Abstract
Background
Sex has been linked to differential outcomes for cardiovascular disease in adults. We examined potential sex differences in outcomes after pediatric cardiac surgery.
Methods and Results
We retrospectively analyzed data from the Pediatric Cardiac Care Consortium (1982–2007) by using logistic regression to evaluate the effects of sex on 30‐day within‐hospital mortality after pediatric (<18 years old) cardiac operations and its interaction with age, risk category, z‐score for weight, and surgical year for the whole cohort. Of 76 312 operations, 55% were in boys. Unadjusted mortality was similar for boys and girls (5.2% versus 5.0%, P=0.313), but boys were more likely to have cardiac surgery as a neonate and to have more complex operations. After adjustment, the overall test of any association between postsurgical mortality and sex was significant (P=0.002), but the overall test of any interaction was not (P=0.503). However, a potential age‐dependent sex effect on postsurgical mortality was observed among infants subjected to high‐risk operations, with girls doing worse during the first 6 months of life.
Conclusions
Patient sex has a significant effect on mortality after pediatric cardiac operations, with an increased risk of death in early infancy for girls after high‐risk cardiac operations. This age‐dependent relationship supports a sex‐related biological effect on postoperative cardiovascular stress.
Keywords: congenital heart defects, pediatrics, sex, surgery
Introduction
Differences in outcomes for diseases and treatments between males and females are increasingly being recognized. For example, women have experienced an increased rate of death after coronary artery bypass graft surgery.1–2 Despite indications that sex differences may be important for outcomes of cardiac operations, investigations into sex‐related influences on operations for congenital heart disease (CHD) are sparse. In children, studies of lesion‐specific outcomes from the Society of Thoracic Surgeons Congenital Heart Surgery Database (STS‐CHSD) and single‐center datasets do not support a sex effect on outcomes for surgical procedures for hypoplastic left heart syndrome,3–4 atrioventricular canal,5–6 and tetralogy of Fallot,7 but these are limited by the relatively small number of patients and deaths. A recent study using risk‐adjusted clinical data (2007–2009) from the STS‐CHSD data center did not find any significant difference in the early postoperative mortality between boys and girls.8 These results conflict with data from large administrative datasets that reported that female sex was associated with 20% to 50% higher odds of death after pediatric cardiac surgery after adjustment for severity of cardiac procedure and age.9–11
The reasons for this discrepancy in mortality from previous studies could be multiple: sample size, data source, quality of clinical versus administrative data, risk adjustment methodology, or analysis strategy. Alternatively, a waning sex effect over time could be explained by changes in medical practice that decreased the overall mortality but had a more profound effect on girls as the most vulnerable group. Of importance, there was no attempt to examine the interaction between a patient's sex and other important factors that contribute to the surgical outcomes such as age and weight at surgery or complexity of the procedure. These risk factors should be considered because they may be quite different between boys and girls at the time of surgery. For example, weight at surgery has been shown to affect outcomes, and the age‐ and sex‐adjusted weight can differ significantly between boys and girls. In addition, recent animal data suggest a significant metabolic disadvantage of the female neonatal heart, making it vulnerable to ischemia and reperfusion injury.12 Looking for interactions with known risk factors affecting surgical outcomes for CHD may explain some of the discrepancies with previous studies. Some of these interactions (age × complexity of operation) were suggested by Marelli et al11 but never tested. Therefore, the issue of the sex effect on outcomes after operations for CHD warrants further investigation.
In this study, we examined the effect of patient sex on risk‐adjusted early (30‐day) and overall in‐hospital mortality after pediatric cardiac surgery, its interactions with other risk factors, and its trends using data from 1982 to 2007 from the Pediatric Cardiac Care Consortium (PCCC).
Materials and Methods
Study Design
This is a retrospective cohort study from a multi‐institutional registry for pediatric cardiac operations.
Data Source: The PCCC
The PCCC is a voluntary clinical registry collecting outcome data from centers performing pediatric cardiac operations and catheter interventions. The registry also includes information for all identifiable genetic conditions at the time of the operation. We used data from years with completed data entry from 1982 to 2007. The registry includes data from 57 centers: 47 in the United States, 6 in Canada, 3 in South America, and 1 in Europe. For this study, we included only operations performed in North American centers (Figure 1). All cardiac operations (except isolated ductal ligation in preterm infants <2.5 kg) are reported prospectively by the centers, with diagnosis and procedure coding at the core facility. The University of Minnesota Institutional Review Board has approved the use of this database for research purposes without the need for informed consent.
Figure 1.
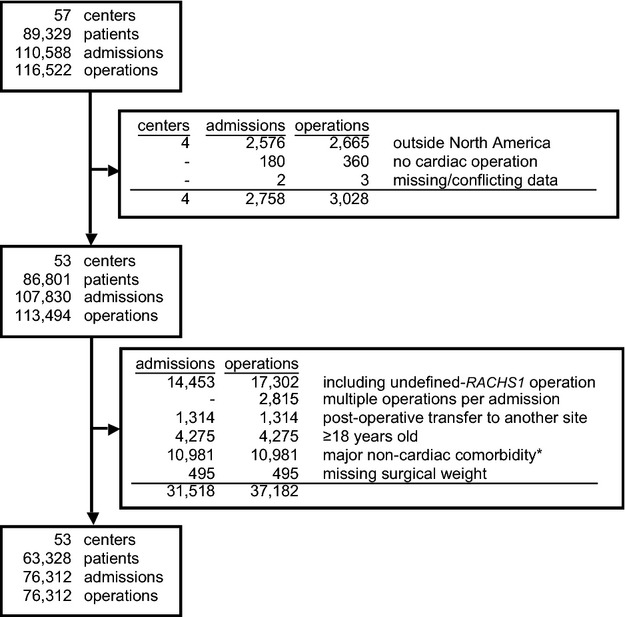
Overview of the study cohort indicating the number of centers, patients, hospital admissions, and cardiac operations within the PCCC and number meeting exclusion criteria. *These include major chromosomal anomalies and genetic syndromes as described in the text. PCCC indicates Pediatric Cardiac Care Consortium; RACHS1, Risk Adjusted Classification for Congenital Heart Surgery system, version 1.
Study Population
The study population includes all patients <18 years of age registered in the PCCC database who underwent a cardiac surgery. For the primary analysis, we excluded hospitalizations containing any procedure not classifiable by the Risk Adjusted Classification for Congenital Heart Surgery system, version 1 (RACHS1) and hospitalizations ending in transfer to another center (1.16% of the total operations) (Figure 1). Multiple surgical admissions per patient were included, and potential correlation among repeated admissions was addressed by using a bootstrap with cluster sampling stratified by hospital to also adjust for any hospital effects. Because of the association of sex with certain genetic conditions, 10 981 patients coded as having major chromosomal anomalies or genetic syndromes were also excluded from the main analysis. These include 8055 patients with trisomy 21, 418 patients with Turner syndrome, 1576 patients with DiGeorge syndrome, and 932 patients with various syndromes and chromosomal defects including trisomies 13 and 18, partial duplications, or deletions in various chromosomes (4, 9, 13, 15, 17, 18, 21, and 22).
Participating Centers and Patients
The PCCC includes data from 113 494 operations (86 801 patients) from 53 North American centers. After applying exclusion criteria (see later) and deleting 495 operations lacking patient weight data, we analyzed 76 312 operations, of which 55% were in boys (n=42 008) and 45% were in girls (n=34 304) (Figure 1, Table 1).
