Abstract
Background
The role of HPV in cutaneous squamous cell carcinoma (cuSCC) is not well defined, with past studies showing conflicting results.
Objective
We sought to determine if there is a significant association between HPV and cuSCC and whether cuSCC from immunosuppressed patients are more likely to carry HPV than cuSCC from immunocompetent patients.
Methods
We performed a systematic review and abstracted data from articles that included: skin samples by biopsy, HPV detection by PCR, and a minimum of 10 cases and 10 controls. Pooled effect size and 95% confidence intervals were calculated using random effects meta-analysis using the inverse variance method.
Results
cuSCC were more likely to carry HPV than normal skin (pooled ES 3.43, 95% CI 1.97–5.98, p<0.0001) in all patients. An increase in HPV prevalence was found in tumors from immunosuppressed patients compared to immunocompetent patients (pooled ES 3.01, 95%CI 2.00–4.52, p<0.0001).
Limitations
The greatest limitation is the heterogeneity of the studies included. The association of higher HPV prevalence in SCC compared to normal skin does not imply causality.
Conclusion
These results contribute to evidence that HPV is associated with cuSCC. Higher HPV burden in tumors from immunosuppressed patients compared to immunocompetent patients may have therapeutic implications.
Keywords: meta-analysis, cutaneous squamous cell carcinoma, human papillomavirus, immunocompetence, immunosuppression, skin cancer
Introduction
Nonmelanoma skin cancer (NMSC) is the most common cancer in the U.S. and its incidence has continued to increase in the past two decades.1 Among immunocompetent individuals, squamous cell carcinoma (SCC) is the second most common type of NMSC. Ultraviolet radiation exposure, fair skin, and immunosuppression are well-known risk factors for development of SCC.2 The clinical behavior and epidemiology of SCC may also suggest a viral etiology given the increased prevalence in organ transplant recipients (OTR) compared to the general population and similar incidence to other virally induced cancers, including Kaposi sarcoma.3
One potential etiologic agent in SCC is human papillomavirus (HPV), as its role in cervical cancer is well established.4,5 HPV also has an established etiologic role in verruca vulgaris, condyloma acuminata, various types of anogenital cancer, and some head and neck SCCs.6,7
However, HPV’s relationship to cutaneous SCC remains in question. Over 100 studies have investigated the relationship between HPV and cutaneous squamous cell carcinoma (cuSCC) using different study populations, sampling techniques, detection methods, and HPV types. Only studies using biopsy specimens with PCR-based detection of HPV were included in the analysis.
The association of β-HPV types with SCC is clearly defined for patients with epidermodysplasia verruciformis (EV), an autosomal recessive disorder characterized by an abnormal susceptibility to β-HPV (5 and 8 mostly).8 However, the relationship between HPV and SCC in the general population is less well defined, as studies have yielded conflicting results. While some studies have failed to find HPV in SCC, most studies report HPV infection in some SCCs (with variable percentages). Variable sampling and detection rates may have led to the wide disparity in prevalence.9
The objective of the current study is to perform a meta-analysis of the literature to determine the association between HPV and cutaneous SCC. We also sought to determine whether there is a higher prevalence of HPV in SCCs from immunosuppressed patients compared to SCCs from immunocompetent patients.
Methods
Search Strategy and Selection Criteria
We systematically searched the biomedical electronic databases Pubmed, Embase, Web of Science, CINAHL and Cochrane Library for all relevant published literature as of June 22, 2012, using the keywords “skin cancer” AND “human papillomavirus” OR “cutaneous squamous cell carcinoma” AND “human papillomavirus”. Duplicate articles were removed, resulting in a total of 3661 records. Titles and abstracts of articles were reviewed to screen for relevance and exclusion criteria. Reference lists from the retrieved studies and reviews were scanned to ensure that all potentially relevant literature was included, but no additional papers were found.
3290 papers were excluded for not being relevant, presenting no unique data, including patients with genodermatoses, including only lips, fingers or genital samples, or unspecified NMSCs rather than cuSCC. An additional 196 articles were not written in English. Our inclusion criteria is included in Table 1. After screening using the aforementioned criteria, 17 articles qualified for the analysis examining tumor samples versus normal skin, and 12 articles were included for analysis of tumors from immunosuppressed individuals versus those from immunocompetent individuals. (Fig. 1). Three articles were used in both analyses as they met criteria for both.
Table 1.
Selection criteria for systematic review of articles
| Exclusion Criteria | Inclusion Criteria |
|---|---|
|
| |
| Not in English Not relevant Reviews Letters to the Editor Case studies with <10 samples (tumor or normal) Studies of patients with comorbid conditions (EV and other genodermatoses) Studies including only lips, fingers, and genitals samples Unspecified NMSC Noncutaneous SCC |
|
Fig 1.
