Abstract
Study Design
A retrospective chart review of cases with congenital vertebral malformations (CVM) and controls with normal spine morphology.
Objective
To determine the relative contribution of maternal environmental factors (MEF) during pregnancy including maternal insulin dependent diabetes mellitus, valproic acid, alcohol, smoking, hyperthermia, twin gestation, assisted reproductive technology, in-vitro fertilization and maternal clomiphene usage to CVM development.
Summary of Background Data
Congenital vertebral malformations (CVM) represent defects in formation and segmentation of somites occurring with an estimated incidence of between 0.13–0.50 per 1000 live births. CVM may be associated with congenital scoliosis, Klippel-Feil syndrome, hemifacial microsomia and VACTERL syndromes, and represent significant morbidity due to pain and cosmetic disfigurement.
Methods
A multicenter retrospective chart review of 229 cases with CVM and 267 controls with normal spine morphology between the ages of 1–50 years was performed in order to obtain the odds ratio (OR) of MEF related to CVM among cases vs. controls. CVM due to an underlying syndrome associated with a known gene mutation or chromosome etiology were excluded. An imputation based analysis was performed in which subjects with no documentation of MEF history were treated as no maternal exposure.” Univariate and multivariate analysis was conducted to calculate the OR.
Results
Of the 229 total cases, 104 cases had single or multiple CVM without additional congenital malformations (CM) (Group 1) and 125 cases had single or multiple CVM and additional CM (Group 2). Nineteen percent of total cases had an identified MEF. The OR (95% CI, P-value) for MEF history for Group 1 was 6.0 (2.4–15.1, P<0.001) in the univariate analysis. The OR for MEF history in Group 2 was 9.1 (95%CI, P-value) (3.8–21.6, P<0.001) in the univariate analysis. The results were confirmed in the multivariate analysis, after adjusting for age, gender, and institution.
Discussion
These results support a hypothesis for an association between the above MEF during pregnancy and CVM and have implications for development of prevention strategies. Further prospective studies are needed to quantify association between CVM and specific MEF.
Introduction
Congenital segmentation defects resulting in congenital vertebral malformations (CVM) are etiologically heterogeneous with both environmental and genetic factors contributing to their occurrence. CVM in humans represent a significant health problem because they may be associated with the occurrence of kyphosis, scoliosis, back and neck pain, disability, pulmonary compromise, cosmetic disfigurement, and functional distress. Although prior estimates indicate prevalence between 0.13- 0.5-1/1000 live births, more recent information indicates that the incidence of CVM in the general population is unknown as many people who are asymptomatic do not present for medical care.1,2
Vertebral malformations may represent an isolated finding, occur in association with other renal, cardiac or spinal cord malformations occur as part of an underlying syndrome or chromosomal abnormality. Frequently encountered syndromes associated with CVM include Alagille syndrome (peripheral pulmonic stenosis, cholestasis, facial dysmorphism), Jarcho Levin syndrome (short trunk dwarfism, multiple vertebral and rib defects with posterior rib fusion), Klippel-Feil syndrome (short neck, low posterior hairline, and fusion of cervical vertebrae), Goldenhar syndrome (associated with craniofacial anomalies including microtia and epibulbar dermoids), and VACTERL association (vertebral malformations, anal atresia, cardiac malformations, tracheo-esophageal fistula, renal and radial anomalies and limb defects). A classification scheme for vertebral malformations was recently proposed by members of the International Consortium for Vertebral Anomalies and Scoliosis. (ICVAS).3 Additional studies are needed to determine whether molecular genetic mechanisms can be associated with various CVM phenotypes.
Occurrence of CVM in animal models and humans has been associated with maternal alcohol use, anticonvulsant medications including valproic acid, hyperthermia, maternal insulin-dependent diabetes mellitus and gestational diabetes.4–6 Various skeletal anomalies have been associated with maternal use of phenytoin during pregnancy.7 Single nucleotide polymorphisms in GLUT1 and other glucose metabolizing genes such as HK1 and LEPR might be expected to associate with patterns of malformations observed in diabetic embryopathy. Cigarette smoking during pregnancy has been reported to be associated with low birth weight, decreases in successive births and behavioral deficits that can be replicated by carbon monoxide alone in animal models.8,9 Anoxic damage to somites and the generation of reactive oxygen species by cigarette smoke could potentially contribute to the development of CVM.
