Abstract
Background
The utility of longitudinal, circumferential, and radial strain and strain rate in determining prognosis in chronic heart failure is not well established.
Methods and Results
In 416 patients with chronic systolic heart failure, we performed speckle‐tracking analyses of left ventricular longitudinal, circumferential, and radial strain and strain rate on archived echocardiography images (30 frames per second). Cox regression models were used to determine the associations between strain and strain rate and risk of all‐cause mortality, cardiac transplantation, and ventricular‐assist device placement. The area under the time‐dependent ROC curve (AUC) was also calculated at 1 year and 5 years. Over a maximum follow‐up of 8.9 years, there were 138 events (33.2%). In unadjusted models, all strain and strain rate parameters were associated with adverse outcomes (P<0.001). In multivariable models, all parameters with the exception of radial strain rate (P=0.11) remained independently associated, with patients in the lowest tertile of strain or strain rate parameter having a ≈2‐fold increased risk of adverse outcomes compared with the reference group (P<0.05). Addition of strain to ejection fraction (EF) led to a significantly improved AUC at 1 year (0.697 versus 0.633, P=0.032) and 5 years (0.700 versus 0.638, P=0.001). In contrast, strain rate did not provide incremental prognostic value to EF alone.
Conclusions
Longitudinal and circumferential strain and strain rate, and radial strain are associated with chronic heart failure prognosis. Strain provides incremental value to EF in the prediction of adverse outcomes, and with additional study may be a clinically relevant prognostic tool.
Keywords: echocardiography, heart failure, strain
Introduction
Heart failure (HF) is a major cause of morbidity and mortality in the United States, representing a leading cause of death and hospitalization. As such, accurate assessment of cardiac function is critical in order to gauge prognosis and guide therapy, particularly in chronic systolic HF. Left ventricular (LV) ejection fraction (EF) is an established predictor of adverse cardiovascular outcomes in these patients,1–2 but its prognostic utility across the full spectrum of HF is limited.3–5 As such, there is an important need to determine the value of novel quantitative imaging measures of cardiac function and mechanics in order to improve upon the identification of HF patients at increased risk of adverse outcomes.
Speckle‐tracking is an emerging technology that enables the noninvasive characterization of regional myocardial strain and strain rate in longitudinal, circumferential, and radial dimensions.6–7 Strain is defined as the change in length of a tissue normalized to its original length ([L−L0]/L0), and strain rate describes the rate at which this change occurs, representing the time‐derivative of strain. These measurements are independent of ultrasound scanline angle and are readily derived using semi‐automated methods.8 Longitudinal strain represents shortening and lengthening of the subendocardial and subepicardial myocardium, which is believed to be more sensitive to early changes such as those induced by subendocardial ischemia.7 Short‐axis function is a composite action of both longitudinal and circumferential fibers, and is characterized by fibers that thicken and thin in the radial direction, and shorten and lengthen in the circumferential direction. These measures are all highly quantitative and provide insight into regional and global cardiac function.
Previous work has shown global longitudinal and circumferential strain or strain rate to be a reproducible and important indicator of prognosis in patients specifically with acute systolic HF, postmyocardial infarction, and ischemic cardiomyopathy.9–12 However, the predictive value of strain and strain rate parameters in all 3 dimensions including longitudinal, circumferential, and radial, as well as the incremental value of these parameters beyond EF, is less well established across a broad spectrum of ambulatory patients with chronic heart failure. In addition, the relationships between strain and strain rate parameters and the relationships with echocardiographic measures of LV remodeling and function have not been fully characterized in chronic systolic HF.
The purpose of this study was to comprehensively evaluate the utility of strain and strain rate as a quantitative measure and risk predictor in a broad cohort of chronic heart failure. We carefully examined the relationships between strain and strain rate parameters and conventional measures of cardiac size and function (EF, LV mass, end‐diastolic volume [EDV], end‐systolic volume [ESV], end‐systolic elastance [Ees], effective arterial elastance [Ea], ventricular‐arterial coupling [Ea/Ees]). Furthermore, we hypothesized that strain analyses would provide incremental value in assessing prognosis beyond EF in this population.
