Abstract
Extinction learning underlies the treatment for a variety of anxiety disorders. Most of what is known about the neurobiology of extinction is based on standard “delay” fear conditioning, in which awareness is not required for learning. Little is known about how complex, explicit associations extinguish, however. “Trace” conditioning is considered to be a rodent model of explicit fear because it relies on both the cortex and hippocampus and requires explicit contingency awareness in humans. Here, we explore the neural circuit supporting trace fear extinction in order to better understand how complex memories extinguish. We first show that the amygdala is selectively involved in delay fear extinction; blocking intra-amygdala glutamate receptors disrupted delay, but not trace extinction. Further, ERK phosphorylation was increased in the amygdala after delay, but not trace extinction. We then identify the retrosplenial cortex (RSC) as a key structure supporting trace extinction. ERK phosphorylation was selectively increased in the RSC following trace extinction and blocking intra-RSC NMDA receptors impaired trace, but not delay extinction. These findings indicate that delay and trace extinction require different neural circuits; delay extinction requires plasticity in the amygdala whereas trace extinction requires the RSC. Anxiety disorders linked to explicit memory may therefore depend on cortical processes that have not been traditionally targeted by extinction studies based on delay fear.
Keywords: Delay fear conditioning, trace fear conditioning, ERK, extinction, NMDA, APV
1. Introduction
The ability to accurately predict and respond to danger signals is critical for an animal's survival. Failure to react in the face of a cue that signals danger can result in harm or death. Conversely, it is also maladaptive for an animal to respond in a disproportionate manner to a nonthreatening cue. In humans, excessive responding to situations and cues that are poor predictors of danger may contribute to anxiety disorders (Davis, 2002; Rothbaum & Davis, 2003).
Anxiety disorders are often treated clinically through behavioral extinction in which the individual is exposed to the threatening stimulus in the absence of an aversive outcome (Barad, 2005; Foa, 2000; Rothbaum & Schwartz, 2002; Wolpe, 1969). Affective reactions to the stimulus are gradually reduced as the person learns that the cue does not predict danger. These exposure-based therapies can be modeled in rodents through fear conditioning and extinction training as a way to understand the neural mechanisms underlying anxiety reduction (Davis, 2002; Milad & Quirk, 2012).
To date, most of the research on the neural mechanisms of extinction learning comes from rodent studies that use delay fear conditioning to model anxiety (for review, see Milad & Quirk, 2012). In delay fear conditioning, an initially neutral conditional stimulus (CS), such as a white noise or tone, is presented contiguously with a naturally aversive unconditional stimulus (UCS), such as a foot shock. Delay fear can be acquired very rapidly and, in humans, can be learned and expressed without awareness of the stimulus relationship (Clark & Squire, 1998; Knight, Nguyen, & Bandettini, 2006) making it a good model for basic, implicit fear memories. Delay fear extinction requires three critical brain structures: the infralimbic medial prefrontal cortex (IL), the hippocampus, and the amygdala (Sierra-Mercado, Padilla-Coreano, & Quirk, 2011). The hippocampus' role in extinguishing fear to a discrete auditory CS is largely restricted to controlling the context specificity of extinction (Corcoran, Desmond, Frey, & Maren, 2005; Hobin, Ji, & Maren, 2006) although more recent evidence points to a central role of the hippocampus in extinction when the most salient predictor of shock is the training context (Fischer et al., 2007; Huh et al., 2009; Radulovic & Tronson, 2010; Schimanski, Wahlsten, & Nguyen, 2002; Tronson et al., 2009; Vianna, Szapiro, McGaugh, Medina, & Izquierdo, 2001). In contrast to the hippocampus, both the IL and amygdala undergo plastic changes during the extinction of an auditory CS previously used in delay fear conditioning. This plasticity in IL and amygdala regions is believed to support the formation of a new extinction memory (Herry et al., 2010; Quirk & Mueller, 2008). Blocking neural activity or general plasticity in the IL (Burgos-Robles, Vidal-Gonzalez, Santini, & Quirk, 2007; Sierra-Mercado et al., 2011) or amygdala (Sierra-Mercado et al., 2011; Sotres-Bayon, Bush, & LeDoux, 2007) is sufficient to disrupt the formation of extinction memory, usually tested the following day.
While the neural mechanisms supporting delay fear extinction have received substantial recent attention, far less is understood about the extinction of more complex associations that may better relate to explicit memory in humans. This is important because anxiety disorders can involve both implicit and explicit associations (Brewin, 2001; Rothbaum & Davis, 2003). One way to investigate the neural basis of explicit memory extinction is to use trace fear conditioning. In trace fear conditioning, the CS and UCS are separated by an empty period of time, called the trace interval. Temporal separation of the two cues makes the association slightly more difficult to learn but significantly alters the circuitry and attentional mechanisms required for acquisition. Whereas delay fear can be acquired without awareness and relies largely on subcortical structures (particularly the amygdala), trace fear conditioning requires awareness of the CS-UCS contingency and relies on hippocampal and cortical participation for acquisition (Gilmartin & Helmstetter, 2010; Gilmartin, Kwapis, & Helmstetter, 2012; Han et al., 2003; Knight et al., 2006; Quinn, Oommen, Morrison, & Fanselow, 2002) in addition to the amygdala (Gilmartin et al., 2012; Kwapis, Jarome, Schiff, & Helmstetter, 2011). Trace conditioning shares a number of important characteristics with human declarative memory. First, as with explicit memory in humans, trace fear conditioning involves learning a relatively complex relationship between multiple stimuli. Second, explicit awareness of the CS-UCS contingency is necessary for human participants to learn trace fear (Knight et al., 2006; Weike, Schupp, & Hamm, 2007). Finally, trace fear conditioning involves structures known to participate in declarative memory, including the hippocampus and cortex (Gilmartin & Helmstetter, 2010; Gilmartin et al., 2012; Han et al., 2003; Quinn et al., 2002; Squire, 1992). Trace fear conditioning is therefore a particularly good paradigm for modeling explicit fear memory in rodents.
Despite the clear value of trace fear conditioning as a model of fear memory, few studies have investigated extinction after this training procedure (Abumaria et al., 2011; Kaczorowski, Davis, & Moyer, 2012). To date, no study has systematically investigated how the circuitry supporting trace extinction differs from the established circuit that supports delay fear extinction. Delay and trace conditioning rely on different structures for acquisition, however, so it is feasible that the circuits required for extinction are also distinct. Structures such as the PL, which is required for trace fear acquisition (Gilmartin & Helmstetter, 2010), and the retrosplenial cortex (RSC), which is involved in contextual and relational associations (Aggleton, 2010; Cooper, Manka, & Mizumori, 2001; Corcoran et al., 2011; Haijima & Ichitani, 2008; Katche, Dorman, Gonzalez, et al., 2013; Katche, Dorman, Slipczuk, Cammarota, & Medina, 2013; Keene & Bucci, 2008a, 2008b; Robinson, Keene, Iaccarino, Duan, & Bucci, 2011), for instance, may supplement or take over the roles of the amygdala, IL, and hippocampus in the extinction of trace fear. The RSC is particularly suitable for supporting explicit associations, as it plays a well-documented role in supporting autobiographical, relational, and spatial memory in humans (Maddock, 1999; Maguire, 2001; Rosenbaum, Ziegler, Winocur, Grady, & Moscovitch, 2004; Steinvorth, Corkin, & Halgren, 2006; Svoboda, McKinnon, & Levine, 2006). Whether the RSC plays a role in trace extinction, however, is unknown. Characterizing the neural circuit that underlies trace fear extinction is an important step towards a comprehensive understanding of anxiety reduction in humans.
Here, we tested whether the circuitry supporting trace fear extinction is the same or different from that of delay extinction. First, we tested whether the amygdala is necessary for trace extinction, as it is with delay. We then measured the phosphorylation of extracellular regulated kinase (pERK) in a number of candidate brain structures in order to identify regions that undergo extinction-related plasticity following trace fear extinction. One region of interest, the retrosplenial cortex, showed elevated pERK following trace, but not delay extinction, suggesting that this region is selectively involved in the extinction of trace fear. In our final study, we directly tested whether the RSC is required for trace, but not delay fear extinction. Together, our results demonstrate that trace fear extinction relies on a different neural circuit than delay extinction.
2. Materials and Methods
2.1. Subjects
Subjects were 238 male Long-Evans rats obtained from Harlan (Madison, WI) weighing approximately 350g. Rats were housed individually and allowed free access to water and rat chow. The colony room was maintained under a 14:10h light/dark cycle and all behavioral tests were conducted during the light portion of this cycle. All animals were handled for 3 days before surgery and 3 days before training. For the western blot study, all animals were handled for 6 days: 3 days of standard handling in the animal room followed by 3 days of transport to another room in the lab (in order to acclimate animals to the transportation cart) followed by handling in that room. All procedures were approved by the university Animal Care and Use Committee and were in compliance with the National Institutes of Health guidelines.
2.2 Surgery
Animals were implanted with bilateral cannulae aimed at either the basolateral nucleus of the amygdala (BLA) or the anterior retrospenial cortex (RSC) (Kwapis, Jarome, Lonergan, & Helmstetter, 2009; Paxinos & Watson, 2007). Before surgery, each rat was anesthetized with 2–4% isoflurane in oxygen and implanted with bilateral stainless steel 26-guage cannulae aimed at the basolateral nucleus of the amygdala (BLA) or dual 26-guage cannulae (1mm center-to-center) aimed at the anterior retrosplenial cortex (RSC). BLA coordinates were 3.0mm posterior, ±5.0mm lateral, 7.2mm ventral relative to bregma. RSC coordinates were 3.5mm posterior, ±0.5mm lateral, 1.8 mm ventral relative to bregma (Paxinos & Watson, 2007). Cannulae were secured to the skull with stainless steel screws, superglue, and dental cement. Following surgery, the incision site was swabbed with a lidocaine and prilocaine solution (2.5%/2.5%) to minimize discomfort during the recovery period. Stainless steel obdurators remained in the cannulae when rats were not being injected to prevent occlusion. Rats were given a recovery period of at least 7d before behavioral testing.
