Abstract
Purpose
Interleukin-18 (IL-18) is a critical mediator of obstruction-induced renal injury. While previous studies have demonstrated that IL-18 participates in a positive feedback loop via the IL-18 receptor (IL-18R) and localized renal IL-18 and IL-18R production to tubular epithelial cells (TEC), the mechanism of IL-18 activation during obstruction remains unclear. We hypothesized that IL-18 activation is dependent on TLR4 signaling during renal obstruction.
Materials and Methods
Male C57BL6 TLR4 knockout (TLR4KO) and WT mice were subjected to UUO vs. sham operation for 1 week. The animals were sacrificed, and renal cortical tissue was harvested and analyzed for TLR4 expression (Western blot), active IL-18 production (ELISA, real time PCR), IL-18 receptor expression (real time PCR), and TLR4/IL-18 vs. IL-18R cellular localization (dual immunofluorescent staining).
Results
Renal TLR4 expression increased significantly in WT mice in response to obstruction, but remained at sham treatment levels in TLR4KO mice. IL-18 and IL-18R gene expression and active IL-18 production were similarly increased in WT mice in response to obstruction, but decreased significantly to sham treatment levels in the absence of TLR4. Dual immunofluorescent staining revealed co-localization of TLR4 and IL-18 to renal TECs during obstruction.
Conclusion
IL-18 production and activation during renal obstruction is dependent on intact TLR4 signaling. Co-localization of IL-18 and TLR4 production to TECs during obstruction suggests that TECs are the primary site of IL-18 production and activation. Further characterization of the pathway may be necessary to develop targeted therapy in obstruction-induced renal injury.
Keywords: IL-18, TLR4, Obstruction, Kidney, IL-18R
INTRODUCTION
Chronic renal obstruction is an important cause of end stage renal disease in children, resulting in progressive tubulointerstitial fibrosis, a loss in renal parenchyma, and ultimately, irreversible renal dysfunction. The pathophysiology of obstruction-induced renal injury is characterized by inflammatory cell infiltration, fibroblast proliferation, and an imbalance in extracellular matrix (ECM) deposition and degradation (1–3). A number of cytokines and growth factors have been implicated in this maladaptive response, including interleukin-18 (IL-18) (3).
Interleukin-18 (IL-18) is a pro-inflammatory mediator that is structurally and functionally related to the IL-1 family, and has been implicated in the pathophysiology of a variety of renal inflammatory diseases including ischemia-reperfusion injury, allograft rejection, autoimmune disease, and most recently, obstructive nephropathy (3–7). Urinary IL-18 levels have been shown to be a sensitive and early marker of renal tubular damage in patients with ischemic and post-transplantation ATN, and circulating IL-18 levels and renal IL-18 receptor (IL-18R) expression have been shown to be elevated in patients with chronic kidney disease (8–10).
IL-18 is synthesized as a biologically inactive precursor (pro-IL-18), which requires cleavage into an active molecule by caspase-1. Caspase-1 is remarkably specific for the precursors of IL-1β and IL-18, making a single initial cut in each pro-cytokine that results in the extracellular secretion of active mature cytokine (11, 12). It appears that Toll like receptor (TLR) activation is necessary for the inflammasome priming that stimulates caspase-1 activation and the subsequent processing of pro-IL-18 into the mature, active IL-18 product (13–18). We have previously demonstrated that active IL-18 stimulates a positive feedback loop through the IL-18 receptor (IL-18R) to increase IL-18 production during renal obstruction (19), and that both IL-18 and TLR4 are important mediators of obstruction-induced renal injury. We therefore hypothesized that IL-18 production and the processing of pro-IL-18 into mature, active IL-18 product during renal obstruction is dependent on intact TLR4 signaling. To study this, we examined renal cortical TLR4 expression, active IL-18 production, IL-18 receptor expression (real time PCR), and TLR4/IL-18 cellular co-localization in male C57B16 WT and TLR4KO mice using a well-established model of UUO.
