsis. Shock. 2005;23(1):11–17. doi: 10.1097/01.shk.0000144134.03598.c5. [DOI] [PubMed] [Google Scholar]
- Deboer T, Fontana A, et al. Tumor necrosis factor (TNF) ligand and TNF receptor deficiency affects sleep and the sleep EEG. J Neurophysiol. 2002;88(2):839–846. doi: 10.1152/jn.2002.88.2.839. [DOI] [PubMed] [Google Scholar]
- Engler H, Doenlen R, et al. Acute amygdaloid response to systemic inflammation. Brain, behavior, and immunity. 2011;25(7):1384–1392. doi: 10.1016/j.bbi.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Fang I, Tooley D, et al. Differential effects of total and upper airway influenza viral infection on sleep in mice. Sleep. 1996;19(4):337–342. [PubMed] [Google Scholar]
- Fang J, Sanborn CK, et al. Influenza viral infections enhance sleep in mice. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine. 1995;210(3):242–252. doi: 10.3181/00379727-210-43945. [DOI] [PubMed] [Google Scholar]
- Forger JM, 3rd, Bronson RT, et al. Murine infection by vesicular stomatitis virus: initial characterization of the H-2d system. Journal of virology. 1991;65(9):4950–4958. doi: 10.1128/jvi.65.9.4950-4958.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken P, Malafosse A, Tafti M. Biochemistry. Genetic variation in EEG activity during sleep in inbred mice. Am J Physiol. 1998;275:R1127–R1137. doi: 10.1152/ajpregu.1998.275.4.R1127. [DOI] [PubMed] [Google Scholar]
- Gourmelon P, Briet D, et al. Sleep alterations in experimental street rabies virus infection occur in the absence of major EEG abnormalities. Brain research. 1991;554(1–2):159–165. doi: 10.1016/0006-8993(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Gourmelon P, Briet D, et al. Electrophysiological and sleep alterations in experimental mouse rabies. Brain research. 1986;398(1):128–140. doi: 10.1016/0006-8993(86)91258-8. [DOI] [PubMed] [Google Scholar]
- Hill AV. The genomics and genetics of human infectious disease susceptibility. Annual review of genomics and human genetics. 2001;2:373–400. doi: 10.1146/annurev.genom.2.1.373. [DOI] [PubMed] [Google Scholar]
- Hode Y, Ratomponirina C, et al. Hypoexpression of benzodiazepine receptors in the amygdala of neophobic BALB/c mice compared to C57BL/6 mice. Pharmacol Biochem Behav. 2000;65(1):35–38. doi: 10.1016/s0091-3057(99)00131-8. [DOI] [PubMed] [Google Scholar]
- Huneycutt BS, Bi Z, et al. Central neuropathogenesis of vesicular stomatitis virus infection of immunodeficient mice. Journal of virology. 1993;67(11):6698–6706. doi: 10.1128/jvi.67.11.6698-6706.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huneycutt BS, I, Plakhov V, et al. Distribution of vesicular stomatitis virus proteins in the brains of BALB/c mice following intranasal inoculation: an immunohistochemical analysis. Brain research. 1994;635(1–2):81–95. doi: 10.1016/0006-8993(94)91426-5. [DOI] [PubMed] [Google Scholar]
- Huygen K, Palfliet K. Strain variation in interferon gamma production of BCG-sensitized mice challenged with PPD II. Importance of one major autosomal locus and additional sexual influences. Cell Immunol. 1984;85(1):75–81. doi: 10.1016/0008-8749(84)90279-x. [DOI] [PubMed] [Google Scholar]
- Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nature reviews Neuroscience. 2009;10(3):199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang CY, Prevec L. Proteins of vesicular stomatitis virus. I. Polyacrylamide gel analysis of viral antigens. Journal of virology. 1969;3(4):404–413. doi: 10.1128/jvi.3.4.404-413.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapas L, Krueger JM. Tumor necrosis factor-beta induces sleep, fever, and anorexia. Am J Physiol. 1992;263(3 Pt 2):R703–707. doi: 10.1152/ajpregu.1992.263.3.R703. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Takahashi S. Thermoregulation and sleep. Closely linked but separable. Annals of the New York Academy of Sciences. 1997;813:281–286. doi: 10.1111/j.1749-6632.1997.tb51706.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Wellman LL, et al. Antagonizing corticotropin-releasing factor in the central nucleus of the amygdala attenuates fear-induced reductions in sleep but not freezing. Sleep. 2011;34(11):1539–1549. doi: 10.5665/sleep.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yang L, et al. GABAergic antagonism of the central nucleus of the amygdala attenuates reductions in rapid eye movement sleep after inescapable footshock stress. Sleep. 2009;32(7):888–896. doi: 10.1093/sleep/32.7.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundh B, Love A, et al. Non-lethal infection of aminergic reticular core neurons: age-dependent spread of ts mutant vesicular stomatitis virus from the nose. Journal of neuropathology and experimental neurology. 1988;47(5):497–506. doi: 10.1097/00005072-198809000-00001. [DOI] [PubMed] [Google Scholar]