Methods
Study Population
The Penn Heart Failure Study (PHFS) is a prospective cohort study of outpatients with primarily chronic systolic heart failure recruited from referral centers at the University of Pennsylvania (Philadelphia, PA), University of Wisconsin (Madison, WI), and Case Western (Cleveland, OH).13–14 The primary inclusion criterion was a clinical diagnosis of heart failure. Participants were excluded only if they had a non‐cardiac condition resulting in an expected mortality of <6 months as judged by the treating physician, or if they were unable or unwilling to provide informed consent. This substudy consisted of subjects recruited from the University of Pennsylvania between 2003 and 2009 that were representative of this clinical site.
At time of study entry, detailed clinical data were obtained using a standardized questionnaire administered to the patient and treating physician, with verification via medical records. Two‐dimensional (2D) transthoracic echocardiography was performed according to standard protocol and digitally archived in all patients at an ICAEL‐accredited laboratory typically within 60 days of study entry.
Follow‐up events including all‐cause mortality, cardiac transplantation, and ventricular assist device (VAD) placement were prospectively ascertained every 6 months through direct patient contact and verified through death certificates, medical records, and contact with patients' family members by dedicated research personnel.
All participants provided written, informed consent, and the PHFS protocol was approved by the Institutional Review Board.
Transthoracic Echocardiography
All 2D transthoracic echocardiograms were digitally archived according to a standardized protocol. Echocardiographic parameters including M‐mode, 2D, and Doppler images were subsequently analyzed by the core laboratory at the Hospital of the University of Pennsylvania using dedicated, commercially available software (TomTec Imaging Systems). Apical 4‐chamber LV end diastolic (EDV) and end systolic (ESV) volumes were obtained using Simpson's method of discs as recommended by the American Society of Echocardiography.15 LV mass was estimated at end‐diastole by digitizing the endocardial and epicardial surfaces of the LV short axis to obtain short axis myocardial areas. LV mass was calculated using the area‐length method (5/6 short axis myocardial area×LV cavity length×myocardial density (1.055)). LV volumes and mass were indexed to body surface area, which was determined using the Dubois formula (0.20247×height(m)0.725×weight(kg)0.425).
Stroke volume (SV) was measured using the difference between EDV and ESV. Ejection fraction was thus calculated as SV divided by the EDV. End systolic pressure (ESP) was estimated as 0.90 × systolic pressure, obtained by manual blood pressure (BP) cuff measurement.16 Effective arterial elastance (Ea) was defined as the ratio of ESP/SV.17 End systolic elastance (Ees) was determined using a modified single‐beat algorithm described by Chen et al using arm‐cuff pressures, stroke volumes (EDV‐ESV), and several timing intervals (isovolumic contraction time, pre‐ejection period, ejection time, total systolic period).16 We computed the Ea/Ees ratio, an index of ventricular‐vascular coupling. All measurements were made by research personnel blinded to patient characteristics and outcomes.
For end‐diastolic volumes, the intra‐observer coefficients of variation (CV) for this measurement in our core lab are 4.5% to 6.3%. The intra‐ and interobserver CV for Ees,sb are 8.2% and 9.8%, and for Ea are 7.9% and 8.6%, respectively.
Strain and Strain Rate Analysis
Longitudinal, circumferential, and radial strain and strain rate measurements were performed on digitally archived images using dedicated, commercially available software (TomTec Imaging System). The LV endocardial border was manually traced at the end‐systolic frame of 1 cardiac cycle from the parasternal short‐axis view at the midpapillary level and apical 4‐chamber view. In individual segments with poor tracking, the borders were manually readjusted. Peak longitudinal, circumferential, and radial strain and strain rate values were computed automatically. As per standard conventions, a greater degree of myocardial shortening is reflected by more negative longitudinal and circumferential strain and strain rate values and more positive radial strain and strain rate values.