MATERIALS AND METHODS
Animals, Experimental Groups, and Operative Techniques
The animal protocol was reviewed and accepted by the Indiana University laboratory animal care and use committee. Male C57BL/6 (WT) or B6.B10ScN-Tlr4lps-del/JthJ (TLR4KO) mice weighing 25–30g (6 animals per group) were acclimated and maintained on a standard pellet diet for 1 week before initiation of the experiments. Mice were anesthetized with isoflurane and the left ureter was isolated and completely ligated with a 5-0 silk suture. Sham treated animals underwent an identical surgical procedure without ureteral ligation. After one week, the animals were re-anesthetized, the left kidneys were removed and snap frozen in liquid nitrogen, and the animals were subsequently euthanized.
Tissue Homogenization
A portion of each renal cortex was homogenized after the samples had been diluted in 10 volumes of homogenate buffer per gram of tissue (10 mM Hepes (pH 7.9), 10 mM KCL, 0.1 mM EGTA, 1 mM DTT, and Complete Protease Inhibitor tabs (Boehringer Mannheim, Indianapolis, IN)) using a vertishear tissue homogenizer. Renal homogenates were then centrifuged at 3,000 × g for 15 minutes, and the supernatants stored at −70°C until the ELISA assays/Western Blots could be performed.
Western Blot Analysis
The protein extracts from homogenized samples (50 mg/lane) were electrophoresed on a Tris-glycine gel (Invitrogen, Carlsbad, CA) and transferred to a PVDF membrane. Immunoblotting was performed by incubating each membrane in 5% dry milk overnight at 4 C, followed by incubation anti-TLR4 antibody (1:200; overnight at 4°C, Santa Cruz Biotechnology, Santa Cruz, CA). After being washed three times in T-PBS, each membrane was incubated for 1 hour at RT with a peroxidase-conjugated secondary antibody (1:1000). The membranes were then developed using enhanced chemiluminescence (Amersham Pharmacia Biotech Inc., Piscataway, NJ). Equivalent protein loading for each lane was confirmed by stripping and re-blotting each membrane for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:20000 for 30 min at RT, secondary 1:20,000 for 30 min at RT; Biodesign International, Saco, ME). The analysis was repeated in triplicate to assure reproducibility of results. The membranes were developed using enhanced chemiluminescence (Amersham Pharmacia Biotech Inc.), and the density of each band determined using NIH image analysis software and expressed as a percentage of GAPDH density.
Renal cortical active IL-18 levels
Renal homogenate active IL-18 levels were determined using an ELISA (mouse IL-18; MBL Int., Woburn, Massachusetts). The ELISA was performed by adding 100μl of each sample to wells in a 96-well plate of a commercially available ELISA kit. The assay was performed according to manufacturer instructions. All samples were tested in duplicate. ELISA results are expressed as pg/mg protein.
Real-Time PCR
Total RNA was extracted from renal cortical tissue by homogenization in TRIzol® (Gibco BRL, Gaithersburg, MD), then isolated by precipitation with chloroform and isopropanol. Total RNA (0.5 μg) was subjected to cDNA synthesis using iScript™ (Bio-Rad, Hercules, CA). cDNA from each sample was analyzed for IL-18 (Mm00434226_m1) and IL-18R (Mm00515180_m1) using a TaqMan® gene expression assay (RT-PCR; Applied Biosystems, Foster City, CA). FAMTM Dye/MGB labeled probes for mouse GAPDH served as endogenous controls.
Cellular Immunolocalization
Renal sections (5 μm) were harvested from each sample, fixed in acetone for 10 minutes, rinsed in PBS 3 times and incubated for 20 minutes in blocking solution containing 10% goat or donkey serum. Slides were incubated with goat polyclonal anti-IL-18 (1:50, Santa cruz) or goat polyclonal anti-Il-18R (1:50, Santa cruz) and mouse anti-TLR4 (1:50, Santa Cruz Biotechnology, Santa Cruz, California) for 60 minutes at room temperature. Slides were washed 3 times in PBS and incubated for 45 minutes at room temperature with secondary antibody (donkey anti-goat (texas red 1:200) or goat anti-mouse FITC (1:200); Santa Cruz Biotechnology). The nuclei were couterstained with bisbenzimide (2 μg/ml). To assess immunostaining specificity, adjacent sections were incubated without primary antibody and processed using identical conditions. Slides were mounted and stored at −4C. Digital photographs were obtained at 400× using a DM IRB fluorescence microscope (Leica, Wetzlar, Germany).
