Abstract
Monosodium urate and tumor necrosis factor-α, are two potent mediators of separate inflammatory response pathways in arthritic joints where inflammation may be accompanied by the loss of chondrocyte vitality via apoptosis. To address this possibility in vitro, chondrocyte cultures were employed to determine the extent to which monosodium urate and recombinant TNF-α altered the frequency of apoptotic chondrocytes. Apoptosis as a function of the activation of p38 kinase, C-Jun-terminal kinase, signal transducer and activator of transcription-3 and/or the activity of xanthine oxidase was also studied. Using normal human chondrocytes, monosodium urate or recombinant tumor necrosis factor-α increased the frequency of apoptosis and activity of xanthine oxidase. However, the xanthine oxidase-specific inhibitor, febuxostat, failed to blunt this response. Monosodium urate, tumor necrosis factor-α or the Janus kinase inhibitor, AG-490, increased the frequency of apoptotic nuclei in macroaggregate pellet cultures initiated from juvenile human chondrocytes, but not in pellet cultures derived from mesenchymal stem cells. In OA chondrocytes, activation of p38, C-Jun-NH2-kinase and signal transducer and activator of transcription-3 preceded apoptosis. Activation of signal transducer and activator of transcription-3 also was seen in pellet cultures initiated from juvenile chondrocytes and MSCs incubated with MSU, recombinant tumor necrosis factor-α or febuxostat, but apoptosis was increased only in the pellet cultures derived from juvenile chondrocytes. Although AG-490 or the combination of AG-490 and febuxostat inhibited signal transducer and activator of transcription-3 activation, apoptosis was unaffected. These results showed that recombinant tumor necrosis factor-α, monosodium urate and AG-490 increased apoptosis in normal human chondrocytes, OA chondrocytes and human juvenile chondrocyte pellet cultures, but not in chondrocyte pellet cultures initiated from MSCs. The increased frequency of apoptotic chondrocytes in response to recombinant tumor necrosis factor-α or monosodium urate was not dependent on either activation of STAT3 or the activity of XO.
Keywords: Apoptosis, Arthritis, Chondrocytes, Human, Monosodium urate, Tumor Necrosis Factor-α (TNF-α)
Introduction
Experimental evidence supports the view that apoptosis plays a critical role in the pathogenesis and progression of various forms of arthritis as well as other autoimmune-mediated and chronic non-inflammatory arthritic disorders of synovial joints [1]. For example, the elevated frequency of apoptotic chondrocytes was shown to partially account for the significant decline in chondrocyte vitality of osteoarthritic (OA) human cartilage [2-5].
Significantly elevated levels of monosodium urate (MSU) monohydrate and tumor necrosis factor-α (TNF-α) in arthritic synovial fluid are thought, in part, to result in reduced chondrocyte vitality and markedly deficient repair of articular cartilage. Thus, defective cartilage repair in arthritis is generally believed to arise from the combined effects of the reduced capacity of articular chondrocytes to survive in the presence of nitric oxide, TNF-α, IL-1β [4-6] or MSU as well as an inadequate chondrocyte response to insulin-like growth factor-1 [7].
This in vitro study was primarily designed to determine the extent to which MSU or recombinant human TNF-α (rhTNF-α) increased the frequency of apoptosis in human chondrocytes. We also investigated the extent to which MSU and rhTNF-α activated the SAPK/MAPK and JAK/STAT pathways. TNF-α-mediated activation of p38 kinase, C-Jun-terminal protein kinase (JNK) and STAT proteins were previously shown to regulate the induction of apoptosis in chondrocytes as well as other cell types [8-11] whereas MSU was reported to primarily activate signaling pathways involving proline-rich kinase and Src kinase in chondrocytes [12].
Importantly, the potential contribution of xanthine oxidase (XO), (the terminal enzyme required for catalyzing the hydroxylation of xanthine to hypoxanthine) and its role in JAK/STAT activation was reported [13]. Therefore, we assessed the potential role of XO in the induction of apoptosis by MSU or rhTNF-α. Previous studies had also shown that modulating the activity of XO attenuated the induction of apoptosis in other cell types [13,14]. In addition, modulation of XO activity could result from activation of JAK/STAT. Indeed, Albuquerque et al. [15] showed that under hypoxic conditions, IL-6 activated JAK/STAT and sequentially JAK/STAT activated XO.
The study of the role of XO activity on chondrocyte apoptosis was facilitated by employing the non-purinergic XO-specific inhibitor, 2-[3-cyano-4-(2-methylpropoxy) phenyl]-4-methyl-thiazole-5-carboxylic acid [16]. This compound, formally known as TEI-6720 and now called febuxostat, was shown to inhibit XO activity in A549 lung cancer cells at a concentration of 16 μM without altering the activity of other purinergic enzymes [17].
Materials and Methods
Cell culture, reagents and antibodies
Culture media, fetal bovine serum and antibiotics were obtained from Invitrogen/Life Technologies (Carlsbad, CA, USA). The JAK2 inhibitor, Tyrphostin B42 (AG-490) was obtained from Cayman Chemical (Ann Arbor, MI, USA). rhTNF-α was purchased from Prospecbio (Rehovot, ISRAEL) or R and D Systems (Minneapolis, MN, USA). An in situ cell death detection kit for use in the Terminal deoxynucleotidyl transferase dUTP Nick End Labeling (TUNEL) assay was purchased from Roche Diagnostic GmbH (Mannheim, Germany). The Vecta-shield™ reagent containing 4’,6’ diamino-2-phenylindole.2HCl (DAPI) was obtained from Vector Laboratories (Servion, SWITZERLAND). (2-[3-cyano-4-(2-methylpropoxy) phenyl]-4-methyl-thiazole-5-carboxylic acid; febuxostat; Lot # 47006CB00) was kindly provided by Takeda Pharmaceuticals of North America, Inc (Deerfield, IL, USA) through a research contract agreement with CJM. The XO activity kit and pronase was purchased from Sigma (St. Louis, MO, USA). To detect p38 kinase, JNK and STAT3 by Western blotting, phosphorylation and non-phosphorylation state-specific polyclonal antibodies (Cell Signaling Technologies, Beverly, MA) were employed. For immunohistochemical detection of STAT3, an anti-STAT3 monoclonal antibody that detects endogenous human, mouse and rat unphosphorylated STAT3 (U-STAT3) and an affinity-purified rabbit anti-STAT3 antibody that detects STAT3 phosphorylated at Y705 (p-STAT3) were obtained from R and D Systems. The anti-mouse/anti-rabbit EnVision System containing the HRP-labeled polymer, DAKO was purchased from Glostrup (Copenhagen, Denmark). A monoclonal antibody reactive with Type II collagen (11-116B3) was obtained from the Developmental Studies Hybridoma Bank (http://dshb.biology.uiowa.edu/). An antibody reactive with Type X collagen was a mouse polyclonal antibody that was generously supplied by Dr. Gary Gibson (Henry Ford Hospital, Detroit, MI). A biotinylated goat anti-mouse antibody was obtained from Cappel Laboratories (Malvern, PA) and streptavidin-peroxidase was purchased from InVitrogen. The Vector VIP™ substrate kit was obtained from Vector Laboratories.
