Abstract
Visual aesthetic perception (“aesthetics”) or the capacity to visually perceive a particular attribute added to other features of objects, such as form, color, and movement, was fixed during human evolutionary lineage as a trait not shared with any great ape. Although prefrontal brain expansion is mentioned as responsible for the appearance of such human trait, no current knowledge exists on the role of prefrontal areas in the aesthetic perception. The visual brain consists of “several parallel multistage processing systems, each specialized in a given task such as, color or motion” [Bartels, A. & Zeki, S. (1999) Proc. R. Soc. London Ser. B 265, 2327–2332]. Here we report the results of an experiment carried out with magnetoencephalography which shows that the prefrontal area is selectively activated in humans during the perception of objects qualified as “beautiful” by the participants. Therefore, aesthetics can be hypothetically considered as an attribute perceived by means of a particular brain processing system, in which the prefrontal cortex seems to play a key role.
It is widely accepted that visual aesthetics, namely the capacity of assigning different degrees of beauty to certain forms, colors, or movements, is a human trait acquired after the divergence of human and ape lineages. Despite the chimpanzees' pictorial activities, art appreciation seems to be an exclusive human attribute, appearing ≈40,000 years ago as confirmed by the “explosion of Art.” A great number of decorative objects appear in Europe at the time (1). The origins of creativity and symbolism, however, go even further back both in Europe (2) and Africa (3). Actually, the perception of beauty (“aesthetics”) could be interpreted as the latest step of an evolutionary increasing of the cognitive capacity in the genus Homo. The earliest specimens of our genus, Homo habilis, already show “marked transverse expansion of the cerebrum, specially the frontal and parieto-occipital parts” (4).
Deacon (5) attributes the symbolic mind and language of Homo sapiens to an expansion of the prefrontal cortex, a hypothesis often quoted (6, 7). However, findings in the research of Semendeferi and Damasio (8) demonstrate a lack of expansion in the frontal area of humans as compared with other Hominoidea. Rilling and Einsel (9) reported some morphological brain differences mainly showing that the human prefrontal cortex is more convoluted and that, therefore, the gyrification index (ratio between the length of the outer cortical surface and the length of the total cortical surface) shows a significant extraalometric increase. So far, an actual relationship between aesthetic perception and prefrontal cortical activity has not been evidenced.
From a neuroscientific perspective, the theory of the multistage integration of visual consciousness “is based on evidence that the visual brain consists of several parallel multistage processing systems, each specialized in a given attribute such as color or motion” (10–13). Among the multiple stages, prefrontal cortex has been associated with the perception of colored objects (14). A locus situated ventrally is activated by normally colored objects, whereas another, located dorsolaterally, is activated by abnormally colored objects (14). In addition, “these two regions are activated by visual stimuli linked to factors such as memory, spatial location and attention” (14). Zeki's research team identified some visual cortical patterns when perceiving some essential compounds of art, like the position and variability of the “color center” in the human brain (V4-complex) (15, 16), and concluded that “the processing system is the same as the perceptual system” (17). Artists would intuitively exploit this processing/perceptual system of forms and colors to promote aesthetic sensation (17) and also some explicit “laws” of aesthetic experience would have been already identified (18–22). However, the research of Zeki and Marini did not take into account the aesthetic variable in the identification of the brain areas in charge of visual perception. Therefore, the eventual implication of prefrontal cortex in aesthetic perception deserves a more detailed study.
Using magnetoencephalography (MEG), we studied the localization of brain areas activated during the visual perception of aesthetic objects. Visual stimuli (pictures) consisting of (i) artworks of very different styles and (ii) natural photographs were presented to participants, who were asked to perform an aesthetic judgment task (they should decide whether each picture was beautiful or not beautiful) by raising a finger. Therefore, for the remainder of this paper, the terms “aesthetic” and “beautiful” will only refer to stimuli considered as such by participants themselves. The participant's brain activity was recorded by means of MEG, and the tracings showed a striking difference between beautiful and not beautiful conditions.
Methods
Subjects. Eight female subjects (average age 20 years) were tested. All were neurobiology students at the Universidad Complutense (Madrid) with no previous training in art or the history of art. All subjects had normal or corrected vision and normal color vision. All were right-handed. The experiment was approved by the Ethical Committee of the Comunitat Autònoma de les Illes Balears (Spain), and all subjects participating in the study gave informed consent.