- Majde JA, Krueger JM. Links between the innate immune system and sleep. The Journal of allergy and clinical immunology. 2005;116(6):1188–1198. doi: 10.1016/j.jaci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Marvaldi JL, Lucas-Lenard J, et al. Cell killing by viruses. IV. Cell killing and protein synthesis inhibition by vesicular stomatitis virus require the same gene functions. Virology. 1977;79(2):267–280. doi: 10.1016/0042-6822(77)90354-3. [DOI] [PubMed] [Google Scholar]
- Mendelson WB. The benzodiazepine receptor and sleep. Psychiatric developments. 1984;2(3):161–177. [PubMed] [Google Scholar]
- Miyawaki T, Sogawa N, et al. Effect of midazolam on interleukin-6 mRNA expression in human peripheral blood mononuclear cells in the absence of lipopolysaccharide. Cytokine. 2001;15(6):320–327. doi: 10.1006/cyto.2001.0940. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Harter DH, et al. Neuropathological and immunofluorescence studies of experimental vesicular stomatitis virus encephalitis in mice. Journal of neuropathology and experimental neurology. 1971;30(2):266–277. doi: 10.1097/00005072-197104000-00008. [DOI] [PubMed] [Google Scholar]
- Mohammed AK, Maehlen J, et al. Persistent changes in behaviour and brain serotonin during ageing in rats subjected to infant nasal virus infection. Neurobiol Aging. 1992;13(1):83–87. doi: 10.1016/0197-4580(92)90013-n. [DOI] [PubMed] [Google Scholar]
- Mohammed AK, Magnusson O, et al. Behavioural deficits and serotonin depletion in adult rats after transient infant nasal viral infection. Neuroscience. 1990;35(2):355–363. doi: 10.1016/0306-4522(90)90089-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U, Steinhoff U, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264(5167):1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Opp MR. Cytokines and sleep. Sleep medicine reviews. 2005;9(5):355–364. doi: 10.1016/j.smrv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Pappenheimer JR, Koski G, et al. Extraction of sleep-promoting factor S from cerebrospinal fluid and from brains of sleep-deprived animals. J Neurophysiol. 1975;38(6):1299–1311. doi: 10.1152/jn.1975.38.6.1299. [DOI] [PubMed] [Google Scholar]
- Prager G, Hadamitzky M, et al. Amygdaloid Signature of Peripheral Immune Activation by Bacterial Lipopolysaccharide or Staphylococcal Enterotoxin B. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2012 doi: 10.1007/s11481-012-9373-0. [DOI] [PubMed] [Google Scholar]
- Rabinowitz SG, Dal Canto MC, et al. Comparison of central nervous system disease produced by wild-type and temperature-sensitive mutants of vesicular stomatitis virus. Infection and immunity. 1976;13(4):1242–1249. doi: 10.1128/iai.13.4.1242-1249.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss CS, I, Plakhov V, et al. Viral replication in olfactory receptor neurons and entry into the olfactory bulb and brain. Annals of the New York Academy of Sciences. 1998;855:751–761. doi: 10.1111/j.1749-6632.1998.tb10655.x. [DOI] [PubMed] [Google Scholar]
- Schwarz MJ, Chiang S, et al. T-helper-1 and T-helper-2 responses in psychiatric disorders. Brain, behavior, and immunity. 2001;15(4):340–370. doi: 10.1006/brbi.2001.0647. [DOI] [PubMed] [Google Scholar]
- Sekellick MJ, Marcus PI. Persistent infection. II. Interferon-inducing temperature-sensitive mutants as mediators of cell sparing: possible role in persistent infection by vesicular stomatitis virus. Virology. 1979;95(1):36–47. doi: 10.1016/0042-6822(79)90399-4. [DOI] [PubMed] [Google Scholar]
- Silverman MN, Pearce BD, et al. Immune modulation of the hypothalamic-pituitary-adrenal (HPA) axis during viral infection. Viral immunology. 2005;18(1):41–78. doi: 10.1089/vim.2005.18.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soilu-Hanninen M, Roytta M, et al. Semliki Forest virus infection leads to increased expression of adhesion molecules on splenic T-cells and on brain vascular endothelium. J Neurovirol. 1997;3(5):350–360. doi: 10.3109/13550289709030749. [DOI] [PubMed] [Google Scholar]