Table 1.
Descriptive Statistics for All Operations in the PCCC Study Cohort (1982–2007)
| Total | Girls (%) | Boys (%) | P Value | |
|---|---|---|---|---|
| 76 312 | 34 304 (45.0%) | 42 008 (55.0%) | ||
| Age at surgery, days | 1181±1613 | 1189±1570 | 1174±1647 | <0.001* |
| Quartiles* for age | 78/387/1633 | 102/442/1661 | 58/341/1604 | |
| Age group | <0.001* | |||
| 0 to 30 days | 14 402 (18.9%) | 5483 (16.0%) | 8919 (21.2%) | <0.001* |
| 31 to 365 days | 23 038 (30.2%) | 10 507 (30.6%) | 12 531 (29.8%) | 0.0172 |
| 1 to 9 years | 30 424 (39.9%) | 14 706 (42.9%) | 15 718 (37.4%) | <0.001* |
| 10 to 18 years | 8448 (11.1%) | 3608 (10.5%) | 4840 (11.5%) | <0.001* |
| LWN (<2.5 kg) | 1605 (2.10%) | 735 (2.14%) | 870 (2.07%) | 0.12 |
| Weight at surgery, kg | 14.1±15.7 | 14±14 | 14±17 | |
| Quartiles* for weight | 4.3/8.5/16.1 | 4/9/16 | 4/8/16 | 0.041 |
| Weight z‐score | −1.11±1.97 | −1.16±2.17 | −1.08±1.79 | <0.001* |
| Quartiles* for weight z‐score | −2/−1/−0.05 | −2/−1/−0.08 | −2/−1/−0.03 | |
| RACHS1 risk category | <0.001* | |||
| 1 | 17 265 (22.6%) | 9260 (27.0%) | 8005 (19.1%) | <0.001* |
| 2 | 25 934 (34.0%) | 11 335 (33.0%) | 14 599 (34.8%) | <0.001* |
| 3 | 24 547 (32.2%) | 10 454 (30.5%) | 14 093 (33.5%) | <0.001* |
| 4 | 5956 (7.80%) | 2301 (6.71%) | 3655 (8.70%) | <0.001* |
| 5 and 6 | 2610 (3.42%) | 954 (2.78%) | 1656 (3.94%) | <0.001* |
| Unassigned | [11 417] (13.0%) | [4862] (12.4%) | [6555] (13.5%) | <0.001* |
| Surgical era | <0.001* | |||
| 1982–1989 | 11 403 (14.9%) | 5187 (15.1%) | 6216 (14.8%) | 0.2122 |
| 1990–1999 | 33 694 (44.2%) | 15 471 (45.1%) | 18 223 (43.4%) | <0.001* |
| 2000–2007 | 31 215 (40.9%) | 13 646 (40.9%) | 17 569 (41.8%) | <0.001* |
| Weekend operation | 1476 (1.93%) | 582 (1.70%) | 894 (2.13%) | <0.001* |
| Time of death, days | 15±33 | 15±33 | 16±34 | |
| Quartiles* for time of death | 0/3/15 | 0/2/15 | 0/3/15 | 0.2701 |
| Length of stay, days | 10.6±19 | 10±20 | 11±18 | |
| Quartiles* for length of stay | 4/6/10 | 4/6/10 | 4/6/11 | <0.001* |
| Surgeries per admission | ||||
| 1 | 74 225 (97.3%) | 33 402 (97.4%) | 40 823 (97.2%) | 0.102 |
| >1 | 2087 (2.73%) | 902 (2.63%) | 1185 (2.82%) | |
| Previous admissions | <0.001* | |||
| 0 | 62 240 (81.6%) | 28 568 (83.3%) | 33 672 (80.2%) | <0.001* |
| 1 | 10 098 (13.2%) | 4155 (12.1%) | 5943 (14.1%) | <0.001* |
| 2 | 3174 (4.16%) | 1287 (3.75%) | 1887 (4.49%) | <0.001* |
| >2 | 800 (1.05%) | 294 (0.857%) | 506 (1.20%) | <0.001* |
| Deaths (<30 days) | 3893 (5.10%) | 1719 (5.01%) | 2174 (5.18%) | 0.302 |
| All deaths | 4558 (5.97%) | 1990 (5.80%) | 2568 (6.11%) | 0.072 |
Numbers after ± represent standard deviations (SD). Numbers with percents are frequencies. Numbers within brackets indicate operations with nonassignable RACHS1 risk category and on patients with syndromes that were excluded from the primary analysis. LWN, low‐weight neonates (<2.5 kg at surgery) were analyzed as a separate category independent of their chronologic age. PCCC indicates Pediatric Cardiac Care Consortium; RACHS1, Risk Adjusted Classification for Congenital Heart Surgery system, version 1.
Wilcoxon rank sum test.
Numbers represent the lower quartile/median/upper quartile for continuous variables.
Pearson χ2 test.
Outcome Measurements
All‐cause postoperative 30‐day within‐hospital mortality was used as the primary outcome. All‐cause within‐hospital mortality regardless of length of stay (LOS) was used as secondary outcome.
Study Variables and Risk Adjustment
Variables available for analysis included patient's sex, age at operation, weight at operation, date of operation, number of surgeries during the same surgical admission, number of prior admissions, date of death or discharge, and surgical risk category. Perioperative risk was assessed by using the RACHS1. The RACHS1 method assigns operations for CHD into 6 risk categories based on expected early mortality rates.13 Risk category 1 operations have the lowest risk of death and category 6 has the highest. For the analysis, we combined risk categories 5 and 6 into a single category (termed “category 5 and 6”) as in previous studies, because of the small number of category 5 (0.2%) operations and the significantly higher risk of these 2 categories compared with all other categories.14 Cases with combinations of procedures during the same admission were placed in the category of the highest‐risk procedure, as in previous studies.11,15 If multiple procedures were in the highest‐risk category, the earliest procedure was used.
Statistical Analysis
Categorical variables were summarized in frequency tables and compared using χ2 tests, while continuous variables were summarized with means, SDs, and quartiles and compared by using the Wilcoxon rank sum test (Tables 1 and 2). Variables used for analysis included sex, age, z‐score for weight, RACHS1 category, number of previous surgical admissions in the PCCC, number of operations during the admission, weekend day of operation, year of operation, and an indicator for low‐weight newborns at surgery (LWN). We adjusted for LWN (defined as <2.5 kg at the time of surgery) because it is a possible confounder with differential morbidity between boys and girls.16 Rather than assuming linearity in the log‐odds of mortality for continuous variables or categorizing continuous variables, we used restricted cubic splines for age, z‐score for weight, and year of operation. Three knots were used for each variable set at the corresponding 10th, 50th, and 90th percentiles. The splines are restricted beyond the outer knots to be linear while the knot at the 50th percentile allows for the relationship to change directions.17 Age groups for reporting in summary tables were predefined and based on typical cut‐points for neonates (0 to 30 days) and infants (31 to 365 days) along with a cut‐point at 9 years of age that was determined by published data as an approximate pubertal cut‐point.18–19 Surgical year was grouped into approximate decades for reporting in summary tables. Surgeries per admission ranged from 1 to 5 and were collapsed to fewer groups because of the small number of admissions requring ≥2 procedures (n=188, 0.2%). Likewise, the number of previous admissions ranged from 0 to 7 and was collapsed to fewer groups because of the small number with >3 previous admissions (n=142, 0.1%).
