Abstract
Delay discounting (the devaluation of delayed rewards) has been studied extensively using animal models with psychophysical adjustment procedures. Similar procedures have been developed to assess delay discounting in humans and these procedures most often use hypothetical rewards and delays. The Experiential Discounting Task (EDT) was developed to assess human delay discounting using real rewards and delays. In the present study we examined the test-retest reliability and construct validity of the EDT. Construct validity was evaluated by comparing it to a standard delay discounting task. The EDT had poor test-retest reliability and discounting rates obtained with this task were uncorrelated with those obtained in the standard delay discounting task. Area under the EDT discounting curve was negatively correlated with scores on a measure of boredom proneness (i.e., individuals prone to boredom more steeply discounted delayed money in the EDT). This correlation may underlie previous reports that discounting in the EDT is correlated with addictions, as some evidence suggests boredom proneness is correlated with gambling, cigarette smoking, alcohol consumption, and sensation-seeking. Boredom proneness scores were correlated with no other measure of discounting. These findings suggest the EDT measures a different construct than that measured by traditional delay discounting tasks.
Keywords: Experiential discounting task, delay discounting, probability discounting, test-retest reliability, boredom proneness scale
Delay discounting is the systematic devaluation of an outcome as the delay to its receipt increases (for review see Madden & Bickel, 2010). Delay discounting is thought to underlie a specific type of impulsive choice (Ainslie, 1975)—one characterized by preference for a smaller-sooner reward (SSR) over a larger-later reward (LLR). For example, pay-day loan services profit from those who choose to obtain a small amount of cash now (SSR) over a larger amount of money dispersed later (LLR), when the next paycheck arrives. Choosing the SSR indicates that the value of the LLR is discounted below the undiscounted value of immediate cash.
The seminal work in delay discounting was conducted in nonhuman animal labs and used titrating procedures borrowed from psychophysics (e.g., Mazur, 1987). In these studies, the shape and steepness of the delay discounting function were investigated by giving rats or pigeons repeated choices between SS and LL amounts of food at a range of delays. At each delay, the animal’s choices were used to quantify the discounted value of the LLR. One commonly used method for accomplishing this is to make choice-dependent adjustments to the amount of the immediate reward until the animal is indifferent between the LLR and SSR (e.g., Green, Myerson & Calvert, 2010; Richards, Mitchell, de Wit, & Seiden, 1997). The rate at which delayed rewards are discounted is reflected in the steepness of the curve relating these indifference points to delay and is quantified as the k-parameter in the hyperbolic discounting equation proposed by Mazur (1987):
| (1) |
where V is the present value of a reward of amount (A), available after a delay (D). Higher k values reflect steeper discounting.
In the last 20 years, a considerable amount of research has explored variants of these methods to examine the shape and steepness of the delay discounting function in humans. Most of these studies employ hypothetical monetary outcomes and an adjusting-amount procedure similar to the one described above (e.g., Rachlin, Raineri & Cross, 1991). However, three concerns have often been raised about the procedures used to study delay discounting in humans: a) the vast majority, if not all, of the rewards used in these studies are hypothetical prospects rather than real consequences of choice (e.g., Lawyer, Schoepflin, Green & Jenks, 2011), b) the delays to the LLRs are verbal descriptions of delays rather than delays that will be experienced (e.g., Lagorio & Madden, 2005), and c) steady-state procedures characterizing the animal literature are not used with humans (e.g., Lagorio & Madden, 2005).
These types of concerns motivated the development of the Experiential Discounting Task (EDT) as an alternative method of assessing human delay discounting (Reynolds & Schiffbauer, 2004). In the EDT, humans make repeated choices involving real SSRs and LLRs, real delays to LLR delivery are imposed, and an adjusting-amount procedure is used to obtain stable indifference points (Reynolds, Penfold & Patak, 2008; Reynolds, Richards & de Wit, 2006; Shiels et al. 2009; Voon et al. 2010). Four findings support the use of the EDT as a measure of delay discounting. First, the EDT yields characteristically hyperbolic discounting curves in a single session. Second, choice under the EDT is sensitive to acute experimental manipulations (e.g., alcohol, Reynolds, 2006; and other drugs, Voon et al., 2010, Shiels et al., 2009), whereas choices made in traditional delay discounting tasks (DDTs) with humans are not (see review by de Wit and Mitchell, 2010). Third, the EDT has differentiated substance-dependent populations from matched controls (Fields, Collins, Leraas, & Reynolds, 2009; Reynolds, 2006), a robust finding when traditional DDTs are used (see MacKillop et al., 2011 for a meta-analysis). Fourth, one study using the EDT (Krishnan-Sarin et al., 2007) revealed that individuals whose choices yielded steeper discounting functions tended to have poorer outcomes in a cigarette-smoking treatment trial (an oft-reported finding when traditional DDTs are used, e.g., MacKillop & Kahler, 2009; Sheffer et al., 2012). While the latter finding with the EDT was not replicated among marijuana-dependent individuals (Peters, Petry, LaPaglia, Reynolds, & Carroll, 2012), the relation between steep delay discounting (as measured by traditional DDTs) and marijuana dependence is not strong (e.g., Johnson et al., 2010; Stea, Hodgins, & Lambert, 2011). Thus, with the exception of the finding that EDT-choice is sensitive to acute experimental manipulations where DDT-choice is not, the EDT and DDT have a similar profile and would appear to measure the same construct – delay discounting. Indeed, the advantage would appear to go to the EDT because it arranges real rewards and real delays.