Stimuli. The stimuli consisted of artistic and natural colored pictures divided into five groups: (i) 40 pictures of abstract art; (ii) 40 pictures of classic art; (iii) 40 pictures of Impressionist art; (iv) 40 pictures of Postimpressionist art; and (v) 160 photographs of landscapes, artifacts, urban scenes, and so forth (true-life pictures from the Master Clips Premium Image Collection, IMSI, San Rafael, CA; the book Boring Postcards, Phaidon Press, London; and photographs taken by us). We used, as a guide, styles selected from the collection Movements in Modern Art of the Tate Gallery, London, adding European XVII and XVIII Centuries and American Popular Art pictures. The objective was to present subjects a variety of artistic styles to increase their choice of aesthetic judgment. To avoid the activation of facial recognition brain mechanisms, pictures containing close views of humans were discarded. All stimuli were adjusted to the same resolution (150 pixels per inch) and dimensions (12 × 9 cm).
Stimuli were homogenized by means of three operations.
First, a behavioral test of semantic judgment was performed to assess the effect of pictorial complexity in aesthetic perception (23–25). In 114 voluntary subjects (undergraduate university students), 711 stimuli were projected in the screen of a Macintosh PowerPC, asking the subjects to score a picture's complexity from 1 to 10. All pictures receiving a mean <4.51 points were discarded.
Second, the color spectrum of the visual stimuli was adjusted. We analyzed 503 stimuli selected in the previous step, measuring their color spectrum by means of photoshop 6 (Adobe Systems, Mountain View, CA) run on a Macintosh Power Mac G4. The screen was calibrated with 9,300 white dot adjustment. Values of extreme illumination and shadow in each picture were adjusted to reach a global tone range allowing the best detail. Stimuli were classified according to their dominant tone (dark, medium, or light), and those with a mean distribution of pixels concentrated in both the left (dark) and right (light) extremes of the histogram were discarded. The selected stimuli were then adjusted to a luminance value between 133 and 134 pixels per inch, being the standard deviation between 55 and 59.9.
Third, the light reflected by stimuli was measured, in a dark room, by means of a Minolta Auto Meter IV F digital photometer placed 40 cm from the screen with an accessory for 40° reflected light. Stimuli under 390 lux (lx) and below 370 lx were discarded. A total of 320 stimuli reasonably homogenized in regards to pictorial complexity, color spectrum, luminosity, and light reflection were thus obtained. Also, four stimuli (two artistic and two natural) were fixed for the participants' training tasks. Four additional stimuli (two artistic and two natural) were projected as initial pictures in the MEG experiment, their results being discarded to avoid the primacy effect.
During the MEG recording, the stimuli were generated by an Apple iBook computer running the superlab application. The pictures were projected through an LCD video projector (Sony VPL-X600E) situated outside of the shielded room onto a series of mirrors located inside, the last of which was suspended ≈1 m above the subject's face. The pictures subtended 1.8° and 3° horizontal and vertical visual angle, respectively. The participants were informed that they would be viewing artistic and natural pictures. They were asked to raise a finger when they considered the picture projected to be beautiful. The answer was counterbalanced; four subjects were asked to raise a finger in the not beautiful condition.
Image Acquisition. The principles underlying MEG data collection and analysis are described in detail elsewhere (26, 27) and are outlined only briefly here. MEG recordings were made with a whole-head neuromagnetometer (Magnes 2500 WH, 4-D Neuroimaging, San Diego) consisting of 148 magnetometer coils. The instrument is housed in a magnetically shielded room designed to reduce environmental magnetic noise that might interfere with biological signals. The signal was filtered online with a bandpass between 0.1 and 50 Hz, digitized for 1,000 ms (254-Hz sampling rate), including a 150-ms prestimulus period, and subjected to an adaptive filtering procedure that is part of the 4-D Neuroimaging signal analysis package. The single trial event-related fields (ERFs) were then averaged together after removing those during which an eye movement or blink had occurred (as indicated by a peak-to-peak amplitude in the electrooculogram channel in excess of 50 μV). A minimum of 90 ERF epochs were collected to calculate each averaged wave-form. Finally, the averaged epochs were digitally filtered with a low-pass 20-Hz filter.