Following recovery from surgery, all animals were transported and handled for 3 days before behavioral testing began. During this handling period, animals were gently restrained with a towel while the infusion pump was activated in order to allow the animals to habituate to its noise. The obdurators were removed from the cannulae during this handling session and the surgical site was cleaned with a cotton swab.
2.3. Apparatus
Fear conditioning was conducted in a set of four identical chambers (Context A). The floor of Context A was composed of stainless steel rods through which footshocks were delivered. Each chamber was illuminated by an overhead 7.5-W bulb and was connected to its own shock generator-scrambler (Grason-Stadler, West Concord, MA). Ventilation fans provided constant background noise (~60dB). Chamber A was cleaned with a solution of 5% ammonium hydroxide between animals.
A second set of chambers (Context B) was used to conduct extinction to the auditory CS. Context B had a number of distinct features, including infrared illumination, a solid and opaque textured floor panel, and a different cleaning solution (5% acetic acid). All tests were conducted in Context B.
2.4. Infusions
Infusions of CNQX, APV, or vehicle were given approximately 5 minutes before extinction training. CNQX (Sigma-Aldrich, St. Louis, Missouri) was dissolved in DMSO to a final concentration of 10 μg/μl. D-APV (Sigma-Aldrich) was diluted in ACSF to a final concentration of 10 μg/μl. The doses used for CNQX and APV were chosen based on their effectiveness in past studies in our lab (Gilmartin & Helmstetter, 2010; Parsons, Gafford, & Helmstetter, 2010) and others (Falls, Miserendino, & Davis, 1992; Maren, Aharonov, Stote, & Fanselow, 1996; Matus-Amat, Higgins, Sprunger, Wright-Hardesty, & Rudy, 2007; Milton, Lee, Butler, Gardner, & Everitt, 2008). We specifically chose the D-APV isomer for this study because it is less likely to interfere with freezing expression than the mixed DL-APV isomer but is still effective in disrupting NMDAR-dependent plasticity in the amygdala (Matus-Amat et al., 2007). In both the BLA and RSC, a volume of 0.5 μl/side was infused over 60s (Kwapis, Jarome, Gilmartin, & Helmstetter, 2012). Injectors remained in place for an additional 90s after infusion to allow for diffusion.
2.5. Fear Conditioning and Extinction
Fear conditioning was conducted in Context A while fear extinction and extinction retention tests were conducted in a novel environment, Context B. All animals were trained on day 1 with strength-matched delay or trace fear conditioning. Previous work from our lab (Kwapis et al., 2011) has shown that 4 trials of delay conditioning with a variable intertrial interval (ITI) of 110s ±20s produces approximately the same level of freezing as 6 trials of trace fear conditioning with a longer ITI of 240 ±20s. For both delay and trace, the CS was a 10s white noise cue (72dB) and the UCS was a 1s footshock (1mA). For delay fear conditioning, the UCS was presented at the moment of CS offset. For trace fear conditioning, the CS and UCS were separated by an empty 20s trace interval. Both training types had a 6-min baseline and a 4-minute postshock period. On day 2, rats were injected with the appropriate drug into the BLA or RSC 5 minutes before extinction training in Context B. Extinction training consisted of 40 presentations of the white noise CS (30s; 72dB; 60s ITI) in the absence of footshock. While the primary purpose of the day 2 extinction session was to extinguish the animals' CS freezing, it also allowed us to test the animals' ability to retrieve and express fear to the CS in the presence of the drug. To this end, an average of the first 8 or 12 extinction trials on day 2 was analyzed to test whether the drug infusion impaired memory retrieval or expression. On day 3, the rats were tested with 8 CS presentations (30s; 72dB; 60s ITI) in Context B. The extinction and test parameters were chosen based on their effectiveness in previous studies (e.g. Parsons et al., 2010). Further, pilot research testing these parameters has confirmed that rats successfully extinguish fear to the CS even when the extinction and test sessions use a longer CS presentation than the CS used during training (data not shown).
For Experiment 3, training was conducted on day 1 as described above. On day 2, animals were left in their homecages (HC), given a 4-trial retrieval session (4T) with 4 unreinforced CS presentations (30s, 72dB, 60sITI), or given the full 40-trial extinction session described above (EXT). Animals were either tested the following day (as described above) or were sacrificed 30 minutes after the end of the session and tissue was processed for western blots or immunofluorescence as described below. We consider the 4T session to be “retrieval” and the 40-trial session to be “extinction” based on behavioral evidence obtained in this study (see Fig. S4) and other studies (Parsons et al., 2010) showing that these protocols produce appropriate behavior consistent with either retrieval or extinction learning. Specifically, in this preparation, 4 CS presentations do not produce reduced CS freezing whereas 40 trials are sufficient to cause reduced freezing characteristic of extinction.
2.6. Histology
To confirm cannulae placements, histology was performed as described previously (Kwapis et al., 2012; Kwapis et al., 2011). Briefly, following behavioral testing, animals were killed with an overdose of isoflurane and transcardially perfused with saline followed by 10% buffered formalin. Heads were submerged in buffered formalin for at least 24h before the brains were removed and soaked in 30% sucrose formalin for cryoprotection. Frozen 40 μm sections from the BLA (experiments 1 and 2) or RSC (experiment 4) were mounted on slides, stained with cresyl violet, and the cannulae placements were confirmed with the help of a rat brain atlas (Paxinos & Watson, 2007). Animals with injection sites outside the appropriate structure were excluded from the analyses.
2.7. Western Blotting
Following overdose with isoflurane, rats were decapitated and brains were immediately frozen (−80°C). Western blots were then conducted as previously described (Jarome, Werner, Kwapis, & Helmstetter, 2011). Brains were dissected by blocking the brain in a rat brain matrix (Harvard Apparatus, Holliston, MA) and the amygdala, dorsal hippocampus, prelimbic medial prefrontal cortex, infralimbic medial prefrontal cortex, and anterior retrosplenial cortex were removed. For the prelimbic and infralimbic cortices, a coronal cut was made at the anterior portion of the mPFC and one at the posterior portion of the mPFC. A cut was then made at the border of the PL and IL to separate the two structures and perpendicular cuts were made along the lateral borders of the PL and IL regions. All tissue above the IL was collected as PL tissue (including the entire PL and some anterior cingulate cortex above it) and only tissue in the IL region was collected as IL tissue. For the amygdala, dorsal hippocampus, and retrosplenial cortex, all tissue was collected from the same slice. A coronal cut was made at anterior tip of the dorsal hippocampus and amygdala and another cut was made at the posterior end of the amygdala. For the amygdala, a cut was made along the external capsule and a diagonal cut was made along the optic tract. For the retrospenial cortex, two vertical cuts were made on each of the lateral borders of the RSC directly above the peaks of the hippocampus. The ventral portion of RSC was separated from the dorsal hippocampus with a single horizontal cut. For the dorsal hippocampus, a scalpel blade was used to gently separate the dorsal hippocampus from its surrounding tissue. Each tissue sample was homogenized in buffer (all in 100ml DDH2O; 0.605g Tris-HCL; 0.25g sodium deoxycholate; 0.876g NaCl; 0.038g EDTA; 0.0042g NaF; 1μg/ml PMSF; 1μg/ml leupeptin; 1μg/ml aprotinin; 10ml 10% SDS, 1 Mm sodium orthovanadate) and immediately placed on dry ice. Samples were stored at −80°C until needed. Samples were thawed and centrifuged at 4000 rpm for 20 min at 4°C. The supernatant was removed and measured using a Bradford protein assay kit (Bio-Rad, Hercules, CA). Protein samples were normalized and loaded on a 7.5% SDS/PAGE gel. Proteins were transferred from the gel to a membrane using a Turbo Transfer system (Bio-Rad). Membranes were incubated in blocking buffer for 1h and then overnight at 4°C in primary antibody (1:500) against phospho-ERK (Cell Signaling Technology, Danvers, MA, USA) or total-ERK (1:500; Cell Signaling). Following primary antibody exposure, membranes were incubated in goat anti-rabbit secondary antibody (1:10,000) (Millipore, Billerica, MA, USA) for 60min at room temperature. Membranes were washed three times, placed in a chemiluminescence solution for 5 min (SuperSignal West Dura; Thermo Scientific, Rockford, IL) and imaged with a camera-based G:Box Chemi system (Syngene, Frederick, MD). Desitometry was performed with the GeneTools analysis software (Syngene).