Although the EDT was designed to approximate the choice procedures arranged in nonhuman laboratories, there are three notable differences between these procedural approaches to quantifying delay discounting. First, in the EDT, LLRs are delivered probabilistically (p = .35) whereas they are delivered with certainty after the delay in the animal laboratory. To remove the effects of the probabilistic reward arrangement from the EDT’s measure of delay discounting, the standard practice is to normalize indifference points obtained at delays greater than zero to the indifference point obtained when the delay is zero. However, the adequacy of this normalization procedure has not been rigorously assessed. Second, the EDT arranges no post-reward inter-trial interval (ITI), a common procedure in nonhuman studies arranging real delays to real rewards (e.g., Evenden & Ryan, 1996; Mazur, 1988; Richards et al., 1997). Omitting the ITI allows EDT participants to obtain several SSRs in the time it takes to obtain a single LLR. As such, choosing the SSR may reflect a) sensitivity to local rates of reward (Logue, Peña-Correal, Rodriguez, & Kabela, 1986), b) boredom proneness (i.e., individuals who perceive the delay to the LLR to be particularly boring may choose the SSR as a means of avoiding having to monitor a blank computer display while waiting for the opportunity to collect the reward), or c) discounting the value of the LLR. Third, the delays arranged in the EDT are delays to the delivery of points or coins which cannot be exchanged for goods/services within the session. Hyten, Madden, and Field (1994) referred to the delay to a non-consumable reward as a “point delay” and found that humans exclusively preferred the LL monetary reward when point delays were in the range used in the EDT (see also Belke, Pierce, & Powell, 1989; Flora & Pavlik, 1992; Logue, King, Chavarro, & Volpe, 1990; Logue et al., 1986). Similarly, Jackson and Hackenberg (1996) reported that pigeons most often chose the LLR (a token reinforcer) when the delay to the reward was a point-delay, but strongly preferred the SSR when the delay was to a consumable LLR. Given these findings, why humans discount monetary rewards across the point delays arranged in the EDT is unclear and raises the possibility that the EDT does not measure delay discounting but a separate, yet potentially important behavioral process.
Given the robust findings in the human delay discounting literature (e.g., orderly effects of delay on reward value, systematic relation between steep delay discounting and substance abuse, steep delay discounting predicts the outcome of substance-abuse treatment trials, see MacKillop et al., 2011; Odum, 2011a, 2011b), it is important to evaluate if the EDT measures the same construct as traditional DDTs. The existing literature addressing this question is mixed. Two studies have reported significant positive correlations between EDT and DDT discounting rates (Reynolds, 2006: r = .52; Reynolds et al., 2008: r = .26) but five others found no significant correlation (Krishnan-Sarin et al., 2007; Melanko, Leraas, Collins, Fields, & Reynolds, 2009; Paloyelis, Asherson, Mehta, Faraone, & Kuntsi, 2010; Reynolds et al., 2006; Shiels et al., 2009). While traditional DDTs are known to produce stable measures of delay discounting across re-testing intervals ranging up to 1 year (Kirby, 2009; Ohmura, Takahashi, Kitamura, & Wehr, 2006; Simpson & Vuchinich, 2000) little is known about the test-retest reliability of the EDT. Acheson, Richards and de Wit (2007) reported that EDT discounting rates did not differ significantly when reassessed 10 hours later. However, the short re-test interval raises the possibility that choices made in the first session influenced those made in the second. In addition, test-retest reliability (i.e., the correlation between test and retest EDT discounting rates) was not reported by Acheson et al.
Given the frequent use of the EDT and the socially significant findings that are emerging from studies using the EDT, it is important to further evaluate the relation between the discounting rates produced by the EDT and those produced by more traditional measures of delay discounting. The present study evaluated the construct validity and one-week test-retest reliability of the EDT. Construct validity was evaluated by comparing EDT performance to that obtained using a standardized DDT (Richards, Zhang, Mitchell, & de Wit, 1999), the same DDT used in the two studies that reported a significant positive correlation between delay discounting rates in the EDT and DDT. To explore the role of probability discounting in EDT decision-making, we also examined the relation to measures obtained from a standard probability discounting task (Richards et al., 1999). Boredom proneness (Farmer & Sundberg, 1986) was also assessed to evaluate the hypothesis that those who find particularly aversive no-stimulation intervals might be those participants who, in the EDT, choose the SSR so as to avoid within-task periods in which they must attend to a computer monitor that provides no stimulation (i.e., during delays to the LLR).
Method
Participants
Forty-nine undergraduate students and community members (33 female) participated (M = 21 years, SD = 2.9). The majority of participants (n = 26) completed two 1-hour sessions (separated by seven days) to assess test-retest reliability. The remaining 23 participants completed one session to evaluate correlations between discounting tasks. Participants were compensated between $7 and $15 per session, depending upon choices made in the EDT. Compensation was provided at the end of each session. All procedures were approved by the Utah State University Institutional Review Board.
Procedure
Sessions were completed in a small room (9.5 ft. by 6.5 ft.) containing two tables, one chair, and a desktop computer. During each session participants completed three different behavioral procedures and one self-report task. Sessions began with either the EDT or the combined delay and probability discounting tasks (DDT/PDT); the order in which these tasks were completed was counterbalanced across participants and, for those who completed two sessions, was the same across sessions. These tasks were completed on a computer using applications programmed in Visual Basic 2008. The Boredom Proneness Scale (BPS) was given last and was completed with paper and pencil.
Experiential discounting task (EDT)
The EDT was programmed and implemented exactly as described by Reynolds and Schiffbauer (2004). Specifically, prior to the EDT task, the experimenter read standardized instructions (see Leraas, Patak, Shroff & Reynolds, 2009) and answered any questions by paraphrasing or referring to the relevant portion of the instructions. The experimenter remained in the room as the participant completed one practice trial block (ranging from 16 to 40 trials with the delay equal to 7 s) for which they received no money. Choices were made by pressing one of two on-screen light-bulb buttons. The alternative on the left (standard) offered $0.30 and was unchanged throughout the session. Pressing the standard-alternative button darkened the light bulbs on both buttons (signaling that further button presses had no programmed consequences) and initiated the delay, after which the reward was either available (p = .35) or the next trial began with the re-illumination of the light bulbs. The button on the right side of the screen was the adjusting alternative; the amount available for pressing this button was initially $0.15 (amount displayed below the button) and adjusted based on the participant’s choices. Pressing the adjusting-alternative button illuminated immediately a button with a picture of a bank on it (with p = 1.0). Clicking the bank button once added the reward to the total within-block earnings that were displayed at the bottom of the screen. On trials in which the standard alternative was selected and the probabilistic reward was to be delivered, the bank icon was presented after the delay.