Magnetic source estimation was performed separately for each hemisphere. Source estimation was attempted only when the surface distribution of magnetic flux was dipolar, in that it consisted of a single region of magnetic outflux and a single region of magnetic influx. This kind of surface distribution usually indicates the presence of a single underlying active cortical region that can be modeled as an equivalent current dipole (ECD) (henceforth referred to as an “activity source”). Occasionally, two distinct dipolar distributions were discerned at a single time point (in one or both hemispheres). In those instances, source estimation was performed for both dipolar distributions independently. To avoid localization errors produced by smearing of the magnetic flux from one source by the flux induced by the other source, two simultaneous source solutions were retained only if the corresponding dipoles were found to be at least 5 cm apart. With this method no more than two sources, located in different anatomical regions, can be computed in each hemisphere at each 4-ms time bin. Therefore, if we suppose one dipole at each 4 ms, a maximum number of (850 ms)/(4 ms) = 212 sources could be computed for each hemisphere and task.
Activity sources were modeled by using the nonlinear Levenberg–Marquardt algorithm (28). For a given point in time, the ECD fitting algorithm was applied to the magnetic flux measurements obtained from a group of 34–38 magnetometers, always including both magnetic flux extremes. The algorithm used in this study searched for the ECD that was most likely to have produced the observed magnetic field distribution at a given point in time. The ECD solutions were considered satisfactory after meeting the following criteria: (i) correlation coefficient of at least 0.9 between the observed and the “best” predicted magnetic field distribution, (ii) a goodness of fit of at least 0.9 or higher, and (iii) a confidence volume <5cm3 (27, 29).
To determine the anatomical regions where the activity sources were located, ECD coordinates were overlaid onto T1 longitudinal relaxation time–weight, magnetic resonance images (repetition time 13.6 ms, echo time 4.8 ms, recording matrix 256 × 256 pixels, 1 excitation, 240-mm field of view, and 1.4-mm slice thickness) obtained from every participant on a separate session. The MEG–MRI overlay was performed by using the program star, which is part of the 4-D Neuroimaging software (see ref. 26 for a detailed description of the co-registration process).
Regions of interest are not established a priori in MEG studies. Modeling of activity sources is performed solely on the basis of the surface distribution of magnetic flux without making hypotheses or placing constraints regarding the anatomical location of the underlying intracranial sources. When activity source locations are co-registered on individual MRI scans, the resulting individual activation profiles are inspected visually (and blindly with respect to experimental condition) to identify brain areas where activity sources are localized consistently across participants (i.e., in at least seven of eight). This process revealed reliable “presence” of activity sources in the following areas: parietal lobe, occipital lobe, medial temporal lobe (including hippocampus), superior temporal lobe, inferior temporal lobe, prefrontal dorsolateral [including Brodmann's areas (BA) 9 and 46], prefrontal orbital (BA 11 and 47), frontal pole (BA 10), anterior cingulate cortex (BA 24 and 32), motor areas (Broca's area, insula, motor supplementary, premotor area; BA 4, 6, and 44), and superior frontal gyrus (BA 8).
The sum of all acceptable sources localized in each region in two time windows extending from 100 to 400 and from 400 to 1,000 ms poststimulus onset served as a metric of the degree of stimulus-locked activation of that area. This measure directly reflects the amount of time that neurophysiological activity can be detected and modeled in a particular brain region as participants process each visual stimulus.
The reliability and validity of the MEG method presented above to evaluate cognitive functions were first tested empirically in a series of language normative studies (27, 29). Further studies in memory, with severely brain damaged patients (30, 31), and normal controls (32) extend the validity of the current analyses protocol for cognitive function.
Results
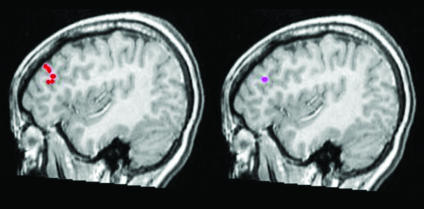
The results of the experiment are displayed in Table 1. The left prefrontal dorsolateral cortex (PDC) was activated when participants perceived beautiful stimuli (either natural or artistic), reaching statistically significant differences (P < 0.01) (Fig. 1). This activation took place at a latency of 400–1,000 ms with corresponding activation of the visual cortex at 130 ms (Fig. 2). Time is crucial, because it shows that in a multistage process “attributes perceived at different times are processed at different sites” (10).