MSU crystal suspension
MSU crystals were a gift from Dr. R. Liu-Bryan (Veteran’s Administration Medical Center, University of California at San Diego, San Diego, CA, USA). 10 μl of a suspension of these MSU crystals contained 0.03 EU/ml as measured by the Limulus assay and was judged to be free of contamination by endotoxin. Of note, an overnight incubation of mouse bone marrow-derived macrophages with this MSU crystal suspension at a concentration of 0.5 mg/ml caused the release of 650 pg/ml of IL-1β into the culture medium (Liu-Bryan, personal communication).
Human chondrocyte cultures
Four sources of human chondrocytes were obtained and cultures prepared for this study.
Osteoarthritic chondrocytes (OA-Hu-C) were dissociated by enzyme digestion from the “resident” cartilage of subjects undergoing joint replacement surgery for end-stage disease. First passage cultures were employed and maintained as previously described [18,19].
Normal human chondrocytes (Hu-C) were purchased from Cell Applications, Inc (Cedarlane Labs, Burlington, Ontario, Canada). Chondrocytes were maintained in DMEM/F12 (1:1) supplemented with 10% fetal bovine serum. Chondrocytes were sub-passaged according to the vendor’s instructions and maintained as previously described [18,19].
Normal juvenile human chondrocytes (N-JHu-C) were enzymatically dissociated from the knee cartilage of a 12 year old subject as previously described [20]. The patient had a diagnosis of osteosarcoma and cartilage was obtained from the amputated femur. N-JHu-C in first passage was maintained as macroaggregate pellet cultures as previously described [21,22].
Chondrocytes were derived from the lineage of bone marrow-derived MSCs (BMD-MSC-C) obtained from 3 subjects, aged 24, 28, and 50 years and maintained in macroaggregate pellet culture as previously described [21,22].
For specific experiments, chondrocyte cultures were initiated at the following cell densities, OA-Hu-C or Hu-C: 105 cells/ml; N-JHu-C and BMD-MSC-C macroaggregate pellet cultures: 2.5×105 cells/ml. OA-Hu-C or Hu-C was maintained until the cells reached the hyperdense state as previously defined [18]. N-JHu-C and BMD-MSC-C macroaggregate pellet cultures were maintained for up to 3 weeks prior to initiating specific experiments.
At the time of initiating specific experiments, chondrocyte cultures were either untreated (control; no additions) or incubated with rhTNF-α (1.5 ng/ml to 50 ng/ml); with MSU (0.5 mg/ml); or combinations of the following: MSU plus AG-490 (50 μM), rhTNF-α or MSU plus AG-490 plus febuxostat (16 μM), or febuxostat (8-33 μM). Cultures were kept under these conditions for up to 48 hrs.
Apoptosis assays
Induction of apoptosis after incubation of OA-Hu-C with rhTNF-α (10 ng/ml) was also determined by electrophoresis on 1.2% agarose gels of intranucleosomal DNA fragments which were then stained with ethidium bromide (EtBr) as previously described [18,23]. Computer-assisted image-enhanced photomicrographs of macroaggregate pellet culture 5 μm cross-sections or microscopic fields of hyperdense Hu-C cultures were employed to determine the frequency of TUNEL-positive chondrocyte nuclei [24,25].
Western blots
Total cytosolic protein lysate (25 μg/lane) was resolved by denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Bio-Rad). Western blots were produced essentially as previously described by Singh et al. [26].
Staining of chondrocyte macroaggregate pellet cultures with Toluidine blue O
The sulfated proteoglycan content of the chondrocyte macroaggregate pellet culture extracellular matrix (ECM) was visualized by staining JHu-C and BMD-MSC-C macroaggregate pellet culture 5 μm cross-sections with Toluidine blue O [19].
Type II collagen and Type X collagen immunohistochemistry
5 μm cross-sections were rehydrated in PBS, followed by incubation with pronase (1 mg/ml) in PBS containing 4 mM CaCl2 for 10 min at room temperature, washed with PBS twice, blocked with 3% BSA/PBS and then incubated with anti-type II collagen antibody (1:200) or anti-type X collagen antibody (1:200) in 1% BSA/PBS for 1.5 hours. The slides were washed and then incubated with 1:200 dilution of biotinylated goat anti-mouse IgG in 1% BSA/PBS for 1 hour at room temperature, washed, and incubated with streptavidin peroxidase for 30 min followed by PBS. The peroxidase enzyme reaction was visualized using the Vector VIP™ substrate kit according to the manufacturer’s instructions. Cross-sections were then counterstained with 0.00125% Fast Green (w/vol) in a mixture of 24% ethanol/1% acetic acid (vol/vol).
U-STAT3/p-STAT3 immunohistochemistry
Indirect immunoperoxidase staining was employed to detect U-STAT3 and p-STAT3 immunoreactive products. An anti-mouse/anti-rabbit EnVision System containing the HRP-labeled polymer, DAKO with diaminobenzidine (DAB) as the chromogen was used with 10% neutral-buffered formalin-fixed paraffin-embedded 5 μM cross-sections of N-JHuC and BMD-MSC-C macroaggregate pellets. Human lung tissue was employed to demonstrate the positive immunoreaction for U-STAT3 and p-STAT3 antibodies, respectively.
Statistical analysis
The number of TUNEL-positive chondrocytes or XO activity was determined in Hu-C, N-JHu-C and BMD-MSC-C by an evaluator blinded to the various treatment groups. For data analysis, the significance of differences in XO activity between the control group and the various treatment groups was performed using the Student’s t-test calculation. The significance of differences in TUNEL values in the various treatment groups were assessed by calculating the critical-F value using analysis of variance and Tukey’s post hoc test. A p-value of <0.05 was considered as the level of statistical significance.
Protection of human subjects
Each experimental protocol for the acquisition of normal human and OA cartilage and/or iliac crest bone marrow samples were approved by the University Hospitals Case Medical Center Institutional Review Board. Experimental protocols and the language in the informed consent documents conformed to the 1990 Declaration of Helsinki governing the ethical conduct of human subject’s research.
Results
Apoptosis in OA-Hu-C
Treatment of OA-Hu-C with rhTNF-α at concentrations ranging from 10 ng/ml to 50 ng/ml for up to 48 hrs resulted in the appearance of intranucleosomal DNA fragments (Figure 1). The migration of these DNA fragments (Figure 1, arrows) was consistent with those fragments produced by apoptotic cells.
Figure 1.