2.8. Immunofluorescence
Following an overdose of isoflurane, rats were perfused transcardially with 0.1M PBS followed by 10% buffered formaldehyde before being decapitated. Brains were immediately removed and soaked in 10% buffered formaldehyde overnight. The following day, the brains were moved to 30% sucrose formalin for cryoprotection until they sunk. The brains were then embedded in OCT medium, rapidly frozen in isopentane and stored at −80°C until slicing. 50μm slices were collected throughout the amygdala and retrosplenial cortex and were floated in 0.1M PBS in 24-well plates. The slices were incubated in 1% sodium borohydride for 15 min, washed twice in PBS for 10 min each, incubated for 30 min in 10% normal goat serum, followed by primary antibody for pERK (1:500, Cell Signaling) and NeuN (1:500; Millipore) overnight at 4°C. The following day, the slices were washed twice in 0.1M PBS (10 minutes each) before being incubated in secondary antibody solution with both anti-rabbit Alexa 594 and anti-mouse Alexa 488 antibodies (1:500 dilution each, Invitrogen) for 2h in the dark. Slices were then rinsed with 0.1M PBS twice and mounted onto unsubbed slides using Ultra Cruz mounting medium (Santa Cruz). Finally, the slides were coverslipped and the edges were sealed with nail polish. The slides were stored at 4°C.
The slices were imaged using a fluorescence microscope (Nikon Eclipse) running NIS-Elements software. NeuN images were used to anatomically match the amygdala and RSC slices. Images were collected using identical exposure parameters and background adjustments were conducted individually to produce equivalent background luminescence to allow punctate pERK staining to be compared between slices.
2.9. Statistical analysis
For behavioral experiments, the average percent time spent freezing was calculated using the FreezeScan 1.0 software (Clever Sys Inc., Reston, VA). The computer scoring parameters were chosen to closely match handscoring methods used previously in our lab to measure freezing behavior. Acquisition data were analyzed on a minute-by-minute basis. For the extinction and test sessions, the data were handscored by a trained observer to correct for resting behavior; rats would commonly lie down during the long extinction training and subsequent test session, which would be automatically scored as freezing behavior by the computer. For both the extinction and test sessions, the percent time spent freezing during the 30s discrete CS presentation was calculated separately from the percent time spent freezing during the 60s ITI periods. T-tests were used to identify drug effects for the average of the first 8 or 12 trials of the extinction session, the average of the first 6 or all 8 trials of the test session, or the corresponding average ITI freezing.
For the western blot experiment, pERK densitometry measurements obtained using the GeneTools software were normalized to total ERK expression and expressed as a percentage of the HC control for each training type. Individual blots were only excluded from analysis if the densitometry measurement was greater than 2 standard deviations from the group mean or if the image was improperly developed. Data were analyzed using ANOVA and planned comparisons where appropriate.
3. Results
3.1. Experiment 1: Amygdala activity is required for delay, but not trace fear extinction
We first tested whether amygdala activity is necessary for the extinction of delay and trace fear. To this end, we infused the AMPA receptor (AMPAR) antagonist CNQX into the amygdala before delay or trace extinction training (Fig. 1A). Blocking AMPARs with CNQX prevents normal postsynaptic depolarization, effectively shutting down excitatory neural transmission (Day, Langston, & Morris, 2003; Izquierdo et al., 1993; Kim, Campeau, Falls, & Davis, 1993; Malenka & Nicoll, 1999). Previous work has demonstrated that a local microinfusion of CNQX quickly and effectively shuts down fast synaptic transmission (Day et al., 2003). On day 1, animals were trained with strength-matched delay or trace fear conditioning to produce approximately equivalent levels of freezing (Kwapis et al., 2011). All animals showed normal acquisition (Fig. S1A). No group differences were observed in the levels of postshock freezing for either delay [t(9) = 0.847, p = 0.419] or trace [t(9) = 0.560, p = 0.589] animals.
Figure 1.
Infusion of the AMPAR antagonist CNQX into the amygdala disrupts freezing expression for both delay and trace fear conditioning during the extinction session. (A) Experimental timeline. (B) Location of injector placements in the amygdala. Figures adapted from (Paxinos & Watson, 2007), copyright 2007. (C) Freezing during the extinction session (Day 2). Both Delay (n=6) and Trace (n=6) rats given CNQX showed impaired freezing during the CS (top) and ITI (bottom) periods of the extinction session compared to vehicle animals (DFC n=6, TFC n=5). Data are expressed as an average of the first 12 trials. *p<0.05. DFC, delay fear conditioning; TFC, trace fear conditioning; AMY, amygdala; CS, conditional stimulus; ITI, intertrial interval.
On day 2, animals were given intra-amygdala infusions of either CNQX or vehicle (DMSO) approximately 5 minutes before extinction training in context B (see Figure 1B for infusion sites). The first 12 trials of the extinction session were analyzed in order to determine whether retrieval or expression of the fear memory was affected by this infusion. Both delay and trace CNQX animals showed impaired freezing expression at the beginning of the extinction session (Fig. 1C; see Figure S2A for full time course). Delay animals given CNQX showed significantly less freezing than delay vehicle animals during the first 12 CS [t(9) = 2.994, p = 0.015] and ITI [t(9) = 3.083, p = 0.013] presentations (Fig. 1C). Similarly, trace CNQX animals showed impaired freezing relative to vehicle controls in both the first 12 CS [t(9) = 2.315, p = 0.046] and ITI [t(9) = 2.560, p = 0.031] presentations (Fig. 1C). This CNQX-induced freezing impairment is consistent with previous data demonstrating that amygdala activity is required for normal freezing expression (Helmstetter, 1992; Helmstetter & Bellgowan, 1994; Maren & Holt, 2004; Muller, Corodimas, Fridel, & LeDoux, 1997; Sierra-Mercado et al., 2011) and suggests that our infusion effectively disrupted amygdala activity during the extinction training session.
On day 3, animals were given a drug-free extinction retention test (Fig. 2) to assess their memory for the extinction training they received in the presence of CNQX or vehicle the previous day. We found that intra-amygdala CNQX impaired extinction memory retention for delay, but not trace fear. No differences in freezing were observed during the pre-CS baseline period of the test session for either delay [t(9) = −0.852, p = 0.416] or trace [t(9) = −0.919, p = 0.382] animals (see Fig. S3A). For the delay group, an average of all 8 trials revealed significantly higher freezing in CNQX animals relative to vehicle controls during the ITI periods [t(9) = 2.421, p = 0.039], with a strong trend for this effect during the CS periods [t(9) = 2.079, p = 0.067] (Fig. 2). When only the first 6 trials were considered, CNQX animals showed significantly more freezing than vehicle animals during both the CS [t(9) = 2.332, p = 0.045] and ITI [t(9) = 2.900, p = 0.018] periods. This finding suggests that amygdala activity is necessary for delay fear extinction. Surprisingly, we observed no effect of CNQX on trace fear extinction. There was no effect for drug during either the CS [8 trials: t(9) = 1.068, p = 0.313; 6 trials: t(9) = 1.585, p = 0.147] or ITI [8 trials: t(9) = 1.861, p = 0.096; 6 trials: t(9) = 1.863, p = 0.095] periods of the test session for trace animals, indicating that trace fear extinction was learned normally despite amygdala inactivity the previous day during the extinction training session. Together, our results confirm that amygdala activity is necessary for normal delay fear extinction, but also suggest that trace extinction may not require AMPAR-mediated amygdala activity.
Figure 2.
Infusion of CNQX into the amygdala during extinction training disrupts extinction retention the following day for delay, but not trace fear conditioning. Freezing during the extinction retention test (Day 3) is shown. Delay animals given CNQX during extinction learning show impaired extinction retention during both the CS (top) and ITI (bottom) periods of the test session. CNQX did not affect extinction for Trace animals. *p<0.05, #p = 0.067. CS, conditional stimulus; ITI, intertrial interval
3.2. Experiment 2: Plasticity in the amygdala is also required for delay, but not trace fear extinction
Our first experiment demonstrated that amygdala activity was only necessary for delay fear extinction; trace extinction was learned normally despite amygdala inactivation. In our second experiment, we tested whether plasticity in the amygdala is required for delay and trace fear extinction. To this end, we used APV to block NMDA receptor (NMDAR) activity during extinction (Fig. 3A). During learning, NMDAR activation allows calcium into the postsynaptic cell, triggering a number of molecular cascades that ultimately result in plasticity that strengthens the synaptic connections supporting the association. Blocking NMDAR activity with a local infusion of APV prevents the induction of long-term potentiation (a form of synaptic plasticity believed to underlie memory formation) without affecting normal synaptic transmission (Day et al., 2003). Blocking NMDAR-dependent plasticity in the amygdala before extinction is known to disrupt extinction retention for delay fear conditioning (Falls et al., 1992; Lee & Kim, 1998; Santini, Muller, & Quirk, 2001; Sotres-Bayon et al., 2007), but it is unknown whether NMDAR-dependent plasticity in the amygdala is necessary for trace fear extinction. If the amygdala is not necessary for trace extinction, NMDAR blockade in the amygdala during extinction should affect delay extinction retention without preventing normal trace fear extinction.
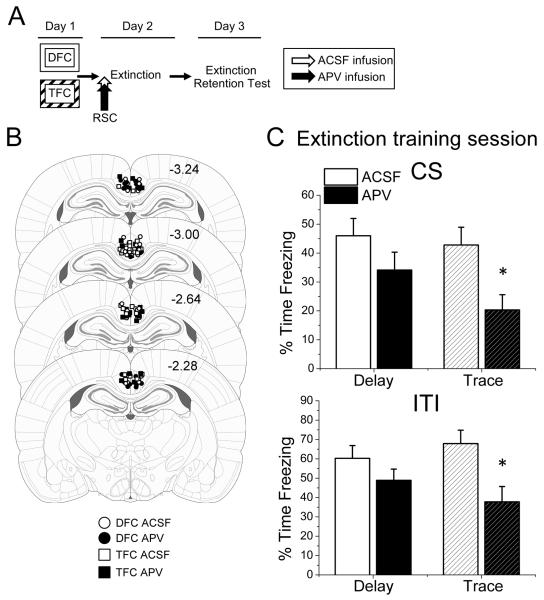
Figure 3.
