Abstract
Unpredictable stress is known to profoundly enhance susceptibility to fear and anxiety while reducing the ability to extinguish fear when threat is no longer present. Accordingly, partial aversive reinforcement, via random exposure to footshocks, induces fear that is resistant to extinction. Here we sought to determine the hippocampal mechanisms underlying susceptibility versus resistance to context fear extinction as a result of continuous (CR) and partial (PR) reinforcement, respectively. We focused on N-methyl-D-aspartate receptor (NMDAR) subunits 2A and B (NR2A and NR2B) as well as their downstream signaling effector, extracellular signal-regulated kinase (ERK), based on their critical role in the acquisition and extinction of fear. Pharmacological inactivation of NR2A, but not NR2B, blocked extinction after CR, whereas inactivation of NR2A, NR2B, or both subunits facilitated extinction after PR. The latter finding suggests that co-activation of NR2A and NR2B contributes to persistent fear following PR. In contrast to CR, PR increased membrane levels of ERK and NR2 subunits after the conditioning and extinction sessions, respectively. In parallel, nuclear activation of ERK was significantly reduced after the extinction session. Thus, co-activation and increased surface expression of NR2A and NR2B, possibly mediated by ERK, may cause persistent fear. These findings suggest that patients with post-traumatic stress disorder (PTSD) may benefit from antagonism of specific NR2 subunits.
Keywords: partial reinforcement, continuous reinforcement, extracellular signal-regulated kinase, surface NMDAR, hippocampus, mice
1. Introduction
Contextual fear conditioning in rodents has long been utilized to model anxiety disorders such as PTSD. Many conditioning paradigms, however, do not take into account the unpredictability that is characteristic of traumatic events that give rise to anxiety disorders. Unpredictability in CS-US parings is known to exacerbate fear responses in humans (Acheson, Forsyth, Prevoneau, & Bouton, 2007; Oka, et al., 2010; Fonteyne, Vervliet, Hermans, Baeyens, & Vansteenwegen, 2009; Vansteenwegen, Iberico, Vervliet, Marescau, & Hermans, 2008; Grillon, Baas, Cornwell, & Johnson, 2006; Acheson, Forsyth, & Moses, 2012), particularly those with PTSD or panic disorders (Grillon, et al., 2008; Gorka, Nelson, & Shankman, 2013; Grillon, et al., 2013). Patients with anxiety disorders also demonstrate impaired extinction (Nevin, 2012; Seligman, 1968) and enhanced stress responses (McGuire, Herman, Horn, Sallee, & Sah, 2010; Gliner, 1972) to unpredictable stimuli. Prior unpredictable stressors have even been shown to impair healthy participants’ later fear learning, even when that fear learning is adaptive (Meulders et al., 2012). Although many animal studies have been utilized to investigate the behavioral consequences of PR, very few have taken advantage of this paradigm to examine signaling mechanisms in the brain that underlie those phenomena.
Both human (Kalisch, et al., 2006; Rauch, Shin, & Phelps, 2006; Milad et al., 2007) and rodent (Fischer, Sananbenesi, Schrick, Spiess, & Radulovic, 2004; Fischer, et al., 2007) studies have implicated the hippocampus as a critical region for fear extinction. Interestingly, fMRI data from humans point to the hippocampus as an encoder of uncertainty in cue-outcome relationships (Vanni-Mercier, Mauguiere, Isnard, & Dreher, 2009), and its activity is modulated both by outcome probabilities (Rodriguez, 2009) and unexpected aversive stimuli (Ploghaus, et al., 2000) during associative learning tasks. Furthermore, PTSD patients have reduced hippocampal volume (Shu, et al., 2013) and decreased hippocampal activation during extinction recall (Milad, et al., 2009). A role of hippocampal mechanisms in fear extinction has also been supported by studies using animal models (Radulovic & Tronson, 2010), which highlight the significance of the MAPK/ERK pathway (Matsuda, et al., 2010; Szapiro, Vianna, McGaugh, Medina, & Izquierdo, 2003). Previous work in our lab suggests that increased somatonuclear phopsho-ERK (pERK) in CA1 pyramidal neurons is indicative of shock expectancy violation and is required for effective fear extinction. Both hippocampal ERK signaling and fear extinction are impaired after PR contextual fear conditioning (Huh, et al., 2009). Together, these data suggest that understanding hippocampal signaling during extinction after PR will lead to identification of potential therapeutic targets for anxiety disorder sufferers.
In this study, we aimed to identify the primary hippocampal mechanism for impaired context fear extinction after PR. Previous studies have demonstrated that activation of hippocampal NMDAR regulates fear extinction (Szapiro, Vianna, McGaugh, Medina, & Izquierdo, 2003; Labrie, et al., 2009; Gomes, et al., 2010), and that systemic NMDAR blockade can prevent fear extinction in a variety of fear-motivated tasks (Cox & Westbrook, 1994; Baker & Azorlosa, 1996; Bevilaqua, et al., 2006, Santini, Muller, & Quirk, 2001; Suzuki et al., 2004; Sotres-Bayon, Bush, & LeDoux, 2007; Parkes & Westbrook, 2010). Because NMDAR bidirectionally regulate ERK activation depending on their subunit composition and synaptic localization (Chandler, Sutton, Dorairaj, & Norwood, 2001; Kim, Dunah, Wang, & Sheng, 2005; Choo, et al., 2012), we hypothesized that recruitment of different NR2 subunits after PR, compared to CR, results in resistance to extinction.