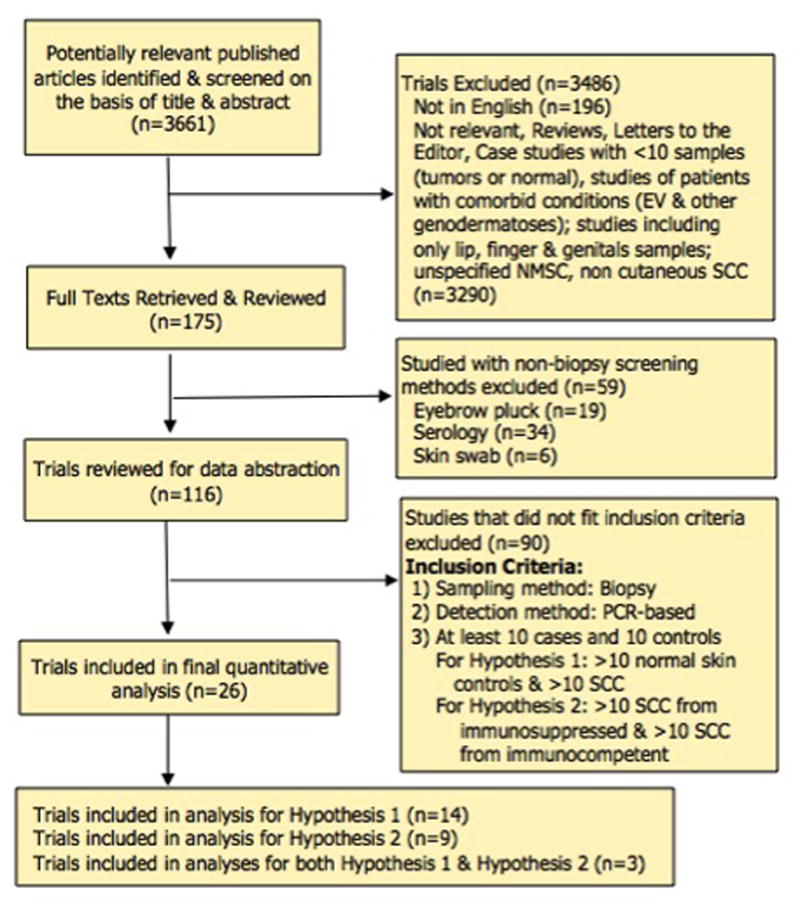
Flow chart of the search strategy and selection criteria
Data Collection and Quality Assessment
A standard data extraction procedure was created by BA, JW, and SA. Each article was checked independently by JW and JY for eligibility, and all relevant data was extracted independently and in duplicate for each paper by BA, JW, and JY. Any discrepancies in the duplicates were settled by SA, BA, JW and JY in group discussion until a consensus was reached.
Data abstracted from each paper included the year of publication, whether the assay was broad-spectrum (detecting more than 10 HPV types) or limited-spectrum (fewer than 10 HPV types), and types of HPV assayed (cutaneous, mucosal, or both). One paper (Gustafsson 2004) reported cutaneous HPV type and mucosal HPV type assays separately but did not give aggregate numbers; these were considered as separate experiments. Numbers of SCC and normal skin from immunocompetent and immunosuppressed patients were extracted, as well as numbers of HPV-positive and -negative specimens in each subgroup. “SCC” included tumors recorded as cutaneous squamous cell carcinomas, keratoacanthomas, Bowen’s Disease and veruccous carcinomas. Bowenoid papillosis was excluded. “Normal skin” included both normal tissue from subjects with cuSCC and normal skin from subjects without any cuSCC. Benign lesions were not included. When given, data was collected on the age, sex, immune status and race of patients and whether the samples were from sun-exposed or sun-protected skin. Sun-exposed includes head/neck, arms and hands. Non-sun exposed includes trunk and legs. Sites of interest exclude lips, fingers, and genital regions. In studies where biopsy sites were recorded, those collected from lips, fingers, and genital regions were excluded; as a result, the numbers in the tables may differ slightly from those published in a particular paper. In studies that did not specify the exact location from which biopsies were taken, all samples were included.