Statistical Analysis
Data are shown as the mean ±SD with differences at the 95% CI considered significant. Experimental groups were compared using ANOVA with the post hoc Bonferroni- Dunn test using JMP®, version 5.0.
RESULTS
Renal TLR4 Expression
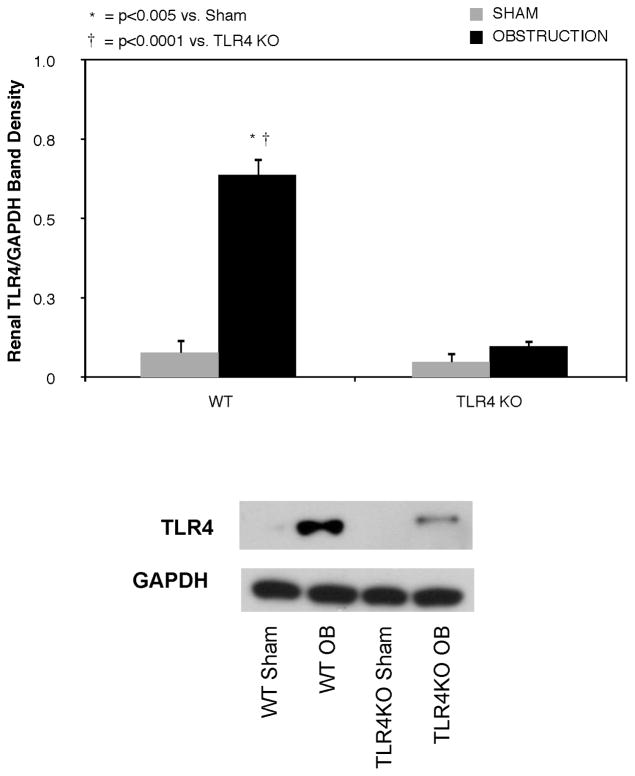
Renal TLR4 expression increased significantly in WT mice in response to one week of ureteral obstruction (Figure 1). In contrast, TLR4 expression remained at sham treatment levels in TLR4KO mice exposed to the same degree of obstruction.
Figure 1. Renal cortical TLR4 expression following UUO.
Gel photograph and densitometric analysis of TLR4 expression represented as a percentage of GAPDH in WT and TLR4KO mice exposed to sham operation or 1 week of UUO.
Active IL-18 Production
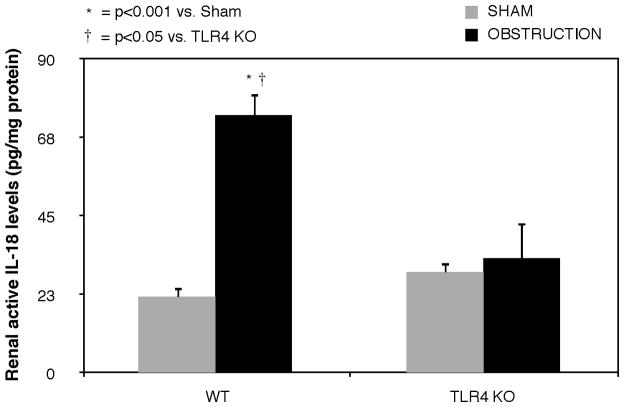
Active IL-18 levels remained low in renal cortical samples from sham treated mice, but increased significantly in response to 1 week of obstruction in WT animals (Figure 2). In contrast, mice deficient in TLR4 signaling exhibited a significant reduction in active IL-18 levels in response to obstruction.
Figure 2. Renal cortical active IL-18 production in response to UUO.
Active IL-18 levels in renal cortical samples obtained from WT and TLR4KO mice exposed to sham operation or 1 week of UUO.