2. Materials and methods
2.1. Subjects
All experiments were conducted on nine-week-old male C57BL6/N mice (Harlan, Indianapolis, IN). Mice were individually housed and maintained on a 12/12 light/dark cycle (lights on at 7 a.m.) with food and water available ad libitum. All animals were treated in compliance with National Institutes of Health standards, and the Northwestern University Animal Care and Use Committee approved all procedures.
2.2. Surgery
Mice were anesthetized with 1.2% Avertin and implanted bilaterally with 26-gauge guide cannulae into dorsal hippocampus (1.5mm posterior, ±1mm lateral, 2mm ventral to Bregma). Animals were allowed to recover for at least three days prior to behavioral experiments.
2.3. Infusions
Intrahippocampal infusions were made using 28-gauge injectors that extended 1mm beyond the tips of the guide cannulae. All infusions were bilateral and delivered in a volume of 0.25 μL/side at a rate of 0.5 μL/min. NR2A were blocked with the preferential antagonist NVP-AAM077 (NVP; Novartis) at a dose of 1 μg/μL in 10% DMSO in artificial cerebrospinal fluid (aCSF). NR2B were blocked with the specific antagonist Ro25-6981 (Ro; Sigma) at a dose of 2 μg/μL in 10% DMSO in aCSF. NMDAR were non-specifically activated by D-serine (200 μg/μL in aCSF, Sigma) whereas NR2 subunits were nonselectively blocked by ((2R)-amino-5-phosphonovaleric acid (APV; 10 μg/μL in aCSF; Sigma). All infusions were made immediately following each daily extinction session, for a total of three to six infusions per mouse (Figures 2 and 1, respectively).
Fig. 2.
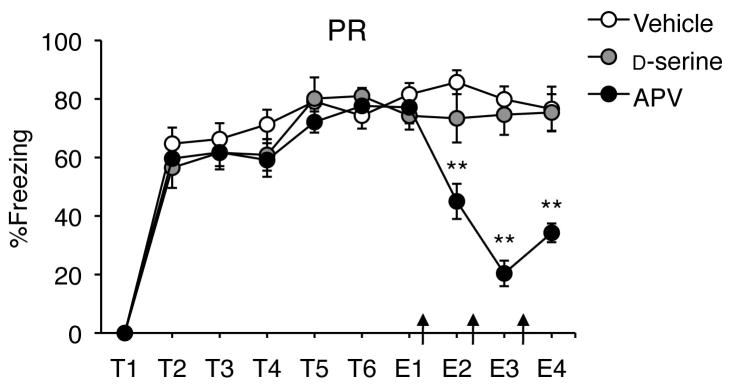
Extinction after PR training with intra-hippocampal infusions of APV or D-serine. NMDAR blockade with APV enhanced extinction after PR, whereas NMDAR activation via D-serine had no effect on fear. ** APV vs. vehicle p < 0.01. Arrows indicate intra-hippocampal infusions.
Fig. 1.
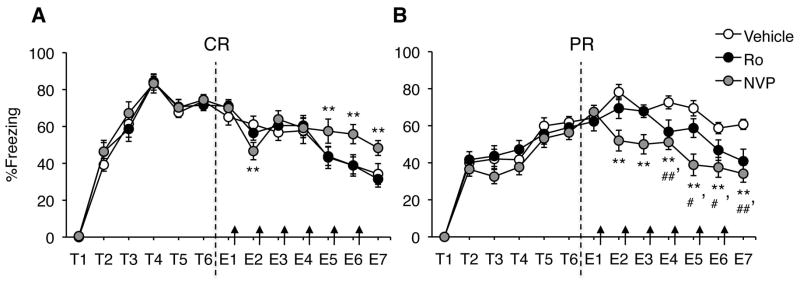
Extinction after CR or PR training paradigms with intra-hippocampal drug infusions. NR2A blockade with NVP prevented extinction after CR (A), whereas NR2A or NR2B inhibition enhanced extinction after PR (B). * NVP vs. vehicle p < 0.05. ** NVP vs. vehicle p < 0.01. ## Ro vs. vehicle p < 0.05. ## Ro vs. vehicle p < 0.01. Arrows indicate intra-hippocampal infusions.
2.4. Fear conditioning and extinction
All fear conditioning and extinction sessions took place in a Plexiglass chamber (35×20×20cm) with a stainless steel rod floor in a sound-attenuating cabinet (TSE, Inc.). The chamber was cleaned with 70% ethanol after every mouse. In the PR training paradigm, mice were placed in the conditioning chamber for three minutes on six consecutive days. Days one, four, and six concluded with a 0.7 mA, 2 s, unscrambled footshock. In the CR training paradigm, mice were placed in the conditioning chamber for three minutes on six consecutive days, and each session concluded with a footshock. To examine extinction processes, mice were placed in the same chambers for three minutes on at least four consecutive days following the last day of training. Freezing behavior was scored using a sampling method by a trained observer unaware of the experimental conditions who manually recorded freezing every fifth second over 180 seconds (a total of 36 data points). Freezing data were expressed as the percentage of total observations that each animal spent motionless, save for respiration.
2.5. Tissue preparation
Dorsal hippocampi were collected either 24 hours after the final training session or 1 hour after the final extinction session. Tissue was immediately frozen in liquid nitrogen and then transferred to storage at −80°C until lysis. ProteoExtract Subcellular Proteome Extraction Kit (EMD Millipore) was used to generate membrane, cytoplasmic, and nuclear fractions.