Congenital scoliosis has been reported to occur in monozygotic twins, and there is an increased risk for congenital malformations in both monozygotic and dizygotic twins.10 Assisted reproductive technology (ART) is linked with occurrence of congenital malformations and syndromes including Prader-Willi, Angelman and Beckwith-Wiedemann syndromes. Epigenetic factors including methyl donor content of the growth media have been suggested as a possible mechanism of their occurrence in ART-assisted pregnancies, and nutritional factors have been implicated for their occurrence in non-ART pregnancies. The relatively sporadic nature of CVM, similar to other birth defects, makes epigenetic factors another plausible mechanism for investigation. Little information exists regarding the relative contribution of environmental factors to the development of CVM and congenital scoliosis. Prior studies have focused on specific environmental exposures and pregnancy outcomes resulting from various maternal environmental factors during pregnancy which were associated with a variety of birth defects including CVM. Our working hypothesis is that maternal risk factors are associated with a significant proportion of CVM. We undertook a retrospective pilot case-control study to determine the odds ratio (OR) of CVM in children who have had maternal exposures during their mother’s pregnancy. We have chosen to highlight maternal exposures based on prior evidence in the literature which would be expected to contribute to CVM, including maternal exposures which have been associated with CVM in experimental animals, and maternal exposures which have the potential of being associated with birth defects including clomid, in-vitro fertilization, and cigarette smoke. Exposure history and maternal risk factors may give more insight into the etiology of CVM and may identify means to help prevent their occurrence.
Materials and Methods
A retrospective chart review of 229 cases with CVM and 267 controls with normal spine morphology between the ages of 1–50 years were seen between the years January 1, 2000 and November 1, 2010. The cases reported were obtained from one of the co-author’s private clinical practice, whereas the cases from another clinic were obtained from subjects who had enrolled in an IRB approved study in which the purpose was to identify genetic factors associated with CVM development.
Individuals with CVM, were identified from these different sites, using ICD9 codes including 754.2 (congenital scoliosis), 756.1 (anomalies of the spine), 756.13 (congenital absence of vertebra), 756.14 (hemivertebra), 756.15 (congenital fusion of spine), 756.16 (Klippel-Feil), 756.19 (supernumerary vertebra), 756.0 (anomalies of skull and face bones), 744.89 (other), and 756.81 (absence of muscle and tendon). CVM due to an underlying syndrome associated with a known gene mutation or chromosome etiology were excluded. CVM cases were classified as either isolated with no additional CM (Group I) or with additional CM (Group II) including VACTERL, hemifacial microsomia, diabetic embryopathy, Klippel-Feil and multiple congenital anomalies (NOS) diagnosis. Controls, defined as individuals with a full spinal evaluation, normal spine morphology and minimal scoliosis, were identified from available medical records.
Controls were identified by use of the ICD 9 codes: 737.43 (scoliosis) and 737.3. (kyphoscoliosis and scoliosis) All lists were unduplicated to ensure no patient was counted twice.
The medical record of all cases and controls was reviewed for documentation of maternal exposures during pregnancy in the available general pediatric, orthopedic surgery, neurosurgery and genetics notes. “Significant exposures” which could be associated with CVM development included maternal insulin dependent diabetes, alcohol, valproic acid, twins, hyperthermia, cigarette smoking, clomiphene and assisted reproductive technology.
Imputation based analysis was performed in which missing exposure was treated as “no exposure.” Separate analyses were performed for “all isolated and multiple CVM” in which patients with “other birth defects” were included in the total (55% of total, data not shown) and “all isolated and multiple CVM” in which patients had no “other birth defects” (45% of the total sample of CVM analyzed, data presented in Tables 1–4 below). To help control for recall bias in older subjects a separate analysis was performed for subjects <20 years of age. Since this was a retrospective case-control study, multivariate logistic regression analysis was also conducted to control for confounding variables including age, gender, and institution. Odds ratios (OR) and the corresponding 95% confidence intervals (CI) were reported for the multivariate (adjusted) analysis, univariate (unadjusted).
Table 1.
