Abstract
Recent evidence of neuropathic pain among adults with sickle cell disease (SCD) reveals a need for adjuvant analgesic treatments for these patients. Ca2+/calmodulin protein kinase IIα (CaMKIIα) has a known role in neuropathic pain and trifluoperazine is a potent CaMKIIα inhibitor. The study aim was to determine trifluoperazine's acute effects, primarily on adverse effects and secondarily on pain intensity reduction, in adults with SCD. In a phase I, open-label study of 6 doses of trifluoperazine (0.5, 1, 2, 5, 7.5, 10 mg), we obtained 7-hourly and 24-hour repeated measures of adverse effects, pain intensity, and supplemental opioid analgesics in 18 adults with SCD (18 hemoglobin SS disease, 15 women, average age 35.8 ± 8.9 years, ranged 23-53) each of whom received a single dose. Data were analyzed with descriptive statistics. Subjects reported moderate to severe sedative effects at 7.5 and 10 mg doses, respectively. Eight subjects reported 50% reduction in chronic pain without severe sedation or supplemental opioid analgesics; one of these subjects had dystonia 24.5 hrs after the 10 mg dose. The analgesic effect lasted for at least 24 hrs in 3 subjects. Sedation resolved with caffeine and dystonia resolved with diphenhydramine. Adults with SCD experienced minimal adverse effects at doses under 10 mg. In this molecular mechanism-driven translational study, trifluoperazine shows promise as an analgesic drug that is worthy of further testing in a randomized controlled study of adults with SCD starting at a dose of 1 mg in repeated doses to determine long-term adverse and analgesic effects.
Keywords: sickle cell disease, neuropathic pain, phase 1 study, safety, trifluoperazine
1. Introduction
Important recent advances in knowledge of the pain experience by adults with sickle cell disease (SCD) is its chronic nature that includes both nociceptive and neuropathic mechanisms (Molokie et al., 2011; Wang et al., 2010; Wilkie et al., 2010). Although opioids are effective for these types of pain and adjuvant analgesics are often more effective than opioids for neuropathic pain in other disease conditions, there are no investigations of adjuvant analgesics for treatment of pain experienced by adults with SCD. The purpose of our study was to determine the safety of trifluoperazine, based on preclinical studies where it exhibited efficacious antinociceptive effects in several rodent models of chronic pain (Chen et al., 2009; Chen et al., 2010; Luo et al., 2008) including sickle cell transgenic mice (Wang et al., 2010).
Inherited disorders of hemoglobin are the most common monogenic diseases in the world (Williams and Weatherall, 2012), with SCD being the most common pathological hemoglobin mutation (Piel et al., 2013). The disease is caused by a point mutation in the sixth codon of the beta-globin chain, replacing glutamate with valine. Though the disease affects virtually every organ system, pain is the most common disease manifestation, a marker of disease severity, and the most common diagnosis associated with hospital admission in SCD (Brousseau et al., 2010). It is estimated that the annual health care costs for all sickle hemoglobinopathies are 2.4 billion United States dollars (Lanzkron et al., 2010), enormous costs especially on a per capita basis for the 100,000 Americans with SCD. In addition to pain managed in the hospital, patients often manage their pain at home on a daily basis (Smith et al., 2008; Wilkie et al., 2010). Nearly 6 out of 10 outpatients report their pain is continuous and occurs in an average of 3.6 sites; 92% also selected neuropathic pain descriptors (Wilkie et al., 2010). These findings and those from the SCD transgenic mouse model suggest that the pain of SCD is much more complex than previously thought, and appears to have both neuropathic and nociceptive mechanisms.
Ca2+/calmodulin protein kinase IIα (CaMKIIα), a multifunctional Ca2+ and calmodulin-activated serine/threonine protein kinase, is ubiquitous in the central nervous system and plays a role in the development of neuropathic pain (Chen et al., 2009; Chen et al., 2010; Luo et al., 2008; Wang et al., 2010). We previously identified trifluoperazine, a clinically used antipsychotic drug, to be a potent CaMKIIα inhibitor (Luo et al., 2008). Inhibition of CaMKIIα by trifluoperazine reverses or prevents complete Freund's adjuvant-induced persistent inflammatory pain (Luo et al., 2008), spinal nerve ligation-induced experimental neuropathic pain (Chen et al., 2009), and paradoxical opioid-induced hyperalgesia (Chen et al., 2010), in rodent models.