2.6. Immunoblot
Membrane and nuclear fractions (10 μg per sample) were reduced in loading buffer with β-mercaptoethanol and boiled for five minutes. Samples were then subjected to SDS polyacrylamide gel electrophoresis and transferred to PVDF membranes (Millipore). Membranes were blocked with I-Block (Tropix), incubated with primary antibody overnight (NR2B, Chemicon 1:200; NR2A, Millipore 1:1000; ERK1/2, Santa Cruz 1:8000; pERK1/2, Sigma 1:5000; Actin, Santa Cruz 1:400) at 4°C and corresponding secondary antibody (Goat Anti-Rabbit or Goat Anti-Mouse 1:10,000; Santa Cruz) for one hour at room temperature. Membranes were then incubated with alkaline phosphatase chemiluminescence enhancer (Nitro-Block II, Tropix) and the substrate CDP Star (Tropix), and then exposed to X-ray film for detection.
2.7. Co-immunoprecipitation
Prepared membrane fractions (three to five samples per condition) were combined to create 160 μg per co-immunoprecipitation. Immunoprecipitations were performed using Catch and Release Kit (Upstate) according to the user’s manual. NR2A- and NR2B-associated complexes were isolated with 4 μg primary antibody (Millipore AB1555 and Chemicon MAB5220, respectively) and 4 μg mouse IgG (Sigma I8765) was used as a negative control. Eluates and inputs were reduced in loading buffer with DTT and boiled for five minutes prior to electrophoresis.
2.8. Histology
At the end of each pharmacological experiment, mice were anesthetized with 1.2% Avertin, and methylene blue was infused through the cannulas at the same volume and flow rate as drug or vehicle. Brains were then extracted, frozen on liquid nitrogen, and stored at −20°C until cryosectioning and examination under a light microscope for cannula placement. Mice with inaccurate placements were excluded from analysis.
2.9. Data Analysis
Freezing data were analyzed with repeated measures analysis of variance (ANOVA). The first day of extinction was excluded from any analyses since freezing during this session preceded drug infusions. When a significant main effect or interaction effect was found (p < 0.05), post-hoc analyses were performed using LSD tests. For immunoblot analyses, ImageJ software (NIH) was used to determine mean optical density for each band, which was then normalized to internal controls (Actin, or total ERK in the case of pERK) and then to the mean optical density of the naïve condition. Data were analyzed with one-way ANOVA with LSD tests for post-hoc analysis when necessary and presented as means ± standard error of the mean.
3. Results
3.1. Behavioral results
After CR, control mice successfully extinguished their fear responses by the third day of extinction training compared to the last day of conditioning (p < 0.05; Fig. 1A). This extinction process was impaired by the NR2A-preferring antagonist NVP but not the NR2B-specific antagonist Ro. Repeated measures ANOVA gave a significant effect of day (F5,125 = 24.13, p < 0.01) and a significant day by drug interaction (F5,125 = 4.82, p < 0.01). Post-hoc analyses revealed that mice in the NVP group froze significantly more than mice in the Ro and vehicle groups on days 5–7 (ps < 0.01).
PR, as expected, resulted in impaired extinction; control mice did not significantly decrease their freezing levels compared to the last day of conditioning. However, this effect was reversed by infusion of either NVP or Ro (Fig. 1B). Repeated measures ANOVA revealed a significant effect of day (F5,90 = 16.58, p < 0.01) and drug (F2,19= 16.49, p < 0.01). Post-hoc analyses indicated that the Ro group froze significantly less than the vehicle group on days 4–7 (ps < 0.05) and the NVP group froze less than the vehicle group on days 2–7 (ps < 0.01). The freezing levels of the Ro and NVP groups on the last day of extinction (40.87% and 34.13%, respectively) resemble those of the vehicle group in the CR training condition (34.26%), indicating that either drug treatment completely rescued the PR-induced extinction deficit. Thus, NR2A activation is required for extinction after CR, whereas activation of NR2A and NR2B results in the impaired extinction seen after PR.
To further validate the inhibitory role of NMDAR in fear extinction after PR, we performed an additional experiment with the NR2 subunit-nonselective antagonist (APV) and a general NMDAR agonist (D-serine). Antagonism of NR2 in hippocampus via APV facilitated extinction after PR (Fig. 2), as revealed by a significant effect of day (F2, 20 = 7.56, p < 0.01) and drug (F2, 40 = 54.92, p < 0.01), and a significant day by drug interaction (F2, 20= 6.99, p < 0.01). The APV group froze less than the vehicle group on days 2–4 (ps < 0.01), whereas agonism of hippocampal NMDAR via D-serine had no effect on extinction after PR (ps > 0.05). Interestingly, APV reduced freezing after PR to 21.13% after the second infusion, whereas NVP and Ro only reduced freezing levels to 40.87% and 34.13%, respectively, after six infusions. These results suggest that, after PR, the effects of NR2A and NR2B blockade on extinction are additive, and that impaired extinction results from the co-activation of NR2A and NR2B.