Table 1. Hemisphere × condition effect on number of activity sources (dorsolateral cortex, 400- to 900-ms interval).
| Hemisphere | Condition | Mean | SD |
|---|---|---|---|
| Left | Aesthetic | 3.375 | 0.680 |
| Nonaesthetic | 1.125 | 0.441 | |
| Right | Aesthetic | 0.500 | 0.500 |
| Nonaesthetic | 0.625 | 0.625 |
Fig. 1.
Activation of the PDC under stimuli qualified as beautiful by participant (Left) and under stimuli qualified as not beautiful (Right).
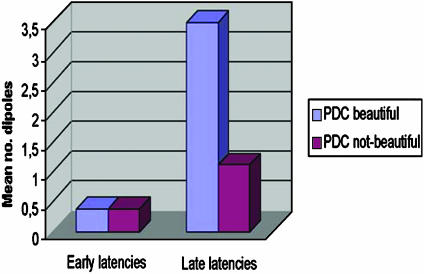
Fig. 2.
PDC dipoles activated in early latencies (100–400 ms) and late latencies (400–1000 ms) when participants see both natural and artistic pictures qualified as beautiful or not beautiful by participants themselves. The results show an activation of PDC under the “beautiful” condition in late latencies, with statistically significant differences.
A series of two-way hemisphere × condition (aesthetic vs. nonaesthetic) ANOVAs were computed for each area on the early latencies (100–300 ms) (a total of 13). No significant differences were found. The same procedure was carried out on the late (400–900 ms) latencies. In this case a significant main effect of hemisphere (F1,7 = 6.5; P < 0.05) and its interaction, hemisphere × condition (F1,7 = 11.3; P < 0.01), were detected for dorsolateral prefrontal area. Global left hemisphere activation (i.e., number of activity sources) was superior compared to right hemisphere, and this specific pattern was clearly affected by the type of decision (aesthetic vs. nonaesthetic) made by the subjects on every single stimulus (Table 1). Aesthetic stimuli elicited a significantly greater number of activity sources in left PDC (t7 = 3.63; P < 0.01, assuming the Bonferroni-corrected significance levels) than nonaesthetic stimuli, whereas no differences emerged from a right-hemisphere comparison. The differences in the left hemisphere, concerning dipole activation number for 400- to 900-ms latency, are also present in each subject considered individually (Table 2).
Table 2. Number of activated dipoles in the left dorsolateral cortex (400- to 900-ms latency) for each subject.
| Subject | Aesthetic | Nonaesthetic |
|---|---|---|
| 1 | 4 | 3 |
| 2 | 1 | 0 |
| 3 | 6 | 1 |
| 4 | 2 | 1 |
| 5 | 5 | 3 |
| 6 | 1 | 0 |
| 7 | 3 | 1 |
| 8 | 5 | 0 |
No significant differences was derived from the late latency analysis in anterior cingulum (P < 0.05).
Discussion
Our purpose was to check, by means of MEG, whether prefrontal areas are implied in the visual perception of aesthetic objects. However, the lack of a precise definition of “aesthetic perception” constitutes an important obstacle for this kind of research. The proposal of a perceptive experience related to beauty and named “aesthetics” comes from the 18th century (33). Since then, aesthetic experience is understood as a sensorial perception (34). Almost all authors, however, agree that aesthetic perception goes further, referring to factors like beauty, art, and hedonistic pleasure. Nevertheless, relationships between beauty, art, and aesthetics are very complex. For instance, artworks are commonly understood as objects produced with the aim of engendering aesthetic experiences (35). But an immediate difference between art and aesthetics becomes evident when taking into account that avant-garde artworks rarely engender any aesthetic experience in the public at large. Moreover, a strong link between art and beauty has its detractors, such as some of the contemporary (and ancient) artists who reject beauty as an essential compound of art and search for other emotions apart from hedonistic pleasure to ground aesthetic experiences (36). Despite these facts, it would be difficult to deny that “people who visit galleries, read poetry and so on, do it, after all, looking for beauty” (37). It is very common to attribute beauty to both artistic and natural objects like, for instance, a landscape.
The main obstacle for an experimental study of aesthetics refers to the complexity of those processes. How could the constellation of aesthetic experiences be reduced to the study of some simple variables? A possible answer is given by the “general factor” of aesthetic experience proposed by Eysenck (38). Several studies (39, 40) have confirmed the presence of a “basic knowledge” of art in participants with little or no formal training in the arts or aesthetics. Human beings seem to share “a common denominator” (22) underlying all artistic experience. However, the multiple factors affecting the aesthetic criteria (social, historical, cultural, biological, pedagogical, and personality) will undoubtedly produce a dispersion of experimental results in studies dealing with aesthetic perception. To check this obstacle, we carried out a study on the perceptive categories “beautiful,” “pleasant,” “original,” and “interesting” (41), by means of semantic differential techniques (42). The research showed the existence of a general factor linked to the “beautiful” dimension, with values higher than 1, explaining a 54% of the total varianza (41).