Trifluoperazine is FDA approved for treatment of psychotic conditions and has been used for more than three decades with an adequate safety profile, but its safety in people with SCD has not been established. We conducted a CaMKIIα-mechanism driven phase I translational study of trifluoperazine to determine safety and potential pain relief effect in adults with SCD and well characterized pain.
2. Materials and Methods
2. 1. Study Design/Setting
In a repeated measures study, we conducted pretest (baseline) and repeated posttest (hourly for 7 hrs and again 24 hrs after trifluoperazine) measures of safety (adverse effects) and pain intensity in adults with SCD. The Institutional Review Board at the University of Illinois at Chicago approved the study. We conducted the study at the adult sickle cell clinic, which is part of the University of Illinois Comprehensive Sickle Cell Center that serves a panel of 500 adults and 250 children with SCD.
2. 2. Sample
Inclusion criteria required that the patient: (a) had a diagnosis of hemoglobin SS disease; (b) had pain ≥ 3 (0-10 scale) related to SCD at baseline; (c) reported chronic pain with ≥ 4 neuropathic pain descriptors; (d) had not consumed drugs metabolized by cytochrome P450, family 1, subfamily A, polypeptide 1 (CYP1A1) within 2.5 half-lives of the drug; (e) spoke and read English; (f) was 18 years or older; (g) was not taking a drug that prolongs the Q-T interval; and (h) had no history of prolonged Q-T interval. Subject exclusion criteria were: (a) legally blind; (b) mentally or physically unable to complete study questionnaires; (c) taking any adjuvant analgesic drugs within three weeks of baseline; (d) being treated for any psychoses; (e) adverse effects at baseline; (f) alanine transaminase (ALT) > 300 IU/L, or albumin < 2.0mg/dL; (g) creatinine > 2.5mg/dL and creatinine clearance < 60ml/min; (h) pregnant or breast feeding; (i) taking herbals-St John's Wort, dong quai, kava kava, gotu kola, valerian; or (j) history of priapism.
A total of 20 patients with hemoglobin SS disease consented to participate; 18 met eligibility criteria and completed the study. The age of the 3 men and 15 women averaged 35.8 ± 8.9 years (ranged from 23 to 53). Sixteen self-reported ethnicity as non-Hispanic black, 1 Hispanic-white, and 1 Hispanic-mixed race. Three subjects completed high school, 4 had vocational training, 8 attended but did not finish college, and 3 had a 4-year college degree.
2.3. Procedures
After verbal consent for screening, a well-trained research nurse (R-RN) completed the screening procedures and scheduled the patient for a convenient time to complete the 24-hr study. On the study day in the sickle cell clinic, the R-RN verified eligibility, obtained written informed consent, documented vital signs, and inserted an intravenous (IV) cannula for blood sampling. Once the patient completed the self-report and observational tools, the R-RN administered the pre-determined dose of trifluoperazine and monitored the patient hourly for 7 hrs with measures of vital signs, adverse effects, pain intensity, and analgesic doses consumed during the previous hour. The R-RN called the patient the following day to collect self-reported adverse effects, pain intensity, and analgesic doses from the 7th hr to 24 hrs after trifluoperazine administration.
2. 4. Intervention
We initially planned to administer six doses (0.5 mg, 1 mg, 2 mg, 5 mg, 10 mg, and 20 mg) as a single trifluoperazine dose to determine the safety of each dose in three subjects. We selected this dose range based on human studies (Marques et al., 2004) and analgesic observations in mouse studies (Tang et al., 2006). A priori rules specified that the minimum toxic dose was defined by adverse effects rated > 2 for two patients at a given dose. Rules also specified that profound analgesic effects at a particular dose, as demonstrated in mice, required that we stop the dose escalation, consider a lower dose, and assess safety and effect on pain in the remaining sample.
2.5. Measures
2.5.1. Adverse effects
We used the Extrapyramidal Symptom Rating Scale (ESRS) (Chouinard and Margolese, 2005) and an adverse effects checklist to document adverse effects of trifluoperazine. The ESRS is a standardized observational tool to measure extrapyramidal symptoms that are common with antipsychotic medications. The ESRS measures drug-induced movement disorders, including akathisia, dystonia, and tardive dyskinesia. A well-trained research nurse completed the observation of the activity series in less than 10 min. The observational tool has documented validity and reliability (Chouinard and Margolese, 2005). The adverse checklist documents the presence and severity (none[0], mild[1], moderate[2], severe[3]) of nine subjective adverse effects (sedation, confusion, agitation, dry mouth, blurred vision, urinary retention, tremors, shaking, and difficulty walking) and other adverse effects that the subject reported.