The EDT was composed of four trial-blocks (not including the practice trial-block), each arranging a different delay to the standard-alternative (0, 7, 14 or 28 seconds); delays were completed in ascending order. These delays were chosen to reflect the most recent EDT methodology (Fields, et al., 2009; Melanko et al., 2009; Peters et al., 2012; Reynolds et al., 2008; Shiels et al., 2009; Voon et al., 2010). Each trial-block included a minimum of 16 choice trials. Following each trial the adjusting-amount was increased (decreased) if the participant selected the standard (adjusting) alternative on the preceding trial. Amount adjustments were made in accordance with the standard titrating procedure used in all EDT studies (Reynolds & Schiffbauer, 2004). Briefly, choosing the adjusting alternative initially decreased the amount of that reward by 15%; further choices of the adjusting alternative decreased the percent of the decrease by 2% each time. Choosing the standard alternative inversely changed the amount of the adjusting alternative according to the same percentage-adjustments. If participants selected an alternative four consecutive times, they were forced to select the dis-preferred alternative (i.e., only one choice button was presented).
In accord with standard EDT procedures, stability was evaluated following trial 16 and after each subsequent choice. Choice was considered stable when the standard alternative was selected on three of the previous six free-choice trials. When stability was achieved, all buttons were darkened for the remainder of the trial-block. The duration of a trial block was equal to the delay to the standard alternative arranged in that trial block (e.g., 28 s) times 20 (e.g., 560 s). The timer controlling trial-block duration elapsed only during delays to the standard alternative and during the post-stability portion of the trial block. If participants chose the standard alternative 20 or more times, the trial block was terminated after stability was reached. At the end of the session, an on-screen message displayed the amount earned in each trial block, the total amount earned in the session, and a message to alert the researcher that the session was over.
Delay and probability discounting tasks (DDT/PDT)
The DDT and PDT replicated the procedures used by Richards et al. (1999; see also Baker, Johnson & Bickel, 2003). These are the most commonly used procedure (as well as amounts, delays and probabilities) in studies comparing EDT, DDT, and PDT performance, as well as the task used when a positive relation was found between EDT and DDT performance (Reynolds, 2006; Reynolds, et al., 2008). Before the task began, the experimenter read aloud the on-screen instructions (specifying the hypothetical nature of the outcomes and to click on the amounts to make a selection) and answered participant questions in the same manner as above. For the DDT, the standard alternative was described as $10 to be delivered after a delay (1 week, 2 weeks, 1 month, 6 months and 1 year). For the PDT, the standard alternative was described as $10 delivered probabilistically (p = 1.0, .9, .75, .5, or .25). The standard alternative was presented on the left side of the screen on all trials. As in previous studies, delay and probability trials were intermixed, presented in a randomized sequence, and were separated by a 1-s ITI. The amount of the adjusting alternative (SSR or smaller-certain reward) was chosen within decreasing-limits as described by Johnson and Bickel (2002). The limits (for each delay and probability) adjusted until the maximum-upper and maximum-lower limits (the two extremes) were within $0.50, the average of which was then taken as the indifference point. When an indifference point had been determined at a given delay or probability, choice alternatives were no longer presented from that delay/probability. As described previously (Richards et al., 1999), following 70 choices, distractor questions were used to disguise the adjusting algorithm. Distractor questions were randomly chosen delays (and probabilities) and amounts; choices in these distractor questions did not influence the adjusting-amount algorithm.
Boredom proneness scale (BPS)
We used a 7-point Likert-scale version of the BPS which has been shown to have good internal consistency and test-retest reliability (Farmer & Sundberg, 1986; Vodanovich & Kass, 1990; Watt & Blanchard, 1994; Watt & Ewing, 1996).
Data Analysis
Indifference points from all trial blocks of the EDT were normalized by dividing by the indifference point obtained in the 0-s delay block (Reynolds & Schiffbauer, 2004). For the DDT and PDT, indifference points were divided by $10 to express as a proportion of the standard alternative. The area under the empirically determined indifference points was calculated for individual participants (AUC; Myerson, Green & Warusawitharana, 2001). A single outlier (EDT z-score > 3) was excluded from all analyses. Repeated measures ANOVAs were used to evaluate the main effects of delay and session (i.e., test & retest) on AUC. Test-retest reliability was evaluated with either Pearson, or given violations of homoscedasticity, Spearman correlation coefficients. Wilcoxon signed-rank tests were used to assess systematic variation across the test and retest sessions that might not be detected by correlations (Rousson, Gasser, & Seifert, 2002). Cohen’s (1988) d was used to quantify between-session changes in behavior. All analyses were conducted using GraphPad software (Ver. 5.01) and SPSS (ver. 19.0, SPSS Inc., Chicago, IL). Boredom proneness scores were calculated as the summed scores for all items (with some items reverse coded); scores could range from 27–189 with higher numbers indicating greater proneness to boredom.
Results
The top panel of Figure 1 shows median normalized EDT indifference points as a function of delay for the 26 participants who completed two sessions. The repeated measures ANOVA applied to indifference points revealed a significant main effect of delay (F(3, 23) = 5.913, p < .001), no main effect of session (p = .09), and no session × delay interaction (p = .55). Similarly, Wilcoxon’s test applied to AUC revealed no significant effect of session (see Table 1).
Figure 1.
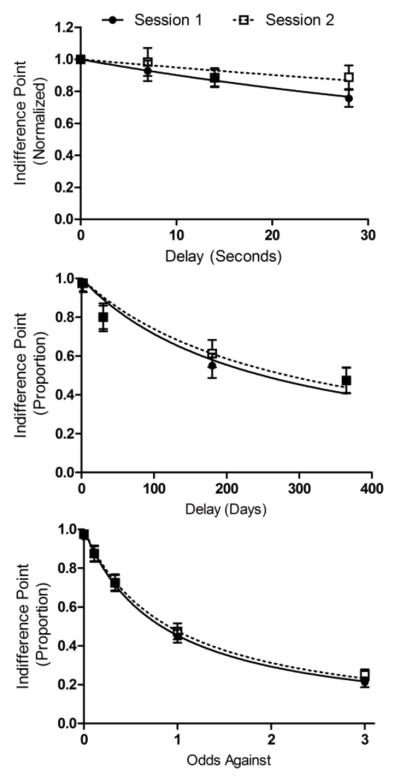
Median indifference points from the discounting tasks in the first and second sessions. Error bars depict standard error of the mean. n = 26.