3.2. NR2A, NR2B, and ERK expression and interactions
3.2.1. Hippocampal membrane expression of NR2A and NR2B 24 hours after fear conditioning and 1 hour after extinction training
ANOVAs revealed that neither the CR nor PR conditioning paradigms resulted in changes in levels of NR2A (F2,11 = 2.05, p = 0.175) or NR2B (F2,11= 1.46, p = 0.275) (Fig. 3A, left). After extinction, however, levels of both NR2A (F2,10= 4.916, p = 0.033) and NR2B (F2,10= 5.145, p = 0.026) were significantly increased over controls (Fig. 3A, right). Post-hoc tests revealed that, only in the PR group, the increases in NR2A and NR2B were significant (ps = 0.013 and 0.008, respectively).
Fig. 3.
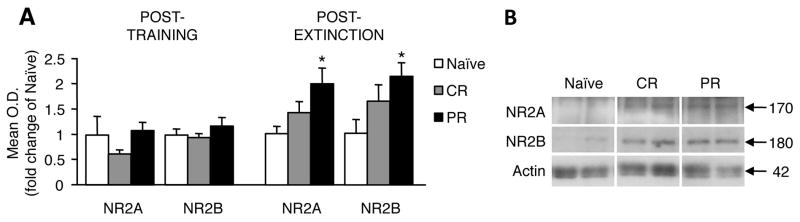
Membrane NR2 subunit expression 24 hr after last training session or 1 hr after last extinction session from naïve, CR-trained, and PR-trained animals. NR2 subunit levels were not affected by training, but both NR2A and NR2B levels were significantly higher after extinction sessions in PR-trained animals compared to naive (A). Representative immunoblots of statistically significant differences are shown in (B). * p < 0.05.
3.2.2. Hippocampal membrane expression of ERK1/2 24 hours after fear conditioning and 1 hour after extinction training
Fear conditioning resulted in changes in ERK1 (F2,11 = 3.97, p = 0.05) and ERK2 (F2,11= 5.612, p = 0.02) membrane expression in the PR group (Fig. 4A, left). Post-hoc analysis revealed that PR mice had significantly higher ERK1 (p = 0.018) and ERK2 (p = 0.007) retained in the membrane compared to naïve controls, whereas CR mice did not. Although ERK levels appeared to be elevated in the PR group after extinction, this difference did not reach significance (ERK1: F2,10= 3.047, p = 0.089; ERK2: F2,10= 3.364, p = 0.072; Fig. 4A, right).
Fig. 4.
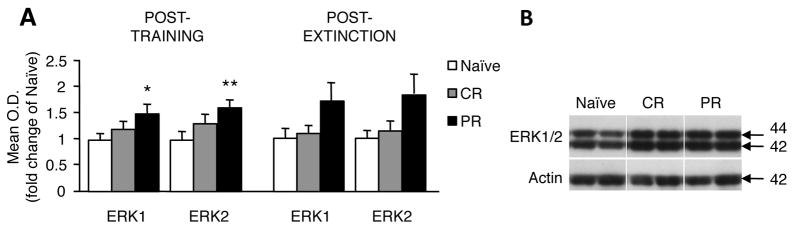
Membrane ERK1/2 expression 24 hr after last training session or 1 hr after last extinction session in naïve, CR-trained, and PR-trained animals. ERK1/2 levels significantly increased post-training in PR but not CR animals compared to naïve (A). Although it appeared that ERK1/2 was retained at the membrane after extinction as well, the difference from naïve was not significant. Representative immunoblots of statistically significant differences are shown in (B). * p < 0.05, ** p < 0.01.
3.2.3. NR2/ERK interactions after CR and PR
Twenty-four hours after the last conditioning session, membrane fractions were isolated and probed with NR2A or NR2B antibodies in order to establish whether ERK1/2 were in the same protein complexes (Fig. 5). ERK1/2 co-immunoprecipitated with both NR2A and NR2B after fear conditioning and in naïve mice. It appeared that ERK1 and ERK2 increased their association with NR2A after PR training, but further experiments are required to quantitatively confirm this finding. pERK2 was in complex with NR2B in the naïve group, but not after either training paradigm, whereas pERK1 did not appear to be in complex with either NR2A or NR2B in any condition. These findings suggest that ERK/NR2 complexes primarily involve inactive, unphosphorylated ERK1/2.
Fig. 5.
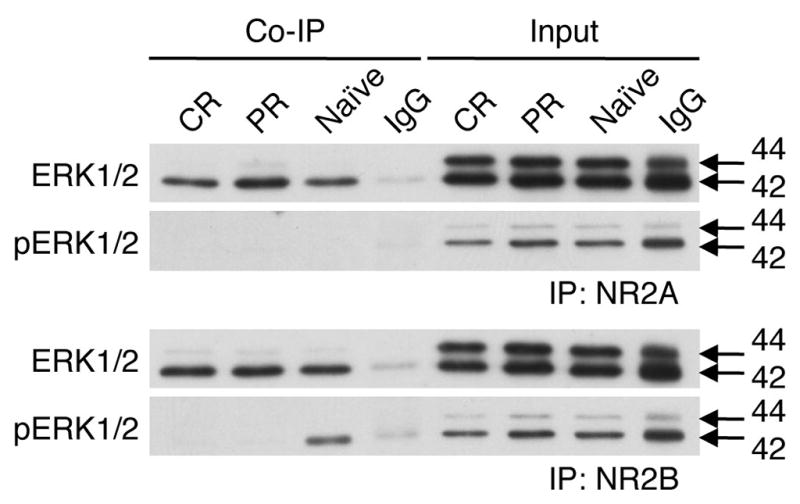
Membrane-associated NR2 protein complexes 24 hr after last training session in naïve, CR-trained, and PR-trained animals. ERK1/2, NR2A, and NR2B were expressed on the membrane and found in complexes. ERK1/2 interacted with NR2A and NR2B regardless of training, and association appeared greater with NR2A after PR. pERK1/2 were not found in complex with NR2 subunits, save for pERK2 bound to NR2B in the naïve state.