Effect of TLR4 Depletion on IL-18 and IL-18R Gene Expression
Relative renal IL-18 and IL-18R gene expression was low in sham treated animals, but increased significantly in response to 1 week of obstruction in WT animals (Figures 3A and B). Mice deficient in TLR4 signaling; however, demonstrated a dramatic reduction in both IL-18 and IL-18R mRNA expression in response to the same degree of obstruction.
Figure 3. Renal cortical IL-18 and IL-18 receptor mRNA expression following UUO.
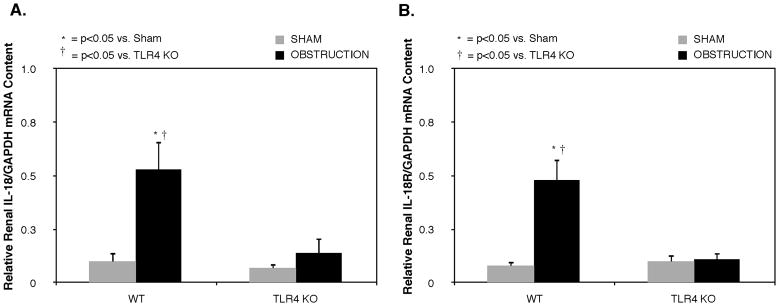
Quantitative IL-18 and IL-18 receptor (IL-18R) mRNA expression represented as a percentage of GAPDH in in WT and TLR4KO mice exposed to sham operation or 1 week of UUO.
TLR4 Dependent Caspase-1 Expression
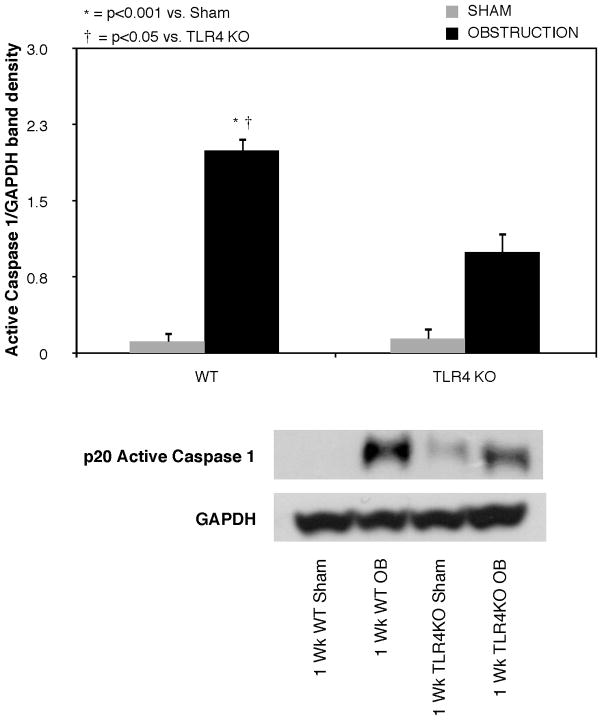
Active caspase-1 expression was low in sham treated animals, but increased significantly in WT animals exposed to 1 week of obstruction (Figure 4). A significant reduction in active caspase-1 expression was detected in the TLR4KO mice exposed to the same degree of obstruction.
Figure 4. Renal cortical active caspase-1 expression following UUO.
Active caspase 1 expression in renal cortical samples obtained from WT and TLR4KO mice exposed to sham operation or 1 week of UUO.