Intra‐observer CV for longitudinal strain and strain rate are 10.5% and 9.9%, for circumferential strain and strain rate 13.0%, and for radial strain and strain rate 24.2% and 18.8%, respectively. Of the patients with analyzable volumes, 3% did not have analyzable longitudinal strain in the 4‐chamber view and 7.7% did not have analyzable radial or circumferential strain in the short‐axis view. The following criteria were used to exclude images from analyses secondary to inadequate image quality: (1) significant foreshortening of the 4‐chamber, ie, when the maximum length of the LV was not displayed or when the apex was out of sector; (2) lack of visualization of a substantial portion of the LV endocardium/myocardium due to undergaining or artifact; (3) oblique short axis views rather than circular, ie, when the LV minor axes were too dissimilar. All measurements were quantified from digital images archived at 30 frames per second and made by a single observer blinded to all other patient characteristics or outcomes.
Laboratory Analyses
N‐terminal pro‐hormone of brain natriuretic peptide (NT‐proBNP) was measured from banked plasma obtained at the time of study entry by a standard electrochemiluminesence immunoassay (Elecsys proBNP, Roche Diagnostics), as previously described.18 The assay range was 20 to 5000 pg/mL. The intra‐ and interassay CV were 2.9% and 6.1%, respectively.
Statistical Methods
Baseline characteristics were summarized for all participants using standard descriptive statistics. Echocardiographic parameters were evaluated according to New York Heart Association (NYHA) functional classification using Kruskal‐Wallis rank sum tests, and the relationship between echocardiographic parameters and NT‐proBNP was assessed using Spearman rank correlation. Cox regression models were used to determine the association of strain and strain rate parameters and EF with risk of the combined outcome of all‐cause death, cardiac transplantation, or VAD placement. To facilitate the comparison of associations across echocardiographic parameters, each parameter was categorized according to tertiles of its distribution. In our study, the first tertile represented better strain and strain rate values whereas the third tertile represented worse strain and strain rate values with respect to adverse outcomes. Adjustment variables were selected based on clinical rationale and included age, gender, race, heart failure etiology, height, weight, heart rate, estimated glomerular filtration rate (GFR), and medication use, including angiotensin converting enzyme inhibitors (ACE‐I), angiotensin receptor blockers (ARB), aldosterone antagonists, and beta‐blockers. Age exhibited nonproportional hazards and was adjusted for using a time‐varying covariate, which was obtained by interacting age with the natural log of time. We also explored the effects of EF on our multivariable association models.
Time‐dependent receiver operating characteristic (ROC) curves were used to quantify the ability of strain and strain rate parameters in combination with EF to discriminate participants with respect to the combined outcome.19 Cox regression models were used to determine a composite marker for EF, longitudinal strain, circumferential strain, and radial strain, and a composite marker for EF, longitudinal strain rate, circumferential strain rate, and radial strain rate.20 A leave‐one‐out jackknife approach was used such that the value of the composite marker for each participant was calculated as a weighted combination of ejection fraction and strain parameters, with weights determined by Cox regression coefficients, which were estimated from a model fit to the data for all other participants. The jackknife approach ameliorates the potential for bias when applying a prognostic score to the same dataset from which it was derived, and avoids arbitrarily splitting the data into derivation and validation cohorts. The area under the ROC curve (AUC) was calculated at 1 and 5 years to quantify both short‐ and long‐term prognostic accuracy. Bootstrap resampling was used to compute standard error estimates upon which to base confidence intervals for the AUC and 1‐sided Wald tests of whether the difference between the AUC for a composite marker and that for EF was equal to 0. Estimates of the AUC and the difference between 2 AUCs were obtained for each resampled dataset, and the standard deviation of the estimates across 1000 resampled datasets was used as the standard error. All analyses were completed using R 3.0.0 (R Development Core Team), including the survivalROC extension package.21
Results
Baseline Characteristics
Baseline clinical and echocardiographic characteristics of the 416 patients who comprised this subcohort analysis are summarized in Table 1. The median age was 56 years and the majority of the participants were male (61%) and Caucasian (72%). The etiology of heart failure was ischemic in 24% of patients, and 45% had NYHA class III or IV heart failure. The median value for LVEF was 26% (interquartile range [IQR] 20%, 36%), for EDV 115 mL/m2 (IQR 88, 150 mL/m2), and for ESV 85 mL/m2 (IQR 58, 118 mL/m2).