Statistical Methods
Pooled effect size and 95% confidence intervals were calculated using random effects meta-analysis using the inverse variance (Dersimonian and Laird) method.10 Heterogeneity between studies was assessed with two indicators, the I2 and Q statistics. The I2 statistic provides an estimate of the percentage of variability in the outcome that is due to differences in exposure-outcome association; a significant Q statistic rejects the null hypothesis of homogeneity and indicates that the true effect size varies from study to study.11
Funnel plots were used to assess publication bias.12 In a funnel plot the estimated effects are plotted against their standard error. When publication bias is absent, the observed studies are expected to be distributed symmetrically around the pooled effect size. Begg’s rank correlation and Egger’s linear correlation tests were used to detect funnel plot asymmetry, with a threshold p value of 0.01. In the analysis of HPV prevalence in SCC vs. normal skin, a trim-and-fill technique was used to adjust the random-effects model for possible publication bias.13
Results
Hypothesis 1: Tumors are more likely to carry HPV than normal skin
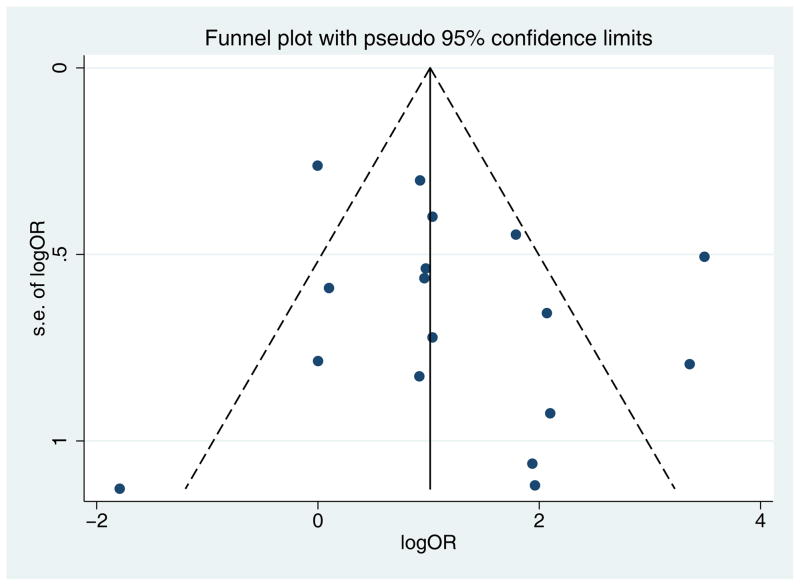
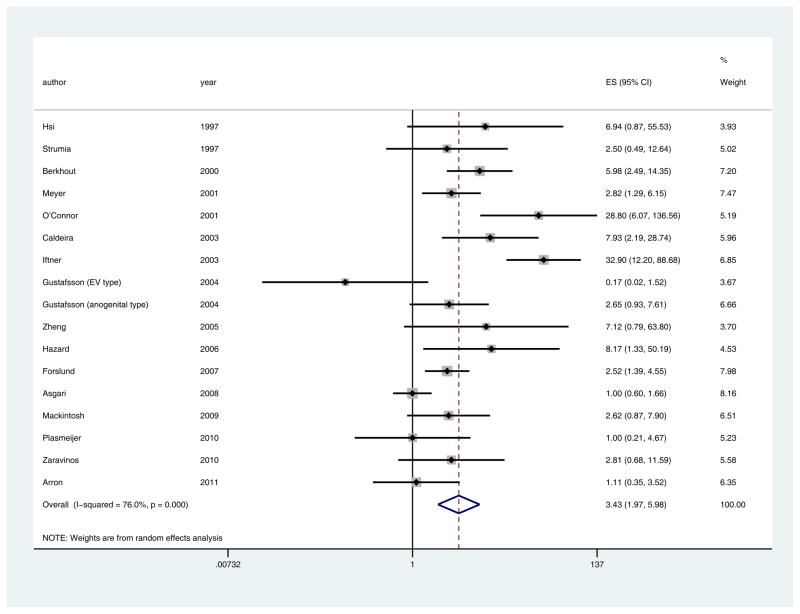
17 papers met inclusion criteria (Table 2).14–29 SCC were more likely to carry HPV than normal skin (pooled ES 3.43, 95% CI 1.97–5.98, p<0.0001). The funnel plot showed no evidence of publication bias (Egger’s p=0.23). (Fig 2).
Table 2.