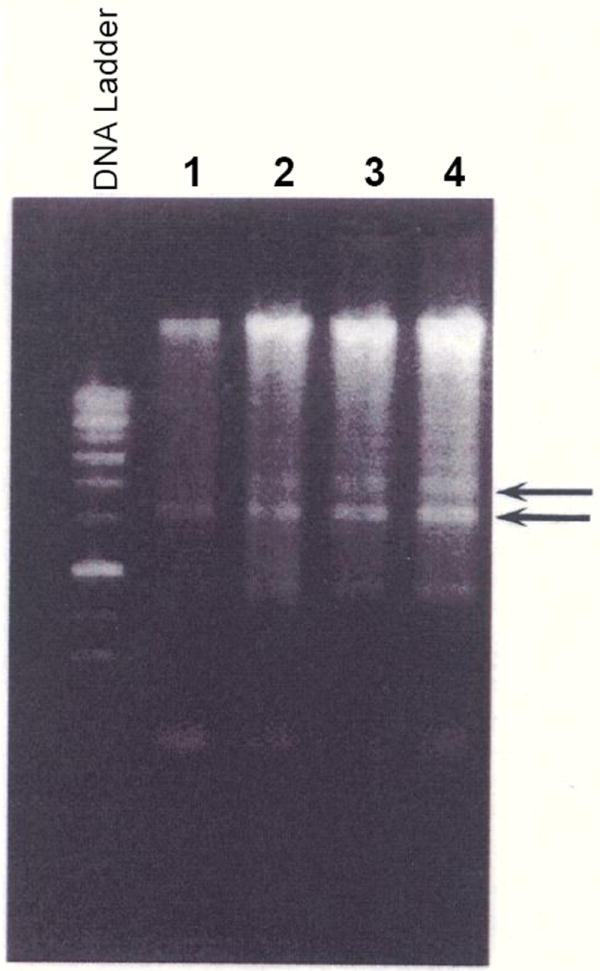
rhTNF-α Increases Apoptosis in OA Chondrocytes. rhTNF-α (10 ng/ml to 50 ng/ml) was incubated with OA chondrocytes for 48 hrs. Intranucleosomal DNA fragments were identified using the manufacturer’s protocol as previously modified by Islam et al. [23]. DNA electrophoresed on a 1.2% agarose gel, stained with ethidium bromide (EtBr) and DNA fragments visualized using a Vilber-Laurmat UV Illuminator [23]. The lane marked DNA Ladder (supplied by the manufacturer) shows the migration of EtBr-positive bands characteristic of intranucleosomal DNA fragments from apoptotic cells. Lane 1: No addition; Lane 2: rhTNF-α (10 ng/ml); Lane 3: rhTNF-α (20 ng/ml); Lane 4: rhTNF-α (50 ng/ml). Note the 2 DNA fragments indicated by the arrows which showed increased EtBr signal intensity as a function of rhTNF-α concentration.
rhTNF-α increased XO activity in OA-Hu-C
The baseline XO activity was 41.6 mU/ml. XO activity increased following incubation of OA-Hu-C with rhTNF-α for 24 hrs and continued to rise from baseline at 48 hours (Table 1) The increase in XO activity from baseline at 48 hours was Δ%=+95.4. At the same time evidence of apoptosis was also seen (Figure 1).
Table 1.
Recombinant Human TNF-α (rhTNF-α) Increases XO Activity in OA-Hu-C.
ng/ml
mU/ml
Baseline XO activity was 41.6 mU/ml
Febuxostat inhibited XO activity in Hu-C
XO activity in Hu-C was determined in the control group (i.e. no additions) or after adding varying concentrations of febuxostat for 24 hours. The change in XO activity from baseline was Δ%=-45% with 8 μM febuxostat (p>0.05), Δ%=-60% (p<0.05) with 16 μM febuxostat and Δ%=-77% (p<0.05) with 33 μM febuxostat.
AG-490, rhTNF-α, or rhTNF-α plus febuxostat increased apoptosis in Hu-C
Treatment of Hu-C with AG-490, rhTNF-α alone or the combination of rhTNF-α plus febuxostat significantly increased the number of TUNEL-positive nuclei (F=7.6544; p=0.008) (Figure 2).
Figure 2.
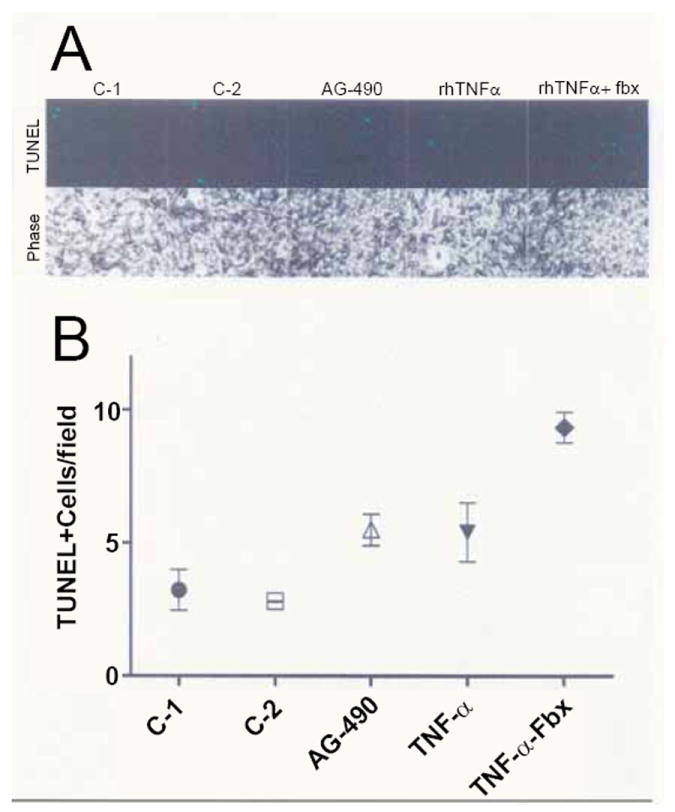
rhTNF-α, AG-490 and rhTNF-α/febuxostat Increase TUNEL-Positive Hu-C. Normal human chondrocytes were grown in 3.8 cm2 microwell culture dishes and incubated either with rhTNF-α (1.5 ng/ml), AG-490 (50 μM) or rhTNF-α (1.5 ng/ml) plus febuxostat (fbx) (16μM) (n=3) for 24 hours. The total number of TUNEL-positive cells in at least 60 individual fields was enumerated. Panel A: TUNEL-positive chondrocytes with matching phase-contrast images. Panel B: The total number of TUNEL-positive chondrocytes in a representative field from the control groups and from each of the experimental groups is shown. There were 2 control groups: C-1 contained medium alone; C-2 contained medium plus DMSO at the concentration used to dissolve AG-490.
XO activity is uncoupled from apoptosis in Hu-C
XO activity was also significantly increased after treatment of Hu-C with rhTNF-α, AG-490 or rhTNF-α plus febuxostat (F=4.1407; p=0.048) with the most significant inhibition of XO activity (Δ%=-64.9%) occurring in the rhTNF-α plus febuxostat treatment group. However, treatment of Hu-C with rhTNF-α plus febuxostat also produced the highest frequency of TUNEL-positive chondrocytes (Figure 2).
Western blotting for phosphorylated and un-phosphorylated p38 kinase, JNK and STAT3 in OA-Hu-C
The immunoreactive products of un-phosphorylated p38 kinase (Figure 3, Panel A), SAPK p46 (JNK-1), SAPK p54 (JNK-2) (Figure 3, Panel B) or STAT3 (Figure 3, Panel C) were unchanged by rhTNF-α. Because the un-phosphorylated kinases were constitutively produced by these chondrocytes, the level of these immunoreactive products also served as a control for the loading of each lane. RhTNF-α (10 ng/ml) activated p38 kinase (phospho-p38-MAPK; Figure 3, Panel A), phospho-SAPK p46 (JNK-1), phospho-SAPK, p54 (JNK-2) (Figure 3, Panel B) and STAT3 (p-STAT3; Figure 3, Panel C). Immunoreactive cytosolic phospho-p38 kinase and p-STAT3 increased up to 30 min decreasing thereafter, whilst the level of phospho-SAPK p46 and phospho-SAPK p54 was increased at 5 min decreasing thereafter.