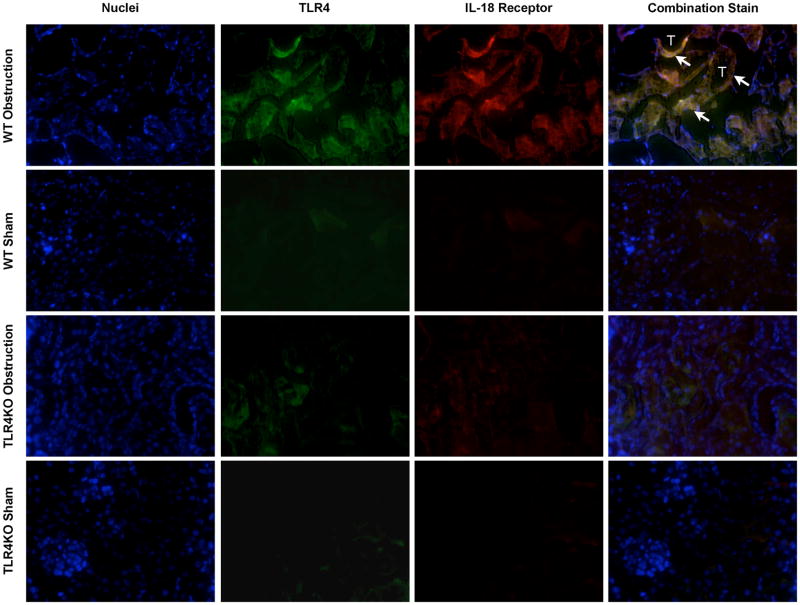
Cellular Localization of IL-18, IL-18R, and TLR4 during Renal Obstruction
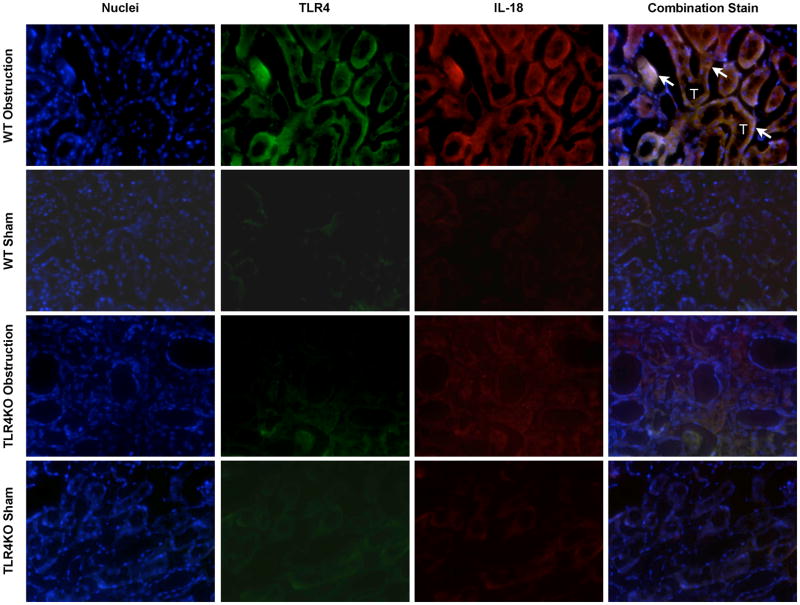
Dual immunofluorescent staining of renal cortical tissue sections demonstrated cellular co-localization of IL-18 and TLR4 (Figure 5), and IL-18 and IL-18R (Figure 6) to renal tubular cells in response to 1 week of obstruction. Minimal staining was observed in sham treated mice and TLR4KO mice exposed to the same degree of obstruction.
Figure 5. Renal cortical dual labeled immunofluorescent staining for IL-18 and TLR4 following UUO.
Photographs (magnification 400×) depicting renal cortical IL-18 production (red) and TLR4 expression (green) in WT and TLR4KO mice exposed to sham operation or 1 week of UUO. White arrows indicate IL-18 overlying TLR4 within renal tubules. T = Tubules.
Figure 6. Renal cortical dual labeled immunofluorescent staining for IL-18 receptor and TLR4 following UUO.
Photographs (magnification 400×) depicting renal cortical IL-18 receptor production (red) and TLR4 expression (green) in WT and TLR4KO mice exposed to sham operation or 1 week of UUO. White arrows indicate IL-18 receptor overlying TLR4 within renal tubules. T = Tubules.
DISCUSSION
IL-18 has previously been shown to mediate multiple aspects of obstruction-induced renal injury, including tubulointerstitial fibrosis, epithelial-mesenchymal transition (EMT), inflammatory cell infiltration, and apoptosis (3, 20). We have previously demonstrated that IL-18 production localizes primarily to renal tubular cells during renal obstruction, and that active IL-18 stimulates a positive feedback loop through the IL-18 receptor that amplifies renal IL-18 production in response to obstruction (19). The mechanism of IL-18 production and activation during renal obstruction; however, has been unclear. This is the first study to demonstrate that IL-18 production during renal obstruction is dependent on intact TLR4 signaling.