3.2.4. Hippocampal nuclear pERK1/2 expression 1 hour after extinction training
Surface NMDAR activity regulates ERK signal propagation from the membrane to the nucleus, which is required for fear extinction. We therefore determined the level of nuclear pERK in CR, PR, and naïve groups (Fig. 6). Nuclear pERK1 (F2,9= 4.95, p = 0.035) and pERK2 (F2,9= 4.56, p = 0.043) were significantly lower after extinction sessions in PR-conditioned animals compared to naïve controls (pERK1: p = 0.013; pERK2: p = 0.018). There were no differences between CR-conditioned mice and controls. This finding replicated earlier observations that impaired nuclear ERK signaling parallels the inability to extinguish fear.
Fig. 6.
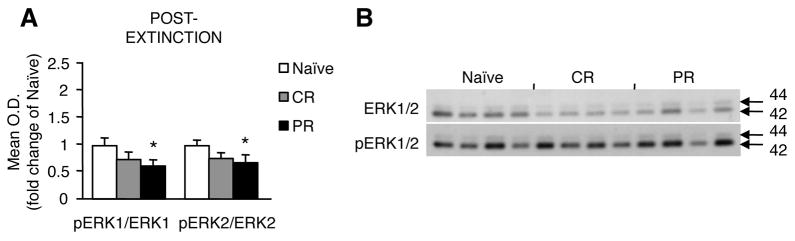
Nuclear pERK expression 1 hr after last extinction session in naïve, CR-trained, and PR-trained animals. pERK1/2 were reduced in PR but not CR animals compared to naïve (A). Representative immunoblots of statistically significant differences are shown in (B). * p < 0.05.
4. Discussion
Our findings reveal unique roles of NR2A and NR2B activation in the regulation of fear extinction. Whereas hippocampal NR2A activity is required for successful extinction after CR, activity of either NR2A- or NR2B-containing NMDARs prevents extinction after PR. This finding highlights a unique role of hippocampal NR2A/B co-activation in conferring resistance to fear extinction. Simultaneous antagonism of NR2A and NR2B with APV enhanced extinction more potently than antagonism of either subunit alone, suggesting additive effects of NR2A and NR2B after PR. Lastly, intra-hippocampal infusion of the NMDAR agonist D-serine, a treatment that typically enhances context fear extinction after CR (Fiorenza, Rose, Izquierdo, & Myskiw, 2012), failed to enhance extinction after PR, further indicating that NMDAR activity after predictable versus unpredictable shock can have different, if not opposing, effects on extinction.
It should be noted that although NVP preferentially inactivates NR2A subunits, it can also block NR2B subunits. This raises the possibility that the effects of NVP after PR training were actually due to NR2B blockade. However, Milton et al. (2013) were able to dissociate the roles of amygdalar NR2A and NR2B on memory reconsolidation using a 10-fold higher dose of NVP than was used here. Moreover, simultaneous antagonism of NR2A and NR2B with APV more potently enhanced extinction than antagonism of either subunit alone, suggesting distinct and additive effects of NR2A and NR2B activity. Thus, impaired extinction after PR most likely results from co-activation of NR2A and NR2B.
We found that hippocampal NR2B antagonism had no effect on extinction of contextual fear after CR, but facilitated extinction after PR. In contrast, systemic or amygdalar NR2B blockade prevents fear extinction after continuous reinforcement conditioning using a discrete cue (Sotres-Bayon, Bush, & LeDoux, 2007; Dalton, Wang, Floresco, & Phillips, 2008), and forebrain overexpression of the NR2B subunit enhances both contextual and cued fear extinction (Tang, Wang, Feng, Kyin, & Tsien, 2001). Collectively, these results emphasize that the role of NR2B in fear extinction is region-specific and depends on the reinforcement schedule used during conditioning.
At a cellular level, CR and PR differentially affect NR2A/B and ERK levels in membrane and nuclear compartments of hippocampal cells. Unlike CR, PR results in increased membrane levels of ERK1/2 post-conditioning. The failure to extinguish after PR is accompanied by increased NR2A/B expression in the membrane and a failure of pERK1/2 signal propagation in the nucleus. Although NR2A antagonism inhibited extinction after CR, no changes in NR2A expression at the membrane were observed after CR conditioning or extinction sessions. It is possible that signaling through basal levels of NR2A is sufficient to support extinction after CR, or that changes in NR2A function were caused by mechanisms not investigated here, such as enhanced receptor phosphorylation or scaffold coupling.
PR resulted in sequestration of ERK at the membrane where, in its inactive, unphosphorylated form, it complexes with NR2A and NR2B. Together with the observation that increased membrane levels of ERK1/2 preceded NR2A and NR2B accumulation, this suggests that ERK1/2 may target NMDAR mobilization to the neuronal surface. ERK1/2 signaling has been shown to enhance AMPA receptor trafficking (Kim, Dunah, Wang, & Sheng, 2005), illustrating the ability of this kinase to regulate receptor expression at the cell membrane. One potential mechanism for ERK1/2 to attract NR2 subunits to the membrane is changes in scaffolding proteins in NMDAR/ERK complexes. IQGAP1, for example, is a scaffolding protein that links NR2A subunits to the ERK pathway, and mice lacking this protein have reduced surface expression of NR2A and impaired ERK signaling (Gao, et al., 2011).