Figure 3.
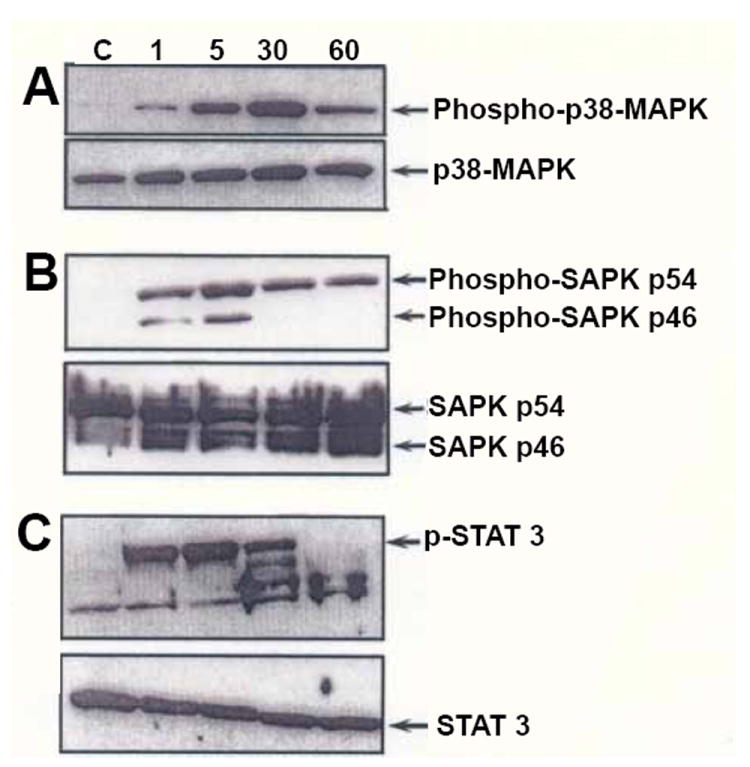
Western blotting for p38 kinase, JNK and STAT3. Human OA chondrocytes were grown to hyperdensity as previously described [18,26] and treated with rhTNF-α (10ng/ml) or not (C) for up to 60 min. Cytosolic protein lysate from the 1, 5, 30 and 60 min incubations were electrophoresed as previously described and Western blotting performed [26].
Panels A and B: Western blots were produced as previously described [26] using anti-phospho-p-38 MAPK antibody or an anti-p38 MAPK antibody; (Panel A), anti-phospho-SAPK p54 (JNK 2) antibody/anti-phospho-SAPK p46 (JNK 1) antibody or (Panel B) anti-SAPK p54 (JNK 2)/anti-SAPK p46 (JNK 1) antibody.
Panel C: A Western blot was produced with the specific anti-U-STAT3 antibody or anti-phospho-STAT3 antibody as indicated in Materials and Methods. The bands migrating faster than the phospho-STAT3 standard have retained reactivity with anti-phospho-STAT3, but have not been fully characterized. These bands may represent degradation products of phospho-STAT3. The fastest migrating band reacting with anti-phospho-STAT3 was also present in the control (C) group.
Histology of N-JHu-C and BMD-MSC-C macroaggregate pellet cultures
N-JHu-C and BMD-MSC-C macroaggregate pellet cultures stained with toluidine blue O showed that the final chondrocyte density achieved in the N-JHu-C macroaggregate pellet cultures (Figure 4A) was lower compared to BMD-MSC-C macroaggregate pellet cultures (Figure 4B). In addition, the ECM of BMD-MSC-C appeared to be more topographically heterogeneous than N-JHu-C. Thus, the metachromatic staining intensity of the ECM in BMD-MSC-C was accompanied by a multilayer of flattened cells at the periphery of the macroaggregate pellet culture cross-section which stained principally orthochromatic (Figure 4C). The results shown in figure 4C were obtained with each of the 3 MSC samples used to generate these macroaggregate pellet cultures. Of note, the metachromatic appearance of the ECM in N-JHu-C and BMD-MSC-C pellet cultures was not altered after treatment with MSU or rhTNF-α (results not shown).
Figure 4.
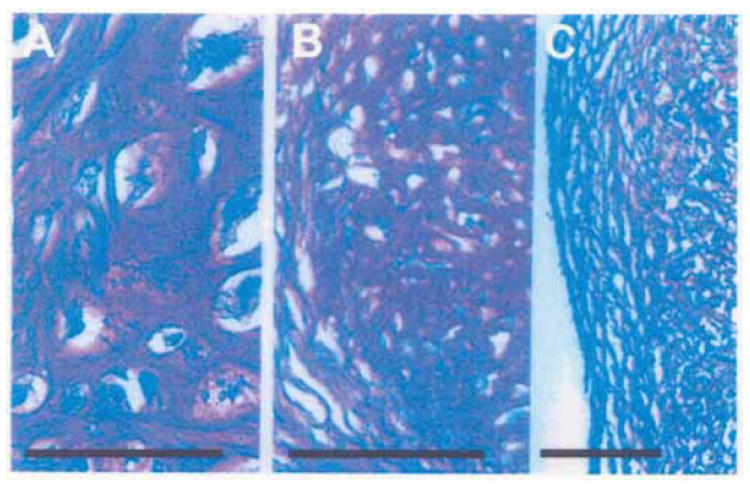
Histology of N-JHu-C and BMD-MSC-C Macroaggregate Pellet Cultures. A 5μm cross-section of normal juvenile chondrocyte pellet cultures (N-JHu-C) and pellet cultures initiated from MSCs (BMD-MSC-C) was stained with Toluidine blue O. A: N-JHu-C; B: BMD-MSC-C; C: BMD-MSC-C; Bar=200 μm.
Collagen immunohistochemistry
The ECM of the N-JHu-C macroaggregate pellet culture contained Type II collagen. In contrast, the ECM of BMD-MSC-C contained both Type II and Type X collagen. BMD-MSC-C incubated with either rhTNF-α or MSU for 60 min did not alter the intensity of the Type II collagen or Type X collagen immunoreactive products. However, BMD-MSC-C treated with AG-490 appeared to increase the intensity of the anti-type X collagen immunoreactive product.
Apoptosis in N-JHu-C macroaggregate pellet cultures
The total number of chondrocytes calculated from summating TUNEL-positive, TUNEL-negative and DAPI-positive nuclei was similar in each of the experimental groups. The number of TUNEL-positive N-JHu-C was increased after treatment for 60 min with MSU or rhTNF-α compared to the control group (Table 2). Furthermore, the frequency of TUNEL-positive nuclei also increased after incubation of N-JHu-C with AG-490 alone. However, the combined treatment of N-JHu-C with rhTNF-α plus AG-490 or MSU plus AG-490 for 60 min did not appreciably increase the number of TUNEL-positive chondrocytes compared to rhTNF-α or MSU alone (Table 2).