Descriptive summary of the complete analysis of all institutions. Demographic and gender information was not available for all cases and controls
| Variable | Case N=229 | Control N=267 | p value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 20.37 | 11.97 | 13.20 | 3.14 | <0.0001 |
| Case N=229 | Control N=267 | p value | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Sex: Male | 102 | 44.74 | 88 | 32.96 | 0.0072 |
| Female | 126 | 55.26 | 179 | 67.04 | |
| Race: Caucasian | 147 | 83.52 | 218 | 99.09 | 0.047 |
| Non-Caucasian | 29 | 16.48 | 2 | 0.91 | |
| Race: Non-Hispanic/Latino | 168 | 94.92 | 218 | 99.54 | 0.086 |
| Hispanic/Latino | 9 | 5.08 | 1 | 0.46 | |
Table 4.
Analysis restricted to subjects 20 years or younger of age
| Unadjusted Analysis | Adjusted Analysis† | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Isolated & Multiple CVM without additional CMs (N=48) | 13.2 | 5.0 – 35.0 | <0.001 | 13.0 | 3.1 – 55.2 | 0.005 |
| Isolated & Multiple CVM with additional CMs (N=88) | 11.0 | 4.5 – 26.9 | <0.001 | 10.3 | 2.9 – 36.9 | <0.001 |
| All Isolated & Multiple CVM (N=137) | 11.8 | 5.1 – 27.3 | <0.001 | 13.0 | 4.3 – 39.7 | <0.001 |
adjusted for gender and institution, using a multivariate logistic regression analysis model
All P values were 2-sided, and P < .05 was used to indicate statistical significance. Statistical analyses were performed using SAS software version 9.2. (SAS Institute, Cary, NC).
Results
A total of 229 cases and 267 controls were identified with a mean age of 20.4 (SD 12) and 13.2 (SD 3.1) years respectively. The data regarding age and ethnic background are indicated in Table 1. Stratification of cases and controls by the 3 separate institutions is indicated in Table 2. A maternal risk factor was identified in 43 of 229 cases (18.9%) and 7 of 267 (2.6 %) controls. The unadjusted and adjusted analysis (multivariate logistic regression) resulted in significant OR for maternal exposure factors (MEF) for all isolated and multiple CVMs was 7.6 (95%, p value) (3.4–17.4, p<0.001) in the unadjusted analysis and 16.8 (5.6–50, p<0.001) in the adjusted analysis (Table 3). Statistically significant ORs were obtained when separate analyses were performed for CVM with and without additional CM (Table 3).
Table 2.
Distribution of cases and controls by institution
| Institution | Cases | Controls |
|---|---|---|
| X-1 | 150 | 46 |
| X-2 | 25 | 221 |
| X-3 | 54 | 0 |
| Total | 229 | 267 |
Table 3.
Odds ratios (95% CI) for significant exposure between CVM cases and controls
| Unadjusted Analysis | Adjusted Analysis† | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Isolated & Multiple CVM without additional CMs (N=104) | 6.0 | 2.4 – 15.1 | <0.001 | 20.2 | 4.8 – 85.4 | <0.001 |
| Isolated & Multiple CVM with additional CM (N=125) | 9.1 | 3.8 – 21.6 | <0.001 | 12.1 | 3.5 – 42.2 | <0.001 |
| All Isolated & Multiple CVM (N=229) | 7.6 | 3.4 – 17.4 | <0.001 | 16.8 | 5.6 – 50.0 | <0.001 |
Unadjusted and adjusted (by age, gender and institution) analysis was conducted.
Missing exposure values in CVM cases were treated as “no exposure”
adjusted for age, gender, and institution, using a multivariate logistic regression analysis model
Clearly, age may be the major confounding factor in this study since the case group includes many older subjects (resulting into different level/type of exposure & diagnostic issues). With this restriction (only subjects younger than 20 years), the distribution of age is balanced between the cases and control group (mean age of ~ 13 years in both groups). As in the original analysis, missing exposure history entries were imputed as “no exposure”. The OR for MEF for all CVM was 11.8 (5.1–27.3, p<0.001) in the unadjusted analysis and 13.0 (4.3–39.7, p<0.001) in the adjusted analysis, as indicated in Table 4. Separate analyses for CVM with and without additional CM resulted in statistically significant ORs (Table 4).