List of articles included in the meta-analysis for tumor versus normal (Hypothesis 1)
| Source | HPV types | # of HPV types | # of HPV+ pts (%) | |
|---|---|---|---|---|
| SCC group | control group | |||
| Strumia et al, 1997 | Mucosal | Limited | 6 (60) | 6 (37.5) |
| Hsi et al, 1997 | Mucosal | Broad | 15 (21.7) | 1 (3.8) |
| Berkhout et al, 2000 | Cutaneous | Broad | 74 (74) | 10 (32.2) |
| O’Connor et al, 2001 | Cutaneous | Broad | 18 (85.7) | 5 (17.2) |
| Meyer et al, 2001 | Both | Broad | 39 (35.1) | 10 (16.1) |
| Caldeira et al, 2003 | Cutaneous | Limited | 12 (46.2) | 4 (9.8) |
| Iftner et al 2003 | Both | Broad | 57 (62) | 5 (4.7) |
| Gustafsson et al, 2004 (EV type) | Cutaneous | Limited | 1 (3.2) | 5 (16.7) |
| Gustafsson et al, 2004 (anogenital type) | Mucosal | Broad | 19 (52.8) | 8 (29.6) |
| Zheng et al, 2005 | Both | Broad | 5 (13.2) | 1 (2.1) |
| Hazard et al, 2006 | Cutaneous | Limited | 3 (5.8) | 2 (0.7) |
| Forslund et al, 2007 | Both | Broad | 21 (25.6) | 42 (12) |
| Asgari et al, 2008 | Both | Broad | 46 (54) | 103 (54.2) |
| Mackintosh et al, 2009 | Cutaneous | Broad | 34 (56.7) | 6 (33.3) |
| Zaravinos et al, 2010 | Both | Broad | 4 (33.3) | 8 (15.1) |
| Plasmeijer et al, 2010 | Cutaneous | Broad | 17 (81) | 17 (81) |
| Arron et al, 2011 | Cutaneous | Broad | 20 (29.9) | 5 (27.8) |
Fig 2.
Funnel plot with pseudo 95% confidence intervals for tumor versus normal (Hypothesis 1) showing no evidence of publication bias (Egger’s p=0.23).
There was significant evidence of statistical heterogeneity in the published studies, suggesting that individual study effect sizes varied based on differences study design (I2=76.0%, Q = 66.65 (d.f. = 16) p <0.0001) (Fig 3). We investigated this with multiple methods including subgroup analyses based on the available covariates of broad vs. limited spectrum PCR assays, cutaneous vs. mucosal HPV types, and immunocompetent vs. immunosuppressed subjects. These investigations yielded similar pooled effect sizes, ranging from odds of 2.61–4.99. Removing outliers at the 25th percentile resolved the heterogeneity to an I2 statistic of 0%, p=0.85, with minimal change in the pooled effect size (3.12, 95% CI 2.28–4.37, p<0.0001).
Fig 3.
Pooled effect size and 95% confidence intervals for tumor versus normal (Hypothesis 1), showing that SCC were more likely to carry HPV than normal skin (pooled ES 3.43, 95% CI 1.97–5.98, p<0.0001). I2 and Q statistics showed significant evidence of heterogeneity in the published studies (I2=76.0%, Q = 66.65 (d.f. = 16) p <0.0001).
Hypothesis 2: SCC from immunosuppressed patients are more likely to carry HPV than SCC from immunocompetent patients
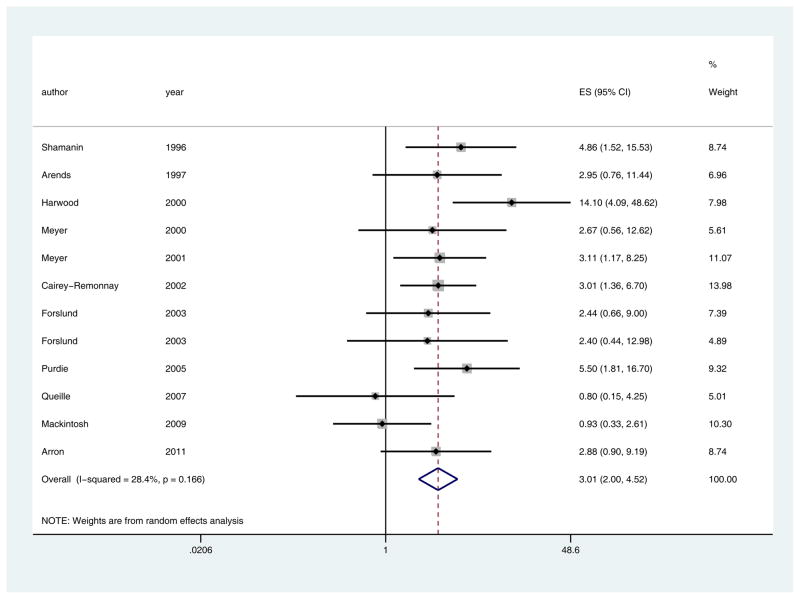
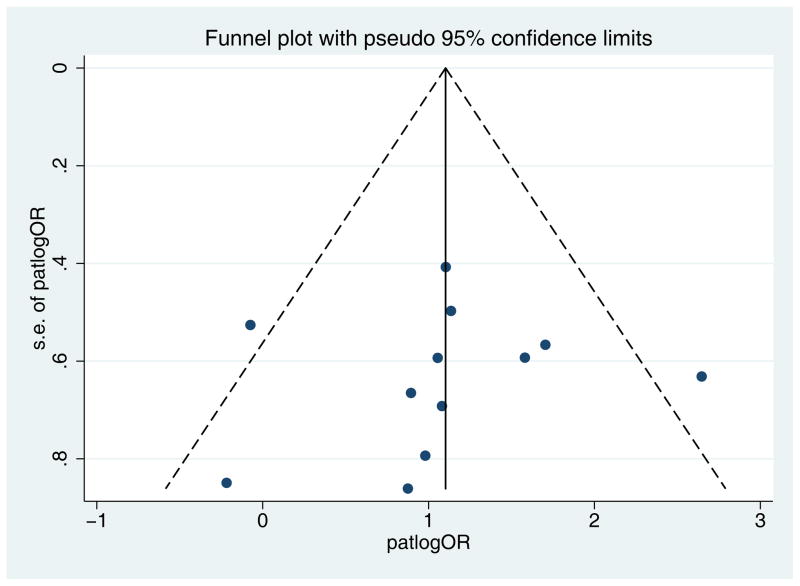
Twelve papers met inclusion criteria (Table 3).14,23,24,30–38 SCC from immunosuppressed patients are more likely to carry HPV than SCC from immunocompetent patients (pooled ES 3.01, 95%CI 2.00–4.52, p<0.0001). There was minimal evidence of heterogeneity (I2=28.4%, Q = 15.37 (d.f. = 11) p = 0.17) (Fig 4). The funnel plot showed no evidence of publication bias (Egger’s p=0.78) (Fig 5).