Table 1.
Mean dependent measures obtained in Sessions 1 and 2 (±SD) in the three different discounting tasks and on the Boredom Proneness Scale. Results of a Wilcoxon Signed-Rank Tests comparing scores across sessions are given by W, p, and Cohen’s d. Pearson or Spearman (bold) between-session correlation coefficients are shown.
| Discounting Task | AUC
|
Wilcoxon’s Test | Test-retest Correlation | ||||
|---|---|---|---|---|---|---|---|
| Session 1 | Session 2 | W | p | d | ρ/r | p | |
| EDT | 0.82 (0.19) | 0.88 (0.26) | −117 | .14 | 0.27 | .32 | .11 |
| DDT | 0.56 (0.29) | 0.53 (0.33) | 43 | .52 | 0.05 | .86 | <.01 |
| PDT | 0.41 (0.13) | 0.47 (0.15) | −143 | .07 | 0.41 | .38 | .06 |
| BPS | 94.0 (19) | 94.0 (17) | 4 | .96 | 0.00 | .89 | <.01 |
The middle and bottom panels of Figure 1 show median indifference points in the DDT and PDT as a function of delay or odds against (i.e., [1−p]/p). The pattern of results was the same across these discounting tasks: main effect of delay (F(3, 23) = 13.32, p < .0001) and of probability (F(3, 23) = 87.5, p < .0001), no main effect of session (DDT: p = .21; PDT: p = .08), and no interaction (DDT: p = .56; PDT: p = .06). The Wilcoxon’s tests indicated that AUC values did not change significantly from the test to the retest (see Table 1).
Table 1 also shows the test-retest correlations obtained in the three discounting tasks as well as on the BPS. Test-retest reliability of the DDT and BPS were significantly positive and classified as “good” (i.e., >.80). By contrast, the one-week test-retest reliability of the EDT and PDT were poor as neither exceeded .40, and neither achieved statistical significance.
The correlation matrix shown in Table 2 addresses the construct validity of the EDT. For these analyses, AUC values obtained with all 49 participants were used. For those participants who completed two sessions, across-session mean AUC-values were used (a procedure that did not affect the qualitative outcomes of the results that follow). As shown in Table 2, and in the top panel of Figure 2, AUC-values obtained in the EDT were not correlated with those obtained in the DDT, nor were they correlated with AUC-values obtained in the PDT (scatterplot not shown). However, choices made in the PDT were positively correlated with indifference points obtained in the 0-sec delay trial block of the EDT (r = .40, p < .01). Thus, when delays were removed from the EDT but the larger reward was probabilistic, participants’ choices in the EDT and PDT were reflective of the same probability-discounting process.
Table 2.
Correlation matrix for all dependent measures
Note.
p < .05.
Bold indicates Spearman’s ρ was used. n = 48.
Figure 2.
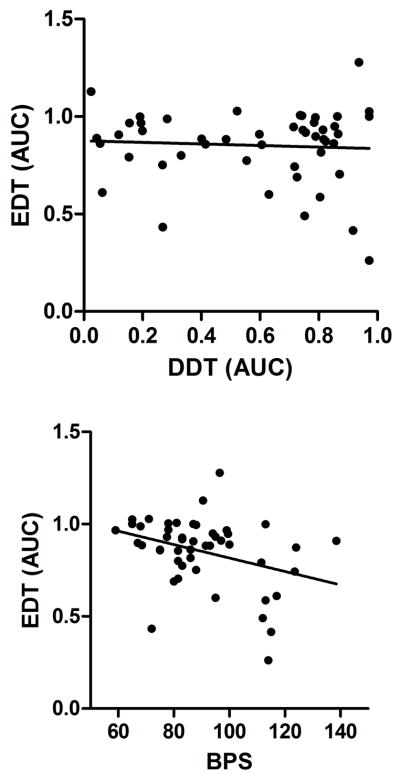
Scatterplot of individual EDT AUC as a function of individual DDT scores (top) and individual boredom proneness scores (bottom) as well as the lines of best-fit. n = 48.
The bottom panel of Figure 2 illustrates that AUC-values from the EDT were significantly negatively correlated with total BPS scores (see Table 2). That is, those who reported that they were generally more susceptible to boredom tended to more often choose the SSR during the EDT. No other significant correlations between BPS scores and the remaining discounting tasks were observed.
Discussion
The goals of the present experiment were to assess the test-retest reliability and the construct validity of the EDT by comparing discounting rates between this task and a widely used and reliable DDT. The 7-day test-retest reliability of the DDT was good (r = .86) whereas it was poor (r = .32) for the EDT. In addition, delay discounting rates (AUC values) in the EDT were not correlated with those obtained in the DDT, despite having ample power to detect such a correlation and using the same DDT used in the two previous studies that have reported a significant correlation (Reynolds, 2006; Reynolds, et al., 2008). When combined with past studies showing that discounting in the EDT is uncorrelated with discounting in a DDT (Krishnan-Sarin et al., 2007; Melanko et al., 2009; Paloyelis et al., 2010; Reynolds et al., 2006; Shiels et al., 2009), the consensus of the evidence suggests that the EDT measures a different construct than is measured by traditional DDTs.