The results shown above were confirmed using various types of imputation techniques for missing exposure values, including the most conservative scenario where all missing exposure entries in the case group were treated as “no exposure” and missing exposure entries in the control group were treated as “exposure”.
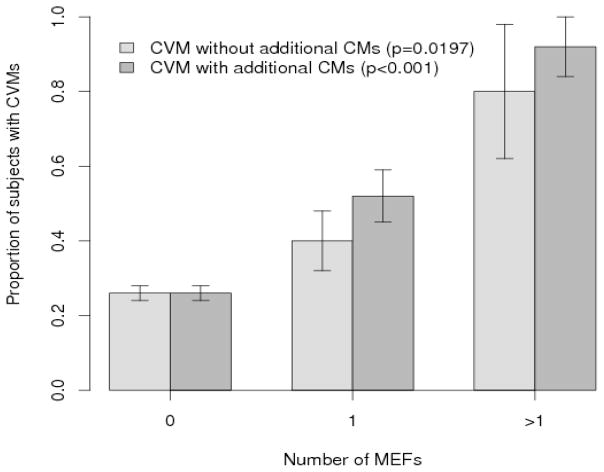
Logistic regression analysis of single or multiple CVMs with respect to number of MEFs (0,1, ≥2) revealed a statistically significant positive dose response (Group 1, p=0.0197; Group 2, p<0.001). (Figure 1) Given the small number of patients with “significant” maternal exposures in each subcategory it was not possible to perform subgroup analysis.
Figure 1.
Dose response graph of number of MEF vs. proportion of cases with CVM in Group I (isolated CVM) and Group II (CVM and additional CM).
Discussion
To our knowledge this represents the first analysis which addresses MEF associated with CVM development. In previous studies CVM have been linked to various maternal exposures (Table 5) but there has not be a systematic effort to begin to delineate MEF associated with CVM occurrence. A surveillance study of 206,244 live births, stillbirths and elective terminations during the years 1972–74 and 1979–2000 identified 27 cases of hemivertebrae in which 5 were associated with maternal insulin dependent diabetes mellitus.11 The present study identified at least one MEF in 19% of the cases analyzed, demonstrating significant association between the MEF and CVM.
Table 5.
Maternal risk factors analyzed for CVM
| Exposure | Mechanism of Action | Reference |
|---|---|---|
| Maternal insulin dependent diabetes | Increased reactive oxygen species (ROS); altered histone deacetylase (HDAC) | 19,20 |
| Alcohol | ROS | 21 |
| Valproic acid | Downregulation of Pax-1 and Paraxis | 22 |
| Twins | Association with midline malformations; multiple factors such as mis-segregation of cytoplasmic material, imprinting; etiology not established | 10,23 |
| Hyperthermia | Heat shock protein activation may alter tissue development or morphogenic processes | 24,25 |
| Cigarette smoking | ROS, anoxic damage to somites | 8,18 |
| Clomiphine | Epigenetic effects | 26 |
| Assisted Reproductive Technology | Epigenetic effects | 26 |
Analyses performed using the entire CVM cohort (N=229) in addition to separate cohorts comprised of cases with isolated CVM and cases with CVM with other CM yielded statistically significant elevated OR. Finally, a separate analysis performed using cases with ages of 20 or less resulted in statistically significant elevated OR.