IL-18 is a pro-inflammatory cytokine that is structurally and functionally related to the IL-1 family. It is synthesized as a biologically inactive precursor (pro-IL-18), that requires cleavage into a mature, active cytokine by the intracellular cysteine protease, caspase-1(21). Capsase-1 is specific for the precursors of both IL-18 and IL-1β, and cleaves both pro-cytokines to result in the secretion of active, mature cytokine. IL-18 gene expression appears to be unlike that of most cytokines, in that its precursor (pro-IL-18) is constitutively expressed, it is produced in a wide range of cells, and it has a relatively long half life as compared to other cytokines (22). The processing requirement of IL-18 by caspase-1 therefore appears to be critical to the control and specificity of IL-18’s downstream effects.
In response to cellular danger, caspase 1 is recruited to intracellular multiprotein complexes, termed inflammasomes, comprised of Nod-like receptor proteins, such as NLRP3, and the adaptor protein ASC (apoptosis-associated speck-like protein) which binds caspase 1. Following activation of the inflammasome by stimuli such as bacterial RNA and danger-associated molecular patterns, caspase 1 is converted to its active form (23). Although the function of NLRP3 on inflammasome activation and the subsequent activation of caspase 1 is well defined (24, 25), there appears to be growing evidence of cross talk between NLRPs and another family of pattern-recognition molecules, toll like receptors (TLRs). In fact, a recent study demonstrates that NLRP3 expression is induced by LPS, a TLR4 agonist, in an NFκB dependent manner (26).
Toll like receptors are transmembrane proteins that are expressed in a variety of cells, including renal tubular epithelial cells. Engagement of a TLR with its specific pathogen associated molecular pattern (PAMP) initiates an intracellular signaling cascade that culminates in the release of cytokines and the expression of cell surface co-stimulatory molecules capable of activating T cells and initiating an adaptive immune response (27). Certain endogenous molecules are now understood to function as PAMPs, including necrotic cells, heat shock proteins, and elements of the extracellular matrix (28–30). We have previously demonstrated that TLR4 contributes to obstruction-induced EMT and renal fibrosis (31), and based on the role of TLR4 agonists on NLRP3 expression, hypothesized that TLR4 may have an integral role in IL-18 activation via caspase 1.
Our data demonstrate that TLR4 is significantly increased in response to one week of renal obstruction, and further, that intact TLR4 signaling is necessary for mature IL-18 production and IL-18 and IL-18 receptor gene expression in response to obstruction. While WT mice exhibited a significant increase in active renal IL-18 production and IL-18/IL-18R gene expression in response to obstruction, active IL-18 production and IL-18/IL-18R gene expression were reduced to sham treatment levels in TLR4 KO mice exposed to the same degree of obstruction. We also observed that obstruction-induced active caspase 1 expression is significantly reduced in mice with deficient TLR4 signaling as compared to WT mice, suggesting that TLR4’s impact on IL-18 production and activation is mediated through alterations in caspase 1 activation.
Dual immunofluorescence staining was subsequently used to co-localize the cellular production of IL-18 and TLR4, and IL-18R and TLR4 during renal obstruction. The data demonstrate that both IL-18/TLR4 and IL-18R/TLR4 localize to renal tubular epithelial cells in response to obstruction, providing further evidence that TEC are the primary site of IL-18 production and activation during renal obstruction, rather than infiltrating macrophage.