Table 1.
Baseline Characteristics; All Summaries Presented as Median (25th and 75th percentile) Unless Otherwise Noted as n (%)
| Entire Cohort (n=416) | |
|---|---|
| Demographic Characteristics | |
| Age, y | 56 (45, 65) |
| Male, n (%) | 254 (61) |
| Race, n (%) | |
| Caucasian | 301 (72) |
| African American | 98 (24) |
| Other | 17 (4) |
| Medical History and Risk Factors | |
| History of hypertension, n (%) | 207 (50) |
| History of diabetes, n (%) | 115 (28) |
| Tobacco use, n (%) | |
| Never | 171 (41) |
| Former | 211 (51) |
| Current | 34 (8) |
| Heart Failure Characteristics | |
| NYHA functional classification, n (%) | |
| I | 57 (14) |
| II | 170 (41) |
| III | 143 (34) |
| IV | 46 (11) |
| Ischemic etiology, n (%) | 101 (24) |
| Cardiac resynchronization therapy, n (%) | 97 (23) |
| Defibrillator, n (%) | 166 (40) |
| Medication Use | |
| ACE inhibitor or ARB, n (%) | 353 (85) |
| Aldosterone antagonist, n (%) | 152 (37) |
| Beta‐blocker, n (%) | 355 (85) |
| Diuretics, n (%) | 333 (80) |
| Clinical Measurements | |
| Body surface area, m2 | 2.0 (1.8, 2.2) |
| Systolic blood pressure, mm Hg | 108 (96, 124) |
| Diastolic blood pressure, mm Hg | 68 (60, 76) |
| Heart rate, beats/min | 74 (64, 84) |
| eGFR, mL/min per 1.73 m2 | 69 (51, 85) |
| Echocardiogram Measurements | |
| Longitudinal strain, % | −8.2 (−10.8, −5.7) |
| Circumferential strain, % | −11.1 (−16.8, −7.1) |
| Radial strain, % | 14.9 (9.4, 22.4) |
| Longitudinal strain rate, 1/s | −0.48 (−0.65, −0.34) |
| Circumferential strain rate, 1/s | −0.70 (−1.08, −0.49) |
| Radial strain rate, 1/s | 0.88 (0.58, 1.22) |
| Ejection fraction, % | 26 (20, 36) |
| LV end‐diastole volume/BSA, mL/m2 | 115 (88, 150) |
| LV end‐systole volume/BSA, mL/m2 | 85 (58, 118) |
| LV mass/BSA, g/m2 | 129 (104, 156) |
| Effective arterial elastance (Ea), mm Hg/mL | 1.63 (1.26, 2.12) |
| End systolic elastance (Eessb), mm Hg/mL | 0.85 (0.61, 1.27) |
| Ventricular‐arterial coupling (Ea/Eessb) | 1.92 (1.47, 2.50) |
ACE indicates angiotensin converting enzyme; ARB, angiotensin receptor blockers; BSA, body surface area; eGFR, estimated glomerular filtration rate; LV, left ventricular; NYHA, New York Heart Association.
The median values for longitudinal, circumferential, and radial strain were −8.2% (IQR −10.8%, −5.7%), −11.1% (IQR −16.8%, −7.1%), and 14.9% (IQR 9.4%, 22.4%), respectively. These were substantially worse compared with the mean reference values in normal, healthy controls, which were −19.7% (95% CI −20.4, −18.9) for longitudinal; −23.3% (95% CI −24.6, −22.1) for circumferential, and 47.3% (95% CI 43.6, 51) for radial.22–23 In our cohort, median values for strain rate were −0.48 1/s (IQR −0.65, −0.34 1/s) for longitudinal, −0.70 1/s (IQR −1.08, −0.49 1/s) for circumferential, and 0.88 1/s (IQR 0.58, 1.22 1/s) for radial.