In the remainder of this paper we will discuss three points about the EDT and what it may and may not measure. First, the EDT arranges probabilistic LLRs and this may influence the measure of reward discounting that it provides. In the present study, participants’ choices in the PDT were positively correlated with their choices in the portion of the EDT in which the larger reward is probabilistic but not delayed. Thus, it is clear that probability discounting processes influence choices made in at least this portion of the EDT. What is not known is how probability- and delay-discounting processes combine to affect choice at non-zero delays in the EDT. The most straightforward interaction would be that proposed by Rachlin, Logue, Gibbon, and Frankel (1986) – that probability and delay discounting are a single process wherein repeated trials until a probabilistic reward is received produce a de facto delay to reward. Mazur (1989) provided empirical support for an amended version of this proposal in experiments with pigeons making choices for delayed and probabilistic food. We will raise two objections to applying the findings of Mazur to the EDT. First, the EDT uses non-consumable rewards and, as noted earlier, when non-consumable reinforcers are arranged, delays to the delivery of these rewards do not have the same function as delays to consumable rewards. That is, non-consumable rewards arranged after the range of delays used in the EDT are not discounted in value by humans (e.g., Hyten et al., 1994) or pigeons (Jackson & Hackenberg, 1996); thus, applying the logic of Rachlin et al. to a procedure that arranges a nonfunctional delay appears inappropriate. Second, studies of delay discounting in nonhumans (including those of Mazur, 1989) arrange choice opportunities to occur at regular intervals that are not influenced by the choices made. Thus, animals cannot obtain several SSRs in the time required to obtain a single LLR. Under these conditions, choosing the SSR may be unambiguously described as “impulsive” because it reflects the forgoing of more in favor of now. One cannot make the same unambiguous interpretation of SSR choices in the EDT because choices are not made at regular experimenter-controlled intervals. As a result, EDT participants need not sacrifice income to obtain a reward now and, therefore SSR choices may reflect reward rate-maximization rather than steep delay discounting. When these procedural differences between the EDT and DDTs used with animals are considered in light of the present lack of a correlation between EDT and DDT discounting rates, it suggests that the EDT does not provide a valid measure of delay discounting.
Our second discussion point is to speculate about what the EDT does measure. This may be an important question to answer because some evidence suggests that the EDT differentiates drug-dependent individuals from controls (Fields et al., 2009; Meda et al., 2009; Melanko et al., 2009) and is predictive of addiction-treatment trials (Krishnan-Sarin et al., 2007; although see Peters et al., 2012). Revealing an additional behavioral process that differentiates these populations holds promise for understanding and treating substance dependence.
In the present study, the significant negative correlation between scores on the BPS and AUC values obtained in the EDT (but not the DDT) reveal a potentially important difference between these putative delay discounting tasks. Delays in the DDT are not experienced and, even if they were, these would be delays in which the participant would be free to go about their daily lives until the delayed reward was delivered, for example, in 6 months. By contrast, the brief delays experienced in the EDT are periods in which the participant must wait for the reward while monitoring the computer screen for the presentation of the bank icon (presented on LLR trials in which this probabilistic reward is delivered) or the start of the next trial. Thus, choices made in the DDT reveal the rate at which the reward is devalued because of the delay to its delivery (with no constraint on behavior during the delay), whereas choices made in the EDT would appear to reveal the rate at which the reward is devalued because of the nominal delay plus the costs associated with waiting (see Paglieri, in press, for a discussion of these costs). One cost of EDT delays is, as discussed above, the opportunity cost of the SSRs forgone while waiting for the LLR. Another cost, related to boredom proneness, is the cost of having to observe a blank computer screen while waiting for the bank icon to appear (or not) after the EDT delay. In the EDT, one can avoid these no-stimulation periods by choosing the SSR1; a pattern of choice interpreted previously as steep delay discounting. However, the significant correlation between EDT discount rates and BPS scores suggests that preference for the SSR in the EDT may have more to do with avoidance of the cost of boredom than the devaluation of rewards because they are delayed.
There is some evidence to suggest that the flip-side of avoiding boredom is sensation-seeking. For example, Dahlen, Martin, Ragan, and Kuhlman (2005) reported that BPS scores were significantly correlated with scores on the Arnett Inventory of Sensation Seeking (Arnett, 1994). Similarly, Kass and Vodanovich (1990) reported that boredom-prone and sensation-seeking individuals (the latter measured with the Sensation Seeking Scale; Zuckerman, Eysenck, & Eysenck, 1978) reported a tendency to seek environments in which frequent and novel stimulus changes occurred. Sensation seeking is known to correlate with addictions (Birkley & Smith, 2011), and the same appears to be true of scores on the BPS and problematic gambling (Blaszczynski, McConaghy, Frankova, 1990; Hopley & Nicki, 2010) and tobacco smoking and alcohol problems (Stoltenberg, Lehmann, Christ, Hersrud, & Davies, 2011). If choices made in the EDT are influenced by the participants’ intolerance of boring situations, then past reports of differences between smokers and nonsmokers (Fields et al., 2009; Melanko et al., 2009) on the EDT may be related to boredom-proneness or sensation-seeking. Future investigations of the construct measured by the EDT should include a validated measure of sensation-seeking.
A second behavioral process that the EDT might measure was suggested by informal questioning of a subset of our participants who had just completed the EDT. Most of these participants (7 of 11) indicated that they discriminated within the EDT the contingent relation between choosing the LLR and the subsequent adjusting-amount of the SSR. These participants reported that they exploited this contingency by choosing the (probabilistic) LLR to drive up the SSR amount. This contingency bears formal similarity to what Herrnstein, Loewenstein, Prelec, and Vaughan (2003) referred to as an “internality;” that is, the consequences of current choices are determined by the distribution of past choices. Heyman and Dunn (2002) arranged a choice task (the Harvard Game) containing an internality such that choosing the currently advantageous reward increased the interval between choice opportunities (worsening the consequences for both alternatives) whereas choosing the currently disadvantageous reward decreased the interval between choice opportunities (improving the consequences for both alternatives). Under this game, when compared to matched control participants, substance abusers were less likely to learn to exploit the internality and, as a result, did not maximize their earnings. If the EDT measures an individual’s ability to detect and exploit an internality, this may account for the ability of the EDT to differentiate drug-dependent from control participants (Fields et al., 2009; Meda et al., 2009; Melanko et al., 2009). Future research should compare EDT performance to an internality-detection task (e.g., Heyman & Dunn).