Table 2.
The frequency of TUNEL-Positive Nuclei in normal juvenile chondrocyte macroaggregate pellet cultures.
| Group | Control | rhTNF-α | AG-490 | rhTNF-α + AG-490 | MSU | MSU + AG-490 |
|---|---|---|---|---|---|---|
| Total Cells | 489 | 522 | 523 | 491 | 548 | 555 |
| TUNEL +ve (%) | 166 | 492 | 463 | 443 | 500 | 530 |
| TUNEL +ve (%) | 33.9 | 94.3 | 88.5 | 90.2 | 91.2 | 95.4 |
| Δ (%) | — | +178% | +161% | +166% | +169% | +181% |
TUNEL positive (TUNEL +ve) chondrocytes were enumerated from computer-assisted enhanced images
Apoptosis in BMD-MSC-C macroaggregate pellet cultures
In contrast to N-JHu-C, neither treatment of BMD-MSC-C with MSU (Figure 5) nor the combined treatment group of MSU plus AG-490+febuxostat (data not shown) increased the number of TUNEL-positive chondrocytes. Treatment with rhTNF-α also failed to increase the number of TUNEL-positive nuclei compared to control (data not shown).
Figure 5.
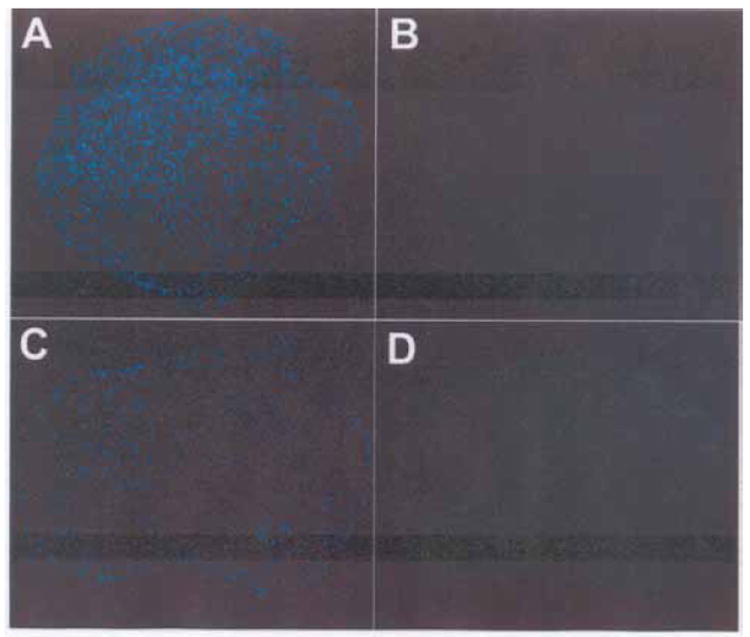
MSU Does Not Increase the Number of TUNEL-Positive Chondrocytes in Macroaggregate Pellet Cultures Initiated from MSCs. A chondrocyte macroaggregate pellet culture initiated from MSCs (BMD-MSC-C) was fixed in formalin and embedded in paraffin. A 5 μm histologic cross-section was prepared from the experimental group that had been incubated for 60 min with MSU (see Materials and Methods for details). Following deparaffinization, the cross-sections were processed for detection of TUNEL-positive chondrocytes and then the chondrocytes were stained with DAPI. A merged DAPI/TUNEL stained cross-section is shown.
Control, No additions, 0 min; Control, No additions, 60 min; MSU, 0 min; MSU, 60 min. A representative of one of the 3 MSC samples obtained for this study is shown
Un-phosphorylated-STAT3 (U-STAT3) and phosphorylated-STAT3 (p-STAT3) in N-JHu-C and BMD-MSC-C
The analysis of changes in U-STAT3 and p-STAT3 by rhTNF-α, MSU, AG-490 and various combinations of these factors plus febuxostat is summarized in Table 3.
Table 3.
U-STAT3 and p-STAT3 in normal juvenile chondrocyte pellet cultures (N-JHu-C) and pellet cultures initiated from MSCs (BMD-MSC-C).
| Macroaggregate PC | Treatment | U-STAT3 | p-STAT3 |
|---|---|---|---|
| N-JHu-C | No Addition | + | - |
| rhTNF-α | ↑ * | ↑ * | |
| MSU | ↑ * | ↑ * | |
| AG-490 | ↑ * | ↑ * | |
| rhTNF-α + AG-490 | ↓ ** | ↓ ** | |
| MSU + AG-490 | ↓ | ↓ ** | |
| BMD-MSC-C | No Addition | + | - |
| MSU | ↔ * | ↔ * | |
| MSU+AG-490 | ↑ ** | ↔ ** | |
| MSU+AG-490+Febuxostat | ↑ ** | ↑ ** | |
| rhTNF-α | ↑ * | ↑ * | |
| rhTNF-α+AG-490+Febuxostat | ↓ ** | ↔ ** |
Increase (↑), Decrease (↓), No Difference (↔) compared to No Addition
Increase (↑), Decrease (↓), No Difference (↔) compared to MSU, AG-490 or rhTNF-α
Treatment of N-JHu-C with rhTNF-α, MSU or AG-490 for 60 min increased the relative number of both U-STAT3 and p-STAT3-positive chondrocytes compared to U-STAT3 and p-STAT3-positive chondrocytes in the control group. However, the combination of rhTNF-α and AG-490 or MSU plus AG-490 reduced the U-STAT3 and p-STAT3 immunoreactive product relative to rhTNF-α, MSU or AG-490 alone. rhTNF-α increased the p-STAT3 and the U-STAT3 immunoreactive product after 60 min compared to control. Although the combination of rhTNF-α plus AG-490 plus febuxostat decreased the p-STAT3 immunoreactive product, treatment with rhTNF-α plus AG-490 and febuxostat did not alter the U-STAT3 immunoreactive product compared to control. Compared to the baseline immunoreactivity of p-STAT3 and U-STAT3, it was primarily U-STAT3 that was increased by MSU plus AG-490 after 60 min. However, this result was not seen with MSU alone after either 30 min (data not shown) or 60 min. The combination of MSU plus AG-490+febuxostat substantially increased both the p-STAT3 and U-STAT3 immunoreactive products at 30 min compared to MSU and AG-490, but by 60 min, the p-STAT3 immunoreactive product in the MSU plus AG-490+febuxostat group was markedly reduced compared to the p-STAT3 immunoreactive product after treatment with MSU plus AG-490 plus febuxostat for 30 min (data not shown).
XO Activity in BMD-MSC-C
Treatment of BMD-MSC-C with rhTNF-α, rhTNF-α plus AG-490, AG-490 alone, MSU alone, or MSU plus AG-490 did not significantly alter the activity of XO (F=0.2812; P=0.9173).
Discussion
It has been speculated that the loss of chondrocyte vitality in arthritic synovial joints occurs principally by apoptosis [1]. In this study, we demonstrated that MSU and rhTNF-α, both of which are significantly elevated in OA, rheumatoid arthritis and gouty arthritic synovial fluid [27-29], could increase the frequency of apoptotic human chondrocytes in vitro.