As illustrated in Table 5, the MEF potentially exert their effects through a variety of different mechanisms including epigenetic effects, reactive oxygen species (ROS) and through regulation of vertebral patterning genes. Logistic regression analysis supports a positive dose response effect for MEF, suggesting that multiple MEF can result in increased susceptibility to CVM. One hypothesis for CVM development is that genetic susceptibility in vertebral patterning genes in association with MEF can result in CVM development. Supporting evidence for this hypothesis includes a patient with sacral agenesis reported by our group, with a c. 1010 C>T mutation resulting in an alanine to valine change at amino acid 338 in the T (Brachyury) gene.12 This patient’s mother had diabetes during pregnancy and had taken valproic acid for seizure control. This mutation had previously been described in another individual with sacral agenesis, with no known history of maternal diabetes.13 This same mutation was observed in two additional patients with CVM reported by our group, including a female child with CVM and maternal exposure to clomiphene and another child with CVM and with no known MEF history. In all three cases in which the c. 1013 C>T mutation was identified, a clinically asymptomatic parent harbored the identical mutation, indicating that the c.1013 C>T mutation alone is not sufficient to result in CVM, suggesting that mutations in vertebral patterning genes may have incomplete penetrance and act in concert with other genes and/or environmental factors to result in the development of CVMs. More recently hypoxia has been shown to increase the phenotypic severity associated with vertebral malformations in Mesp2+/− and Hes7 +/− mice through disruption of FGF signaling.14
This study has several limitations. Since this is a retrospective case-control study, there was no opportunity to directly interview families more rigorously for MEF during pregnancy. This could potentially result in an underestimation of cases. Approximately 25 % of patients and/or families were interviewed for MEF as part of clinical care study by two co-investigators for participation in a clinical genetic study related to the genetic basis for CVM occurrence. Since the majority of controls were obtained from a co-investigator’s hospital and their respective families were also interviewed for MEF it is unlikely that there is an ascertainment bias for MEF in controls vs. cases. Ability to link the maternal record during pregnancy to the affected child and/or control medical record would control for recall bias, but this was not feasible. Because of the relative small number of cases it was difficult to perform meaningful subgroup analysis.
Another limitation is that there could be additional unobserved confounders which could lead to biased results when comparing significant exposure between cases and controls.
We have attempted to identify the more obvious MEF candidates, at the expense of failing to include other MEF which are more difficult to ascertain. For instance CVM have been observed in laboratory animals exposed to I (Kr)-blockers (class III anti-arrhythmic agent), fumonisins, (environmental toxins produced Fusarium moniliforme (F. verticilliodes), F. proliferatum, and other Fusarium species of molds) zinc deficient diet, and the organophosphate pesticide chlopyrifos, during pregnancy. Exposure of juvenile fourhorn sculpin, Myoxocephalus quadricornis L. to tetrachloro-1, 2-benzoquinone, a component in bleached kraft mill effluents, resulted in fish with vertebral deformities and abnormal mechanical vertebral properties.15 Alterations in HOX-mediated gene expression are mediated by exposure to carbon monoxide16 and boric acid.17 Inhibition of nitric oxide (NO) production or addition of NO to developing chick embryos results in increased axial skeletal defects and area correlated with apoptosis.18 These results have translational relevance. Identification of causes for CVM is of major importance for families presenting to scoliosis clinics, as they are often in search of an explanation for their child’s condition and have questions regarding recurrence risks and prevention strategies. Identification of mutations in patterning genes for vertebral development may assist families in the identification of MEF which should be avoided during pregnancy. Future studies will be aimed at interviewing mothers of affected children with CVMs in a larger, separate patient cohort. This will also be instrumental in facilitating subgroup analysis and obtaining a better understanding as to how different environmental agents may contribute to CVM occurrence.
Key Points.
Maternal exposure factors (MEF) during pregnancy were identified in approximately 20% of pregnancies associated with the occurrence of congenital vertebral malformations(s) (CVM) in a multicenter retrospective case control study.
The odds ratio (OR) for MEF during pregnancy among individuals with isolated CVM as compared to individuals with normal spine morphology was 6.0 (2.4–15.1, P<0.001).
There is supporting evidence for a dose-response for MEF contributing to CVM.
Further studies will be instrumental in the development of prevention strategies for CVM occurrence.
Acknowledgments
Source of Funding
Human subject’s permission from the IRB at the University of Wisconsin, Hospital for Special Surgery and Marshfield Clinic has been obtained for this study.
We appreciate the assistance of Gail Pearsall in preparation of this manuscript, and Bob Gordon for his skills on the artwork. We are grateful for the assistance of Lisa Riseberg, Terrie R. Sentry, Deb Melanz, Akilah King, Laura Birkeland for assistance with the identification of cases and controls for review. We also acknowledge the assistance of the Department of Pediatrics Computer support team Philip Doll III and Sandon Jurowski for assistance with our study database. We also acknowledge Marie Fleisner of the Marshfield Clinic’s Office of Scientific Writing and Publication for editorial assistance in the revision of this manuscript.