2.5.2. Pain intensity
We used a computerized version of the Visual Analogue Scale for pain intensity (VAS-P) as ratio level data for subjective pain intensity. The VAS-P is a horizontal, 10-cm line anchored on the left with “no pain” and on the right with “pain as bad as it could be” (Wilkie et al., 1990). Standardized instructions, validity (r = .64 to .81) (Kremer and Atkinson, 1981; Wilkie et al., 1990), reliability (r =.99) (Kremer et al., 1981; Scott and Huskisson, 1979), and sensitivity of the VAS-pain intensity have been estimated previously (Huskisson, 1983), and extended to computer format (Jamison et al., 2002). Subjects completed the VAS in less than 1 minute (Wilkie et al., 1990).
2.5.3. Demographic variables
Demographics included age, gender, ethnicity, and education included in PAINReportIt® (Wilkie et al., 2003). Subjects completed items in less than 5 minutes.
2.5.4. Confounding variable
If patients required analgesics during the 24-hr study period, we documented each dose. Although it would be desirable that patients not consume any opioid analgesics during the study period, we considered it unethical to withhold analgesics for moderate to severe chronic pain.
2.6. Statistical Analysis
Analysis of safety and pain intensity included change from baseline and attainment of threshold criteria within and between subjects. Within subjects, the threshold criterion for adverse effect was a rating of 3 (severe) for any adverse effect. Between subjects who received the same drug dose, two subjects with an adverse-effect rating of 3 determined the minimum toxic dose. The threshold criterion for profound analgesia was a 50% reduction from the baseline pain intensity and sustained for 3 hrs (4 measurements). Descriptive statistics, graphs, and plots provided approaches for interpretation of results for the three subjects at each drug dose.
3. Results
None of the subjects reached the adverse-effect threshold at doses of 0.5, 1, 2, and 5 mg, but two subjects reached the adverse-effect threshold at the 10 mg dose, which identified the minimum toxic dose. After this finding, we added a 7.5 mg dose and none of the three subjects at this dose reached the adverse-effect threshold. Other details of the safety and pain outcomes appear in the following sections.
3.1. Safety
We observed no adverse effects up to a dose of 5 mg. At baseline with 5 mg, one subject had a mild sedation, which returned to none 3 hrs after taking the study drug. At 7.5 mg, two subjects had moderate sedation; one at the 7th hr and the other at the 3rd, 4th, 6th, and 7th hr.
At 10 mg, one subject had severe sedation at the 6th hr along with moderate increase in difficulty ambulating due to the sedation; the study was terminated at the 6th hr due to sedation. Another subject had moderate sedation at the 4th hr. The third subject experienced dystonia 24.5 hrs after the trifluoperazine. Sedation resolved with caffeine and dystonia resolved with diphenhydramine.
No subject had confusion, blurred vision, urinary retention problem, tremors, or shaking. Subjects who reported mild agitation (n=2), dry mouth (n=4), or sedation (n=1) at baseline and all but one with dry mouth reported resolution of these effects after the trifluoperazine.
Observed adverse effects included mild posture impairment, hypokinisia, anteroplusion, lateral pulsion, and retropulsion in the same subject who experienced severe sedation with the 10 mg dose. The subject who experienced dystonia also had observed gait stiffness at baseline that resolved after the 10 mg dose. Mild impaired gait resolved after baseline for one subject (0.5 mg dose), and there was a notation of improved gait that had been noted as impaired (but not a mild level) at baseline for three subjects (2 at 1 mg, 1 at 5 mg doses). Unknown at the time, one subject who received a 2 mg dose had been non-adherent to her prescribed anti-seizure medication for two days and had an observed seizure before the 7th hr; this event was not attributed to the trifluoperazine because of the patient's seizure disorder and the patient's non-adherence to anti-seizure medications.