With respect to normal and OA human chondrocytes maintained as hyperdense cultures, the major findings of this study were that rhTNF-α-induced apoptosis in these cultures which was preceded by activation of p38 kinase, JNK and STAT3 as well as increased XO activity. The increased frequency of apoptosis in OA chondrocytes in response to rhTNF-α was preceded by the appearance of p-STAT3 in the cytosol as early as 1 min after adding rhTNF-α but without changing the content of U-STAT3, even over the 60 min incubation period. Thus, these results are likely to reflect the transport of p-STAT3 from the cytosol to the nucleus over the course of 60 min. Of note, there was no evidence for activation of any other STAT proteins after OA chondrocytes were incubated with rhTNF-α under these conditions (data not shown).
Although MSU or rhTNF-α increased the frequency of TUNEL-positive nuclei in N-JHu-C macroaggregate pellet cultures, neither MSU nor rhTNF-α increased the frequency of TUNEL-positive chondrocytes in BMD-MSC-C. Additionally, MSU or rhTNF-α increased p-STAT3 in N-JHu-C and BMD-MSC-C, but apoptotic chondrocytes only increased in N-JHu-C. This result suggested that the increase in the frequency of apoptotic nuclei in juvenile chondrocyte macroaggregate pellet cultures occurred independently of MSU or rhTNF-α-mediated STAT3 activation.
The failure of either MSU or rhTNF-α to increase the frequency of TUNEL positive nuclei in chondrocyte pellet cultures initiated from MSCs may have come about from what has been termed “inadequate apoptosis” [30]. The analysis of Type II versus Type X collagen in these chondrocyte macroaggregate pellet cultures was employed, in part, to determine the extent to which the differential apoptosis response seen between pellet cultures from juvenile chondrocytes and MSCs in response to rhTNF-α or MSU reflected their developmental characteristics. Thus, the finding of Type II and Type X collagen isotype expression in the pellet cultures initiated from MSCs suggested a chondrogenic phenotype that was more closely aligned with epiphyseal growth plate development and fracture callus, whereas the absence of Type X collagen in the pellet cultures initiated from authentic juvenile chondrocytes suggested an articular cartilage phenotype. In that regard, it is generally viewed that during growth plate cartilage development and fracture callus maturation into mineralized bone, metalloproteinase activity and apoptosis precede the de-repression of the type X collagen gene [31,32]. Therefore it is possible that apoptosis could no longer be induced by MSU or rhTNF-α in the pellet cultures initiated from MSCs after the Type X collagen gene was expressed.
RhTNF-α plus febuxostat or AG-490 alone increased the number of TUNEL-positive normal human chondrocytes. However, XO activity was attenuated after treatment of normal human chondrocytes with AG-490 or a combination of rhTNF-α plus febuxostat.
Interestingly, in none of the treatment groups, including the groups in which chondrocytes were treated with rhTNF-α, rhTNF-α plus AG-490, AG-490, MSU or MSU plus AG-490 altered the activity of XO in pellet cultures initiated from MSCs. Although the overarching significance of this latter result remains unclear in view of the finding that MSU or rhTNF-α also failed to increase the number of TUNEL-positive chondrocytes, this data suggested that “apoptosis resistance” to these treatments likely did not involve XO as a potential regulator of STAT activation [13,14] or XO as a contributor to chondrocyte apoptosis.
To our knowledge, our finding that MSU increased the frequency of apoptotic normal juvenile chondrocytes maintained in a three-dimensional ECM in vitro has not been previously reported. A previous study had shown that MSU-induced apoptosis in human chondrocyte monolayer cultures occurred as a result of MSU-stimulated production of NO [33]. Apoptosis induced by MSU under those conditions was also found to be dependent on the activation of proline-rich tyrosine kinase and Src kinase [12]. Since we did not determine the extent to which MSU altered NO levels in the normal juvenile chondrocyte pellet cultures, it remains to be determined if the MSU-mediated increase in TUNEL-positive chondrocytes also involved NO. This may prove to be critical because NO is also a potent inducer of chondrocyte apoptosis [1,10,28].
Inhibition of JAK/STAT pathway activation by AG-490 was previously shown to be the mechanism responsible for induction of apoptosis in a myeloma cell line [34]. In line with that finding, AG-490 increased the frequency of TUNEL-positive chondrocytes in juvenile chondrocyte pellet cultures as well as in normal human chondrocytes. However, in the present study, AG-490-induced apoptosis in the juvenile chondrocyte pellet cultures was not associated with a reduction in the immunoreactive p-STAT3 product. Although we did not determine the extent to which AG-490 directly blocked JAK2 activity, previously published studies using lung microvascular epithelial cells [13] showed that the enhanced phosphorylation of STAT3/STAT5 induced by hypoxia was significantly blocked by AG-490 at a similar concentration of AG-490 used in this study. Thus, it appears that AG-490-induced apoptosis and inhibition of STAT3 activation in juvenile chondrocyte pellet cultures and normal human chondrocytes is comparable to previously reported findings with other cell types. Although treating chondrocyte pellet cultures initiated from MSCs with AG-490 also increased type X collagen deposition without affecting apoptosis, the overall significance of this result remains to be further investigated.
The combination of MSU plus AG-490 plus febuxostat increased the p-STAT3 and U-STAT3 immunoreactive product in N-JHu-C at 30 min compared to MSU and AG-490 alone. Of note, febuxostat (16 μM) significantly inhibited chondrocyte XO activity. Therefore, inhibition of XO may, in part, be responsible for increasing U-STAT 3 and p-STAT3.
We must also consider the possibility that induction of apoptosis in response to MSU may actually also involve an increase in the expression of TNF-α. In that regard, Islam et al. [18] reported that increased TNF-α mRNA accompanied the induction of apoptosis in human OA chondrocyte cultures in response to hydrostatic pressure. Furthermore, di Giovine et al. [35] showed that TNF-α mRNA levels were elevated after human blood monocytes or RA synoviocytes were treated in vitro with MSU, but not after treatment with calcium pyrophosphate or hydroxyapatite.
We also propose that another possible mechanism for the increase in TUNEL-positive chondrocytes in response to MSU is that MSU upregulated chondrocyte-derived IL-1β. In that regard, the MSU crystal suspension at the 0.5 mg/ml concentration used in these chondrocyte experiments also increased the production of macrophage IL-1β (Liu-Bryan, personal communication). Furthermore, IL-1β is a strong inducer of human chondrocyte apoptosis [1,2,36-40].
One limitation of this study was that unlike our analysis of STAT3 activation, a pharmacologic approach to determining the contribution of p38 kinase and JNK-1/JNK-2 activation by rhTNF-α to apoptosis in OA chondrocytes was not performed. Although a previous study demonstrated that activation of SAPKs/MAPKs was associated with the induction of chondrocyte apoptosis [36-38], it cannot yet be concluded that inhibiting p38 kinase, JNK, or for that matter, ERK 1/2, would also blunt apoptosis in OA chondrocytes in response to rhTNF-α.