Infusion of the NMDAR antagonist APV into the amygdala does not disrupt freezing expression for either delay or trace animals. (A) Experimental timeline. (B) Location of injector placements in the amygdala. Figures adapted from (Paxinos & Watson, 2007), copyright 2007. (C) Freezing during the extinction session (Day 2). Neither Delay (n=7) nor Trace (n=7) rats given APV showed significant impairments in freezing during either the CS (top) or ITI (bottom) periods of the extinction session compared to vehicle controls (DFC n=8, TFC n=7). Data are expressed as an average of the first 8 trials. DFC, delay fear conditioning; TFC, trace fear conditioning; AMY, amygdala; CS, conditional stimulus; ITI, intertrial interval.
Here, we infused APV into the amygdala before delay or trace extinction training (Fig. 3B). On day 1, animals were trained with strength-matched delay or trace fear conditioning. All animals showed normal acquisition (Fig. S1B) with no differences observed between groups during the postshock period for either delay [t(12) = 0.111, p = 0.914] or trace [t(13) = 1.154, p = 0.269] animals.
On day 2, animals were given intra-amygdala infusions of APV or vehicle (ACSF) approximately 5 minutes before extinction training in context B (Fig. 3A). We first analyzed the first 8 trials of the extinction session in order to determine whether the retrieval or expression of the fear memory was affected by this infusion. All animals showed normal levels of freezing at the beginning of the extinction session, with APV dampening freezing levels slightly, though there was no statistical difference between APV- and ACSF-infused animals during the CS [Delay: t(12) = 0.498, p = 0.627; Trace: t(13) = 1.710, p = 0.111] or ITI [Delay: t(12) = 1.791, p = 0.099; Trace: t(13) = 1.575, p = 0.139] periods (Figures 3C and S2B). This demonstrates that APV, unlike CNQX, did not impair freezing behavior at the start of extinction.
The following day, animals were given a drug-free extinction retention test (Fig. 4). No differences in freezing were observed during the pre-CS baseline period of the test session for either delay [t(12) = −0.123, p = 0.904] or trace [t(13) = 1.268, p = 0.227] animals (see Fig. S3B). During the CS, however, intra-amygdala infusion of APV impaired extinction memory retention in delay, but not trace animals. Specifically, we observed significantly higher freezing for delay APV animals compared to delay vehicle animals during both the CS [t(12) = 6.111, p < 0.001] and ITI [t(12) = 4.092, p = 0.001] periods of the retention test (Fig. 4). Trace animals, on the other hand, showed good extinction regardless of whether they were administered APV or vehicle before extinction training; no effect of drug was observed during either the CS [t(13) = 1.217, p = 0.245] or ITI [t(13) = 1.935, p = 0.075] periods of the test (Fig. 4). These results demonstrate that NMDAR-dependent plasticity in the amygdala is necessary for delay, but not trace fear extinction. Together with Experiment 1, our research suggests that the amygdala plays a crucial role in the extinction of delay fear associations but trace fear extinction may occur without amygdala activity (Experiment 1) or plasticity (Experiment 2).
Figure 4.
Infusion of APV into the amygdala during extinction training disrupts extinction retention the following day for delay, but not trace fear conditioning. Freezing during the extinction retention test (Day 3) is shown. Delay animals given APV during extinction learning show impaired extinction retention during both the CS (top) and ITI (bottom) periods of the test session. APV did not affect extinction for Trace animals. *p<0.05. CS, conditional stimulus; ITI, intertrial interval.
3.3. Experiment 3: ERK phosphorylation is increased in the amygdala after delay extinction and in the retrosplenial cortex after trace extinction
Our results show that trace extinction was learned normally even when activity (Experiment 1) or plasticity (Experiment 2) was blocked in the amygdala, suggesting that other structures mediate trace fear extinction. In order to identify brain structures important for the extinction of trace conditioning, we performed a western blot study to measure the phosphorylation of ERK/MAPK (pERK) in a number of candidate structures following delay or trace extinction. We chose pERK as our marker of extinction-related plasticity based on a wealth of studies demonstrating involvement of pERK in a number of learning paradigms, including auditory fear conditioning (Berman, Hazvi, Rosenblum, Seger, & Dudai, 1998; Blum, Moore, Adams, & Dash, 1999; Duvarci, Nader, & LeDoux, 2005; Kelly, Laroche, & Davis, 2003; Schafe et al., 2000; Zhang, Okutani, Inoue, & Kaba, 2003) and delay fear retrieval (Duvarci et al., 2005; Parsons et al., 2010). Importantly, ERK phosphorylation has been shown to be upregulated in both the amygdala and mPFC following delay fear extinction training (Herry, Trifilieff, Micheau, Luthi, & Mons, 2006; Hugues, Deschaux, & Garcia, 2004; Kim, Hamlin, & Richardson, 2009; Parsons et al., 2010). Increased ERK phosphorylation, therefore, is consistent with a structure undergoing plastic changes characteristic of extinction learning.
On day 1 animals were trained with strength-matched delay or trace fear conditioning. On day 2, animals were not extinguished (NE), were given a 4-trial retrieval session (4T), or were given the full 40-trial extinction session (EXT). Thirty minutes later, half of the animals were killed for western blots and half of the animals were given an extinction retention test on day 3 (Figures 5A and S4A). We first demonstrated that our protocols (NE, 4T, and EXT) were effective in producing the appropriate behavior (Fig. S4B–C). Planned comparisons demonstrated that the animals given the 40-trial extinction session showed significantly reduced freezing at test relative to the NE and 4T groups for both delay [p = 0.015] and trace [p = 0.001] animals (Fig. S4C). This indicates that 40T animals successfully acquired extinction. Further, we observed that the 4T group was not significantly different than the NE group during the test session for either delay [p = 0.172] or trace [p = 0.443] animals, indicating that the 4-trial retrieval procedure did not produce extinction. Importantly, the animals showed no difference in freezing levels during the baseline period for the extinction ([Delay: t(43) = −0.316, p = 0.754; Trace: t(44) = −0.583, p = 0.563]; Fig. S4C) or test ([Delay: F(2,30) = 0.161, p = 0.852; Trace: F(2,30) = 1.730, p = 0.195]; Fig. S3C) sessions. Further, the 4T and 40T groups showed equivalent freezing during the first 4 CS presentations of the extinction session [Delay: t(43) = −0.505, p = 0.616; Trace: t(44) = −1.769, p = 0.084], indicating that they showed similar fear levels at the beginning of the day 2 session. Together, these results indicate that our NE, 4T and 40T protocols produced the desired behavior.
Figure 5.
ERK phosphorylation is selectively increased in the amygdala following delay extinction and in the retrosplenial cortex following trace extinction. (A) Experimental design. Animals were sacrificed 30 minutes after extinction or retrieval on Day 2 (n=9–10 per group.) (B) ERK phosphorylation is upregulated in the amygdala following retrieval (4T) and extinction (EXT) of delay fear conditioning, but no change in ERK phosphorylation was observed following trace retrieval or extinction. (C) In the infralimbic cortex, ERK phosphorylation was upregulated following both delay and trace extinction. (D) No changes in ERK phosphorylation were observed in the dorsal hippocampus. (E) ERK phosphorylation in the prelimbic cortex was slightly increased following trace fear extinction, although this effect was not significant. No changes were observed in delay animals. (F) ERK phosphorylation was significantly increased in the retrosplenial cortex following trace fear extinction, but no change was observed following delay extinction. *p < 0.05, #p = 0.054, ^p = 0.533, not significant. DFC, delay fear conditioning; TFC, trace fear conditioning; pERK, phosphorylated ERK; totERK, total ERK.
Once we confirmed that only the EXT protocol produced extinction, we ran western blots on tissue samples from the amygdala, dorsal hippocampus (DH), infralimbic medial prefrontal cortex (IL), prelimbic medial prefrontal cortex (PL), and retrosplenial cortex (RSC) and blotted for pERK and total ERK expression. In the amygdala, ERK phosphorylation was increased following delay, but not trace fear extinction (Fig. 5B). For delay extinction, planned comparisons demonstrated that ERK phosphorylation was increased for the 4T and EXT groups relative to the NE control group [p = 0.023]. For trace extinction, no significant differences were observed between groups and a planned comparison between the NE control and the 4T and EXT groups revealed no significant difference in ERK phosphorylation levels [p = 0.932]. We confirmed these results with immunofluorescence, which qualitatively demonstrated more punctate staining in the lateral nucleus of the amygdala following 4T and EXT for delay animals only (Fig. S5). These results are consistent with the idea that the amygdala undergoes plasticity to support delay, but not trace fear extinction.
In the IL, a structure known to support extinction consolidation (Burgos-Robles et al., 2007; Hugues et al., 2004; Quirk & Mueller, 2008; Quirk, Russo, Barron, & Lebron, 2000; Sierra-Mercado et al., 2011; Sotres-Bayon et al., 2007), we observed increases in ERK phosphorylation following both delay and trace extinction (Fig. 5C). For delay animals, phosphorylated ERK levels were significantly higher in the EXT group than the NE controls [p = 0.031]. Similarly, for trace animals, EXT animals showed a strong trend towards more ERK phosphorylation compared to NE control animals [p = 0.054]. This suggests that plasticity in the IL normally occurs following both delay and trace fear extinction and is consistent with the known role of the IL in inhibiting fear-related output in the amygdala during delay extinction (Burgos-Robles et al., 2007; Herry et al., 2010; Quirk & Mueller, 2008; Sierra-Mercado et al., 2011).