Dr. Philip Giampietro and Dr. Cathleen Raggio had full access to all of the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflict of Interest: No Conflicts of Interest were reported by any of the authors.
Contributor Information
Jennifer Hesemann, Email: hesemanj@gmail.com, UW Madison, Madison, WI.
Emily Lauer, Email: esabo@uwalumni.com, UW Madison, Madison, WI.
Stephen Ziska, Email: sziska12@regis-nyc.org, Hospital for Special Surgery, New York, NY.
Kenneth Noonan, Email: noonan@ortho.wisc.edu, UW Madison, Madison, WI.
Blaise Nemeth, Email: nemeth@ortho.wisc.edu, UW Madison, Madison, WI.
Jessica Scott-Schwoerer, Email: jscottschwoerer@pediatrics.wisc.edu, UW Madison, Madison, WI.
Catherine McCarty, Email: cmccarty@eirh.org, Essential Institute of Rural Health, Duluth, MN.
Kristen Rasmussen, Email: rasmussen.kristen@marshfieldclinic.org, Marshfield Clinic, Marshfield, WI.
Jacob M. Goldberg, Email: jlgoldberg@wisc.edu, UW Madison, Madison, WI
Sarah Sund, Email: sund@ortho.wisc.edu, UW Madison, Madison, WI.
Jens Eickhoff, Email: eickhoff@biostat.wisc.edu, UW Madison, Madison, WI.
Cathleen L. Raggio, Email: raggioc@hss.edu, Hospital for Special Surgery, New York, NY.
Philip F. Giampietro, Email: pfgiampietro@pediatrics.wisc.edu, UW Madison, Madison, WI
References
- 1.Brand MC. Examination of the newborn with congenital scoliosis: focus on the physical. Adv Neonatal Care. 2008;8:265–73. doi: 10.1097/01.ANC.0000338016.03040.6b. quiz 74–5. [DOI] [PubMed] [Google Scholar]
- 2.Wynne-Davies R. Congenital vertebral anomalies: aetiology and relationship to spina bifida cystica. J Med Genet. 1975;12:280–8. doi: 10.1136/jmg.12.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Offiah A, Alman B, Cornier A, et al. Pilot Assessment of a Radiologic Classification System for Segmentation Defects of the Vertebrae. Am J Med Genet. 2010 doi: 10.1002/ajmg.a.33361. [DOI] [PubMed] [Google Scholar]
- 4.Aberg A, Westbom L, Kallen B. Congenital malformations among infants whose mothers had gestational diabetes or preexisting diabetes. Early Hum Dev. 2001;61:85–95. doi: 10.1016/s0378-3782(00)00125-0. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Frias ML, Bermejo E, et al. Epidemiological analysis of outcomes of pregnancy in gestational diabetic mothers. American Journal of Medical Genetics. 1998;78:140–5. doi: 10.1002/(sici)1096-8628(19980630)78:2<140::aid-ajmg8>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.Passarge E, Lenz W. Syndrome of caudal regression in infants of diabetic mothers observations of further cases. Pediatrics. 1966;37:672–5. [PubMed] [Google Scholar]
- 7.Loughnan PM, Gold H, Vance JC. Phenytoin teratogenicity in man. Lancet. 1973;1:70–2. doi: 10.1016/s0140-6736(73)90467-4. [DOI] [PubMed] [Google Scholar]
- 8.Bnait KS, Seller MJ. Ultrastructural changes in 9-day old mouse embryos following maternal tobacco smoke inhalation. Exp Toxicol Pathol. 1995;47:453–61. doi: 10.1016/S0940-2993(11)80327-1. [DOI] [PubMed] [Google Scholar]
- 9.Fichtner RR, Sullivan KM, Zyrkowski CL, et al. Racial/ethnic differences in smoking, other risk factors, and low birth weight among low-income pregnant women, 1978–1988. MMWR CDC Surveill Summ. 1990;39:13–21. [PubMed] [Google Scholar]
- 10.Corsello G, Piro E. The world of twins: an update. J Matern Fetal Neona. 2010;23 (Suppl 3):59–62. doi: 10.3109/14767058.2010.508218. [DOI] [PubMed] [Google Scholar]
- 11.Holmes LB. Common Malformations. Oxford University Press; 2012. Vertebral Anomalies: Hemivertebrae; pp. 283–9. [Google Scholar]