Table 2.
Mortality by Sex Within the PCCC Cohort
| Girls | Boys | |||||
|---|---|---|---|---|---|---|
| N | 30‐Day Mortality | Overall Mortality | N | 30‐Day Mortality | Overall Mortality | |
| Age group | ||||||
| 0 to 30 days | 5483 | 858 (15.6%) | 1006 (18.3%) | 8919 | 1163 (13.0%) | 1408 (15.8%) |
| 31 to 365 days | 10 507 | 502 (4.78%) | 594 (5.65%) | 12 531 | 571 (4.56%) | 671 (5.35%) |
| 1 to 9 years | 14 706 | 296 (2.01%) | 320 (2.18%) | 15 718 | 373 (2.37%) | 418 (2.66%) |
| 10 to 18 years | 3608 | 63 (1.75%) | 70 (1.94%) | 4840 | 67 (1.38%) | 71 (1.47%) |
| LWN (<2.5 kg) | 735 | 178 (24.2%) | 200 (27.2%) | 870 | 181 (20.8%) | 226 (26.0%) |
| Risk category | ||||||
| 1 | 9260 | 45 (0.486%) | 58 (0.626%) | 8005 | 41 (0.512%) | 53 (0.662%) |
| 2 | 11 335 | 226 (1.99%) | 259 (2.28%) | 14 599 | 257 (1.76%) | 305 (2.09%) |
| 3 | 10 454 | 715 (6.84%) | 837 (8.01%) | 14 093 | 849 (6.02%) | 1021 (7.24%) |
| 4 | 2301 | 371 (16.1%) | 432 (18.8%) | 3655 | 498 (13.6%) | 594 (16.25%) |
| 5 and 6 | 954 | 362 (38.0%) | 404 (42.4%) | 1656 | 529 (31.9%) | 595 (35.9%) |
| Unassigned | [4862] | [274] (5.64%) | [392] (8.06%) | [6555] | [339] (5.17%) | [445] (6.79%) |
| Surgical era | ||||||
| 1982–1989 | 5187 | 430 (8.29%) | 473 (9.12%) | 6216 | 551 (8.86%) | 604 (9.72%) |
| 1990–1999 | 15 471 | 889 (5.75%) | 1004 (6.49%) | 18 233 | 1120 (6.14%) | 1273 (6.98%) |
| 2000–2007 | 13 646 | 400 (2.93%) | 513 (3.76%) | 17 569 | 503 (2.86%) | 691 (3.93%) |
| Surgeries per admission | ||||||
| 1 | 33 402 | 1540 (4.61%) | 1725 (5.16%) | 40 823 | 1971 (4.83%) | 2239 (5.48%) |
| >1 | 902 | 179 (19.8%) | 265 (29.4%) | 1185 | 203 (17.1%) | 329 (27.8%) |
| Previous admissions | ||||||
| 0 | 28 568 | 1463 (5.12%) | 1700 (5.95%) | 33 672 | 1803 (5.35%) | 2147 (6.38%) |
| 1 | 4155 | 198 (4.77%) | 223 (5.37%) | 5943 | 280 (4.71%) | 311 (5.23%) |
| 2 | 1287 | 42 (3.26%) | 50 (3.89%) | 1887 | 70 (3.71%) | 82 (4.35%) |
| >2 | 294 | 16 (5.44%) | 17 (5.78%) | 506 | 21 (4.15%) | 28 (5.53%) |
| Weekend operation | ||||||
| Yes | 582 | 93 (16.0%) | 105 (18.0%) | 894 | 123 (13.8%) | 142 (15.9%) |
| No | 33 722 | 1626 (4.82%) | 1885 (5.59%) | 41 114 | 2051 (4.99%) | 2426 (5.90%) |
| Total | 34 304 | 1719 (5.01%) | 1990 (5.80%) | 42 008 | 2174 (5.18%) | 2568 (6.11%) |
N indicates number of operations analyzed, while brackets indicate operations with nonassignable RACHS1 risk category or on patients with syndromes that were excluded from the multivariable analysis; LWN, low‐weight neonates were analyzed as a separate category independent of their chronologic age. PCCC indicates Pediatric Cardiac Care Consortium; RACHS1, Risk Adjusted Classification for Congenital Heart Surgery system, version 1.
The primary analysis consisted of a prespecified multivariable logistic regression model with interactions used to compare the odds of 30‐day within‐hospital mortality for girls versus boys by age, z‐score for weight, risk category, and operation year while adjusting for the variables: weekend operation (yes or no), LWN (yes or no), surgeries per admission (1 or >1), and previous admissions (0, 1, 2, or >2). Statistical significance for the overall sex effect, the overall test of any interaction, individual interactions, and the linear and nonlinear components of the model were tested using Wald χ2 statistics. Because the data are hierarchically clustered (potentially repeated measures on a patient within hospital), a stratified bootstrap with cluster sampling was used to stratify the bootstrap by hospital and cluster sample with replacement by patient. Thus, all hospitals were resampled without replacement and patients within a hospital were sampled with replacement where all observations for a patient were selected if that patient was drawn. The bootstrap is conditional on hospital, to account for potential hospital effects, while accounting for intracorrelation among multiple admissions for a patient. Nonparametric bootstrap confidence limits were computed after 1000 samples were drawn, and coefficients were estimated from the original working independence model. The accuracy of the model was reported using the C‐statistic and the Nagelkerke R2 and validated using 500 bootstrap samples of equivalent size sampled with replacement to account for bias due to potential model overfitting.
We used prespecified quantile regression model to evaluate whether a sex effect was associated with median LOS after surgery. In addition to the variables described in the logistic regression model, a variable for within‐hospital mortality was included along with 4 separate interactions between risk category and within‐hospital mortality, surgical year and age, and sex and mortality. The mortality variable was included to account for the shorter LOS due to within‐hospital mortality. The first 3 interactions were included because the differences in LOS were thought to vary by risk category. The final interaction between sex and mortality was included to test whether the LOS differed by sex depending on whether a patient was discharged or died. The Wald statistics testing the overall effects, interactions, and nonlinearity for LOS were calculated using 1000 bootstraps to compute corrected standard errors as described in the logistic regression.
Analyses were performed using R version 2.15.2 (R Foundation for Statistical Computing) including the packages Hmisc and rms. No corrections were made for multiple comparisons.
Sensitivity Analysis
To assess any potential changes when including unassigned RACHS1 operations or patients carrying major genetic condition, multivariable logistic regression models were rerun with these cases included as a separate risk category.
Results
Summary of Patient Characteristics and Unadjusted Statistics
Patient characteristics are presented in Table 1. Boys were more likely to be operated on as newborns (21% versus 16%, P<0.001) and at a younger age (median 341 versus 442 days, P<0.001) and had fewer surgeries in the lowest ‐risk category 1 (19% versus 27%, P<0.001). The distribution of patient deaths by different risk categories, age categories, surgical era, or weekend surgeries is presented in Table 2. Overall in‐hospital mortality was 6.1% and 5.8% for males and girls, respectively (P=0.073). Girls had a higher risk of death within 30 days associated with the highest‐risk categories 5 and 6 operations (37.9% versus 31.9%, respectively).