To summarize, MSU and TNF-α, 2 important mediators of synovial joint inflammation, increased the frequency of TUNEL-positive nuclei in juvenile chondrocyte macroaggregate pellet cultures and in hyperdense normal human and OA chondrocyte cultures. However, rhTNF-α did not increase the frequency of TUNEL-positive nuclei in human chondrocyte macroaggregate pellet cultures derived from bone marrow MSCs. The finding that MSU or rhTNF-α failed to induce apoptosis in chondrocyte macroaggregate pellet cultures derived from bone marrow MSCs even though treatment with MSU and rhTNF-α resulted in STAT3 activation combined with the finding that febuxostat, a specific inhibitor of XO, activated STAT3 when added to MSU plus AG-490-treated chondrocyte macroaggregate pellet cultures derived from bone marrow MSCs, but also failed to increase TUNEL-positive nuclei provided direct evidence that phosphorylation of STAT3 was not required for increasing chondrocyte apoptosis under these defined in vitro conditions.
Finally, although rhTNF-α-induced apoptosis in human normal and OA chondrocyte hyperdense cultures correlated with increased XO activity and febuxostat partially inhibited normal chondrocyte XO in a concentration-dependent manner, the finding that rhTNF-α plus febuxostat actually increased the frequency of TUNEL-positive nuclei compared to rhTNF-α alone provided support for the conclusion that increased apoptosis in response to rhTNF-α in normal human chondrocyte cultures was not dependent on full XO activity.
Acknowledgments
This study was supported, in part, by an Investigator-Initiated Project Award (#MA-F-021) from Takeda Pharmaceuticals of North America, Inc (Deerfield, IL) to CJM, a grant from the National Institutes of Dental Research to JED (R01-DE53322) and a grant from the National Eye Institute (R01-EY14362) to EP. This study was also supported by a Visual Sciences Center Core Grant from the National Eye Institute (P30-EY11373). The authors thank Ms. Meenakshi Shukla, Ms. Dawn Smith and Scott Howell, Ph.D. for their excellent technical assistance. We also are grateful to Tariq M. Haqqi, Ph.D. and Jean F. Welter, M.D., Ph.D. for helpful discussions.
References
- 1.Malemud CJ, Gillespie HJ. The role of apoptosis in arthritis. Curr Rheum Rev. 2005;1:131–142. [Google Scholar]
- 2.Malemud CJ, Islam N, Haqqi TM. Pathophysiological mechanisms in osteoarthritis lead to novel therapeutic strategies. Cells Tissues Organs. 2003;174:34–48. doi: 10.1159/000070573. [DOI] [PubMed] [Google Scholar]
- 3.Weng LH, Wang CJ, Ko JY, Sun YC, Su YS, et al. Inflammation induction of Dickkopf-1 mediates chondrocyte apoptosis in osteoarthritic joint. Osteoarthritis Cartilage. 2009;17:933–943. doi: 10.1016/j.joca.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Pérez HE, Luna MJ, Rojas ML, Kouri JB. Chondroptosis: an immunohistochemical study of apoptosis and Golgi complex in chondrocytes from human osteoarthritic cartilage. Apoptosis. 2005;10:1105–1110. doi: 10.1007/s10495-005-0649-1. [DOI] [PubMed] [Google Scholar]
- 5.Cherng YG, Chang HC, Lin YL, Kuo ML, Chiu WT, et al. Apoptotic insults to human chondrocytes induced by sodium nitroprusside are involved in sequential events, including cytoskeletal remodeling, phosphorylation of mitogen-activated protein kinase kinase kinase-1/c-Jun N-terminal kinase, and Bax-mitochondria-mediated caspase activation. J Orthop Res. 2008;26:1018–1026. doi: 10.1002/jor.20578. [DOI] [PubMed] [Google Scholar]
- 6.Caramés B, López-Armada MJ, Cillero-Pastor B, Lires-Dean M, Vaamonde C, et al. Differential effects of tumor necrosis factor-a and interleukin-1ß on cell death in human articular chondrocytes. Osteoarthritis Cartilage. 2008;16:715–722. doi: 10.1016/j.joca.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 7.van de Loo FA, Veenbergen S, van den Berg WB. Targeting growth factors in arthritis: a rational for restoring the IGF-1 response in chondrocytes. Curr Rheum Rev. 2008;4:266–276. [Google Scholar]
- 8.Malemud CJ, Pearlman E. Targeting JAK/STAT signaling pathway in inflammatory diseases. Curr Signal Transduct Ther. 2009;4:201–221. [Google Scholar]
- 9.Malemud CJ. Suppression of autoimmune arthritis by small molecule inhibitors of the JAK/STAT pathway. Pharmaceuticals. 2010;3:1446–1455. doi: 10.3390/ph3051446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malemud CJ. Dysfunctional immune-mediated inflammation in rheumatoid arthritis dictates that development of anti-rheumatic disease drugs target multiple intracellular signaling pathways. Anti-Inflammatory Anti-Allergy Agents Med Chem. 2011;10:78–84. [PubMed] [Google Scholar]
- 11.Wisler BA, Dennis JE, Malemud CJ. New organ-specific pharmacological strategies interfering with signaling pathways in inflammatory disorders/autoimmune disorders. Curr Signal Transduct Ther. 2011;6:279–291. [Google Scholar]
- 12.Liu R, Lioté F, Rose DM, Merz D, Terkeltaub R. Proline-rich tyrosine kinase 2 and Src kinase signaling transduce monosodium urate crystal-induced nitric oxide production and matrix metalloproteinase 3 expression in chondrocytes. Arthritis Rheum. 2004;50:247–258. doi: 10.1002/art.11486. [DOI] [PubMed] [Google Scholar]
- 13.Wang G, Qian P, Jackson FR, Qian G, Wu G. Sequential activation of JAKs, STATs and xanthine dehydrogenase/oxidase by hypoxia in lung microvascular endothelial cells. Int J Biochem Cell Biol. 2008;40:461–470. doi: 10.1016/j.biocel.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumagai A, Kodama H, Kumagai J, Fukuda J, Kawamura K, et al. Xanthine oxidase inhibitors suppress testicular germ cell apoptosis induced by experimental cryptorchidism. Mol Hum Reprod. 2002;8:118–123. doi: 10.1093/molehr/8.2.118. [DOI] [PubMed] [Google Scholar]
- 15.Albuquerque RG, Sanson AJ, Malangoni MA. Allopurinol protects enterocytes from hypoxia-induced apoptosis in vivo. J Trauma. 2002;53:415–420. doi: 10.1097/00005373-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Hair PI, McCormack PL, Keating GM. Febuxostat. Drugs. 2008;68:1865–1874. doi: 10.2165/00003495-200868130-00006. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto T, Moriwaki Y, Fujimura Y, Takahashi S, Tsutsumi Z, et al. Effect of TEI-6720, a xanthine oxidase inhibitor, on the nucleoside transport in the lung cancer cell line A549. Pharmacology. 2000;60:34–40. doi: 10.1159/000028344. [DOI] [PubMed] [Google Scholar]