We also measured ERK phosphorylation in the dorsal hippocampus (DH). The DH is important for controlling the context specificity of extinction for a delay CS (Corcoran et al., 2005; Hobin et al., 2006), for the extinction of context fear (Fischer et al., 2007; Huh et al., 2009; Radulovic & Tronson, 2010; Schimanski et al., 2002; Tronson et al., 2009; Vianna et al., 2001), and also for the acquisition of trace fear conditioning (Gilmartin & McEchron, 2005a; McAlonan, Wilkinson, Robbins, & Everitt, 1995; McEchron, Bouwmeester, Tseng, Weiss, & Disterhoft, 1998; McEchron, Tseng, & Disterhoft, 2000; Misane et al., 2005; Quinn, Loya, Ma, & Fanselow, 2005; Quinn et al., 2002; Tsaltas, Preston, & Gray, 1983). The DH may therefore be involved in trace extinction learning. Surprisingly, we observed no changes in ERK phosphorylation in the DH following either delay or trace fear extinction (Fig. 5D). Planned comparisons demonstrated that there was no significant difference between delay EXT and NE animals [p = 0.874], nor was there a difference between trace EXT and NE animals [p = 0.509]. Thus, the DH did not appear to undergo ERK-related plastic changes following either delay or trace extinction.
The PL has been implicated in both the acquisition (Baeg et al., 2001; Gilmartin & Helmstetter, 2010; Gilmartin et al., 2012; Gilmartin & McEchron, 2005b) and storage (Quinn, Ma, Tinsley, Koch, & Fanselow, 2008; Runyan, Moore, & Dash, 2004) of trace fear associations. We therefore hypothesized that the PL would play a key role in trace extinction, as well. In the PL, we observed a slight, nonsignificant increase in ERK phosphorylation following trace, but not delay fear extinction (Fig. 5E). For delay animals, we observed no increases in pERK following retrieval or extinction [p = 0.533]. On the other hand, for trace animals, we observed a slight increase in ERK phosphorylation following extinction [p = 0.123]. Although this upregulation of pERK was not significant, this pattern is consistent with the idea that the PL may play a role in trace fear extinction.
We also measured ERK phosphorylation in the retrosplenial cortex following delay and trace extinction. The involvement of the RSC in complex, explicit, spatial, and relational associations in both humans and rodents (Aggleton, 2010; Cooper et al., 2001; Corcoran et al., 2011; Haijima & Ichitani, 2008; Katche, Dorman, Gonzalez, et al., 2013; Katche, Dorman, Slipczuk, et al., 2013; Keene & Bucci, 2008a, 2008b; Maddock, 1999; Maguire, 2001; Robinson et al., 2011; Rosenbaum et al., 2004; Steinvorth et al., 2006; Svoboda et al., 2006) makes it a good candidate for participating in trace fear extinction. Consistent with this, we observed upregulated ERK phosphorylation in the RSC following trace, but not delay fear extinction (Fig. 5F). For delay animals, there was no significant difference in ERK phosphorylation between NE and EXT animals [p = 0.931]. For the trace group, EXT animals had significantly higher ERK phosphorylation levels than NE animals [p = 0.028]. Immunofluorescence demonstrated this same pattern; we observed little evidence of punctate staining in the RSC except in the trace extinction group, which showed a large upregulation of pERK-expressing cells in the RSC (Fig. S6). This indicates that the RSC undergoes ERK-related plasticity following trace, but not delay fear extinction.
Our western blot study demonstrated three key points. First, as with Experiments 1 and 2, it suggested that plasticity in the amygdala is important for the extinction of delay, but not trace fear conditioning. Secondly, we observed increases in ERK phosphorylation in the IL following both delay and trace fear extinction, suggesting that the IL plays a key role in extinction learning regardless of the specific type of conditioning. Finally, we identified the RSC as a structure that undergoes plasticity following trace, but not delay extinction. The RSC, therefore, may specifically participate in trace fear extinction.
3.4. Experiment 4: Plasticity in the retrosplenial cortex is required for trace, but not delay fear extinction
Our western blot study identified the RSC as a structure that may be involved in the extinction of trace, but not delay fear. To test whether plasticity in the RSC is necessary for delay or trace extinction, we infused the NMDAR antagonist APV into the anterior RSC before delay or trace extinction training (Figure 6A). On day 1, animals were trained with strength-matched delay or trace fear conditioning. All animals showed normal acquisition (Fig. S1C) and no differences were observed between groups during the postshock period for either delay [t(25) = 0.458, p = 0.651] or trace [t(23) = 0.002, p = 0.998] animals.
Figure 6.
Infusion of the NMDAR antagonist APV into the retrosplenial cortex disrupts trace (but not delay) memory retrieval during the extinction session. (A) Experimental timeline. (B) Location of injector placements in the anterior retrosplenial cortex. Figures adapted from (Paxinos & Watson, 2007), copyright 2007. (C) Freezing during the extinction session (Day 2). Trace animals given APV (n=12) show significant impairments in freezing relative to ACSF controls (n=13) during both the CS (top) and ITI (bottom) periods of the extinction session. Delay animals given APV (n=13) showed no drug impairments during either period relative to vehicle delay animals (n=14). Data are expressed as an average of the first 12 trials. *p < 0.05. DFC, delay fear conditioning; TFC, trace fear conditioning; RSC, retrosplenial cortex; CS, conditional stimulus; ITI, intertrial interval.
On day 2, animals were infused into the anterior RSC with APV or vehicle (ACSF) before extinction (Fig. 6B). The first 12 trials of the extinction session were analyzed in order to determine whether the retrieval or expression of the fear memory was affected by this infusion (Fig. 6C). Delay animals showed normal levels of freezing at the beginning of the extinction session; APV animals and ACSF animals froze at similar levels during both the first 12 CS [t(25) = 1.372, p = 0.181] and ITI [t(25) = 1.291, p = 0.209] periods. The trace animals, however, showed significantly reduced freezing during both the first 12 CS [t(23) = 2.752, p = 0.011] and ITI [t(23) = 2.867, p = 0.009] periods of the extinction session (Figures 6C & S2C). Thus, blocking NMDA receptors in the RSC only disrupted freezing for the trace animals. As delay animals were able to express normal levels of freezing in the presence of the same manipulation, this finding suggests that inhibiting NMDA receptors in the RSC does not generally impair freezing behavior but may more selectively block the retrieval of trace fear memory.
On day 3, animals were given a drug-free extinction retention test (Figure 7). APV infusion into the RSC impaired trace extinction memory without affecting extinction memory retention in the delay group. No differences in freezing were observed during the pre-CS baseline period of the test session for either delay [t(25) = 0.776, p = 0.445] or trace [t(23) = −0.685, p = 0.500] animals (see Fig. S3D). For delay animals, no differences in freezing levels were observed during either the CS [t(25) = 0.554, p = 0.585] or ITI [t(25) = 0.244, p = 0.809] periods of the test session. For the trace group, however, APV-infused animals showed significantly higher freezing during both the CS [t(23) = 2.059, p = 0.051] and ITI [t(23) = 2.464, p = 0.022] periods than vehicle animals. These results demonstrate that NMDARs in the RSC are important for both the retrieval of trace fear memory (Fig. 6C) and the extinction of trace fear (Fig. 7). The RSC does not appear to play a key role in either the retrieval or the extinction of delay fear associations, however.
Figure 7.
Infusion of APV into the RSC during extinction training disrupts extinction retention the following day for trace, but not delay fear conditioning. Freezing during the extinction retention test (Day 3) is shown. Trace animals given APV during extinction learning showed impaired extinction retention during both the CS (top) and ITI (bottom) periods of the test session. APV did not affect extinction for Delay animals. *p < 0.05. CS, conditional stimulus; ITI, intertrial interval
4. Discussion
The current study demonstrates that the amygdala and RSC play critical and dissociable roles in the extinction of two different forms of fear memory. Delay fear extinction appears to require the amygdala while trace fear extinction requires the RSC. In our first two experiments, we demonstrated that blocking either AMPAR-dependent neural activity or NMDAR-dependent plasticity in the amygdala was sufficient to disrupt delay, but not trace fear extinction. Next, in order to identify structures that specifically support trace extinction learning, we ran a series of western blots using ERK phosphorylation as a general marker of extinction-related plasticity. Consistent with the results of Experiments 1 and 2, we observed upregulation of ERK phosphorylation in the amygdala following delay, but not trace fear extinction. The RSC showed the opposite pattern, however; in the RSC, we observed increased ERK phosphorylation following trace, but not delay fear extinction. This finding indicates that the RSC may be an important structure in the extinction of trace, but not delay fear memories. To test this, in our final study, we inhibited NMDAR-dependent plasticity before delay or trace fear extinction and demonstrated that only trace extinction was affected. Together, our results demonstrate that the amygdala is crucial for delay, but not trace fear extinction and for the first time identify a novel role for the RSC in the extinction of trace, but not delay associations.
The main goal of this study was to identify the neural circuit supporting trace fear extinction in order to better understand fear reduction in humans in response to explicit cues. Trace fear conditioning can be considered a rodent model for explicit fear because it is both cortex- and hippocampus-dependent and requires explicit contingency awareness in humans. It is therefore important to characterize how the neural circuit supporting trace fear extinction differs from that of standard delay fear extinction. Our results suggest that trace fear relies on cortical, rather than subcortical structures for extinction. While delay fear associations are extinguished through the coordinated actions of the amygdala, IL, and hippocampus, trace extinction requires the retrosplenial cortex, rather than amygdala participation. Delay and trace fear extinction therefore have distinct neural circuits, with trace extinction primarily requiring the participation of cortical regions, particularly the RSC.