3. 2. Pain
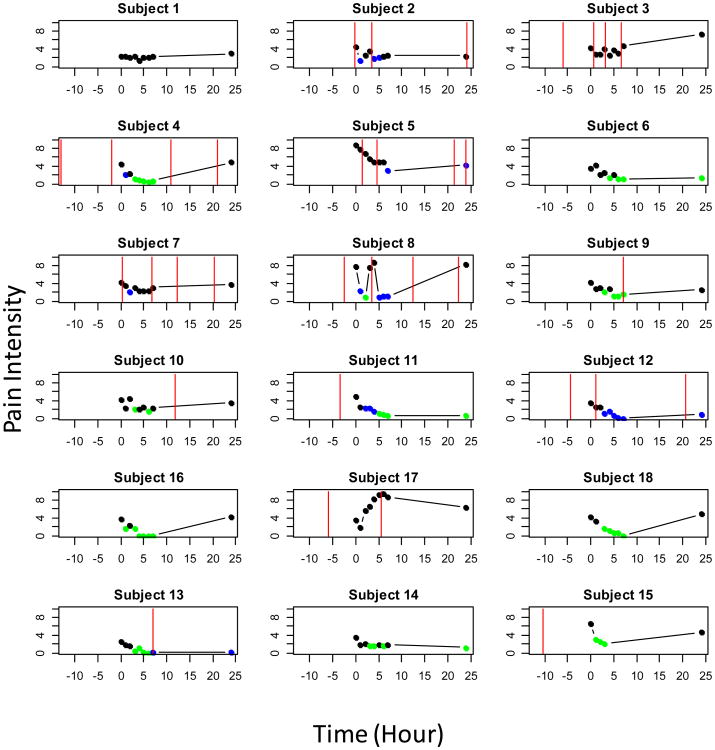
Table 1 presents analgesia outcomes for the 18 subjects by trifluoperazine dose. After the trifluoperazine dose for at least one measurement, 15 subjects (83%) reported 50% reduction in their VAS pain intensity score (Fig. 1), 11 of these subjects' (61%) pain reduction was likely the outcome of the trifluoperazine dose (Table 1 and Fig. 1). At more than 3 time points (not necessarily consecutively), 8 subjects (44%) reported pain that was at least 50% lower than baseline and attributable to trifluoperazine, that is, after the expected peak of the study drug (∼3 hr) and not the effect of any supplemental analgesics (Table 1 and Fig. 1). Of these 8 subjects (44%), 2 received 1 mg, 1 received 2 mg, 1 received 5 mg, 2 received 7.5 mg, and 2 received 10 mg of trifluoperazine.
Table 1. Effects of Trifluoperazine by Subject: Visual Analogue Scale (VAS) Pain Intensity Scores, Supplemental Oral Morphine Equivalent Dose (OMED) Taken, Maximum Side Effect, and Conclusion.
| Trifluoperazine dose (mg) | Subject number | VAS pain Baseline | VAS pain 24 hr | OMED (mg) taken within 12 hr before study dose | OMED (mg) taken within 7 hr of study dose | OMED (mg) taken 8 to 24 hr after study dose | Maximum side effect | Conclusion |
|---|---|---|---|---|---|---|---|---|
| 0·5 | 1 | 2.4 | 2.9 | 0 | 0 | 0 | 0 | No relief |
| 2 | 4.4 | 2.4 | 8 | 16 | 10 | 0 | C | |
| 3 | 4.1 | 7.4 | 75 | 30 | 15 | 0 | C | |
| 1.0 | 4 | 4.4 | 4.9 | 120 | 0 | 120 | 0 | Good relief |
| 5 | 8.7 | 4.3 | 0 | 105 | 105 | 0 | C | |
| 6 | 3.4 | 1.3 | 0 | 0 | 0 | 0 | Good relief | |
| 2.0 | 7 | 4.2 | 3.8 | 0 | 60 | 90 | 0 | C |
| 8 | 7.8 | 8.3 | 30 | 30 | 60 | 0 | C | |
| 9 | 4.2 | 2.5 | 0 | 0 | 60 | 0a | Good relief | |
| 5.0 | 10 | 4.1 | 3.6 | 0 | 0 | 15 | 0 | Some relief |
| 11 | 4.8 | 0.6 | 15 | 0 | 0 | 0 | Good relief | |
| 12 | 3.4 | 0.9 | 30 | 30 | 30 | 0 | C | |
| 7.5 | 16 | 3.7 | 4.3 | 0 | 0 | 0 | 2 | Good relief |
| 17 | 3.4 | 6.3 | 46 | 0 | 16 | 2 | No relief | |
| 18 | 4.2 | 4.8 | 0 | 0 | 0 | 0 | Good relief | |
| 10 | 13 | 2.5 | 0.1 | 0 | 0 | 16 | 0b | Good relief |
| 14 | 3.4 | 1.2 | 0 | 0 | 0 | 2 | Good relief | |
| 15 | 6.7 | 4.6 | 30 | 0 | 0 | 3c | Good relief |
C: Contaminated by supplemental analgesics;
Seizure not attributed to trifluoperazine;
Dystonia (rated 3) 24.5 hr after study dose;
Sedation
Fig. 1.