Model Performance
The multivariable model fit assessment for the primary analysis using 30‐day within‐hospital mortality as the outcome resulted in a validated C‐statistic of 0.852 and a validated Nagelkerke R2 of 0.268. The C‐statistic is used to assess the predictive ability of the model where a value of 0.5 indicates that the model is no better than predicting by chance and a value of 1.0 indicates perfect prediction. Typically, a value >0.7 is considered reasonable and a value >0.8 is considered strong.20 The Nagelkerke R2 is a measure of the explanatory power of the model and ranges from 0 to 1, with 1 indicating the model fully accounts for the total likelihood of mortality.21–22
Sex Has an Effect on Risk for Postsurgical Mortality That May Vary With Age
The bootstrap adjusted tests indicated an overall association between patient's sex and both 30‐day and overall within‐hospital mortality (P=0.002 and P=0.001, respectively, Table 3). The overall test for any interaction with the effect of sex was not statistically significant in either model (P=0.503 and P=0.149, respectively). Of the 4 interactions tested (sex × age, sex × weight z‐score, sex × surgical year, and sex × risk category), only the effect of sex × age appeared to approach statistical significance (P=0.080 and P=0.039, for 30‐day and overall within‐hospital mortality, respectively). Because of the potentially complex associations between the sex effect and mortality due to the interactions in the model, the relationships are presented both graphically (Figure 2A) and as a table of ORs with 95% CIs for various age time points (Table 4). In both the figures and the tables, the remaining variables in the respective models were set at their mode for categorical variables and median for continuous variables without adjustments for multiple comparisons. Females have increased odds of death in early infancy (<6 months old) and for higher‐risk procedures (risk categories 3 to 6) compared with males. The log‐odds of mortality decreased over time for each risk category and the lines for boys and girls were approximately parallel, indicating that their odds of death remained proportional over time (Figure 2B).
Table 3.
Wald Statistics for 30‐Day and Overall Mortality for Each Factor Plus Higher Order Factors, Interactions, and Nonlinearity After Bootstrapping
| 30‐Day Mortality | Overall Mortality | |||||
|---|---|---|---|---|---|---|
| χ2 | df | P Value | χ2 | df | P Value | |
| Factors, including joint tests of model parameters | ||||||
| Sex* | 29.0 | 11 | 0.002 | 30.4 | 11 | 0.001 |
| Risk category* | 2996.5 | 8 | <0.001 | 2948.5 | 8 | <0.001 |
| Age, days* | 418.7 | 4 | <0.001 | 489.3 | 4 | <0.001 |
| Nonlinear* | 135.8 | 2 | <0.001 | 193.4 | 2 | <0.001 |
| Surgical weight z‐score* | 112.0 | 4 | <0.001 | 100.3 | 4 | <0.001 |
| Nonlinear* | 16.7 | 2 | <0.001 | 10.2 | 2 | 0.006 |
| Surgical year* | 1051.9 | 4 | <0.001 | 897.2 | 4 | <0.001 |
| Nonlinear* | 29.8 | 2 | <0.001 | 13.8 | 2 | 0.001 |
| Weekend* | 14.3 | 1 | <0.001 | 14.0 | 1 | <0.001 |
| LWN* | 82.2 | 1 | <0.001 | 109.5 | 1 | <0.001 |
| Surgeries per admission* | 53.1 | 1 | <0.001 | 282.0 | 1 | <0.001 |
| Number of prior admissions* | 18.5 | 3 | <0.001 | 24.3 | 3 | <0.001 |
| Interactions | ||||||
| All interactions* | 9.3 | 10 | 0.503 | 14.6 | 10 | 0.149 |
| Sex × risk category* | 1.00 | 4 | 0.910 | 1.70 | 4 | 0.791 |
| Sex × age (days)* | 5.04 | 2 | 0.080 | 6.48 | 2 | 0.039 |
| Nonlinear* | 5.03 | 1 | 0.025 | 6.39 | 1 | 0.012 |
| Sex × surgical weight z‐score* | 0.36 | 2 | 0.834 | 2.67 | 2 | 0.263 |
| Nonlinear* | 0.00 | 1 | 0.986 | 0.67 | 1 | 0.413 |
| Sex × surgical year* | 0.01 | 2 | 0.9943 | 0.80 | 2 | 0.671 |
| Nonlinear* | 0.01 | 1 | 0.928 | 0.05 | 1 | 0.823 |
The model analyzed for 30‐day and overall mortality was logit{death=yes | χβ}=β0+β1(sex=male)+β2(RACHS score=2)+β3(RACHS score=3)+β4(RACHS score=4)+β5(RACHS score=5+6)+β6(surgical year)+β7(surgical year)′+β8(surgical age)+β9(surgical age)′+β10(surgical weight z‐score)+β11(surgical weight z‐score)′+β12(weekend surgery=no)+β13(low weight infant=yes)+β14(sex=male)(surgical age)+β15(sex=male)(surgical age)′+β16(sex=male)(surgical year)+β17(sex=male)(surgical year)′+β18(sex=male)(surgical weight z‐score)+β19(sex=male)(surgical weight z‐score)′+β20(surgiers per admission=>1)+β21(previous admissions=1)+β22(previous admissions=2)+β23(previous admissions=>2)+β24(sex=male)(RACHS score=2)+β25(sex=male)(RACHS score=3)+β26(sex=male)(RACHS score=4)+β27(sex=male)(RACHS score=5+6) where (…)′ represents (x−t1)+3− (x−t2)+3(t3−t1)/(t3−t2)+(x−t3)+3(t2−t1)/(t3−t2) for 3 knots: t1, t2, and t3.17 Tests of nonlinearity were for the coefficients of the (…)′ terms that are the nonlinear part of the restricted cubic splines. df indicates degrees of freedom; LWN, low‐weight neonates; RACHS1, Risk Adjusted Classification for Congenital Heart Surgery system, version 1.
Joint test of the factor, all interactions with the factor (if interactions with the factor are present), and all nonlinear components (if a continuous factor).
Joint test of all the nonlinear components for continuous factors.
Joint test of all the interaction components with the sex factor including nonlinear components.
Test of the interaction including nonlinear components (if a continuous factor).
Test of the nonlinear components of an interaction for continuous factors.
Figure 2.
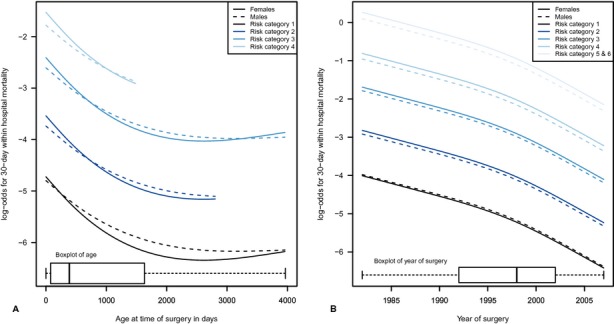
A, Plots of the log‐odds for postoperative death in males and females against age at time of surgery after adjustment for all variables. A separate curve is presented for each risk category for ages within 1.5 times the interquartile range of the first and third quartile for each risk category resulting in truncated curves with a maximum of ≈4000 days (11 years) of age. No curve is provided for category 5 and 6, because most of these operations occurred within first month of life. A boxplot at the bottom of the graph summarizes age at surgery for all risk categories. B, Plots of the log‐odds for postoperative death in boys (males) and girls (females) against year of operation show similar reduction in the relative odds for both sexes over time. A boxplot at the bottom of the graph summarizes year of operation for all patients.