Table 3.
List of articles included in the meta-analysis for immunosuppressed tumors versus immunocompetent tumors (Hypothesis 2)
| Source | HPV types | # of HPV types | # of HPV positive SCCs (%) | |
|---|---|---|---|---|
| Immunosuppressed | Immunocompetent | |||
| Shamanin et al, 1996 | Both | Broad | 17 (70.8) | 10 (33) |
| Arends et al, 1997 | Both | Broad | 15 (51.7) | 4 (26.7) |
| Harwood et al, 2000 | Both | Broad | 37 (84.1) | 6 (27.3) |
| Meyer et al, 2000 | Both | Broad | 16 (76.2) | 6 (54.5) |
| Meyer et al, 2001 | Both | Broad | 12 (57.1) | 27 (30) |
| Cairey-Remonnay et al, 2002 | Both | Broad | 34 (64.2) | 19 (37.3) |
| Forslund et al, 2003 | Cutaneous | Broad | 33 (55) | 4 (33) |
| Forslund et al, 2003 (2) | Cutaneous | Broad | 6 (54.5) | 4 (33) |
| Purdie et al, 2005 | Cutaneous | Broad | 63 (75) | 6 (35.3) |
| Queille et al, 2007 | Both | Broad | 15 (79) | 14 (82.4) |
| Mackintosh et al, 2009 | Cutaneous | Broad | 19 (56) | 15 (57.7) |
| Arron et al, 2011 | Cutaneous | Broad | 15 (38.5) | 5 (17.9) |
Fig 4.
Pooled effect size and 95% confidence intervals for immunosuppressed tumors versus immunocompetent tumors (Hypothesis 2), showing that SCC from immunosuppressed patients are more likely to carry HPV than SCC from immunocompetent patients (pooled ES 3.01, 95%CI 2.00–4.52, p<0.0001). I2 and Q statistics showed minimal evidence of heterogeneity (I2=28.4%, Q = 15.37 (d.f. = 11) p = 0.17).
Fig 5.
Funnel plot with pseudo 95% confidence intervals for immunosuppressed tumors versus immunocompetent tumors (Hypothesis 2) showing no evidence of publication bias (Egger’s p=0.78).
Comment
The role of HPV as an etiologic agent for cuSCC remains a topic of controversy. Our objectives in this study were to perform a meta-analysis of studies attempting to define the association between HPV and cuSCC, and to determine whether cuSCCs from immunosuppressed patients are more likely to carry HPV that cuSCC from immunocompetent patients.
In the pooled data, cuSCC were more likely to carry HPV than normal skin (pooled ES 3.43, 95% CI 1.97–5.98, p<0.0001). Tumors were more likely to carry HPV compared to normal skin in both immunosuppressed and immunocompetent patients; this relationship was slightly stronger for immunocompetent patients. Most of the studies analyzed employed broad spectrum PCR techniques (13/20). The types of HPV analyzed in the studies varied from mucosal, to cutaneous, to both mucosal and cutaneous. Sample sizes varied from 10 to 159 tumors with about half of the studies including over 50 samples. About three quarters of the studies were relatively recent (within the last 10 years) and none were over 20 years old. In general, the newer studies were broad spectrum and able to detect more HPV types.