The theory of multistage integration (TMI) in the visual brain (10–12) holds that different areas, stages, or “nodes” constitute crucial parts of a parallel system processing various perceptive inputs that act asynchronously. Our experimental results sustain the concept of TMI in several important aspects. Firstly, they confirm the existence of different stages in the visual perception of forms. These distinct stages are processed in different temporal epochs. Secondly, an activation of brain areas beyond the visual cortex is confirmed. Moreover, our experiments suggest the eventual existence in the PDC of a perceptive node for aesthetics in addition to the more basic perception of forms and colors. It is interesting to realize that this area was activated in the experiments of Zeki and Marini (14) when participants did see abnormally colored objects. The authors did not ask participants to perform any cognitive task of aesthetic judgment, nor did they explicitly added the aesthetic variable to their research. However, as Zeki and Marini say, their study was inspired by the “liberation of color” of fauvists “to give it greater emotional and expressive power.” Our results would suggest that participants in the experiments of Zeki and Marini (14) grasped the aesthetic attribute induced by this “liberation of color.”
PDC is a brain region that participates, as a center of perception–action interface, in multiple brain functions. Incoming information from posterior parietal and occipital areas is processed in the PDC in a more complex fashion that is required to plan a corresponding action. In this way a particular set of perceptual stimuli engenders a particular set of actions (43). Actually, PDC is critical “for the monitoring of multiple events in working memory” (44) and plays a key role “in making decisions that call for the consideration of multiple sources of information” (45). Consequently, PDC has a significant role in functions related to decision making (46, 47) and visuospatial working memory (48). The activation of PDC in an aesthetic task should not surprise us because when deciding about the “beautiful” condition of stimuli, a judgment requiring visuospatial memory is needed. PDC and cingulated cortex are known to activate during judgment tasks (49). Although the cingulated cortex is activated in both conditions (“beautiful” and “not beautiful”), probably as a result of judgment decisions, the PDC was selectively activated by stimuli considered beautiful. This could not represent an artifact due to the behavioral response (raising the finger), because this condition was counterbalanced (see Methods). The PDC activation cannot have any connection with either judgments regarding perceived objects or their spatial characteristics. Otherwise, this area would be activated in all responses to beautiful and not beautiful pictures. It seems, therefore, that the aesthetic perception of beauty is related to activation of the prefrontal dorsolateral area.
Our experimental results clearly show that cortical activity in the PDC relates with aesthetic perception. Prefrontal activity associated with aesthetic judgment is observed in both aesthetic and nonaesthetic conditions during 400- to 900-ms latency (“aesthetic” and “nonaesthetic” referring to participants' judgment). However, this activity is greater in the left hemisphere under the aesthetic condition. Our results also support the hypotheses that a phylogenetic change in the prefrontal cortex could give way to the decorative and artistic profusion in modern aspect humans and, in more limited cases, in Neandertals. Furthermore, all subjects participating in this study were right-handed, and the aesthetic perception of beauty was related to a specific activation of the left PDC, very likely in dominant hemisphere territory. This finding raises the possibility that aesthetic judgment might be a function of cerebral dominance and could lend some neurobiological input to the phylogeny of such derived characters, like language and aesthetics, whose evolutionary journey started with Homo habilis.
Do the results of our experiment imply the identification of a “brain center of aesthetics” related to a perception of beauty? If we mean a center in which information coming from all other involved sensorial channels converge, the visual system seems to lack that kind of organization. “Anatomical evidence shows that there is no single area to which all of the specialized visual areas connect, which would enable it to act as an integrator capable of binding signals coming from all of the different visual sources” (50). Nevertheless, the theory of multistage integration holds “that activity at any stage of a given multistage processing system is perceptually explicit—in other words, that it requires no further processing to generate a conscious experience” (50). The prefrontal dorsolateral node could be part of a neural network intrinsically related to conscious aesthetic perception. This assumption, however, deserves further study. It is necessary to understand how variables like artistic training modify the aesthetic perception, how the latter is changed by perception of natural vs. artistic or abstract vs. realistic inputs, and how and when subcortical systems are recruited, thus engaging in a cortical–subcortical functional network subserving conscious aesthetic perception.