Table 4.
ORs and 95% CIs for 30‐Day Within‐Hospital Mortality of Girls Versus Boys at Various Ages for All Operations*
| Age | RACHS1 Risk Category | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 and 6 | |
| 0 days | 1.08 (0.67 to 1.74) | 1.22 (0.99 to 1.5) | 1.22 (1.04 to 1.42) | 1.29 (1.07 to 1.54) | 1.31 (1.09 to 1.58) |
| 30 days | 1.07 (0.66 to 1.72) | 1.21 (0.98 to 1.48) | 1.21 (1.04 to 1.41) | 1.27 (1.06 to 1.52) | 1.3 (1.08 to 1.57) |
| 2 months | 1.06 (0.66 to 1.7) | 1.2 (0.97 to 1.47) | 1.19 (1.03 to 1.39) | 1.26 (1.05 to 1.51) | — |
| 3 months | 1.05 (0.65 to 1.68) | 1.18 (0.97 to 1.45) | 1.18 (1.02 to 1.37) | 1.25 (1.05 to 1.49) | — |
| 6 months | 1.02 (0.64 to 1.62) | 1.15 (0.94 to 1.4) | 1.15 (0.99 to 1.33) | 1.21 (1.02 to 1.45) | — |
| 1 year | 0.96 (0.61 to 1.52) | 1.09 (0.89 to 1.33) | 1.09 (0.94 to 1.27) | 1.15 (0.96 to 1.38) | — |
| 2 years | 0.88 (0.56 to 1.39) | 1 (0.8 to 1.25) | 1 (0.83 to 1.2) | 1.05 (0.85 to 1.3) | — |
| 9 years | 0.89 (0.55 to 1.42) | — | 1 (0.78 to 1.28) | — | — |
| 12 years | 1.03 (0.62 to 1.72) | — | 1.16 (0.88 to 1.55) | — | — |
| 15 years | 1.2 (0.67 to 2.16) | — | — | — | — |
RACHS1 indicates Risk Adjusted Classification for Congenital Heart Surgery system, version 1.
Predicted values are presented for ages that were within 1.5 times the interquartile range of the first and third quartile for each risk category.
Figures for overall within‐hospital mortality were similar to the figures for 30‐day within‐hospital mortality and are not presented. Sensitivity analyses that included operations with undefined RACHS1 risk category and patients with genetic conditions were performed and did not change the results of the main analysis (data not shown).
Length of Stay
The median LOS, including deaths, was 6 days for both males and girls. The median time of death was 3 days for males and 2 days for girls (Table 1). LOS was analyzed as secondary outcome using the quantile regression model described in the statistical analysis section. The bootstrap adjusted tests indicated a strong overall association between the effect of sex and LOS (P<0.0001) (Table 5). The overall test for any interaction with the effect of sex was also statistically significant (P<0.0001). Of the 5 interactions that included the effect of sex, those of sex × age and sex × risk category were statistically significant (P=0.0011 and P<0.0001, respectively). As a result, the association of the patient's sex with LOS is complex and changes depending on patients' age and risk category. No significant difference was found for the median length of stay when testing the interaction of sex with mortality, surgical year, and z‐score for weight. Again, because of the complex associations with LOS due to the restricted cubic splines, the effect of age × sex is presented both graphically (Figure 3) for each risk category and as a table of ORs with 95% CIs for various age time points (Table 6). The remaining variables were set at their mode for categorical variables and median for continuous variables. Females had statistically significantly shorter LOS only for the lowest risk category 1 and not for the other categories.
Table 5.
Wald Statistics for LOS for Each Factor Plus Higher Order Factors, Interactions, and Nonlinearity After Bootstrapping
| LOS | |||
|---|---|---|---|
| χ2 | df | P Value | |
| Factors, including joint tests of model parameters | |||
| Sex* | 175.57 | 11 | <0.0001 |
| Risk category* | 11 873.77 | 28 | <0.0001 |
| Age (days)* | 5065.01 | 12 | <0.0001 |
| Nonlinear* | 2484.77 | 6 | <0.0001 |
| Surgical weight z‐score* | 191.22 | 4 | <0.0001 |
| Nonlinear* | 32.87 | 2 | <0.0001 |
| Surgical year* | 3091.23 | 12 | <0.0001 |
| Nonlinear* | 961.57 | 6 | <0.0001 |
| Interactions | |||
| All sex interactions* | 73.08 | 10 | <0.0001 |
| Sex × risk category* | 51.58 | 4 | <0.0001 |
| Sex × age (days)* | 13.62 | 2 | 0.0011 |
| Nonlinear* | 3.27 | 1 | 0.0706 |
| Sex × surgical weight z‐score* | 3.2 | 2 | 0.2019 |
| Nonlinear* | 0.00 | 1 | 0.9917 |
| Sex × surgical year* | 4.31 | 2 | 0.1161 |
| Nonlinear* | 0.22 | 1 | 0.6376 |
The model analyzed was median LOS=β0+β1(death=no)+β2(sex=male)+β3(RACHS score=2)+β4(RACHS score=3)+β5(RACHS score=4)+β6(RACHS score=5+6)+β7(surgical year)+β8(surgical year)′+β9(surgical age)+β10(surgical age)′+β11(surgical weight z‐score)+β12(surgical weight z‐score)′+β13(sex=male)(surgical age)+β14(sex=male)(surgical age)′+β15(sex=male)(surgical year)+β16(sex=male)(surgical year)′+β17(surgeries per admission=>1)+β18(previous admissions=1)+β19(previous admissions=2)+β20(previous admissions=>2)+β21(RACHS score=2)(surgical year)+β22(RACHS score=2)(surgical year)′+β23(RACHS score=3)(surgical year)+β24(RACHS score=3)(surgical year)′+β25(RACHS score=4)(surgical year)+β26(RACHS score=4)(surgical year)′+β27(RACHS score=5+6)(surgical year)+β28(RACHS score=5+6)(surgical year)′+β29(RACHS score=2)(surgical age)+β30(RACHS score=2)(surgical age)′+β31(RACHS score=3)(surgical age)+β32(RACHS score=3)(surgical age)′+β33(RACHS score=4)(surgical age)+β34(RACHS score=4)(surgical age)′+β35(RACHS score=5+6)(surgical age)+β36(RACHS score=5+6)(surgical age)′+β37(sex=male)(RACHS score=2)+β38(sex=male)(RACHS score=3)+ β39(sex=male)(RACHS score=4)+β40(sex=male)(RACHS score=5+6)+β41(sex=male)(surgical weight z‐score)+β42(sex=male)(surgical weight z‐score)′+β43(death=no)(RACHS score=2)+β44(death=no)(RACHS score=3)+β45(death=no)(RACHS score=4)+β46(death=no)(RACHS score=5+6)+β47(death=no)(sex=male) where (…)′ represents (x−t1)+3−(x−t2)+3(t3−t1)/(t3−t2)+(x−t3)+3(t2−t1)/(t3−t2) for 3 knots: t1, t2, and t3.17 Tests of nonlinearity were for the coefficients of the (…)′ terms that are the nonlinear part of the restricted cubic splines. df indicates degrees of freedom; LOS, length of stay; RACHS1 indicates Risk Adjusted Classification for Congenital Heart Surgery system, version 1.