Compared to the immunocompetent population, an increased prevalence of HPV was found in tumors from immunosuppressed patients (pooled ES 3.01, 95%CI 2.00–4.52, p<0.0001). This is not unexpected given that these patients harbor a greater burden of verruca vulgaris, which is known to be related to HPV infection.
The greatest limitation to analyzing the literature is the great degree of heterogeneity. As discussed previously,9 variations in HPV type, sampling methods, and viral detection techniques all present challenges to grouped study analysis.
The association of higher HPV prevalence in SCC compared to normal skin does not imply causality. Lack of HPV presence in all SCCs may imply involvement in the initiation of oncogenesis, rather than tumor promotion or maintenance. Studies have demonstrated that HPV may not be necessary for the maintenance of SCC.14 Also, not all SCCs may harbor the same genetic or cellular mutations. SCC may arise from actinic keratoses (AK) in the classic multi-step model of oncogenesis. TP53 mutations caused by UV light-induced DNA damage, activating mutations in HRAS and loss-of-function mutations in Notch receptors that regulate normal squamous cell differentiation can all be seen in different tumors.39 HPV may play a role in one pathway, but not others, accounting for its presence in only a fraction of the total SCCs.
HPV may act as a co-carcinogen with other factors to amplify the risk of developing cuSCC. In patients with EV, SCC develops on sun-exposed skin. Studies have shown that HPV DNA is more prevalent in sun exposed versus non-sun exposed skin, also suggesting a link between the two factors.15 HPV could disturb cellular DNA repair or apoptosis mechanisms lending the cells more susceptible to UV induced damage. Conversely, UV light may have a transient immunosuppressive effect on skin allowing HPV to evade the immune system. Lastly, it has been postulated that HPV may be merely an innocent bystander and is not a factor in the pathogenesis of cutaneous SCC.40 It may be merely a marker of immunosuppression and a cofounder in the analysis.
Establishing a link between HPV and SCC may have diagnostic and/or therapeutic implications. For example, understanding the mechanism by which KS oncogenesis occurs has allowed for better targeting of treatments toward the HHV-8 genome and signaling pathway. Furthermore, the clear relationship between HPV infection and cervical cancer has led to the development of an effective HPV vaccine. HPV vaccines have been shown to be effective against the development of cervical cancer, by producing antibodies that neutralize the virus and prevent HPV infection.41 If HPV is shown to be involved in a subset of tumors then early vaccination against the offending subtypes may prove of benefit before advance tumor burden or before iatrogenic immunosuppression.
Further research focusing on the natural history of the HPV induced tumors and their responsiveness to different treatment modalities may be of therapeutic benefit. The case may be similar to head and neck, penile, and vulvar SCC in that HPV association predicts better prognosis or response to therapy. On the contrary, in the skin, HPV induced tumors may require more aggressive treatment given the immunosupressed hosts. Longitudinal studies of UV exposure, viral load, specific HPV types in patients and the subsequent development of SCC would be beneficial. Elucidation of the mechanism in cuSCC may lead to more targeted treatment modalities, reduction of disease burden, and better patient outcomes.
Acknowledgments
Funding Sources: None.
Abbreviations
- NMSC
non-melanoma skin cancer
- cuSCC
cutaneous squamous cell carcinoma
- HPV
human papilloma virus
- EV
epidermodysplasia verruciformis
Footnotes
Abstract has been presented at the American Society for Dermatologic Surgery Annual Meeting on October 12, 2013
The authors report no potential conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Archives of dermatology. 2010 Mar;146(3):283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 2.Kiviat NB. Papillomaviruses in non-melanoma skin cancer: epidemiological aspects. Seminars in cancer biology. 1999 Dec;9(6):397–403. doi: 10.1006/scbi.1999.0143. [DOI] [PubMed] [Google Scholar]
- 3.Vajdic CM, McDonald SP, McCredie MR, et al. Cancer incidence before and after kidney transplantation. JAMA: the journal of the American Medical Association. 2006 Dec 20;296(23):2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 4.Pett MR, Alazawi WO, Roberts I, et al. Acquisition of high-level chromosomal instability is associated with integration of human papillomavirus type 16 in cervical keratinocytes. Cancer research. 2004 Feb 15;64(4):1359–1368. doi: 10.1158/0008-5472.can-03-3214. [DOI] [PubMed] [Google Scholar]