One interesting finding was that retrieval of the trace fear memory (assessed by averaging the first 12 trials of the day 2 extinction training session) was impaired when APV was infused into the RSC while delay memory retrieval was not affected. (Fig. 6C). As RSC infusions did not affect freezing in delay animals, it seems that intra-RSC NMDA receptor blockade does not generally impair the animals' ability to freeze. This suggests that inhibiting NMDARs in the RSC selectively prevented trace memory retrieval. This finding is similar to the results of previous studies in which RSC manipulations (either lesions or NMDA receptor blockade) in animals trained with delay fear conditioning were shown to impair context memory retrieval without affecting fear to the discrete delay CS cue (Corcoran et al., 2011; Keene & Bucci, 2008a, 2008b). In particular, Keene & Bucci (2008a) demonstrated that lesions of the RSC one day after delay fear conditioning impaired freezing to the context without affecting fear to the auditory delay CS. It therefore appears that retrieving fear to the discrete CS in trace conditioning relies on cortical structures that also participate in context fear retrieval. Delay fear, on the other hand, does not seem to require this complex circuit for retrieval. Together, these studies suggest that NMDARs in the RSC are required for the retrieval of complex contextual and trace fear associations but are not required for retrieval of CS fear for delay fear conditioning.
As retrieval of trace fear was impaired when APV was infused into the RSC, one might argue that the animals were unable to learn fear extinction because they were unable to express freezing during the extinction session. This does not seem to be the case, however, as our first experiment demonstrated that trace animals with a near-complete blockade of freezing during the extinction session were still able to learn extinction normally (Fig. 1C, Fig. 2). Animals can therefore acquire extinction even when they are unable to express freezing during the extinction training session. Therefore, our finding that intra-RSC NMDA receptor blockade impairs extinction in trace animals cannot simply be explained as the result of impaired freezing expression for these animals during extinction.
In our final experiment, we observed that intra-RSC APV was sufficient to impair extinction in trace animals despite a comparatively small effect size. One important note is that our extinction was relatively weak in this experiment compared to experiments 1 and 2, even though identical training and extinction procedures were used throughout. One possible reason for this reduced extinction is the cannulation procedure itself; cannulation in the anterior RSC necessarily damaged some dorsal retrosplenial tissue, which may have reduced extinction learning. In order to control for nonspecific effects of cannulation, we compared APV animals to vehicle control animals that experienced the same tissue damage. As we observed a significant difference in freezing levels between the APV and vehicle animals for the trace group, we can conclude that intra-RSC APV impaired extinction in trace animals despite the relatively small effect size.
Our western blot study revealed a number of interesting findings that should be further explored in future studies. First, in the amygdala, we observed increased pERK expression following both retrieval and extinction of delay fear conditioning. This suggests that reactivating a delay fear association is sufficient to drive pERK-dependent plasticity in the amygdala. This is not altogether surprising, as previous studies have shown that the amygdala is a crucial site of plasticity during the reconsolidation period (Jarome et al., 2011; Nader, Schafe, & Le Doux, 2000; Parsons, Gafford, Baruch, Riedner, & Helmstetter, 2006) and increased ERK phosphorylation has previously been observed in the amygdala following delay memory retrieval (Parsons et al., 2010). pERK, therefore, marks both extinction- and reconsolidation-related plasticity in the amygdala.
Another interesting observation was the upregulation of ERK phosphorylation in the IL following both delay and trace fear extinction. The IL is known to play a crucial role in extinction learning (Burgos-Robles et al., 2007; Hugues et al., 2004; Quirk & Mueller, 2008; Sierra-Mercado et al., 2011; Sotres-Bayon et al., 2007), as it projects to the intercalated cell (ITC) layer of the amygdala and inhibits amygdalar output by silencing the central nucleus (Herry et al., 2010; Pare, Quirk, & Ledoux, 2004; Sierra-Mercado et al., 2011). According to this circuitry, the IL would be expected to drive extinction-related learning regardless of the type of training. Indeed, we observed increases in pERK in the IL following both delay and trace fear extinction, suggesting that the IL is involved in extinction regardless of the specific form of training used; both implicit and explicit associations appear to require the IL for extinction. Interestingly, we also observed a trend towards increased ERK phosphorylation in the IL following trace memory retrieval, indicating that the IL may play some role in retrieving trace fear memory, as well.
No significant changes in ERK phosphorylation were observed in either the DH or the PL following delay or trace fear extinction. In the hippocampus, we observed no evidence of changes in pERK expression. As the hippocampus is involved in modulating the contextual components of learning and extinction to a discrete CS, rather than storing the memory itself (Bangasser, Waxler, Santollo, & Shors, 2006; Clark & Squire, 1998, 2004; Frohardt, Guarraci, & Bouton, 2000; Ji & Maren, 2005; Wilson, Brooks, & Bouton, 1995), this finding suggests that the DH does not require plasticity to support extinction of a discrete CS. The DH may play more of a modulatory role, rather storing or extinguishing the memory for both delay and trace fear conditioning. The PL, on the other hand, showed a nonsignificant increase in ERK phosphorylation following trace, but not delay fear extinction. Although this increase was not statistically significant, this pattern suggests that some plasticity may occur in PL neurons to support trace, but not delay fear extinction. As the PL is required for the acquisition and consolidation of trace fear associations (Baeg et al., 2001; Gilmartin & Helmstetter, 2010; Gilmartin et al., 2012; Gilmartin & McEchron, 2005b; Quinn et al., 2002; Runyan & Dash, 2004; Runyan et al., 2004), it may be involved, to some extent, in the extinction of trace conditioning, as well. While our results do not demonstrate that the PL is involved in trace fear extinction, they also do not rule out the possibility that the PL is a key structure. Future studies should test what role, if any, the PL plays in trace fear extinction.
Why does trace fear extinction rely on a different neural circuit than delay fear extinction? There are a number of possibilities. One attractive explanation is that the site of memory storage dictates where extinction-related plasticity needs to occur. While delay fear memory relies on the amygdala for permanent storage (Gale et al., 2004; Kwapis et al., 2009; Maren, 2001; Maren, Aharonov, & Fanselow, 1996; Serrano et al., 2008), trace fear associations may be stored in cortical regions, including the mPFC (Blum, Hebert, & Dash, 2006; Runyan & Dash, 2004; Runyan et al., 2004). When extinction occurs, it is likely that the synapses supporting the original fear memory are updated so that the new information about the CS-UCS relationship can be incorporated into the memory trace. From this perspective, the amygdala would need to undergo plastic changes during delay extinction whereas cortical areas, including the mPFC, would need to undergo plasticity during trace extinction in order to update the original memory. Our findings show that plasticity occurs in the mPFC and RSC (but not in the amygdala) during trace fear extinction, which may represent the modification of a trace fear association stored in a distributed cortical manner. This hypothesis requires that the trace CS-UCS association is stored in cortical areas, however, rather than the amygdala. Recent studies have shown that the amygdala is required for the successful acquisition and consolidation of trace fear conditioning (Gilmartin et al., 2012; Guimarais, Gregorio, Cruz, Guyon, & Moita, 2011; Kwapis et al., 2011), but it is unclear whether this plasticity supports the storage of the CS-UCS association itself or whether it represents strengthening of the amygdala outputs to downstream structures that control the fear response. Additional work will be needed to determine whether the mPFC, RSC, and other cortical areas are involved in storing the trace fear association, as hypothesized.
Alternatively, it is possible that the amygdala is not necessary for trace extinction in the presence of cortical input. Specifically, the RSC and mPFC may support extinction of trace fear under normal circumstances, making the amygdala unnecessary for extinction acquisition. If these forebrain structures are inhibited before extinction, however, the amygdala may again become necessary for trace fear extinction. Research from the Mauk laboratory indicates that this may be the case for trace eyeblink conditioning; the basic cerebellar circuitry necessary for delay eyeblink extinction was only required for trace eyeblink conditioning if upstream forebrain input was blocked (Kalmbach & Mauk, 2012). Our results suggest that this is not the case for trace fear extinction, however. First, we observed increased expression of pERK in cortical areas, but not in the amygdala following trace extinction. If the amygdala serves a “backup” role in the extinction of trace fear conditioning, however, one might expect some evidence of plasticity in this location following trace fear extinction. Further, if the amygdala can support extinction in the absence of upstream input, extinction should be able to occur normally despite cortical inhibition. In our study, however, inhibiting NMDARs in the RSC was sufficient to block extinction learning in trace animals. Finally, we inhibited NMDA receptors, rather than general activity in the RSC. While plasticity is impaired by this manipulation, general synaptic transmission should be intact, so any signal generated in the RSC should still be propagated to downstream structures. NMDA receptor blockade does not usually activate compensatory neural circuits, as the neurons are still actively communicating with other brain regions (see Fanselow, 2010 for review). Our results therefore suggest that plasticity in cortical regions, particularly the RSC, is necessary for trace fear extinction.
Our study clearly demonstrates that the neural circuit supporting trace fear extinction is different than that supporting delay extinction. While delay extinction requires the amygdala, trace extinction requires the RSC. As our current understanding of the neural mechanisms of exposure-based therapy is largely based on the extinction of implicit delay fear associations, this finding highlights the importance of studying explicit trace fear memory, also, as the two types of extinction may drastically differ.