Joint test of the factor, all interactions with the factor (if interactions with the factor are present), and all nonlinear components (if a continuous factor).
Joint test of all the nonlinear components for continuous factors.
Joint test of all the interaction components with the sex factor including nonlinear components.
Test of the interaction including nonlinear components (if a continuous factor).
Test of the nonlinear components of an interaction for continuous factors.
Figure 3.
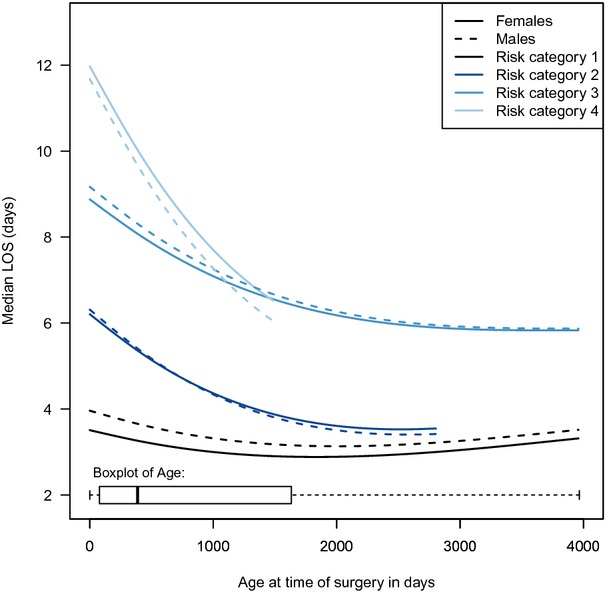
Plots of the postoperative length of stay (LOS) in boys (males) and girls (females) against age at time of surgery after adjustment for all variables. A separate curve is presented for each risk category for ages within 1.5 times the interquartile range of the first and third quartile for each risk category resulting in truncated curves with a maximum of ≈4000 days (11 years) of age. No curve is provided for “category 5 and 6,” because most of these operations occurred within first month of life. A boxplot at the bottom of the graph summarizes age at surgery for all risk categories.
Table 6.
Median Difference and 95% CIs for Length of Stay of Girls Versus Boys at Various Ages for All Operations*
| Age | RACHS1 Risk Category | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 and 6 | |
| 0 days | −0.42 (−0.57 to −0.29) | −0.07 (−0.21 to 0.05) | −0.26 (−0.46 to −0.08) | 0.33 (−0.13 to 0.8) | 0.06 (−0.69 to 0.89) |
| 30 days | −0.42 (−0.56 to −0.29) | −0.07 (−0.2 to 0.05) | −0.26 (−0.46 to −0.08) | 0.33 (−0.13 to 0.8) | 0.06 (−0.69 to 0.89) |
| 2 months | −0.41 (−0.55 to −0.29) | −0.06 (−0.2 to 0.05) | −0.25 (−0.45 to −0.08) | 0.34 (−0.12 to 0.8) | — |
| 3 months | −0.41 (−0.54 to −0.29) | −0.06 (−0.19 to 0.05) | −0.25 (−0.44 to −0.07) | 0.34 (−0.12 to 0.81) | — |
| 6 months | −0.39 (−0.52 to −0.28) | −0.05 (−0.17 to 0.06) | −0.23 (−0.42 to −0.07) | 0.36 (−0.11 to 0.82) | — |
| 1 year | −0.36 (−0.47 to −0.27) | −0.02 (−0.14 to 0.08) | −0.2 (−0.38 to −0.05) | 0.39 (−0.08 to 0.84) | — |
| 2 years | −0.32 (−0.4 to −0.24) | 0.03 (−0.07 to 0.12) | −0.16 (−0.33 to 0) | 0.43 (−0.02 to 0.89) | — |
| 9 years | −0.18 (−0.26 to −0.11) | — | −0.02 (−0.18 to 0.13) | — | — |
| 12 years | −0.16 (−0.27 to −0.08) | — | 0 (−0.17 to 0.13) | — | — |
| 15 years | −0.15 (−0.29 to −0.03) | — | — | — | — |
RACHS1 indicates Risk Adjusted Classification for Congenital Heart Surgery system, version 1.
Predicted values are presented for ages that were within 1.5 times the interquartile range of the first and third quartile for each risk category.
Discussion
There are wide biological differences between males and females that may affect myocardial remodeling, response to myocardial stress or ischemia‐reperfusion injury, inflammatory response after exposure to CPB, propensity for arrhythmias, and pulmonary or systemic vascular tone, all of which potentially affect outcomes after cardiac operations.12,23 Sex‐related differences in incidence and outcomes have been documented for ischemic heart disease, stroke, heart failure, valve disease, and pulmonary hypertension.24–27 In relation to CHD, sex‐related differences in incidence have been reported for several lesions such as transposition of the great arteries and left‐sided obstructive lesions (more common in males) and atrial septal defects and Ebstein's anomaly (more common in females).28–29
Evidence of sex disparities in outcomes after pediatric cardiac surgery has been conflicting and possibly confounded by the differential incidence and severity of CHD between the sexes. Although studies on the outcomes of specific lesions did not identify any sex disparities, other studies using risk‐adjustment methodology suggested that female sex was an independent risk factor for postoperative death. The first such report came from the analysis of a single state (California) administrative dataset9 from 1995 and 1997, which analyzed the outcomes of 6593 operations and demonstrated a higher risk for in‐hospital death, for female patients (OR 1.51). An extension of this study confirmed these findings after linking the state's administrative data from 1989–1999 (N=25 402 patients) with the death registry to account for 30‐day postdischarge mortality.10 This finding did not appear to be related to racial or socioeconomic differences or differential referral and resource utilization pattern,9,30 but was mostly attributed to sex‐differential outcomes in high‐risk procedures in early infancy. Studies from an independent multistate registry came to similar conclusions using data for 2000, 2003, and 2006 (N=33 848).11 This last study used a risk‐adjusted methodology and subgroup analysis to provide an increased OR for overall in‐hospital postsurgical death of 1.21 for girls on account of the subgroup with high‐risk procedures who were <1 year of age (OR 1.39). Recently, a report from the STS‐CHSD using data from 2007–2009 (N=20 399) challenged these findings and found similar risk‐adjusted mortality between males and girls.8
Our study analyzed the largest available clinical dataset over a 25‐year period and demonstrated a complex and clinically important sex effect on mortality after pediatric cardiac surgery that remained unchanged during the 25‐year study period. As in previous studies, we found that boys underwent higher‐risk procedures more frequently and at a younger age, so that the disparity in outcomes became apparent only after adjustment for various risk factors. The inclusion of interaction terms is the most important difference between our and previous studies on the subject.