Our final discussion point concerns delay-discounting tasks other than the EDT that arrange real rewards and real delays. The EDT was designed to address critiques of DDTs that arrange hypothetical rewards and delays. The present findings suggest the EDT does not provide an unambiguous means of assessing delaying discounting and, as a result, the search for a practical real-outcome delay-discounting preparation will continue. Two procedures warrant comment. The first of these is referred to as the Quick Discounting Operant Task (QDOT; Johnson, 2012). The QDOT, arranges real monetary rewards (delivered with certainty) following real delays. Like the EDT, the QDOT employs point delays (Hyten et al., 1994) and does not arrange post-reward ITIs. As a result, it will be difficult to interpret QDOT scores as reflective of delay discounting instead of reward rate-maximization and/or boredom-proneness/sensation-seeking. Johnson reported that QDOT and EDT discounting rates were significantly correlated, but neither outcome was correlated with discounting rates in a DDT similar to the one used in the present study. Future research should explore the relation between QDOT scores and boredom-proneness/sensation-seeking.
The second real-outcome delay-discounting procedure involves brief periods of liquid deprivation and subsequent choices between SS and LL liquid rewards (Jimura, Myerson, Hilgard, Braver, & Green, 2009). In this procedure, delays to the LLRs are experienced and choice-opportunities are presented at regular inter-trial intervals regardless of the outcome selected. By using an efficient adjusting-amount algorithm, Jimura et al. (2009) obtained complete hyperbolic delay discounting curves in a single session. Thus, the Jimura et al. procedure closely mirrors the procedures used with nonhumans (real delays within a range commonly employed with nonhumans, rewards that are consumed at the moment they are received, post-reward ITI). In a second study using this task, Jimura et al. (2011) reported that discount rates obtained under this liquid-reward discounting task were uncorrelated with rates obtained under a traditional hypothetical-outcome DDT. Jimura et al. (2011) interpreted this lack of a correlation as evidence that delay discounting rates vary across domains (e.g., commodity type). Other interpretations are possible but these are beyond the scope of the present paper. Of primary importance for the present purposes is that a domain-independence argument cannot be applied to the present findings because the rewards employed in the EDT and the DDT were both monetary.
In sum, the EDT has proven sensitive to decision-making differences between, for example, smokers and nonsmokers, and it may be sensitive to decision-making differences that underlie success in drug-treatment. The present findings suggest that the EDT measures a construct other than delay discounting, with the strongest evidence suggesting that boredom proneness, or perhaps sensation-seeking, influences EDT choice.
Acknowledgments
The experiment was completed in partial fulfillment of the degree of Master of Science by the first author and was partially supported by a grant from the National Institute on Drug Abuse (DA029100) awarded to the penultimate author (ALO). All authors contributed significantly to the conduct of this research and the writing of this manuscript. All authors have read and approved the final manuscript.
The authors would like to thank Timothy A. Shahan for useful comments on an earlier version of the manuscript.
Footnotes
The EDT uses fixed-duration trial-blocks; thus, any delays avoided during the choice portion of the EDT are experienced at the end of the trial block. One might question why boredom-prone participants would choose to avoid boredom now only to experience boredom later. A discounting of delayed aversive events argument could be invoked but it may simply be that choice in the EDT is influenced by within-trial events and not by waiting periods experienced when no choices can be made. Mazur (1989) has reported the same general finding in pigeons – i.e., choice is influenced by choice-dependent delays but not by choice-independent inter-trial intervals.
Disclosures
The authors have no potential or real conflicts of interest to report.
Contributor Information
Rochelle R. Smits, Department of Psychology, Utah State University
Jeffrey S. Stein, Department of Psychology, Utah State University
Patrick S. Johnson, Department of Psychology, Utah State University
Amy L. Odum, Department of Psychology, Utah State University
Gregory J. Madden, Department of Psychology, Utah State University
References
- Acheson A, Richards JB, de Wit H. Effects of sleep deprivation on impulsive behaviors in men and women. Physiology and Behavior. 2007;91:579–587. doi: 10.1016/j.physbeh.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Ainslie G. Specious reward: A behavioral theory of impulsiveness and impulse control. Psychological Bulletin. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Arnett J. Sensation seeking: A new conceptualization and a new scale. Personality & Individual Differences. 1994;16:289–296. [Google Scholar]
- Baker F, Johnson MW, Bickel WK. Delay discounting in current and never-before cigarette smokers: Similarities and differences across commodity, sign and magnitude. Journal of Abnormal Psychology. 2003;112:382–392. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- Belke TW, Pierce WD, Powell RA. Determinants of choice for pigeons and humans on concurrent chains schedules of reinforcement. Journal of the Experimental Analysis of Behavior. 1989;52:97–109. doi: 10.1901/jeab.1989.52-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkley EL, Smith GT. Recent advances in understanding the personality underpinnings of impulsive behavior and their role in risk for addictive behaviors. Current Drug Abuse Reviews. 2011;4:215–227. doi: 10.2174/1874473711104040215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaszczynski A, McConaghy N, Frankova A. Boredom proneness in pathological gambling. Psychological Reports. 1990;67:36–42. doi: 10.2466/pr0.1990.67.1.35. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Dahlen ER, Martin RC, Ragan K, Kuhlman MM. Driving anger, sensation seeking, impulsiveness, and boredom proneness in the prediction of unsafe driving. Accident Analysis & Prevention. 2005;37:341–348. doi: 10.1016/j.aap.2004.10.006. [DOI] [PubMed] [Google Scholar]