- 12.Ghebranious N, Blank RD, Raggio CL, et al. A missense T (Brachyury) mutation contributes to vertebral malformations. J Bone Miner Res. 2008;23:1576–83. doi: 10.1359/jbmr.080503. [DOI] [PubMed] [Google Scholar]
- 13.Papapetrou C, Drummond F, Reardon W, et al. A genetic study of the human T gene and its exclusion as a major candidate gene for sacral agenesis with anorectal atresia. J Med Genet. 1999;36:208–13. [PMC free article] [PubMed] [Google Scholar]
- 14.Sparrow DB, Chapman G, Smith AJ, et al. A mechanism for gene-environment interaction in the etiology of congenital scoliosis. Cell. 2012;149:295–306. doi: 10.1016/j.cell.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 15.Bengtsson BE, Larsson A, Bengtsson A, et al. Sublethal effects of tetrachloro-1,2-benzoquinone--a component in bleachery effluents from pulp mills--on vertebral quality and physiological parameters in fourhorn sculpin. Ecotoxicol Environ Saf. 1988;15:62–71. doi: 10.1016/0147-6513(88)90043-7. [DOI] [PubMed] [Google Scholar]
- 16.Farley FA, Loder RT, Nolan BT, et al. Mouse model for thoracic congenital scoliosis. J Pediatr Orthop. 2001;21:537–40. [PubMed] [Google Scholar]
- 17.Wery N, Narotsky MG, Pacico N, et al. Defects in cervical vertebrae in boric acid-exposed rat embryos are associated with anterior shifts of hox gene expression domains. Birth Defects Res A Clin Mol Teratol. 2003;67:59–67. doi: 10.1002/bdra.10031. [DOI] [PubMed] [Google Scholar]
- 18.Alexander PG, Chau L, Tuan RS. Role of nitric oxide in chick embryonic organogenesis and dysmorphogenesis. Birth Defects Res A Clin Mol Teratol. 2007;79:581–94. doi: 10.1002/bdra.20386. [DOI] [PubMed] [Google Scholar]
- 19.Fine EL, Horal M, Chang TI, et al. Evidence that elevated glucose causes altered gene expression, apoptosis, and neural tube defects in a mouse model of diabetic pregnancy. Diabetes. 1999;48:2454–62. doi: 10.2337/diabetes.48.12.2454. [DOI] [PubMed] [Google Scholar]
- 20.Zabihi S, Loeken MR. Understanding diabetic teratogenesis: where are we now and where are we going? Birth Defects Res A Clin Mol Teratol. 2010;88:779–90. doi: 10.1002/bdra.20704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong J, Sulik KK, Chen SY. Nrf2-mediated transcriptional induction of antioxidant response in mouse embryos exposed to ethanol in vivo: implications for the prevention of fetal alcohol spectrum disorders. Antioxidants & Redox Signaling. 2008;10:2023–33. doi: 10.1089/ars.2007.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes GL, Jr, Mariani BD, Tuan RS. Valproic acid-induced somite teratogenesis in the chick embryo: relationship with Pax-1 gene expression. Teratology. 1996;54:93–102. doi: 10.1002/(SICI)1096-9926(199606)54:2<93::AID-TERA5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Kaspiris A, Grivas TB, Weiss HR. Congenital scoliosis in monozygotic twins: case report and review of possible factors contributing to its development. Scoliosis. 2008;3:17. doi: 10.1186/1748-7161-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buckiova D, Kubinova L, Soukup A, et al. Hyperthermia in the chick embryo: HSP and possible mechanisms of developmental defects. The International Journal of Developmental Biology. 1998;42:737–40. [PubMed] [Google Scholar]
- 25.Walsh D, Grantham J, Zhu XO, et al. The role of heat shock proteins in mammalian differentiation and development. Environmental medicine: annual report of the Research Institute of Environmental Medicine, Nagoya University. 1999;43:79–87. [PubMed] [Google Scholar]
- 26.Niemitz EL, Feinberg AP. Epigenetics and assisted reproductive technology: a call for investigation. Am J Hum Genet. 2004;74:599–609. doi: 10.1086/382897. [DOI] [PMC free article] [PubMed] [Google Scholar]