CONCLUSION
While IL-18 has been determined to be a critical mediator of obstruction-induced renal injury, this is the first study to demonstrate that obstruction-induced renal IL-18 production and activation are dependent on intact TLR4 signaling. Our results further suggest that TLR4’s impact on IL-18 production is mediated through alterations in caspase 1 activation. An oral caspase 1 inhibitor, pralnacasan, has recently been developed and is currently in clinical trials for the treatment of rheumatoid arthritis. It is therefore plausible that caspase 1 inhibition may emerge as a potential therapeutic option for the management of obstructive nephropathy and fibrotic renal disease. Further characterization of the role of TLR4 on the inflammasome and caspase 1 activation; however, are necessary in order to fully define this signaling pathway.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klahr S. Progression of chronic renal disease. Heart Dis. 2001;3:205–209. doi: 10.1097/00132580-200105000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Eddy AA. Molecular insights into renal interstitial fibrosis. J Am Soc Nephrol. 1996;7:2495–2508. doi: 10.1681/ASN.V7122495. [DOI] [PubMed] [Google Scholar]
- 3.Bani-Hani AH, Leslie JA, Asanuma H, Dinarello CA, Campbell MT, Meldrum DR, Zhang H, Hile K, Meldrum KK. IL-18 neutralization ameliorates obstruction-induced epithelial-mesenchymal transition and renal fibrosis. Kidney Int. 2009;76:500–511. doi: 10.1038/ki.2009.216. [DOI] [PubMed] [Google Scholar]
- 4.Striz I, Krasna E, Honsova E, Lacha J, Petrickova K, Jaresova M, Lodererova A, Bohmova R, Valhova S, Slavcev A, Vitko S. Interleukin 18 (IL-18) upregulation in acute rejection of kidney allograft. Immunol Lett. 2005;99:30–35. doi: 10.1016/j.imlet.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Faust J, Menke J, Kriegsmann J, Kelley VR, Mayet WJ, Galle PR, Schwarting A. Correlation of renal tubular epithelial cell-derived interleukin-18 up-regulation with disease activity in MRL-Faslpr mice with autoimmune lupus nephritis. Arthritis Rheum. 2002;46:3083–3095. doi: 10.1002/art.10563. [DOI] [PubMed] [Google Scholar]
- 6.Dinarello CA. Interleukin-18 and the pathogenesis of inflammatory diseases. Seminars in nephrology. 2007;27:98–114. doi: 10.1016/j.semnephrol.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Daemen MA, van’t Veer C, Wolfs TG, Buurman WA. Ischemia/reperfusion-induced IFN-gamma up-regulation: involvement of IL-12 and IL-18. J Immunol. 1999;162:5506–5510. [PubMed] [Google Scholar]
- 8.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis. 2004;43:405–414. doi: 10.1053/j.ajkd.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 9.Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005;16:3046–3052. doi: 10.1681/ASN.2005030236. [DOI] [PubMed] [Google Scholar]
- 10.Chiang CK, Hsu SP, Pai MF, Peng YS, Ho TI, Liu SH, Hung KY, Tsai TJ, Hsieh BS. Plasma interleukin-18 levels in chronic renal failure and continuous ambulatory peritoneal dialysis. Blood Purif. 2005;23:144–148. doi: 10.1159/000083620. [DOI] [PubMed] [Google Scholar]
- 11.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T, Flavell RA, Sato V, Harding MW, Livingston DJ, Su MS. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 12.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R, Tracey D, Allen H. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto M, Yaginuma K, Tsutsui H, Sagara J, Guan X, Seki E, Yasuda K, Yamamoto M, Akira S, Nakanishi K, Noda T, Taniguchi S. ASC is essential for LPS-induced activation of procaspase-1 independently of TLR-associated signal adaptor molecules. Genes Cells. 2004;9:1055–1067. doi: 10.1111/j.1365-2443.2004.00789.x. [DOI] [PubMed] [Google Scholar]