- de Wit H, Mitchell SH. Drug effects on delay discounting. In: Madden GJ, Bickel WK, editors. Impulsivity: The Behavioral and Neurological Science of Discounting. Washington, DC: American Psychological Association (APA); 2010. pp. 213–241. [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behavior in rats: The effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Farmer R, Sundberg ND. Boredom proneness—The development and correlates of a new scale. Journal of Personality Assessment. 1986;50:4–17. doi: 10.1207/s15327752jpa5001_2. [DOI] [PubMed] [Google Scholar]
- Fields S, Collins C, Leraas K, Reynolds B. Dimensions of impulsive behavior in adolescent smokers and nonsmokers. Experimental and Clinical Psychopharmacology. 2009;17:302–311. doi: 10.1037/a0017185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora SR, Pavlik WB. Human self-control and the density of reinforcement. Journal of the Experimental Analysis of Behavior. 1992;57:201–208. doi: 10.1901/jeab.1992.57-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J, Calvert AL. Pigeons discounting of probabilistic and delayed reinforcers. Journal of the Experimental Analysis of Behavior. 2010;94:113–123. doi: 10.1901/jeab.2010.94-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrnstein RJ, Loewenstein GF, Prelec D, Vaughan W. Utility maximization and melioration: Internalities in individual choice. Journal of Behavioral Decision Making. 1993;6:149–185. [Google Scholar]
- Heyman GM, Dunn B. Decision biases and persistent illicit drug use: An experimental study of distributed choice and addiction. Drug and Alcohol Dependence. 2002;67:193–203. doi: 10.1016/s0376-8716(02)00071-6. [DOI] [PubMed] [Google Scholar]
- Hopley AB, Nicki RM. Predictive factors of excessive online poker playing. Cyperpsychology, Behavior, and Social Networking. 2010;13:379–385. doi: 10.1089/cyber.2009.0223. [DOI] [PubMed] [Google Scholar]
- Hyten C, Madden GJ, Field DP. Exchange delays and impulsive choice in adult humans. Journal of the Experimental Analysis of Behavior. 1994;62:225–233. doi: 10.1901/jeab.1994.62-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson K, Hackenberg TD. Token reinforcement, choice, and self-control in pigeons. Journal of the Experimental Analysis of Behavior. 1996;66:29–49. doi: 10.1901/jeab.1996.66-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K, Myerson J, Hilgard J, Braver TS, Green L. Are humans really more patient than other animals? Evidence from human discounting of real liquid rewards. Psychonomic Bulletin & Review. 2009;16:1071–1075. doi: 10.3758/PBR.16.6.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K, Myerson J, Hilgard J, Keighley J, Braver TS, Green L. Domain independence and stability in young and older adult’s discounting of delayed rewards. Behavioural Processes. 2011;87:253–259. doi: 10.1016/j.beproc.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW. An efficient operant choice procedure for assessing delay discounting in humans: Initial validation in cocaine-dependent and control individuals. Experimental and Clinical Psychopharmacology. 2012;20:191–204. doi: 10.1037/a0027088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. Journal of the Experimental Analysis of Behavior. 2002;77:129–146. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK, Baker F, Moore BA, Badger GJ, Budney AJ. Delay discounting in current and former marijuana-dependent individuals. Experimental and Clinical Psychopharmacology. 2010;18:99–107. doi: 10.1037/a0018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass SJ, Vodanovich SJ. Boredom proneness: Its relation to Type A behavior pattern and sensation seeking. Psychology: A Journal of Human Behavior. 1990;27:7–16. [Google Scholar]
- Kirby KN. One-year temporal stability of delay-discount rates. Psychonomic Bulletin & Review. 2009;16:457–462. doi: 10.3758/PBR.16.3.457. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, Cavallo DA, Carroll KM, Potenza M. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug and Alcohol Dependence. 2007;88:79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagorio CH, Madden GJ. Discounting of real and hypothetical rewards III: Steady-state assessments, forced-choice trials and all real rewards. Behavioral Processes. 2005;69:173–187. doi: 10.1016/j.beproc.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Lawyer SR, Schoepflin F, Green R, Jenks C. Discounting of hypothetical and potentially real outcomes in nicotine-dependent and nondependent samples. Experimental and Clinical Psychopharmacology. 2011;4:263–274. doi: 10.1037/a0024141. [DOI] [PubMed] [Google Scholar]
- Leraas K, Patak M, Shroff P, Reynolds B. Experiential Discounting Task (EDT)(Version4.01) Center for Biobehavioral Health, The Ohio State University, The Research Institute at Nationwide Children’s Hospital; Columbus, Ohio: 2009. manual. [Google Scholar]
- Logue AW, King GR, Chavarro A, Volpe JS. Matching and maximizing in a self-control paradigm using human subject. Learning and Motivation. 1990;21:340–368. [Google Scholar]
- Logue AW, Peña-Correal TE, Rodriguez ML, Kabela E. Self-control in adult humans: Variation in positive reinforcer amount and delay. Journal of the Experimental Analysis of Behavior. 1986;46:159–173. doi: 10.1901/jeab.1986.46-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR. Delayed reward discounting and addictive behavior: A meta-analysis. Psychopharmacology. 2011;216:305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Kahler CW. Delayed reward discounting predicts treatment response for heavy drinkers receiving smoking cessation treatment. Drug and Alcohol Dependence. 2009;104:197–203. doi: 10.1016/j.drugalcdep.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Bickel WK, editors. Impulsivity: The Behavioral and Neurological Science of Discounting. Washington, DC: American Psychological Association (APA); 2010. [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative Analyses of Behavior: Vol. 5. The effect of delay and of intervening events on reinforcement value. Hillsdale, NJ: Erlbaum; 1987. pp. 55–73. [Google Scholar]