Visual Analogue Scores by Subject (1-18) during the 24-hour Study.
The dose appears in order of administration: 0.5 mg for subjects 1-3, 1mg for subjects 4-6, 2 mg for subjects 7-9, 5 mg for subjects 10-12, 7,5 mg for subjects 16-18, and 10 mg for subjects 13-15. The green symbols represent 50% reduction from baseline (hr 0), blue symbols represent 50% from baseline but contaminated by a supplemental analgesic, which is represented by red vertical lines.
3.3. Supplemental analgesics
Twelve subjects consumed no supplemental opioid analgesics between baseline and the end of the 7-hr acute monitoring period, 7 of whom also reported that they consumed no opioid analgesics during the entire 24-hr study (Table 1). Eleven of the 18 subjects required an opioid analgesic dose during the 24-hr study period as presented in Fig. 1. Out of these 11, 9 (82%) had 50% reduction at least once and 6 (55%) did so 3 times or more. Nine subjects consumed an opioid analgesic dose within 12 hrs prior to the study (Table 1). We did not attribute any pain reduction to trifluoperazine if an opioid analgesic effect overlapped (consumed before and during) the 7-hr acute monitoring period.
4. Discussion
Based on preclinical studies (Chen et al., 2009; Chen et al., 2010; Luo et al., 2008; Wang et al., 2010), it was highly promising that trifluoperazine would show efficacy in humans. Here is the first report of safety and analgesic effects of trifluoperazine administered to adults with SCD. We identified 10 mg as the minimum toxic dose based on severe sedation and dystonia, identified using a well-accepted dose escalation plan for phase 1 drug studies designed to determine tolerable doses (Ferte et al., 2011). From a safety perspective, in this open-labeled, single-dose study, the most common moderate to severe adverse effect was sedation, which 4 subjects experienced. All the other adverse effects were mild with the exception of one subject who had difficulty walking, mild at baseline and severe after the 10 mg dose, which was likely caused by the subject's severe sedation. These data suggest that 10 mg was the threshold for triggering severe adverse effects. Pain intensity reduction (≥ 50% of baseline) without supplemental analgesics or severe sedation was evident for 8 subjects, 6 of whom received doses less than the 10 mg (e.g., 1 mg, 2 mg, 5 mg, 7.5 mg). The safety profile and these pain outcomes are encouraging and warrant a larger phase I/II study with a randomized placebo controlled design to determine the long-term safety and efficacy of trifluoperazine for adults with SCD who report pain descriptors consistent with neuropathic pain.
It is now clear that the pain experience of those with SCD is much more complex and frequent than the early studies suggested (Dampier et al., 2002a; Dampier et al., 2002b; Maikler et al., 2001; Platt et al., 1994; Platt et al., 1991; Shapiro et al., 1995). Not only do many adults with SCD select verbal descriptors associated with neuropathic pain (Wilkie et al., 2010), but some also experience allodynia and hyperalgesia in cold weather (Molokie et al., 2011) and with the thermal and mechanical stimuli used for quantitative sensory testing (Ezenwa et al., In review). These three findings are consistent with the central or peripheral nervous system sensitization that typifies neuropathic pain. The neuropathic pain among adults with SCD perhaps results from persistent, recurrent nervous system activation, or synaptic plasticity in the spinal dorsal horn, which also explain the clinical findings of pain hypersensitivity, a key finding of neuropathic pain (von Hehn et al., 2012; Woolf, 1983).
The mechanisms leading to and maintaining central sensitization are complex. As a versatile protein kinase, CaMKIIα may interact with the N-methyl-D-aspartate (NMDA) receptors that are essential for the generation of long-term potentiation, learning, and memory. CaMKIIα phosphorylates the NMDA receptors, leading to the latter's activation. Ca2+ influx through the activated NMDA receptors, in turn, causes autophosphorylation of CaMKIIα and subsequent full activation of the kinase. A similar feed-forward mechanism may be found between CaMKIIα and the transient receptor potential vanilloid 1 receptor (TRPV1) (Wang et al., 2010). In mouse models, we found that the inhibition of CaMKIIα using several CaMKIIα inhibitors including trifluoperazine, prevented or reversed experimental neuropathic pain (Chen et al., 2009).