Supplementary Material
Acknowledgments
Funding This research was supported by the National Institutes of Mental Health (NIMH) grant R01MH069558 to Fred J. Helmstetter and NIMH grant F31MH090685 to Janine L. Kwapis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abumaria N, Yin B, Zhang L, Li XY, Chen T, Descalzi G, Liu G. Effects of elevation of brain magnesium on fear conditioning, fear extinction, and synaptic plasticity in the infralimbic prefrontal cortex and lateral amygdala. Journal of Neuroscience. 2011;31:14871–14881. doi: 10.1523/JNEUROSCI.3782-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP. Understanding retrosplenial amnesia: Insights from animal studies. Neuropsychologia. 2010;48:2328–2338. doi: 10.1016/j.neuropsychologia.2009.09.030. [DOI] [PubMed] [Google Scholar]
- Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cerebral Cortex. 2001;11:441–451. doi: 10.1093/cercor/11.5.441. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Waxler DE, Santollo J, Shors TJ. Trace conditioning and the hippocampus: The importance of contiguity. Journal of Neuroscience. 2006;26:8702–8706. doi: 10.1523/JNEUROSCI.1742-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barad M. Fear extinction in rodents: basic insight to clinical promise. Current Opinion in Neurobiology. 2005;15:710–715. doi: 10.1016/j.conb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Berman DE, Hazvi S, Rosenblum K, Seger R, Dudai Y. Specific and differential activation of mitogen-activated protein kinase cascades by unfamiliar taste in the insular cortex of the behaving rat. Journal of Neuroscience. 1998;18:10037–10044. doi: 10.1523/JNEUROSCI.18-23-10037.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum S, Hebert AE, Dash PK. A role for the prefrontal cortex in recall of recent and remote memories. Neuroreport. 2006;17:341–344. doi: 10.1097/01.wnr.0000201509.53750.bc. [DOI] [PubMed] [Google Scholar]
- Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. Journal of Neuroscience. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin CR. A cognitive neuroscience account of posttraumatic stress disorder and its treatment. Behaviour Research and Therapy. 2001;39:373–393. doi: 10.1016/s0005-7967(00)00087-5. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Clark RE, Squire LR. Classical conditioning and brain systems: the role of awareness. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- Clark RE, Squire LR. The importance of awareness for eyeblink conditioning is conditional: theoretical comment on Bellebaum and Daum (2004) Behavioral Neuroscience. 2004;118:1466–1468. doi: 10.1037/0735-7044.118.6.1466. [DOI] [PubMed] [Google Scholar]
- Cooper BG, Manka TF, Mizumori SJ. Finding your way in the dark: The retrosplenial cortex contributes to spatial memory and navigation without visual cues. Behavioral Neuroscience. 2001;115:1012–1028. doi: 10.1037//0735-7044.115.5.1012. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. Journal of Neuroscience. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Donnan MD, Tronson NC, Guzman YF, Gao C, Jovasevic V, Radulovic J. NMDA receptors in retrosplenial cortex are necessary for retrieval of recent and remote context fear memory. Journal of Neuroscience. 2011;31:11655–11659. doi: 10.1523/JNEUROSCI.2107-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Role of NMDA receptors and MAP kinase in the amygdala in extinction of fear: clinical implications for exposure therapy. European Journal of Neuroscience. 2002;16:395–398. doi: 10.1046/j.1460-9568.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- Day M, Langston R, Morris RG. Glutamate-receptor-mediated encoding and retrieval of paired-associate learning. Nature. 2003;424:205–209. doi: 10.1038/nature01769. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Nader K, LeDoux JE. Activation of extracellular signal-regulated kinase- mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. European Journal of Neuroscience. 2005;21:283–289. doi: 10.1111/j.1460-9568.2004.03824.x. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJD, Davis M. Extinction of fear-potentiated Startle: Blockade by infusion of an NMDA antagonist into the amygdala. Journal of Neuroscience. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. From contextual fear to a dynamic view of memory systems. Trends Cogn Sci. 2010;14:7–15. doi: 10.1016/j.tics.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Radulovic M, Schrick C, Sananbenesi F, Godovac-Zimmermann J, Radulovic J. Hippocampal Mek/Erk signaling mediates extinction of contextual freezing behavior. Neurobiology of Learning and Memory. 2007;87:149–158. doi: 10.1016/j.nlm.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB. Psychosocial treatment of posttraumatic stress disorder. Journal of Clinical Psychiatry. 2000;61(Suppl 5):43–48. discussion 49–51. [PubMed] [Google Scholar]
- Frohardt RJ, Guarraci FA, Bouton ME. The effects of neurotoxic hippocampal lesions on two effects of context after fear extinction. Behavioral Neuroscience. 2000;114:227–240. doi: 10.1037//0735-7044.114.2.227. [DOI] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, Fanselow MS. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. Journal of Neuroscience. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Helmstetter FJ. Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learning and Memory. 2010;17:289–296. doi: 10.1101/lm.1597410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Kwapis JL, Helmstetter FJ. Trace and contextual fear conditioning are impaired following unilateral microinjection of muscimol in the ventral hippocampus or amygdala, but not the medial prefrontal cortex. Neurobiology of Learning and Memory. 2012;97:452–464. doi: 10.1016/j.nlm.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the dentate gyrus and CA1 of the hippocampus exhibit inverse patterns of encoding during trace fear conditioning. Behavioral Neuroscience. 2005a;119:164–179. doi: 10.1037/0735-7044.119.1.164. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behavioral Neuroscience. 2005b;119:1496–1510. doi: 10.1037/0735-7044.119.6.1496. [DOI] [PubMed] [Google Scholar]
- Guimarais M, Gregorio A, Cruz A, Guyon N, Moita MA. Time determines the neural circuit underlying associative fear learning. Front Behav Neurosci. 2011;5:89. doi: 10.3389/fnbeh.2011.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haijima A, Ichitani Y. Anterograde and retrograde amnesia of place discrimination in retrosplenial cortex and hippocampal lesioned rats. Learning and Memory. 2008;15:477–482. doi: 10.1101/lm.862308. [DOI] [PubMed] [Google Scholar]
- Han CJ, O'Tuathaigh CM, van Trigt L, Quinn JJ, Fanselow MS, Mongeau R, Anderson DJ. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13087–13092. doi: 10.1073/pnas.2132313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ. Contribution of the amygdala to learning and performance of conditional fear. Physiology and Behavior. 1992;51:1271–1276. doi: 10.1016/0031-9384(92)90320-2. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Bellgowan PS. Effects of muscimol applied to the basolateral amygdala on acquisition and expression of contextual fear conditioning in rats. Behavioral Neuroscience. 1994;108:1005–1009. doi: 10.1037//0735-7044.108.5.1005. [DOI] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A. Neuronal circuits of fear extinction. European Journal of Neuroscience. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Herry C, Trifilieff P, Micheau J, Luthi A, Mons N. Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. European Journal of Neuroscience. 2006;24:261–269. doi: 10.1111/j.1460-9568.2006.04893.x. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16:174–182. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- Hugues S, Deschaux O, Garcia R. Postextinction infusion of a mitogen-activated protein kinase inhibitor into the medial prefrontal cortex impairs memory of the extinction of conditioned fear. Learning and Memory. 2004;11:540–543. doi: 10.1101/lm.77704. [DOI] [PubMed] [Google Scholar]
- Huh KH, Guzman YF, Tronson NC, Guedea AL, Gao C, Radulovic J. Hippocampal Erk mechanisms linking prediction error to fear extinction: roles of shock expectancy and contextual aversive valence. Learning and Memory. 2009;16:273–278. doi: 10.1101/lm.1240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo I, Bianchin M, Silva MB, Zanatta MS, Walz R, Ruschel AC, Medina JH. CNQX infused into rat hippocampus or amygdala disrupts the expression of memory of two different tasks. Behavioral and Neural Biology. 1993;59:1–4. doi: 10.1016/0163-1047(93)91061-q. [DOI] [PubMed] [Google Scholar]
- Jarome TJ, Werner CT, Kwapis JL, Helmstetter FJ. Activity dependent protein degradation is critical for the formation and stability of fear memory in the amygdala. PLoS One. 2011;6:e24349. doi: 10.1371/journal.pone.0024349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Maren S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learning and Memory. 2005;12:270–276. doi: 10.1101/lm.91705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski CC, Davis SJ, Moyer JR., Jr. Aging redistributes medial prefrontal neuronal excitability and impedes extinction of trace fear conditioning. Neurobiology of Aging. 2012;33:1744–1757. doi: 10.1016/j.neurobiolaging.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Kalmbach BE, Mauk MD. Multiple sites of extinction for a single learned response. Journal of Neurophysiology. 2012;107:226–238. doi: 10.1152/jn.00381.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katche C, Dorman G, Gonzalez C, Kramar CP, Slipczuk L, Rossato JI, Medina JH. On the role of retrosplenial cortex in long-lasting memory storage. Hippocampus. 2013 doi: 10.1002/hipo.22092. [DOI] [PubMed] [Google Scholar]
- Katche C, Dorman G, Slipczuk L, Cammarota M, Medina JH. Functional integrity of the retrosplenial cortex is essential for rapid consolidation and recall of fear memory. Learning and Memory. 2013;20:170–173. doi: 10.1101/lm.030080.112. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behavioral Neuroscience. 2008a;122:89–97. doi: 10.1037/0735-7044.122.1.89. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Neurotoxic lesions of retrosplenial cortex disrupt signaled and unsignaled contextual fear conditioning. Behavioral Neuroscience. 2008b;122:1070–1077. doi: 10.1037/a0012895. [DOI] [PubMed] [Google Scholar]