In the primary analysis using the logistic regression model, the potential interaction between sex and age supports the involvement of an underlying biological factor.18–19,31 Specifically, we found that female sex is associated with increased risk of death in neonates and young infants (<6 months old). This age‐dependent sex disparity appears to exist only in infants with higher‐risk procedures typically associated with exposure to significant ischemia‐reperfusion. The particular vulnerability of girls in this group of operations may be related to the metabolic disadvantage of female newborn hearts because of lower baseline high‐energy phosphates (ATP and creatine phosphate) and lower myocardial glycogen reserves compared with boys, as documented in animal studies.12 This may lead to increased susceptibility to ischemic injury and impaired myocardial function after reperfusion. In children undergoing surgery for CHD, lower levels of ATP were associated with postoperative myocardial dysfunction and longer intensive care unit stay.32 In the pediatric population, low cardiac output following myocardial ischemia is the most common cause of mortality and morbidity after cardiac surgery. In this setting, sex‐specific differences in cellular energy levels during ischemia may offer a potential explanation for differences in operative outcomes in male versus female infants subjected to high‐risk operations for CHD.33 It is noteworthy that a similarly increased risk of in‐hospital mortality death was reported in women undergoing surgery for CHD (OR 1.3).23,34–36 In that regard, a potentially important factor contributing to early postoperative death in female subjects is the inflammatory response after exposure to CPB, which may be differentially modulated by circulating sex hormones. In a previous study of CHD, female sex increased the odds of death by a factor of 5.43 among patients requiring postoperative extracorporeal membrane oxygenation,37 a therapy that can provoke intense inflammatory response. Finally, besides the age‐dependent metabolic and hormonal differences between males and females, there are other possible biological explanations such as the reported sex‐ and age‐related dimorphism in gene expression in the failing human heart.38
Our secondary analysis of LOS demonstrated also a strong but complex relationship between sex and LOS with statistically significant interactions between sex and risk category and between sex and age at surgery. Specifically, we found that females had a shorter LOS after low‐risk procedures at all ages compared with males, but this trend was not observed for higher‐risk procedures. These results describe a complex relationship between sex and LOS than previously reported and support a differential response of males and females after exposure to ischemia‐reperfusion.8
Strengths and Limitations
The PCCC is the longest existing registry of pediatric cardiac surgery and the only one that uses a centralized coding process. The centralized coding ensures consistency over time and between centers in contrast to other registries that use codes entered directly by the individual institutions. This approach, although valuable to ensure high‐quality data, is time consuming, and therefore detailed analysis of data after 2007 is not yet available. However, the parallel trend of the log‐odds for mortality for both sexes makes it unlikely that these trends have suddenly changed in the subsequent years. The PCCC contains mostly data from small and medium‐sized centers; however, there is no evidence or reason to believe that the sex distribution of patients with CHD differs between different‐size centers. In addition, the mortality and case‐mix of the PCCC dataset are comparable with that reported from other datasets and major pediatric cardiac centers.39
The PCCC has consistently used the RACHS1 risk classification. The use of RACHS1 enriched by the other covariables has one of the highest reported discrimination indices for operations for CHD but leaves 12.9% of the procedures unassigned. Inclusion of these cases in a sensitivity analysis did not change the findings or the implications of this study.
The major limitations of this study are its retrospective nature and the limited information in the PCCC database, which does not include race and socioeconomic data. Although race has been shown to affect postoperative outcomes for CHD, there is no evidence that there are significant sex differences in the distribution of CHD between different races. In addition, data from previous studies suggest that health insurance status and home income level do not affect outcomes.9–10
Conclusions, Implications, and Future Directions
Our data suggest a significant effect of patient's sex on outcomes after CHD surgery, which may be age dependent, particularly for high‐risk operations. Although sex is a nonmodifiable risk factor, there may be circumstances in which this differential risk pattern may be of importance for the care and outcome of a patient with CHD.
This observational study supports the need for further research focused on sex‐related differences in biomarkers of cardiac dysfunction/injury or inflammation that may be helpful to provide insights to the mechanisms of the failing postoperative physiology. It also provides the basis to further explore the variable response of promising cardiovascular medications with differential effect between males and females such as the calcium sensitizer levosimendan.40 This knowledge ultimately may benefit patients of both sexes. A further understanding of the role of sex as a risk factor may lead to modification of treatment strategies regarding timing and preoperative preparation for congenital heart operations such as the use of remote ischemic preconditioning in vulnerable children undergoing repair of CHD.41
Sources of Funding
Research reported in this publication was supported by a National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) Award (UL1TR000114). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.......
Disclosures
None.
Acknowledgments
We thank the program directors and data collection coordinators from the participating PCCC institutions; without their effort and dedication, this work could not have been completed.
References
- 1.Vaccarino V. Women and outcomes of coronary artery bypass surgery: do we have an answer? Am Heart J. 2003; 146:935-937 [DOI] [PubMed] [Google Scholar]
- 2.Gorman Koch C, Mora Mangano C, Schwann N, Vaccarino V. Is it gender, methodology, or something else? J Thorac Cardiovasc Surg. 2003; 126:932-935 [DOI] [PubMed] [Google Scholar]
- 3.Gaynor JW, Mahle WT, Cohen MI, Ittenbach RF, DeCampli WM, Steven JM, Nicolson SC, Spray TL. Risk factors for mortality after the Norwood procedure. Eur J Cardiothorac Surg. 2002; 22:82-89 [DOI] [PubMed] [Google Scholar]
- 4.Ashburn DA, McCrindle BW, Tchervenkov CI, Jacobs ML, Lofland GK, Bove EL, Spray TL, Williams WG, Blackstone EH. Outcomes after the Norwood operation in neonates with critical aortic stenosis or aortic valve atresia. J Thorac Cardiovasc Surg. 2003; 125:1070-1082 [DOI] [PubMed] [Google Scholar]
- 5.Morris CD, Magilke D, Reller M. Down's syndrome affects results of surgical correction of complete atrioventricular canal. Pediatr Cardiol. 1992; 13:80-84 [DOI] [PubMed] [Google Scholar]
- 6.Azakie A, Russell IA. Gender differences in pediatric cardiac surgery: the surgeon's perspective. J Thorac Cardiovasc Surg. 2004; 128:4-6 [DOI] [PubMed] [Google Scholar]