- Mazur JE. Estimation of indifference points with an adjusting-delay procedure. Journal of the Experimental Analysis of Behavior. 1988;49:37–47. doi: 10.1901/jeab.1988.49-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE. Theories of probabilistic reinforcement. Journal of the Experimental Analysis of Behavior. 1989;51:87–99. doi: 10.1901/jeab.1989.51-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda SA, Stevens MC, Potenza MN, Pittman B, Gueorguieva R, Andrews MM, Pearlson GD. Investigating the behavioral and self-report constructs of impulsivity domains using principal component analysis. Behavioral Pharmacology. 2009;20:390–399. doi: 10.1097/FBP.0b013e32833113a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melanko S, Leraas K, Collins C, Fields S, Reynolds B. Characteristics of psychopathy in adolescent nonsmokers and smokers: Relations to delay discounting and self-reported impulsivity. Experimental and Clinical Psychopharmacology. 2009;17:258–265. doi: 10.1037/a0016461. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. Journal of the Experimental Analysis of Behavior. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL. Delay discounting: I’m a k, you’re a k. Journal of the Experimental Analysis of Behavior. 2011a;96:427–439. doi: 10.1901/jeab.2011.96-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL. Delay discounting: Trait variable? Behavioural Processes. 2011b;87:1–9. doi: 10.1016/j.beproc.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmura Y, Takahashi T, Kitamura N, Wehr P. Three-month stability of delay and probability discounting measures. Experimental and Clinical Psychopharmacology. 2006;14:318–328. doi: 10.1037/1064-1297.14.3.318. [DOI] [PubMed] [Google Scholar]
- Paglieri F. The costs of delay. Waiting vs. postponing in intertemporal choice. Journal of the Experimental Analysis of Behavior. doi: 10.1002/jeab.18. (in press) [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Asherson P, Mehta MA, Faraone SV, Kuntsi J. DAT1 and COMT effect on delay discounting and trait impulsivity in male adolescents with attention deficit/hyperactivity disorder and healthy controls. Neuropsychopharmacology. 2010;124:1–13. doi: 10.1038/npp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters EN, Petry NM, LaPaglia DM, Reynolds B, Carroll KM. Delay discounting in adults receiving treatment for marijuana dependence. Experimental and Clinical Psychopharmacology. 2012 doi: 10.1037/a0030943. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H, Logue AW, Gibbon J, Frankel M. Cognition and behavior in studies of choice. Psychological Review. 1986;93:33–45. [Google Scholar]
- Rachlin H, Raineri A, Cross D. Subjective probability and delay. Journal of the Experimental Analysis of Behavior. 1991;55:233–244. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. The Experiential Discounting Task is sensitive to cigarette-smoking status and correlates with a measure of delay discounting. Behavioural Pharmacology. 2006;17:133–142. doi: 10.1097/01.fbp.0000190684.77360.c0. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Penfold PB, Patak M. Dimensions of impulsive behavior in adolescents: Laboratory behavioral assessments. Experimental and Clinical Psychopharmacology. 2008;16:124–131. doi: 10.1037/1064-1297.16.2.124. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, de Wit H. Acute-alcohol effects on the Experiential Discounting Task (EDT) and a question based measure of delay discounting. Pharmacology Biochemistry and Behavior. 2006;83:194–202. doi: 10.1016/j.pbb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Schiffbauer R. Measuring state changes in human delay discounting: An experiential discounting task. Behavioural Processes. 2004;67:343–356. doi: 10.1016/j.beproc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Richards JB, Mitchell SH, de Wit H, Seiden LS. Determination of discount functions in rats with an adjusting-amount procedure. Journal of the Experimental Analysis of Behavior. 1997;67:353–366. doi: 10.1901/jeab.1997.67-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior. Journal of the Experimental Analysis of Behavior. 1999;71:121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousson V, Gasser T, Siefert B. Assessing intrarater, interrater and test-retest reliability of continuous measurements. Statistics in Medicine. 2002;21:3439–3446. doi: 10.1002/sim.1253. [DOI] [PubMed] [Google Scholar]
- Sheffer C, MacKillop J, McGeary J, Landes R, Carter L, Yi R, Jones B, Chistensen D, Stitzer M, Jackson L, Bickel W. Delay discounting, locus of control, and cognitive impulsiveness independently predict tobacco dependence treatment outcomes in a highly dependent, lower socioeconomic group of smokers. The American Journal on Addictions. 2012;21:221–232. doi: 10.1111/j.1521-0391.2012.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels K, Hawk LW, Reynolds B, Mazzullo RJ, Rhodes JD, Pelham WE, Waxmonsky JG, Ganloff BP. Effects of Methylphenidate on discounting of delayed rewards in attention deficit/hyperactivity disorder. Experimental and Clinical Psychopharmacology. 2009;17:291–301. doi: 10.1037/a0017259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CA, Vuchinich RE. Reliability of a measure of temporal discounting. The Psychological Record. 2000;50:3–16. [Google Scholar]
- Stea JN, Hodgins DC, Lambert MJ. Relations between delay discounting and low to moderate gambling, cannabis, and alcohol problems among university students. Behavioural Processes. 2011;88:202–205. doi: 10.1016/j.beproc.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Stoltenberg SF, Lehmann MK, Christ CC, Hersud SL, Davies GE. Associations among types of impulsivity, substance use problems and neurexin–3 polymorphisms. Drug and Alcohol Dependence. 2011;119:e31–e38. doi: 10.1016/j.drugalcdep.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodanovich SJ, Kass SJ. A factor analytic study of the boredom proneness scale. Journal of Personality Assessment. 1990;55:115–123. doi: 10.1207/s15327752jpa8503_05. [DOI] [PubMed] [Google Scholar]
- Voon V, Reynolds B, Brezing C, Gallea C, Skaljic M, Ekanayake V, Fernandez H, Potenza MN, Dolan RJ, Hallett M. Impulsive choice and response in dopamine agonist-related impulse control disorders. Psychopharmacology. 2010;207:645–659. doi: 10.1007/s00213-009-1697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt JD, Blanchard MJ. Boredom proneness and the need for cognition. Journal of Research in Personality. 1994;28:44–51. doi: 10.1006/jrpe.1994.1005. [DOI] [Google Scholar]
- Watt JD, Ewing JE. Toward the development and validation of a measure of sexual boredom. The Journal of Sex Research. 1996;33:57–66. [Google Scholar]
- Zuckerman M, Eysenck S, Eysenck HJ. Sensation seeking in England and America: Cross-cultural, age and sex comparisons. Journal of Consulting and Clinical Psychology. 1978;46:139–149. doi: 10.1037//0022-006x.46.1.139. [DOI] [PubMed] [Google Scholar]