Trifluoperazine-like phenothiazines were first used in animal trials during the late 1880s by Paul Ehrich (Ohlow and Moosmann, 2011). In the 1950s, phenothiazine derivatives were developed and used in anesthesia, as antihistamines, and in schizophrenic, agitated and hyperactive psychiatric patients (Ohlow and Moosmann, 2011). Today, they are still among the most widely used psychotropic drugs in the world (Ohlow and Moosmann, 2011; Sudeshna and Parimal, 2010). Besides trifluoperazine, several other members of the phenothiazine drug class also show antinociceptive properties for chronic, but not acute pain, when tested in preclinical models (Yang et al., 2011; Wang et al., unpublished data). In a recent review, Marques et al. (2004) stated that typical daily doses of trifluoperazine are 12 mg to 50 mg and common adverse effects include dopaminergic, cholinergic, adrenergic, and histaminergic effects. In addition to antagonizing dopamine D2 receptors, phenothiazine inhibited calmodulin (Jaszczyszyn et al., 2012; Sudeshna and Parimal, 2010), CaMKIIα (Luo et al., 2008), and likely other yet to be determined targets.
Our findings suggest that analgesia may be possible with much smaller doses than are typical for psychiatric patients, which could reduce the risk for long-term side effects such as tardive dyskinesia. The first dose with 50% reduction in pain intensity was 1 mg, and it would be a prudent starting dose to test in a randomized double-blinded placebo controlled study with repeated doses and long-term follow-up of adverse and analgesic effects. Repeated doses of 2, 2.5, 5 or 7.5 mg could also be tested given the safety profile observed in our study. The double-blind study design is important because expectations (Benedetti et al., 2003) of either the patient or the research nurse could influence study outcomes. Since safety was our primary outcome we did not include a control group in this study, but control of the placebo response is important for a future study with pain as a primary outcome. Additionally, it would prudent to measure monocyte CaMKII activity (Guest et al., 2008) as a surrogate indicator of the mechanism by which trifluoperazine exerts analgesic effects in humans especially since other mechanisms are possible.
Interestingly, in 1961 Bounameaux and colleagues (Serjeant, 1974) suggested use of phenothiazines for SCD based on their in vitro effect to inhibit sickling. In 1963, Lewis again suggested them as a treatment based on the idea that inhibition of glucose-6-phosphate dehydrogenase would result in less oxygen consumption in the red blood cell and, therefore, less hemoglobin polymerization (Raper, 1968). Heller (1966) suggested that phenothiazines could be useful in controlling painful crisis because of their sedative effect. A double-blinded trial of a phenothiazine to reduce acute painful events, however, yielded negative results (Mahmood, 1969). Despite these early notions, based on the CaMKIIα mechanism and pathophysiological role of CaMKIIα in chronic pain, we expect trifluoperazine to be most effective in chronic, not acute, types of SCD pain (Chen et al., 2009; Chen et al., 2010; Luo et al., 2008).
5. Conclusions
We observed moderate sedative effect at the 7.5 mg dose and severe sedative effect at 10 mg, the minimum toxic dose. However, the preliminary data are encouraging that 8 subjects also reported significant alleviation of chronic pain without severe sedation. The effect lasted for at least 24 hr in 3 subjects. In this initial translational study, trifluoperazine shows promise as an analgesic drug that is worthy of further testing in a randomized placebo controlled study of adults with SCD.
Acknowledgments
This publication was made possible by Grant Number 1R01 HL098141 from the National Institutes of Health (NIH), National Heart Lung and Blood Institute (NHLBI). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI. The final peer-reviewed manuscript is subject to the NIH Public Access Policy. The authors thank the patients with sickle cell disease for participating in this study, and the staff at the Comprehensive Sickle Cell Center for their continuous support of the study.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest related to this study. Drs. Molokie and Wilkie are co-investigators on an unrelated study funded by Pfizer.