- Steel CD, Hahto SM, et al. Peripheral dendritic cells are essential for both the innate and adaptive antiviral immune responses in the central nervous system. Virology. 2009 doi: 10.1016/j.virol.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel CD, Stephens AL, et al. Comparison of the lateral tail vein and the retro-orbital venous sinus as routes of intravenous drug delivery in a transgenic mouse model. Lab Anim (NY) 2008;37(1):26–32. doi: 10.1038/laban0108-26. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCarley R. Brainstem Control of Wakefulness and Sleep. New York: Plenum Press; 1990. [Google Scholar]
- Tan X, Uchida S, et al. Benzodiazepine effects on human sleep EEG spectra: a comparison of triazolam and flunitrazepam. Life sciences. 1998;63(8):675–684. doi: 10.1016/s0024-3205(98)00318-x. [DOI] [PubMed] [Google Scholar]
- Tang X, Sanford LD. Telemetric recording of sleep and home cage activity in mice. Sleep (Abstract Supplement) 2002;25(6):691–699. [PubMed] [Google Scholar]
- Torres-Harding S, Sorenson M, et al. Evidence for T-helper 2 shift and association with illness parameters in chronic fatigue syndrome (CFS) Bulletin of the IACFS/ME. 2008;16(3):19–33. [PMC free article] [PubMed] [Google Scholar]
- Toth LA. Strain differences in the somnogenic effects of interferon inducers in mice. J Interferon Cytokine Res. 1996;16(12):1065–1072. doi: 10.1089/jir.1996.16.1065. [DOI] [PubMed] [Google Scholar]
- Toth LA, Opp MR. Cytokine- and microbially induced sleep responses of interleukin-10 deficient mice. Am J Physiol Regul Integr Comp Physiol. 2001;280(6):R1806–1814. doi: 10.1152/ajpregu.2001.280.6.R1806. [DOI] [PubMed] [Google Scholar]
- Toth LA, Rehg JE, et al. Strain differences in sleep and other pathophysiological sequelae of influenza virus infection in naive and immunized mice. J Neuroimmunol. 1995;58(1):89–99. doi: 10.1016/0165-5728(94)00193-r. [DOI] [PubMed] [Google Scholar]
- Toth LA, Tolley EA, et al. Sleep as a prognostic indicator during infectious disease in rabbits. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine. 1993;203(2):179–192. doi: 10.3181/00379727-203-43590. [DOI] [PubMed] [Google Scholar]
- Toth LA, Williams RW. A quantitative genetic analysis of slow-wave sleep in influenza-infected CXB recombinant inbred mice. Behav Genet. 1999;29(5):339–348. doi: 10.1023/a:1021661901196. [DOI] [PubMed] [Google Scholar]
- Touret M, Valatx JL, et al. The locus coeruleus: a quantitative and genetic study in mice. Brain research. 1982;250(2):353–357. doi: 10.1016/0006-8993(82)90430-9. [DOI] [PubMed] [Google Scholar]
- Trammell RA, Liberati TA, et al. Host genetic background and the innate inflammatory response of lung to influenza virus. Microbes and infection/Institut Pasteur. 2012;14(1):50–58. doi: 10.1016/j.micinf.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Trammell RA, Toth LA. Genetic susceptibility and resistance to influenza infection and disease in humans and mice. Expert review of molecular diagnostics. 2008;8(4):515–529. doi: 10.1586/14737159.8.4.515. [DOI] [PubMed] [Google Scholar]
- Tuite A, Gros P. The impact of genomics on the analysis of host resistance to infectious disease. Microbes and infection/Institut Pasteur. 2006;8(6):1647–1653. doi: 10.1016/j.micinf.2005.11.016. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Dalton KP, et al. Relative neurotropism of a recombinant rhabdovirus expressing a green fluorescent envelope glycoprotein. Journal of virology. 2002;76(3):1309–1327. doi: 10.1128/JVI.76.3.1309-1327.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH. Cerebral activity and behavior: control by central cholinergic and serotonergic systems. Int Rev Neurobiol. 1988;30:225–340. doi: 10.1016/s0074-7742(08)60050-1. [DOI] [PubMed] [Google Scholar]
- Wilms H, Claasen J, et al. Involvement of benzodiazepine receptors in neuroinflammatory and neurodegenerative diseases: evidence from activated microglial cells in vitro. Neurobiology of disease. 2003;14(3):417–424. doi: 10.1016/j.nbd.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Yasenkov R, Deboer T. Interrelations and circadian changes of electroencephalogram frequencies under baseline conditions and constant sleep pressure in the rat. Neuroscience. 2011;180:212–221. doi: 10.1016/j.neuroscience.2011.01.063. [DOI] [PubMed] [Google Scholar]