The functional consequence of NR2A and NR2B subunit co-expression at the membrane may be inhibition of ERK signal propagation to the nucleus. Previous work has shown that simultaneous activation of synaptic and extrasynaptic NMDAR, or activation of extrasynaptic NMDAR alone, inhibits ERK signaling (Gao, et al., 2010; Ivanov, et al., 2006; Karpova, et al., 2013). Impaired extinction is likely the results from confinement of ERK1/2 to the membrane, which prevents this extinction-critical kinase from responding to prediction error during extinction trials. Conversely, after CR conditioning, signaling through basal levels of synaptic, NR2A-containing NMDAR likely activates the ERK cascade. Whether the novel role of NR2A in preventing extinction after PR is due to a functional change in downstream NR2A-associated signaling in addition to receptor trafficking (perhaps to extrasynaptic sites) remains to be established.
The implications for this research are primarily relevant to pharmacological treatment of anxiety disorders with NMDAR-targeting drugs. We propose that NMDAR blockade may be beneficial for anxiety patients presenting with impaired extinction responses, particularly after repeated unpredictable traumatic experiences. At first glance, this may appear contradictory to findings that D-cycloserine, a partial agonist of NMDAR at the glycine-binding domain, improves symptoms of PTSD in humans (de Kleine, Hendriks, Kusters, Broekman, & van Minnen, 2012; Heresco-Levy, et al., 2002) and facilitates fear extinction in rodent models (Yamamoto, et al., 2008; Gupta, et al., 2013). The extinction-enhancing effects of D-cycloserine may be due to partial agonism actually reducing, rather than enhancing NMDAR signaling. Another possibility is that NMDAR agonism by D-cycloserine facilitates extinction only when fear is the result of predictable aversive events. This explanation is in line with our findings that NMDAR antagonism impaired extinction after CR and produced an opposite effect after PR, and that D-serine administration failed to enhance extinction after PR. Accordingly, in veterans with PTSD as a result of combat trauma, which is similar to PR in the unpredictability of aversive events, D-cycloserine used as an adjunct to exposure therapy results in poorer patient outcomes (Litz, et al., 2012). In rodents, intrahippocampal D-cycloserine infusions facilitate extinction after CR training, and also enhance hippocampal NR2B expression (Ren, et al., 2013). Because NR2B activity prevents extinction after PR, this may be a mechanism for D-cycloserine’s adverse effect in humans who have suffered unpredictable trauma. Different outcomes of predictable and unpredictable stress on fear extinction have also been found in human models of expectancy and evaluative learning, which result in susceptibility and resistance to extinction, respectively (Baeyens, Eelen, & Van den Bergh, 1990; Vansteenwegen, Francken, Vervliet, De Clercq, & Eelen, 2006; Blechert, Michael, Williams, Purkis, & Wilhelm, 2008). These models can be used to further validate our observations in human studies and identify novel effective treatments for persistent fear.
Acknowledgments
We would like to thank Dr. Jolanda Herzig (Novartis Pharma, AG) for providing the NVP-AAM077. This work was supported by NIMH grants 2T32MH067564 to KL and R01MH078064 to JR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson DT, Forsyth JP, Moses E. Interoceptive fear conditioning and panic disorder: the role of conditioned stimulus-unconditioned stimulus predictability. Behavior Therapy. 2012;43:174–189. doi: 10.1016/j.beth.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Acheson DT, Forsyth JP, Prenoveau JM, Bouton ME. Interoceptive fear conditioning as a learning model of panic disorder: an experimental evaluation using 20% CO(2)-enriched air in a non-clinical sample. Behaviour Research and Therapy. 2007;45:2280–2294. doi: 10.1016/j.brat.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Baeyens F, Eelen P, Van den Bergh O. Contingency awareness in evaluative conditioning: A case for unaware affective-evaluative learning. Cognition & Emotion. 1990;4:3–18. [Google Scholar]
- Baker JD, Azorlosa JL. The NMDA antagonist MK-801 blocks the extinction of Pavlovian fear conditioning. Behavioral Neuroscience. 1996;110:618–620. doi: 10.1037//0735-7044.110.3.618. [DOI] [PubMed] [Google Scholar]
- Blechert J, Michael T, Williams SL, Purkis HM, Wilhelm FH. When two paradigms meet: Does evaluative learning extinguish in differential conditioning? Learning & Motivation. 2008;39:58–70. [Google Scholar]
- Chandler LJ, Sutton G, Dorairaj NR, Norwood D. N-methyl D-aspartate receptor-mediated bidirectional control of extracellular signal-regulated kinase activity in cortical neuronal cultures. The Journal of Biological Chemistry. 2001;276:2627–2636. doi: 10.1074/jbc.M003390200. [DOI] [PubMed] [Google Scholar]
- Choo AM, Geddes-Klein DM, Hockenberry A, Scarsella D, Mesfin MN, Singh P, et al. NR2A and NR2B subunits differentially mediate MAP kinase signaling and mitochondrial morphology following excitotoxic insult. Neurochemistry International. 2012;60:506–516. doi: 10.1016/j.neuint.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J, Westbrook RF. The NMDA receptor antagonist MK-801 blocks acquisition and extinction of conditioned hypoalgesic responses in the rat. Quarterly Journal of Experimental Psychology B. 1994;47:187–210. [PubMed] [Google Scholar]