- 5.zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009 Feb 20;384(2):260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 6.Cardoso JC, Calonje E. Cutaneous manifestations of human papillomaviruses: a review. Acta dermatovenerologica Alpina, Panonica, et Adriatica. 2011 Sep;20(3):145–154. [PubMed] [Google Scholar]
- 7.Syrjanen S. The role of human papillomavirus infection in head and neck cancers. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2010 Oct;21(Suppl 7):vii243–245. doi: 10.1093/annonc/mdq454. [DOI] [PubMed] [Google Scholar]
- 8.Arron ST, Jennings L, Nindl I, et al. Viral oncogenesis and its role in nonmelanoma skin cancer. The British journal of dermatology. 2011 Jun;164(6):1201–1213. doi: 10.1111/j.1365-2133.2011.10322.x. [DOI] [PubMed] [Google Scholar]
- 9.Aldabagh B, Angeles JG, Cardones AR, Arron ST. Cutaneous squamous cell carcinoma and human papillomavirus: is there an association? Dermatologic surgery: official publication for American Society for Dermatologic Surgery [et al.] 2013 Jan;39(1 Pt 1):1–23. doi: 10.1111/j.1524-4725.2012.02558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986 Sep;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003 Sep 6;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997 Sep 13;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000 Jun;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 14.Arron ST, Ruby JG, Dybbro E, Ganem D, Derisi JL. Transcriptome sequencing demonstrates that human papillomavirus is not active in cutaneous squamous cell carcinoma. The Journal of investigative dermatology. 2011 Aug;131(8):1745–1753. doi: 10.1038/jid.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asgari MM, Kiviat NB, Critchlow CW, et al. Detection of human papillomavirus DNA in cutaneous squamous cell carcinoma among immunocompetent individuals. The Journal of investigative dermatology. 2008 Jun;128(6):1409–1417. doi: 10.1038/sj.jid.5701227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berkhout RJ, Bouwes Bavinck JN, ter Schegget J. Persistence of human papillomavirus DNA in benign and (pre)malignant skin lesions from renal transplant recipients. Journal of clinical microbiology. 2000 Jun;38(6):2087–2096. doi: 10.1128/jcm.38.6.2087-2096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caldeira S, Zehbe I, Accardi R, et al. The E6 and E7 proteins of the cutaneous human papillomavirus type 38 display transforming properties. Journal of virology. 2003 Feb;77(3):2195–2206. doi: 10.1128/JVI.77.3.2195-2206.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forslund O, Iftner T, Andersson K, et al. Cutaneous human papillomaviruses found in sun-exposed skin: beta-papillomavirus species 2 predominates in squamous cell carcinoma. Journal of Infectious Diseases. 2007;196(6):876–883. doi: 10.1086/521031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gustafsson AC, Ren ZP, Asplund A, Ponten F, Lundeberg J. The role of p53 codon 72 and human papilloma virus status of cutaneous squamous cell carcinoma in the Swedish population. Acta dermato-venereologica. 2004;84(6):439–444. doi: 10.1080/00015550410021673. [DOI] [PubMed] [Google Scholar]
- 20.Hazard K, Eliasson L, Dillner J, Forslund O. Subtype HPV38b[FA125] demonstrates heterogeneity of human papillomavirus type 38. International journal of cancer. Journal international du cancer. 2006 Sep 1;119(5):1073–1077. doi: 10.1002/ijc.21920. [DOI] [PubMed] [Google Scholar]
- 21.Hsi ED, SvobodaNewman SM, Stern RA, Nickoloff BJ, Frank TS. Detection of human papillomavirus DNA in keratoacanthomas by polymerase chain reaction. Am J Dermatopath. 1997 Feb;19(1):10–15. doi: 10.1097/00000372-199702000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Iftner A, Klug SJ, Garbe C, et al. The prevalence of human papillomavirus genotypes in nonmelanoma skin cancers of nonimmunosuppressed individuals identifies high-risk genital types as possible risk factors. Cancer research. 2003 Nov 1;63(21):7515–7519. [PubMed] [Google Scholar]
- 23.Mackintosh LJ, de Koning MN, Quint WG, et al. Presence of beta human papillomaviruses in nonmelanoma skin cancer from organ transplant recipients and immunocompetent patients in the West of Scotland. The British journal of dermatology. 2009 Jul;161(1):56–62. doi: 10.1111/j.1365-2133.2009.09146.x. [DOI] [PubMed] [Google Scholar]
- 24.Meyer T, Arndt R, Christophers E, Nindl I, Stockfleth E. Importance of human papillomaviruses for the development of skin cancer. Cancer detection and prevention. 2001;25(6):533–547. [PubMed] [Google Scholar]