- Kelly A, Laroche S, Davis S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. Journal of Neuroscience. 2003;23:5354–5360. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Hamlin AS, Richardson R. Fear extinction across development: The involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. Journal of Neuroscience. 2009;29:10802–10808. doi: 10.1523/JNEUROSCI.0596-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Campeau S, Falls WA, Davis M. Infusion of the non-NMDA receptor antagonist CNQX into the amygdala blocks the expression of fear-potentiated startle. Behavioral and Neural Biology. 1993;59:5–8. doi: 10.1016/0163-1047(93)91075-x. [DOI] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. The role of awareness in delay and trace fear conditioning in humans. Cogn Affect Behav Neurosci. 2006;6:157–162. doi: 10.3758/cabn.6.2.157. [DOI] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Gilmartin MR, Helmstetter FJ. Intra-amygdala infusion of the protein kinase Mzeta inhibitor ZIP disrupts foreground context fear memory. Neurobiology of Learning and Memory. 2012;98:148–153. doi: 10.1016/j.nlm.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lonergan ME, Helmstetter FJ. Protein kinase Mzeta maintains fear memory in the amygdala but not in the hippocampus. Behavioral Neuroscience. 2009;123:844–850. doi: 10.1037/a0016343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Schiff JC, Helmstetter FJ. Memory consolidation in both trace and delay fear conditioning is disrupted by intra-amygdala infusion of the protein synthesis inhibitor anisomycin. Learning and Memory. 2011;18:728–732. doi: 10.1101/lm.023945.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Kim JJ. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. Journal of Neuroscience. 1998;18:8444–8454. doi: 10.1523/JNEUROSCI.18-20-08444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: New insights from functional neuroimaging of the human brain. Trends in Neurosciences. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Maguire EA. Neuroimaging studies of autobiographical event memory. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2001;356:1441–1451. doi: 10.1098/rstb.2001.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Maren S. Is there savings for pavlovian fear conditioning after neurotoxic basolateral amygdala lesions in rats? Neurobiology of Learning and Memory. 2001;76:268–283. doi: 10.1006/nlme.2001.4042. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Retrograde abolition of conditional fear after excitotoxic lesions in the basolateral amygdala of rats: absence of a temporal gradient. Behavioral Neuroscience. 1996;110:718–726. doi: 10.1037//0735-7044.110.4.718. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Stote DL, Fanselow MS. N-methyl-D-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditional fear in rats. Behavioral Neuroscience. 1996;110:1365–1374. doi: 10.1037//0735-7044.110.6.1365. [DOI] [PubMed] [Google Scholar]
- Maren S, Holt WG. Hippocampus and Pavlovian fear conditioning in rats: muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behavioral Neuroscience. 2004;118:97–110. doi: 10.1037/0735-7044.118.1.97. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Sprunger D, Wright-Hardesty K, Rudy JW. The role of dorsal hippocampus and basolateral amygdala NMDA receptors in the acquisition and retrieval of context and contextual fear memories. Behavioral Neuroscience. 2007;121:721–731. doi: 10.1037/0735-7044.121.4.721. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Wilkinson LS, Robbins TW, Everitt BJ. The effects of AMPA-induced lesions of the septo-hippocampal cholinergic projection on aversive conditioning to explicit and contextual cues and spatial learning in the water maze. European Journal of Neuroscience. 1995;7:281–292. doi: 10.1111/j.1460-9568.1995.tb01064.x. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Tseng W, Disterhoft JF. Neurotoxic lesions of the dorsal hippocampus disrupt auditory-cued trace heart rate (fear) conditioning in rabbits. Hippocampus. 2000;10:739–751. doi: 10.1002/1098-1063(2000)10:6<739::AID-HIPO1011>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annual Review of Psychology. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Butler VJ, Gardner R, Everitt BJ. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. Journal of Neuroscience. 2008;28:8230–8237. doi: 10.1523/JNEUROSCI.1723-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misane I, Tovote P, Meyer M, Spiess J, Ogren SO, Stiedl O. Time-dependent involvement of the dorsal hippocampus in trace fear conditioning in mice. Hippocampus. 2005;15:418–426. doi: 10.1002/hipo.20067. [DOI] [PubMed] [Google Scholar]
- Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behavioral Neuroscience. 1997;111:683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. Journal of Neurophysiology. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Baruch DE, Riedner BA, Helmstetter FJ. Long-term stability of fear memory depends on the synthesis of protein but not mRNA in the amygdala. European Journal of Neuroscience. 2006;23:1853–1859. doi: 10.1111/j.1460-9568.2006.04723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Helmstetter FJ. Regulation of extinction-related plasticity by opioid receptors in the ventrolateral periaqueductal gray matter. Front Behav Neurosci. 2010:4. doi: 10.3389/fnbeh.2010.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed CA: Academic Press; San Diego: 2007. [Google Scholar]
- Quinn JJ, Loya F, Ma QD, Fanselow MS. Dorsal hippocampus NMDA receptors differentially mediate trace and contextual fear conditioning. Hippocampus. 2005;15:665–674. doi: 10.1002/hipo.20088. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Ma QD, Tinsley MR, Koch C, Fanselow MS. Inverse temporal contributions of the dorsal hippocampus and medial prefrontal cortex to the expression of long-term fear memories. Learning and Memory. 2008;15:368–372. doi: 10.1101/lm.813608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Oommen SS, Morrison GE, Fanselow MS. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus. 2002;12:495–504. doi: 10.1002/hipo.10029. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. Journal of Neuroscience. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J, Tronson NC. Molecular specificity of multiple hippocampal processes governing fear extinction. Reviews in the Neurosciences. 2010;21:1–17. doi: 10.1515/revneuro.2010.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Keene CS, Iaccarino HF, Duan D, Bucci DJ. Involvement of retrosplenial cortex in forming associations between multiple sensory stimuli. Behavioral Neuroscience. 2011;125:578–587. doi: 10.1037/a0024262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum RS, Ziegler M, Winocur G, Grady CL, Moscovitch M. “I have often walked down this street before”: fMRI studies on the hippocampus and other structures during mental navigation of an old environment. Hippocampus. 2004;14:826–835. doi: 10.1002/hipo.10218. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Annals of the New York Academy of Sciences. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Schwartz AC. Exposure therapy for posttraumatic stress disorder. American Journal of Psychotherapy. 2002;56:59–75. doi: 10.1176/appi.psychotherapy.2002.56.1.59. [DOI] [PubMed] [Google Scholar]
- Runyan JD, Dash PK. Intra-medial prefrontal administration of SCH-23390 attenuates ERK phosphorylation and long-term memory for trace fear conditioning in rats. Neurobiology of Learning and Memory. 2004;82:65–70. doi: 10.1016/j.nlm.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Runyan JD, Moore AN, Dash PK. A role for prefrontal cortex in memory storage for trace fear conditioning. Journal of Neuroscience. 2004;24:1288–1295. doi: 10.1523/JNEUROSCI.4880-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. Journal of Neuroscience. 2001;21:9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of Pavlovian fear conditioning. Journal of Neuroscience. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimanski LA, Wahlsten D, Nguyen PV. Selective modification of short-term hippocampal synaptic plasticity and impaired memory extinction in mice with a congenitally reduced hippocampal commissure. Journal of Neuroscience. 2002;22:8277–8286. doi: 10.1523/JNEUROSCI.22-18-08277.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano P, Friedman EL, Kenney J, Taubenfeld SM, Zimmerman JM, Hanna J, Fenton AA. PKMzeta maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biol. 2008;6:2698–2706. doi: 10.1371/journal.pbio.0060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology. 2007;32:1929–1940. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Steinvorth S, Corkin S, Halgren E. Ecphory of autobiographical memories: an fMRI study of recent and remote memory retrieval. Neuroimage. 2006;30:285–298. doi: 10.1016/j.neuroimage.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Schrick C, Guzman YF, Huh KH, Srivastava DP, Penzes P, Radulovic J. Segregated populations of hippocampal principal CA1 neurons mediating conditioning and extinction of contextual fear. Journal of Neuroscience. 2009;29:3387–3394. doi: 10.1523/JNEUROSCI.5619-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaltas E, Preston GC, Gray JA. The effects of dorsal bundle lesions on serial and trace conditioning. Behavioural Brain Research. 1983;10:361–374. doi: 10.1016/0166-4328(83)90040-2. [DOI] [PubMed] [Google Scholar]
- Vianna MR, Szapiro G, McGaugh JL, Medina JH, Izquierdo I. Retrieval of memory for fear-motivated training initiates extinction requiring protein synthesis in the rat hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12251–12254. doi: 10.1073/pnas.211433298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weike AI, Schupp HT, Hamm AO. Fear acquisition requires awareness in trace but not delay conditioning. Psychophysiology. 2007;44:170–180. doi: 10.1111/j.1469-8986.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- Wilson A, Brooks DC, Bouton ME. The Role of the Rat Hippocampal System in Several Effects of Context in Extinction. Behavioral Neuroscience. 1995;109:828–836. doi: 10.1037//0735-7044.109.5.828. [DOI] [PubMed] [Google Scholar]
- Wolpe J. Basic principles and practices of behavior therapy of neuroses. American Journal of Psychiatry. 1969;125:1242–1247. doi: 10.1176/ajp.125.9.1242. [DOI] [PubMed] [Google Scholar]
- Zhang JJ, Okutani F, Inoue S, Kaba H. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase signaling pathway leading to cyclic AMP response element-binding protein phosphorylation is required for the long-term facilitation process of aversive olfactory learning in young rats. Neuroscience. 2003;121:9–16. doi: 10.1016/s0306-4522(03)00392-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.