Compound List: trifluoperazine, phenothiazine
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J Neurosci. 2003;23:4315–4323. doi: 10.1523/JNEUROSCI.23-10-04315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brousseau DC, Owens PL, Mosso AL, Panepinto JA, Steiner CA. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303:1288–1294. doi: 10.1001/jama.2010.378. [DOI] [PubMed] [Google Scholar]
- Chen Y, Luo F, Yang C, Kirkmire CM, Wang ZJ. Acute inhibition of Ca2+/calmodulin-dependent protein kinase II reverses experimental neuropathic pain in mice. J Pharmacol Exp Ther. 2009;330:650–659. doi: 10.1124/jpet.109.152165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yang C, Wang ZJ. Ca2+/calmodulin-dependent protein kinase II alpha is required for the initiation and maintenance of opioid-induced hyperalgesia. J Neurosci. 2010;30:38–46. doi: 10.1523/JNEUROSCI.4346-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard G, Margolese HC. Manual for the Extrapyramidal Symptom Rating Scale (ESRS) Schizophr Res. 2005;76:247–265. doi: 10.1016/j.schres.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Dampier C, Ely B, Brodecki D, O'Neal P. Characteristics of pain managed at home in children and adolescents with sickle cell disease by using diary self-reports. J Pain. 2002a;3:461–470. doi: 10.1054/jpai.2002.128064. [DOI] [PubMed] [Google Scholar]
- Dampier C, Ely E, Brodecki D, O'Neal P. Home management of pain in sickle cell disease: a daily diary study in children and adolescents. J Pediatr Hematol Oncol. 2002b;24:643–647. doi: 10.1097/00043426-200211000-00008. [DOI] [PubMed] [Google Scholar]
- Ezenwa MO, Molokie RE, Wang ZJ, Yao Y, Suarez ML, Pullum C, Fillingim RB, Wilkie DJ. Safety and Utility of Quantitative Sensory Testing among Adults with Sickle Cell Disease: Indicators of Neuropathic pain. doi: 10.1111/papr.12279. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferte C, Soria JC, Penel N. Dose-levels and first signs of efficacy in contemporary oncology phase 1 clinical trials. PloS One. 2011;6:e16633. doi: 10.1371/journal.pone.0016633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest CB, Deszo EL, Hartman ME, York JM, Kelley KW, Freund GG. Ca2+/calmodulin-dependent kinase kinase alpha is expressed by monocytic cells and regulates the activation profile. PloS one. 2008;3:e1606. doi: 10.1371/journal.pone.0001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller P. Hemoglobinopathic dysfunction of the red cell. Am J Med. 1966;41:799–814. doi: 10.1016/0002-9343(66)90038-6. [DOI] [PubMed] [Google Scholar]
- Huskisson EC. Visual analogue scales. In: Melzack R, editor. Pain Assessment and Management. Raven press; New York: 1983. pp. 33–37. [Google Scholar]
- Jamison RN, Gracely RH, Raymond SA, Levine JG, Marino B, Herrmann TJ, Daly M, Fram D, Katz NP. Comparative study of electronic vs. paper VAS ratings: a randomized, crossover trial using healthy volunteers. Pain. 2002;99:341–347. doi: 10.1016/s0304-3959(02)00178-1. [DOI] [PubMed] [Google Scholar]
- Jaszczyszyn A, Gasiorowski K, Swiatek P, Malinka W, Cieslik-Boczula K, Petrus J, Czarnik-Matusewicz B. Chemical structure of phenothiazines and their biological activity. Pharmacol Rep. 2012;64:16–23. doi: 10.1016/s1734-1140(12)70726-0. [DOI] [PubMed] [Google Scholar]
- Kremer E, Atkinson JH, Ignelzi RJ. Measurement of pain: patient preference does not confound pain measurement. Pain. 1981;10:241–248. doi: 10.1016/0304-3959(81)90199-8. [DOI] [PubMed] [Google Scholar]
- Kremer E, Atkinson JH., Jr Pain measurement: construct validity of the affective dimension of the McGill Pain Questionnaire with chronic benign pain patients. Pain. 1981;11:93–100. doi: 10.1016/0304-3959(81)90142-1. [DOI] [PubMed] [Google Scholar]
- Lanzkron S, Carroll CP, Haywood C., Jr The burden of emergency department use for sickle-cell disease: an analysis of the national emergency department sample database. Am J Hematol. 2010;85:797–799. doi: 10.1002/ajh.21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Yang C, Chen Y, Shukla P, Tang L, Wang LX, Wang ZJ. Reversal of chronic inflammatory pain by acute inhibition of Ca2+/calmodulin-dependent protein kinase II. J Pharmacol Exp Ther. 2008;325:267–275. doi: 10.1124/jpet.107.132167. [DOI] [PubMed] [Google Scholar]
- Mahmood A. A double-blind trial of a phenothiazine compound in the treatment of clinical crisis of sickle cell anaemia. Br J Haematol. 1969;16:181–184. doi: 10.1111/j.1365-2141.1969.tb00391.x. [DOI] [PubMed] [Google Scholar]