- 18.Islam N, Haqqi TM, Jepsen KJ, Kraay M, Welter JF, et al. Hydrostatic pressure induces apoptosis in human chondrocytes from osteoarthritic cartilage through up-regulation of tumor necrosis factor-alpha, inducible nitric oxide synthase, p53, c-myc, and bax-alpha, and suppression of bcl-2. J Cell Biochem. 2002;87:266–278. doi: 10.1002/jcb.10317. [DOI] [PubMed] [Google Scholar]
- 19.Wenger R, Hans MG, Welter JF, Solchaga LA, Sheu YR, et al. Hydrostatic pressure increases apoptosis in cartilage-constructs produced from human osteoarthritic chondrocytes. Front Biosci. 2006;11:1690–1695. doi: 10.2741/1914. [DOI] [PubMed] [Google Scholar]
- 20.Tallheden T, Dennis JE, Lennon DP, Sjögren-Jansson E, Caplan AI, et al. Phenotypic plasticity of human articular chondrocytes. J Bone Joint Surg Am. 2003;85-85A(Suppl 2):93–100. doi: 10.2106/00004623-200300002-00012. [DOI] [PubMed] [Google Scholar]
- 21.Dennis JE, Carbillet JP, Caplan AI, Charbord P. The STRO-1+ marrow cell population is multipotential. Cells Tissues Organs. 2002;170:73–82. doi: 10.1159/000046182. [DOI] [PubMed] [Google Scholar]
- 22.Naumann A, Dennis JE, Aigner J, Coticchia J, Arnold J, et al. Tissue engineering of autologous cartilage grafts in three-dimensional in vitro macroaggregate culture system. Tissue Eng. 2004;10:1695–1706. doi: 10.1089/ten.2004.10.1695. [DOI] [PubMed] [Google Scholar]
- 23.Islam S, Islam N, Kermode T, Johnstone B, Mukhtar H, et al. Involvement of caspase-3 in epigallocatechin-3-gallate-mediated apoptosis of human chondrosarcoma cells. Biochem Biophys Res Commun. 2000;270:793–797. doi: 10.1006/bbrc.2000.2536. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y, Fox T, Adhikary G, Kester M, Pearlman E. Inhibition of corneal inflammation by liposomal delivery of short-chain, C-6 ceramide. J Leukoc Biol. 2008;83:1512–1521. doi: 10.1189/jlb.0108076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Y, Pearlman E. Inhibition of corneal inflammation by the TLR4 antagonist Eritoran tetrasodium (E5564) Invest Ophthalmol Vis Sci. 2009;50:1247–1254. doi: 10.1167/iovs.08-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh R, Ahmed S, Malemud CJ, Goldberg VM, Haqqi TM. Epigallocatechin-3-gallate selectively inhibits interleukin-1beta-induced activation of mitogen activated protein kinase subgroup c-Jun N-terminal kinase in human osteoarthritis chondrocytes. J Orthop Res. 2003;21:102–109. doi: 10.1016/S0736-0266(02)00089-X. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237–246. [PubMed] [Google Scholar]
- 28.Goldring MB, Berenbaum F. The regulation of chondrocyte function by proinflammatory mediators: prostaglandins and nitric oxide. Clin Orthop Relat Res. 2004;(427 Suppl):S37–S46. doi: 10.1097/01.blo.0000144484.69656.e4. [DOI] [PubMed] [Google Scholar]
- 29.Polzer K, Schett G, Zwerina J. The lonely death: chondrocyte apoptosis in TNF-induced arthritis. Autoimmunity. 2007;40:333–336. doi: 10.1080/08916930701356721. [DOI] [PubMed] [Google Scholar]
- 30.Tak PP, Firestein GS. Apoptosis in rheumatoid arthritis. In: Winkler JD, editor. Apoptosis and Inflammation- Progress in Inflammation Research. Basel, Birkhauser; 1999. pp. 149–162. [Google Scholar]
- 31.Huang JI, Kazmi N, Durbhakula MM, Hering TM, Yoo JU, et al. Chondrogenic potential of progenitor cells derived from human bone marrow and adipose tissue: a patient-matched comparison. J Orthop Res. 2005;23:1383–1389. doi: 10.1016/j.orthres.2005.03.008.1100230621. [DOI] [PubMed] [Google Scholar]
- 32.Tchetina EV, Kobayashi M, Yasuda T, Meijers T, Pidoux I, et al. Chondrocyte hypertrophy can be induced by a cryptic sequence of type II collagen and is accompanied by the induction of MMP-13 and collagenase activity: implications for development and arthritis. Matrix Biol. 2007;26:247–258. doi: 10.1016/j.matbio.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Liu-Bryan R, Pritzker K, Firestein GS, Terkeltaub R. TLR2 signaling in chondrocytes drives calcium pyrophosphate dihydrate and monosodium urate crystal-induced nitric oxide generation. J Immunol. 2005;174:5016–5023. doi: 10.4049/jimmunol.174.8.5016. [DOI] [PubMed] [Google Scholar]
- 34.De Vos J, Jourdan M, Tarte K, Jasmin C, Klein B. JAK2 tyrosine kinase inhibitor tyrphostin AG490 downregulates the mitogen-activated protein kinase (MAPK) and signal transducer and activator of transcription (STAT) pathways and induces apoptosis in myeloma cells. Br J Haematol. 2000;109:823–828. doi: 10.1046/j.1365-2141.2000.02127.x. [DOI] [PubMed] [Google Scholar]
- 35.di Giovine FS, Malawista SE, Thornton E, Duff GW. Urate crystals stimulate production of tumor necrosis factor alpha from human blood monocytes and synovial cells. Cytokine mRNA and protein kinetics, and cellular distribution. J Clin Invest. 1991;87:1375–1381. doi: 10.1172/JCI115142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malemud CJ. Protein kinases in chondrocyte signaling and osteoarthritis. Clin Orthop Relat Res. 2004;427(Suppl):S145–S151. doi: 10.1097/01.blo.0000143802.41885.50. [DOI] [PubMed] [Google Scholar]
- 37.Chun JS. Expression, activity, and regulation of MAP kinases in cultured chondrocytes. Methods Mol Med. 2004;100:291–306. doi: 10.1385/1-59259-810-2:291. [DOI] [PubMed] [Google Scholar]
- 38.Malemud CJ. MAP kinases. OA, Inflammation and Degradation: A Continuum. In: Buckwalter J, Lotz M, Stoltz J-F, editors. Biomedical and Health Research. Vol. 70. Amsterdam: IOS Press; 2007. pp. 99–117. [Google Scholar]
- 39.López-Armada MJ, Caramés B, Lires-Deán M, Cillero-Pastor B, Ruiz-Romero C, et al. Cytokines, tumor necrosis factor-alpha and interleukin-1beta, differentially regulate apoptosis in osteoarthritis cultured human chondrocytes. Osteoarthritis Cartilage. 2006;14:660–669. doi: 10.1016/j.joca.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Kim J, Xu M, Xo R, Mates A, Wilson GL, et al. Mitochondrial DNA damage is involved in apoptosis caused by pro-inflammatory cytokines in human OA chondrocytes. Osteoarthritis Cartilage. 2010;18:424–432. doi: 10.1016/j.joca.2009.09.008. [DOI] [PubMed] [Google Scholar]