Acknowledgments
We thank two anonymous referees for their comments and suggestions. This work was supported by grants from the Conselleria d'Innovacio i Energia, Govern Balear, and the Spanish Ministerio de Ciencia y Tecnología (Research and Development Project BSO2000-1116-C04).
Abbreviations: ECD, equivalent current dipole; MEG, magnetoencephalography; PDC, prefrontal dorsolateral cortex.
References
- 1.Appenzeller, T. (1998) Science 282, 1451-1454. [Google Scholar]
- 2.Bednarik, R. G. (1997) Hum. Evol. 12, 147-168. [Google Scholar]
- 3.McBrearty, S. & Brooks, A. S. (2000) J. Hum. Evol. 39, 453-563. [DOI] [PubMed] [Google Scholar]
- 4.Tobias, P. V. (1987) J. Hum. Evol. 6, 741-761. [Google Scholar]
- 5.Deacon, H. J. (1997) The Symbolic Species (Norton, New York).
- 6.Mithen, S. (1996) The Prehistory of the Mind (Thames & Hudson, London).
- 7.Noble, W. & Davidson, I. (1996) Human Evolution, Language and Mind (Cambridge Univ. Press, Cambridge, U.K.).
- 8.Semendeferi, K. & Damasio, H. (2000) J. Hum. Evol. 38, 317-332. [DOI] [PubMed] [Google Scholar]
- 9.Rilling, J. K. & Insel, T. R. (1999) J. Hum. Evol. 37, 191-223. [DOI] [PubMed] [Google Scholar]
- 10.Bartels, A. & Zeki, S. (1999) Proc. R. Soc. London Ser. B 265, 2327-2332. [Google Scholar]
- 11.Zeki, S. & Bartels, A. (1999) Proc. R. Soc. London Ser. B 265, 1583-1585. [Google Scholar]
- 12.Zeki, S. (1999) The Neuroscientist 4, 365-372. [Google Scholar]
- 13.Moutoussis, K. & Zeki, S. (2002) Proc. Natl. Acad. Sci. USA 99, 9527-9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeki, S. & Marini, L. (1998) Brain 121, 1669-1885. [DOI] [PubMed] [Google Scholar]
- 15.McKeefry, D. J. & Zeki, S. (1997) Brain 120, 2229-2242. [DOI] [PubMed] [Google Scholar]
- 16.Zeki, S. & Bartels, A. (1999) Philos. Trans. R. Soc. London B 1371-1382. [DOI] [PMC free article] [PubMed]
- 17.Bartels, A. & Zeki, S. (2000) Eur. J. Neurosci. 12, 172-193. [DOI] [PubMed] [Google Scholar]
- 18.Gage, J. (1999) Colour and Meaning: Art, Science and Symbolism (Thames & Hudson, London).
- 19.Eibl Eibesfeldt, I. (1988) in Beauty and the Brain: Biological Aspects of Aesthetics, eds. Rentschler, I., Herzberger, B. & Epstein, D. (Birkhauser, Basel), pp. 29-68.
- 20.Latto, R. (1995) in The Artful Eye, eds. Gregory, R. L., Harris, J., Heard, P. & Rose, D. (Oxford Univ. Press, Oxford), pp. 66-94.
- 21.Ramachandran, V. S. & Hirstein, W. (1999) J. Consciousness Stud. 6, 15-51. [Google Scholar]
- 22.Zeki, S. (1999) Inner Vision: An Exploration of Art and the Brain (Oxford Univ. Press, Oxford).