- 25.O’Connor DP, Kay EW, Leader M, Atkins GJ, Murphy GM, Mabruk MJ. p53 codon 72 polymorphism and human papillomavirus associated skin cancer. Journal of clinical pathology. 2001 Jul;54(7):539–542. doi: 10.1136/jcp.54.7.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plasmeijer EI, Neale RE, Buettner PG, et al. Betapapillomavirus infection profiles in tissue sets from cutaneous squamous cell-carcinoma patients. International Journal of Cancer. 2010 Jun 1;126(11):2614–2621. doi: 10.1002/ijc.24991. [DOI] [PubMed] [Google Scholar]
- 27.Strumia R, Roveggio C, Rotola A, Monini P, Cassai E. Keratoacanthomas: human papillomavirus and herpes simplex virus associated? J Eur Acad Dermatol. 1997 Apr;8(2):130–136. [Google Scholar]
- 28.Zaravinos A, Kanellou P, Spandidos DA. Viral DNA detection and RAS mutations in actinic keratosis and nonmelanoma skin cancers. The British journal of dermatology. 2010 Feb 1;162(2):325–331. doi: 10.1111/j.1365-2133.2009.09480.x. [DOI] [PubMed] [Google Scholar]
- 29.Zheng S, Adachi A, Shimizu M, et al. Human papillomaviruses of the mucosal type are present in some cases of extragenital Bowen’s disease. The British journal of dermatology. 2005 Jun;152(6):1243–1247. doi: 10.1111/j.1365-2133.2005.06643.x. [DOI] [PubMed] [Google Scholar]
- 30.Arends MJ, Benton EC, McLaren KM, Stark LA, Hunter JAA, Bird CC. Renal allograft recipients with high susceptibility to cutaneous malignancy have an increased prevalence of human papillomavirus DNA in skin tumours and a greater risk of anogenital malignancy. Brit J Cancer. 1997;75(5):722–728. doi: 10.1038/bjc.1997.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cairey-Remonnay S, Humbey O, Mougin C, et al. TP53 polymorphism of exon 4 at codon 72 in cutaneous squamous cell carcinoma and benign epithelial lesions of renal transplant recipients and immunocompetent individuals: lack of correlation with human papillomavirus status. The Journal of investigative dermatology. 2002 Jun;118(6):1026–1031. doi: 10.1046/j.1523-1747.2002.01787.x. [DOI] [PubMed] [Google Scholar]
- 32.Forslund O, DeAngelis PM, Beigi M, Schjolberg AR, Clausen OPF. Identification of human papillomavirus in keratoacanthomas. J Cutan Pathol. 2003 Aug;30(7):423–429. doi: 10.1034/j.1600-0560.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 33.Forslund O, Ly H, Reid C, Higgins G. A broad spectrum of human papillomavirus types is present in the skin of Australian patients with non-melanoma skin cancers and solar keratosis. The British journal of dermatology. 2003 Jul;149(1):64–73. doi: 10.1046/j.1365-2133.2003.05376.x. [DOI] [PubMed] [Google Scholar]
- 34.Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. Journal of medical virology. 2000 Jul;61(3):289–297. doi: 10.1002/1096-9071(200007)61:3<289::aid-jmv2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 35.Meyer T, Arndt R, Christophers E, Stockfleth E. Frequency and spectrum of HPV types detected in cutaneous squamous-cell carcinomas depend on the HPV detection system: a comparison of four PCR assays. Dermatology. 2000;201(3):204–211. doi: 10.1159/000018489. [DOI] [PubMed] [Google Scholar]
- 36.Purdie KJ, Surentheran T, Sterling JC, et al. Human papillomavirus gene expression in cutaneous squamous cell carcinomas from immunosuppressed and immunocompetent individuals. The Journal of investigative dermatology. 2005 Jul;125(1):98–107. doi: 10.1111/j.0022-202X.2005.23635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Queille S, Luron L, Spatz A, et al. Analysis of skin cancer risk factors in immunosuppressed renal transplant patients shows high levels of UV-specific tandem CC to TT mutations of the p53 gene. Carcinogenesis. 2007 Mar;28(3):724–731. doi: 10.1093/carcin/bgl191. [DOI] [PubMed] [Google Scholar]
- 38.Shamanin V, zur Hausen H, Lavergne D, et al. Human papillomavirus infections in nonmelanoma skin cancers from renal transplant recipients and nonimmunosuppressed patients. Journal of the National Cancer Institute. 1996 Jun 19;88(12):802–811. doi: 10.1093/jnci/88.12.802. [DOI] [PubMed] [Google Scholar]
- 39.Ratushny V, Gober MD, Hick R, Ridky TW, Seykora JT. From keratinocyte to cancer: the pathogenesis and modeling of cutaneous squamous cell carcinoma. The Journal of clinical investigation. 2012 Feb 1;122(2):464–472. doi: 10.1172/JCI57415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feltkamp MC, de Koning MN, Bavinck JN, Ter Schegget J. Betapapillomaviruses: innocent bystanders or causes of skin cancer. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2008 Dec;43(4):353–360. doi: 10.1016/j.jcv.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012 Nov 20;30( Suppl 5):F123–138. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]