- Dalton GL, Wang YT, Floresco SB, Phillips AG. Disruption of AMPA receptor endocytosis impairs the extinction, but not acquisition of learned fear. Neuropsychopharmacology. 2008;33:2416–2426. doi: 10.1038/sj.npp.1301642. [DOI] [PubMed] [Google Scholar]
- de Kleine RA, Hendriks GJ, Kusters WJ, Broekman TG, van Minnen A. A randomized placebo-controlled trial of D-cycloserine to enhance exposure therapy for post-traumatic stress disorder. Biological Psychiatry. 2012;71:962–968. doi: 10.1016/j.biopsych.2012.02.033. [DOI] [PubMed] [Google Scholar]
- Fiorenza NG, Rose J, Izquierdo I, Myskiw JC. Modulation of the extinction of two different fear-motivated tasks in three distinct brain areas. Behavioural Brain Research. 2012;232:210–216. doi: 10.1016/j.bbr.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Fischer A, Radulovic M, Schrick C, Sananbenesi F, Godovac-Zimmermann J, Radulovic J. Hippocampal Mek/Erk signaling mediates extinction of contextual freezing behavior. Neurobiology of Learning and Memory. 2007;87:149–158. doi: 10.1016/j.nlm.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. Distinct roles of hippocampal de novo protein synthesis and actin rearrangement in extinction of contextual fear. The Journal of Neuroscience. 2004;24:1962–1966. doi: 10.1523/JNEUROSCI.5112-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonteyne R, Vervliet B, Hermans D, Baeyens F, Vansteenwegen D. Reducing chronic anxiety by making the threatening event predictable: An experimental approach. Behaviour Research and Therapy. 2009;47:830–839. doi: 10.1016/j.brat.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Gao C, Frausto SF, Guedea AL, Tronson NC, Jovasevic V, Leaderbrand K, et al. IQGAP1 regulates NR2A signaling, spine density, and cognitive processes. The Journal of Neuroscience. 2011;31:8533–8542. doi: 10.1523/JNEUROSCI.1300-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Gill MB, Tronson NC, Guedea AL, Guzman YF, Huh KH, et al. Hippocampal NMDA receptor subunits differentially regulate fear memory formation and neuronal signal propagation. Hippocampus. 2010;20:1072–1082. doi: 10.1002/hipo.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliner JA. Predictable vs. unpredictable shock: Preference behavior and stomach ulceration. Physiology & Behavior. 1972;9:693–698. doi: 10.1016/0031-9384(72)90036-4. [DOI] [PubMed] [Google Scholar]
- Gomes GM, Mello CF, da Rosa MM, Bochi GV, Ferreira J, Barron S, et al. Polyaminergic agents modulate contextual fear extinction in rats. Neurobiology of Learning and Memory. 2010;93:589–595. doi: 10.1016/j.nlm.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Gorka SM, Nelson BD, Shankman SA. Startle response to unpredictable threat in comorbid panic disorder and alcohol dependence. Drug and Alcohol Dependence. 2013 doi: 10.1016/j.drugalcdep.2013.02.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas JM, Cornwell B, Johnson L. Context conditioning and behavioral avoidance in a virtual reality environment: Effect of predictability. Biological Psychiatry. 2006;60:752–759. doi: 10.1016/j.biopsych.2006.03.072. [DOI] [PubMed] [Google Scholar]
- Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. The American Journal of Psychiatry. 2008;165:898–904. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biological Psychiatry. 2009;66:47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Hillman BG, Prakash A, Ugale RR, Stairs DJ, Dravid SM. Effect of D-cycloserine in conjunction with fear extinction training on extracellular signal-regulated kinase activation in the medial prefrontal cortex and amygdala in rat. The European Journal of Neuroscience. 2013;37:1811–1822. doi: 10.1111/ejn.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heresco-Levy U, Kremer I, Javitt DC, Goichman R, Reshef A, Blanaru M, et al. Pilot-controlled trial of D-cycloserine for the treatment of post-traumatic stress disorder. International Journal of Neuropsychopharmacology. 2002;5:301–307. doi: 10.1017/S1461145702003061. [DOI] [PubMed] [Google Scholar]
- Huh KH, Guzman YF, Tronson NC, Guedea AL, Gao C, Radulovic J. Hippocampal Erk mechanisms linking prediction error to fear extinction: roles of shock expectancy and contextual aversive valence. Learning and Memory. 2009;16:273–278. doi: 10.1101/lm.1240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, et al. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. The Journal of Physiology. 2006;572:789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. The Journal of Neuroscience. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova A, Mikhaylova M, Bera S, Bar J, Reddy PP, Behnisch T, et al. Encoding and transducing the synaptic or extrasynaptic origin of NMDA receptor signals to the nucleus. Cell. 2013;152:1119–1133. doi: 10.1016/j.cell.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Labrie V, Duffy S, Wang W, Barger SW, Baker GB, Roder JC. Genetic inactivation of D-amino acid oxidase enhances extinction and reversal learning in mice. Learning and Memory. 2009;16:28–37. doi: 10.1101/lm.1112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litz BT, Salters-Pedneault K, Steenkamp MM, Hermos JA, Bryant RA, Otto MW, et al. A randomized placebo-controlled trial of D-cycloserine and exposure therapy for posttraumatic stress disorder. Journal of Psychiatric Research. 2012;46:1184–1190. doi: 10.1016/j.jpsychires.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Matsuzawa D, Nakazawa K, Sutoh C, Ohtsuka H, Ishii D, et al. D-serine enhances extinction of auditory cued fear conditioning via ERK1/2 phosphorylation in mice. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34:895–902. doi: 10.1016/j.pnpbp.2010.04.013. [DOI] [PubMed] [Google Scholar]