- 23.Looft, W. R. & Baranowski, M. (1971) J. Gen. Psychol. 85, 307-313. [DOI] [PubMed] [Google Scholar]
- 24.Frith, C. D. & Nias, D. K. B. (1974) J. Gen. Psychol. 91, 163-173. [PubMed] [Google Scholar]
- 25.Chevrier, J. & Delorme, A. (1980) Percept. Motor Skills 50, 839-849. [DOI] [PubMed] [Google Scholar]
- 26.Maestú, F., Ortiz, T., Fernandez, A., Amo, C., Martin, P., Fernandez, S. & Sola, R. G. (2002) NeuroImage 17, 1579-1586. [DOI] [PubMed] [Google Scholar]
- 27.Papanicolaou, A. C., Simos, P. G., Breier, J. I., Zouridakis, G., Willmore L. J., Wheless, J. W., Constantinou, J. E. C., Maggio, W. W. & Gormley, W. B. (1999) J. Neurosurg. 90, 85-93. [DOI] [PubMed] [Google Scholar]
- 28.Sarvas, J. (1987) Phys. Med. Biol. 32, 11-22. [DOI] [PubMed] [Google Scholar]
- 29.Breier, J. I., Simos, P. G., Papanicolaou, A. C., Zouridakis, G., Wilmore, L. J., Wheless, J. W., Constantinou, J. C. & Maggio, W. W. (1999) Neurology 22, 938-945. [DOI] [PubMed] [Google Scholar]
- 30.Maestú, F., Fernandez, A., Simos, P. G., Gil, P., Amo, C., Rodriguez, R., Arrazola, J. & Ortiz, T. (2001) NeuroReport 12, 3917-3922. [DOI] [PubMed] [Google Scholar]
- 31.Maestú, F., Arrazola, J., Fernández, A., Simos, P. G., Amo, C., Gil-Gregorio, P., Fernández, S., Papanicolaou, A. C. & Ortiz, T. (2003) J. Neurol. Neurosurg. Psychiatry 74, 208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maestú, F., Simos, P. G., Campo, P., Fernández, A., Amo, A., Paul, N., González-Marqués, J. & Ortiz, T. (2003) NeuroImage 20, 1110-1121. [DOI] [PubMed] [Google Scholar]
- 33.Baumgarten, A. (1735) Meditationes Philosophicae de Nonnullis ad Poema Pertinentibus (Grunert, Halle, Germany), reprinted (1983) (Meiner, Hamburg).
- 34.Collinson, D. (1992) in Philosophical Aesthetics, ed. Hanfling, O. (Blackwell, Oxford), pp. 111-178.
- 35.Carroll, N. (1999) Philosophy of Art (Routledge, London).
- 36.Collingwood, R. G. (1938) The Principles of Art (Oxford Univ. Press, Oxford).
- 37.Hanfling, O. (1992b) in Philosophical Aesthetics, ed. Hanfling, O. (Blackwell, Oxford), pp. 41-73.
- 38.Eysenck, H. J. (1940) Br. J. Psychol. 31, 94-102. [Google Scholar]
- 39.Seifert, L. S. (1992) J. Psychol. 126, 73-78. [Google Scholar]
- 40.Cupchik, G. C., Winston, A. S. & Herz, R. S. (1992) Vis. Arts Res. 14, 37-50. [Google Scholar]
- 41.Marty, G., Cela-Conde, C. J., Munar, E., Rosselló, J., Roca, M. & Escudero, J. T. (2003) Psicothema 14, 478-483. [Google Scholar]
- 42.Osgood, C. E., Suci, G. J. & Tannebaum, P. H. (1957) The Measurement of Meaning (Univ. of Illinois Press, Urbana).
- 43.Otani, S. (2002) Biol. Rev. Cambridge Philos. Soc. 77, 563-577. [DOI] [PubMed] [Google Scholar]
- 44.Petrides, M. (2000) Exp. Brain Res. 133, 44-54. [DOI] [PubMed] [Google Scholar]
- 45.Krawczyk, D. C. (2002) Neurosci. Biobehav. Rev. 26, 631-664. [DOI] [PubMed] [Google Scholar]
- 46.Paulus, M. P., Hozack, N., Frank, L., Brown, G. G. & Schuckit, M. A. (2003) Biol. Psychiatry 53, 65-74. [DOI] [PubMed] [Google Scholar]
- 47.Petrides, M., Alivisiatos, B. & Frey, S. (2002) Proc. Natl. Acad. Sci. USA 99, 5649-5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glahn, D. C., Kim, J., Cohen, M. S., Poutanen, V. P., Therman, S., Bava, S., Van Erp, T. G., Manninen, M., Huttunen, M., Lonnqvist, J., et al. (2002) NeuroImage 17, 201-213. [DOI] [PubMed] [Google Scholar]
- 49.Dehaene, S. & Changeux, J. P. (2000) Prog. Brain Res. 126, 217-229. [DOI] [PubMed] [Google Scholar]
- 50.Zeki, S. & Bartels, A. (1999) Conscious. Cognit. 8, 225-259. [DOI] [PubMed] [Google Scholar]