- 14.Stehlik C, Lee SH, Dorfleutner A, Stassinopoulos A, Sagara J, Reed JC. Apoptosis-associated speck-like protein containing a caspase recruitment domain is a regulator of procaspase-1 activation. J Immunol. 2003;171:6154–6163. doi: 10.4049/jimmunol.171.11.6154. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Alavez M, Bartfai T. It all happens between Toll receptors and caspase 1. Proc Natl Acad Sci U S A. 2007;104:7733–7734. doi: 10.1073/pnas.0702505104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozoren N, Masumoto J, Franchi L, Kanneganti TD, Body-Malapel M, Erturk I, Jagirdar R, Zhu L, Inohara N, Bertin J, Coyle A, Grant EP, Nunez G. Distinct roles of TLR2 and the adaptor ASC in IL-1beta/IL-18 secretion in response to Listeria monocytogenes. J Immunol. 2006;176:4337–4342. doi: 10.4049/jimmunol.176.7.4337. [DOI] [PubMed] [Google Scholar]
- 17.Miggin SM, Palsson-McDermott E, Dunne A, Jefferies C, Pinteaux E, Banahan K, Murphy C, Moynagh P, Yamamoto M, Akira S, Rothwell N, Golenbock D, Fitzgerald KA, O’Neill LA. NF-kappaB activation by the Toll-IL-1 receptor domain protein MyD88 adapter-like is regulated by caspase-1. Proc Natl Acad Sci U S A. 2007;104:3372–3377. doi: 10.1073/pnas.0608100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Creagh EM, O’Neill LA. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006;27:352–357. doi: 10.1016/j.it.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Vanderbrink BA, Asanuma H, Hile K, Zhang H, Rink RC, Meldrum KK. Interleukin-18 Stimulates a Positive Feedback Loop During Renal Obstruction via Interleukin-18 Receptor. J Urol. 2011;186:1502–1508. doi: 10.1016/j.juro.2011.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Hile KL, Asanuma H, Vanderbrink B, Franke EI, Campbell MT, Meldrum KK. IL-18 mediates proapoptotic signaling in renal tubular cells through a Fas ligand-dependent mechanism. Am J Physiol Renal Physiol. 2011;301:F171–178. doi: 10.1152/ajprenal.00339.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leslie JA, Meldrum KK. The role of interleukin-18 in renal injury. J Surg Res. 2008;145:170–175. doi: 10.1016/j.jss.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 22.Tone M, Thompson SA, Tone Y, Fairchild PJ, Waldmann H. Regulation of IL-18 (IFN-gamma-inducing factor) gene expression. J Immunol. 1997;159:6156–6163. [PubMed] [Google Scholar]
- 23.Taniguchi S, Sagara J. Regulatory molecules involved in inflammasome formation with special reference to a key mediator protein, ASC. Seminars in immunopathology. 2007;29:231–238. doi: 10.1007/s00281-007-0082-3. [DOI] [PubMed] [Google Scholar]
- 24.van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol. 2011;32:110–116. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Correa-Costa M, Braga TT, Semedo P, Hayashida CY, Bechara LR, Elias RM, Barreto CR, Silva-Cunha C, Hyane MI, Goncalves GM, Brum PC, Fujihara C, Zatz R, Pacheco-Silva A, Zamboni DS, Camara NO. Pivotal role of Toll-like receptors 2 and 4, its adaptor molecule MyD88, and inflammasome complex in experimental tubule-interstitial nephritis. PloS one. 2011;6:e29004. doi: 10.1371/journal.pone.0029004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiao Y, Wang P, Qi J, Zhang L, Gao C. TLR-induced NF-kappaB activation regulates NLRP3 expression in murine macrophages. FEBS letters. 2012;586:1022–1026. doi: 10.1016/j.febslet.2012.02.045. [DOI] [PubMed] [Google Scholar]
- 27.Gay NJ, Gangloff M. Structure and function of Toll receptors and their ligands. Annu Rev Biochem. 2007;76:141–165. doi: 10.1146/annurev.biochem.76.060305.151318. [DOI] [PubMed] [Google Scholar]
- 28.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 30.Li M, Carpio DF, Zheng Y, Bruzzo P, Singh V, Ouaaz F, Medzhitov RM, Beg AA. An essential role of the NF-kappa B/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J Immunol. 2001;166:7128–7135. doi: 10.4049/jimmunol.166.12.7128. [DOI] [PubMed] [Google Scholar]
- 31.Campbell MT, Hile KL, Zhang H, Asanuma H, Vanderbrink BA, Rink RR, Meldrum KK. Toll-like receptor 4: a novel signaling pathway during renal fibrogenesis. J Surg Res. 2011;168:e61–69. doi: 10.1016/j.jss.2009.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]