- McGuire J, Herman JP, Horn PS, Sallee FR, Sah R. Enhanced fear recall and emotional arousal in rats recovering from chronic variable stress. Physiology & Behavior. 2010;101:474–482. doi: 10.1016/j.physbeh.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulders A, Vervliet B, Fonteyne R, Baeyens F, Hermans D, Vansteenwegen D. Preexposure to (un)predictable shock modulates discriminative fear learning between cue and context: an investigation of the interaction between fear and anxiety. International Journal of Psychophysiology. 2012;84:180–187. doi: 10.1016/j.ijpsycho.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Milton AL, Merlo E, Ratano P, Gregory BL, Dumbreck JK, Everitt BJ. Double dissociation of the requirement for GluN2B- and GluN2A-containing NMDA receptors in the destabilization and restabilization of a reconsolidating memory. Journal of Neuroscience. 2013;33:1109–1115. doi: 10.1523/JNEUROSCI.3273-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA. Resistance to extinction and behavioral momentum. Behavioural Processes. 2012;90:89–97. doi: 10.1016/j.beproc.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka S, Chapman CR, Kim B, Shimizu O, Noma N, Takeichi O, et al. Predictability of painful stimulation modulates subjective and physiological responses. The Journal of Pain. 2010;11:239–246. doi: 10.1016/j.jpain.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Parkes SL, Westbrook RF. The basolateral amygdala is critical for the acquisition and extinction of associations between a neutral stimulus and a learned danger signal but not between two neutral stimuli. Journal of Neuroscience. 2010;30:12608–12618. doi: 10.1523/JNEUROSCI.2949-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Clare S, Gati JS, Rawlins JN, Matthews PM. Learning about pain: the neural substrate of the prediction error for aversive events. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:9281–9286. doi: 10.1073/pnas.160266497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J, Tronson NC. Molecular specificity of multiple hippocampal processes governing fear extinction. Reviews in the Neurosciences. 2010;21:1–17. doi: 10.1515/revneuro.2010.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Li X, Zhang X, Li M, Wang Y, Ma Y. The effects of intra-hippocampal microinfusion of D-cycloserine on fear extinction, and the expression of NMDA receptor subunit NR2B and neurogenesis in the hippocampus in rats. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2013;44:257–264. doi: 10.1016/j.pnpbp.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biological Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Rodriguez PF. Stimulus-outcome learnability differentially activates anterior cingulate and hippocampus at feedback processing. Learning and Memory. 2009;16:324–331. doi: 10.1101/lm.1191609. [DOI] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. Journal of Neuroscience. 2001;21:9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman ME. Chronic fear produced by unpredictable electric shock. Journal of Comparative and Physiological Psychology. 1968;66:402–411. doi: 10.1037/h0026355. [DOI] [PubMed] [Google Scholar]
- Shu XJ, Xue L, Liu W, Chen FY, Zhu C, Sun XH, et al. More vulnerability of left than right hippocampal damage in right-handed patients with post-traumatic stress disorder. Psychiatry Research. 2013;212:237–244. doi: 10.1016/j.pscychresns.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology. 2007;32:1929–1940. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. Journal of Neuroscience. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szapiro G, Vianna MR, McGaugh JL, Medina JH, Izquierdo I. The role of NMDA glutamate receptors, PKA, MAPK, and CAMKII in the hippocampus in extinction of conditioned fear. Hippocampus. 2003;13:53–58. doi: 10.1002/hipo.10043. [DOI] [PubMed] [Google Scholar]
- Tang YP, Wang H, Feng R, Kyin M, Tsien JZ. Differential effects of enrichment on learning and memory function in NR2B transgenic mice. Neuropharmacology. 2001;41:779–790. doi: 10.1016/s0028-3908(01)00122-8. [DOI] [PubMed] [Google Scholar]
- Vanni-Mercier G, Mauguiere F, Isnard J, Dreher JC. The hippocampus codes the uncertainty of cue-outcome associations: an intracranial electrophysiological study in humans. The Journal of Neuroscience. 2009;29:5287–5294. doi: 10.1523/JNEUROSCI.5298-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansteenwegen D, Francken G, Vervliet B, De Clercq A, Eelen P. Resistance to extinction in evaluative conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:71–79. doi: 10.1037/0097-7403.32.1.71. [DOI] [PubMed] [Google Scholar]
- Vansteenwegen D, Iberico C, Vervliet B, Marescau V, Hermans D. Contextual fear induced by unpredictability in a human fear conditioning preparation is related to the chronic expectation of a threatening US. Biological Psychology. 2008;77:39–46. doi: 10.1016/j.biopsycho.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Morinobu S, Fuchikami M, Kurata A, Kozuru T, Yamawaki S. Effects of single prolonged stress and D-cycloserine on contextual fear extinction and hippocampal NMDA receptor expression in a rat model of PTSD. Neuropsychopharmacology. 2008;33:2108–2116. doi: 10.1038/sj.npp.1301605. [DOI] [PubMed] [Google Scholar]