- Maikler VE, Broome ME, Bailey P, Lea G. Childrens' and adolescents' use of diaries for sickle cell pain. J Soc Pediatr Nurs. 2001;6:161–169. doi: 10.1111/j.1744-6155.2001.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Marques LO, Lima MS, Soares BG. Trifluoperazine for schizophrenia. Cochrane Database Syst Rev. 2004:CD003545. doi: 10.1002/14651858.CD003545.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molokie RE, Wang ZJ, Wilkie DJ. Presence of neuropathic pain as an underlying mechanism for pain associated with cold weather in patients with sickle cell disease. Med Hypotheses. 2011;77:491–493. doi: 10.1016/j.mehy.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlow MJ, Moosmann B. Phenothiazine: the seven lives of pharmacology's first lead structure. Drug Discov Today. 2011;16:119–131. doi: 10.1016/j.drudis.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M, Temperley WH, Williams TN, Weatherall DJ, Hay SI. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381:142–151. doi: 10.1016/S0140-6736(12)61229-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, Kinney TR. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325:11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- Raper AB. A commentary on the anti-sickling effect of the phenothiazine drugs. Trans R Soc Trop Med Hyg. 1968;62:84–91. doi: 10.1016/0035-9203(68)90035-7. [DOI] [PubMed] [Google Scholar]
- Scott J, Huskisson EC. Vertical or horizontal visual analogue scales. Ann Rheum Dis. 1979;38:560. doi: 10.1136/ard.38.6.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serjeant GR. The painful crisis. In: Serjeant GR, editor. The clinical features of sickle cell disease. North-Holland Publishing Company; New York: 1974. pp. 137–146. [Google Scholar]
- Shapiro BS, Dinges DF, Orne EC, Bauer N, Reilly LB, Whitehouse WG, Ohene-Frempong K, Orne MT. Home management of sickle cell-related pain in children and adolescents: natural history and impact on school attendance. Pain. 1995;61:139–144. doi: 10.1016/0304-3959(94)00164-A. [DOI] [PubMed] [Google Scholar]
- Smith WR, Penberthy LT, Bovbjerg VE, McClish DK, Roberts JD, Dahman B, Aisiku IP, Levenson JL, Roseff SD. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148:94–101. doi: 10.7326/0003-4819-148-2-200801150-00004. [DOI] [PubMed] [Google Scholar]
- Sudeshna G, Parimal K. Multiple non-psychiatric effects of phenothiazines: a review. Eur J Pharmacol. 2010;648:6–14. doi: 10.1016/j.ejphar.2010.08.045. [DOI] [PubMed] [Google Scholar]
- Tang L, Shukla PK, Wang ZJ. Trifluoperazine, an orally available clinically used drug, disrupts opioid antinociceptive tolerance. Neurosci Lett. 2006;397:1–4. doi: 10.1016/j.neulet.2005.11.050. [DOI] [PubMed] [Google Scholar]
- von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. 2012;73:638–652. doi: 10.1016/j.neuron.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZJ, Wilkie DJ, Molokie R. Neurobiological mechanisms of pain in sickle cell disease. Hematology Am Soc Hematol Educ Program. 2010;2010:403–408. doi: 10.1182/asheducation-2010.1.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie D, Lovejoy N, Dodd M, Tesler M. Cancer pain intensity measurement: concurrent validity of three tools--finger dynamometer, pain intensity number scale, visual analogue scale. Hospice Journal. 1990;6:1–13. doi: 10.1080/0742-969x.1990.11882662. [DOI] [PubMed] [Google Scholar]
- Wilkie DJ, Judge MK, Berry DL, Dell J, Zong S, Gilespie R. Usability of a computerized PAINReportIt in the general public with pain and people with cancer pain. J Pain Symptom Manage. 2003;25:213–224. doi: 10.1016/s0885-3924(02)00638-3. [DOI] [PubMed] [Google Scholar]
- Wilkie DJ, Molokie R, Boyd-Seal D, Suarez ML, Kim YO, Zong S, Wittert H, Zhao Z, Saunthararajah Y, Wang ZJ. Patient-reported outcomes: descriptors of nociceptive and neuropathic pain and barriers to effective pain management in adult outpatients with sickle cell disease. J Natl Med Assoc. 2010;102:18–27. doi: 10.1016/s0027-9684(15)30471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TN, Weatherall DJ. World distribution, population genetics, and health burden of the hemoglobinopathies. Cold Spring Harb Perspect Med. 2012;2:a011692. doi: 10.1101/cshperspect.a011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Yang C, Chen Y, Tang L, Wang ZJ. Haloperidol disrupts opioid-antinociceptive tolerance and physical dependence. J Pharmacol Exp Ther. 2011;338:164–172. doi: 10.1124/jpet.110.175539. [DOI] [PMC free article] [PubMed] [Google Scholar]