- 7.Cho JM, Puga FJ, Danielson GK, Dearani JA, Mair DD, Hagler DJ, Julsrud PR, Ilstrup DM. Early and long‐term results of the surgical treatment of tetralogy of Fallot with pulmonary atresia, with or without major aortopulmonary collateral arteries. J Thorac Cardiovasc Surg. 2002; 124:70-81 [DOI] [PubMed] [Google Scholar]
- 8.Dibardino DJ, Pasquali SK, Hirsch JC, Benjamin DK, Kleeman KC, Salazar JD, Jacobs ML, Mayer JE, Jacobs JP. Effect of sex and race on outcome in patients undergoing congenital heart surgery: an analysis of the society of thoracic surgeons congenital heart surgery database. Ann Thorac Surg. 2012; 94:2054-2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang RK, Chen AY, Klitzner TS. Female sex as a risk factor for in‐hospital mortality among children undergoing cardiac surgery. Circulation. 2002; 106:1514-1522 [DOI] [PubMed] [Google Scholar]
- 10.Klitzner TS, Lee M, Rodriguez S, Chang RK. Sex‐related disparity in surgical mortality among pediatric patients. Congenit Heart Dis. 2006; 1:77-88 [DOI] [PubMed] [Google Scholar]
- 11.Marelli A, Gauvreau K, Landzberg M, Jenkins K. Sex differences in mortality in children undergoing congenital heart disease surgery: a United States population‐based study. Circulation. 2010; 122Suppl:S234-S240 [DOI] [PubMed] [Google Scholar]
- 12.Wittnich C, Quaglietta D, Tan L, Belanger MP. Sex differences in newborn myocardial metabolism and response to ischemia. Pediatr Res. 2011; 70:148-152 [DOI] [PubMed] [Google Scholar]
- 13.Jenkins KJ. Risk adjustment for congenital heart surgery: the RACHS‐1 method. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004; 7:180-184 [DOI] [PubMed] [Google Scholar]
- 14.Vinocur JM, Menk JS, Connett J, Moller JH, Kochilas LK. Surgical volume and center effects on early mortality after pediatric cardiac surgery: 25‐year North American experience from a multi‐institutional registry. Pediatr Cardiol. 2013; 34:1226-1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkins KJ, Gauvreau K. Center‐specific differences in mortality: preliminary analyses using the Risk Adjustment in Congenital Heart Surgery (RACHS‐1) method. J Thorac Cardiovasc Surg. 2002; 124:97-104 [DOI] [PubMed] [Google Scholar]
- 16.Shepard CW, Kochilas LK, Rosengart RM, Brearley AM, Bryant R, III, Moller JH, St Louis JD. Repair of major congenital cardiac defects in low‐birth‐weight infants: is delay warranted? J Thorac Cardiovasc Surg. 2010; 140:1104-1109 [DOI] [PubMed] [Google Scholar]
- 17.Harrell FE., Jr Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. 2001New York, NY: Springer‐Verlag [Google Scholar]
- 18.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969; 44:291-303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970; 45:13-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosmer DWLS. Applied Logistic Regression. 20002nd edNew York, NY: John Wiley & Sons [Google Scholar]
- 21.Nagelkerke N. A note on a general definition of the coefficient of multiple determination. Biometrika. 1991; 78:691-692 [Google Scholar]
- 22.Ash A, Shwartz M. R2: a useful measure of model performance when predicting a dichotomous outcome. Stat Med. 1999; 18:375-384 [DOI] [PubMed] [Google Scholar]
- 23.Piro M, Della Bona R, Abbate A, Biasucci LM, Crea F. Sex‐related differences in myocardial remodeling. J Am Coll Cardiol. 2010; 55:1057-1065 [DOI] [PubMed] [Google Scholar]
- 24.Rankin JS, Hammill BG, Ferguson TB, Jr, Glower DD, O'Brien SM, DeLong ER, Peterson ED, Edwards FH. Determinants of operative mortality in valvular heart surgery. J Thorac Cardiovasc Surg. 2006; 131:547-557 [DOI] [PubMed] [Google Scholar]
- 25.Bondy CA. Aortic coarctation and coronary artery disease: the XY factor. Circulation. 2012; 126:5-7 [DOI] [PubMed] [Google Scholar]
- 26.Engelfriet PM, Duffels MG, Moller T, Boersma E, Tijssen JG, Thaulow E, Gatzoulis MA, Mulder BJ. Pulmonary arterial hypertension in adults born with a heart septal defect: the Euro Heart Survey on adult congenital heart disease. Heart. 2007; 93:682-687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warnes CA. Sex differences in congenital heart disease: should a woman be more like a man? Circulation. 2008; 118:3-5 [DOI] [PubMed] [Google Scholar]
- 28.Samanek M. Boy:girl ratio in children born with different forms of cardiac malformation: a population‐based study. Pediatr Cardiol. 1994; 15:53-57 [DOI] [PubMed] [Google Scholar]
- 29.Miller‐Hance WC, Tacy TA. Gender differences in pediatric cardiac surgery: the cardiologist's perspective. J Thorac Cardiovasc Surg. 2004; 128:7-10 [DOI] [PubMed] [Google Scholar]
- 30.Chang RK, Rodriguez S, Lee M, Klitzner TS. Risk factors for deaths occurring within 30 days and 1 year after hospital discharge for cardiac surgery among pediatric patients. Am Heart J. 2006; 152:386-393 [DOI] [PubMed] [Google Scholar]
- 31.Faiman C, Winter JS. Sex differences in gonadotrophin concentrations in infancy. Nature. 1971; 232:130-131 [DOI] [PubMed] [Google Scholar]
- 32.Najm HK, Wallen WJ, Belanger MP, Williams WG, Coles JG, Van Arsdell GS, Black MD, Boutin C, Wittnich C. Does the degree of cyanosis affect myocardial adenosine triphosphate levels and function in children undergoing surgical procedures for congenital heart disease? J Thorac Cardiovasc Surg. 2000; 119:515-524 [DOI] [PubMed] [Google Scholar]
- 33.Cheung MM, Smallhorn JF, Vogel M, Van Arsdell G, Redington AN. Disruption of the ventricular myocardial force‐frequency relationship after cardiac surgery in children: noninvasive assessment by means of tissue Doppler imaging. J Thorac Cardiovasc Surg. 2006; 131:625-631 [DOI] [PubMed] [Google Scholar]
- 34.Gabel SA, Walker VR, London RE, Steenbergen C, Korach KS, Murphy E. Estrogen receptor beta mediates gender differences in ischemia/reperfusion injury. J Mol Cell Cardiol. 2005; 38:289-297 [DOI] [PubMed] [Google Scholar]
- 35.Karamlou T, Diggs BS, Person T, Ungerleider RM, Welke KF. National practice patterns for management of adult congenital heart disease: operation by pediatric heart surgeons decreases in‐hospital death. Circulation. 2008; 118:2345-2352 [DOI] [PubMed] [Google Scholar]
- 36.Zomer AC, Verheugt CL, Vaartjes I, Uiterwaal CS, Langemeijer MM, Koolbergen DR, Hazekamp MG, van Melle JP, Konings TC, Bellersen L, Grobbee DE, Mulder BJ. Surgery in adults with congenital heart disease. Circulation. 2011; 124:2195-2201 [DOI] [PubMed] [Google Scholar]
- 37.Johnson TR, Schamberger MS, Hart JC, Turrentine MW, Brown JW. After repair, atrioventricular valve regurgitation during cardiac extracorporeal membrane oxygenation predicts survival. Ann Thorac Surg. 2003; 76:848-852 [DOI] [PubMed] [Google Scholar]
- 38.Fermin DR, Barac A, Lee S, Polster SP, Hannenhalli S, Bergemann TL, Grindle S, Dyke DB, Pagani F, Miller LW, Tan S, Dos Remedios C, Cappola TP, Margulies KB, Hall JL. Sex and age dimorphism of myocardial gene expression in nonischemic human heart failure. Circ Cardiovasc Genet. 2008; 1:117-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vinocur J, Moller JH, Kochilas LK. Putting the pediatric cardiac care consortium in context: evaluation of scope and case mix compared to other reported surgical datasets. Circ Cardiovasc Qual Outcomes. 2012; 5:577-579 [DOI] [PubMed] [Google Scholar]
- 40.Akar F, Manavbasi Y, Parlar AI, Ulus AT, Katircioglu SF. The gender differences in the relaxation to levosimendan of human internal mammary artery. Cardiovasc Drugs Ther. 2007; 21:331-338 [DOI] [PubMed] [Google Scholar]
- 41.Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, Holtby HM, Cox PN, Smallhorn JF, Van Arsdell GS, Redington AN. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006; 47:2277-2282 [DOI] [PubMed] [Google Scholar]