The Relationships Between Strain, Strain Rate, and Parameters of Heart Failure Severity
Worsening of each individual strain and strain rate parameter was associated with higher NYHA class (P<0.001) and greater NT‐proBNP levels (P<0.001; Table 2). Strain and strain rate correlated strongly with LV volumes, but more modestly with LV mass (Figure 1). Interestingly, strain and strain rate were associated weakly with measures of load (Ea), and more strongly with measures of chamber elastance and contractility (Ees,sb). The correlations between these conventional measures of cardiac remodeling and function and strain and strain rate parameters were similar in all dimensions. In comparison, EF demonstrated strong correlations with LV volumes and mass, a modest correlation with arterial load (Ea), and lack of correlation with Ees,sb. Strain, strain rate, and EF all demonstrated moderate correlations with ventricular‐arterial coupling, as assessed by Ea/Ees,sb.
Table 2.
Summary Statistics for Echocardiography‐Derived Parameters by NYHA Functional Classification, Presented as Median (25th, 75th percentile), and Spearman Rank Correlation With NT‐proBNP
| Echocardiographic Parameter | NYHA Functional Classification* | Correlation With NT‐proBNP* (n=293) | |||
|---|---|---|---|---|---|
| I (n=57) | II (n=170) | III (n=143) | IV (n=46) | ||
| Longitudinal strain, % | −10.9 (−13.0, −9.1) | −8.8 (−11.3, −6.8) | −6.8 (−9.1, −4.9) | −6.1 (−7.8, −4.2) | 0.53 |
| Circumferential strain, % | −16.4 (−19.7, −12.4) | −12.3 (−17.5, −7.7) | −9.3 (−14.1, −6.4) | −7.7 (−9.5, −5.3) | 0.46 |
| Radial strain, % | 22.2 (16.8, 27.9) | 16.1 (11.4, 23.8) | 12.1 (7.9, 17.5) | 9.6 (5.5, 16.0) | −0.43 |
| Longitudinal SR, 1/s | −0.63 (−0.71, −0.51) | −0.51 (−0.70, −0.39) | −0.41 (−0.54, −0.32) | −0.39 (−0.55, −0.29) | 0.45 |
| Circumferential SR, 1/s | −1.00 (−1.36, −0.72) | −0.77 (−1.17, −0.51) | −0.61 (−0.87, −0.44) | −0.52 (−0.71, −0.41) | 0.44 |
| Radial SR, 1/s | 1.15 (0.84, 1.48) | 0.94 (0.64, 1.24) | 0.74 (0.52, 1.02) | 0.68 (0.44, 1.08) | −0.41 |
| Ejection fraction, % | 36 (27, 42) | 28 (22, 36) | 24 (18, 32) | 20 (18, 27) | −0.40 |
NT‐proBNP indicates N‐terminal pro‐hormone of brain natriuretic peptide; NYHA, New York Heart Association; SR, strain rate.
All P values from Kruskal‐Wallis rank sum test <0.001.
All P values <0.001.
Figure 1.
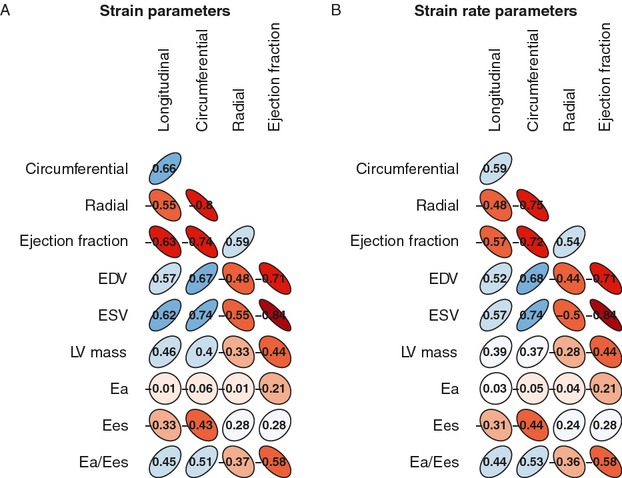
Spearman rank correlation between echocardiogram measurements: Relationships between quantitative parameters of cardiac size and function and strain and strain rate. Blue hue indicates positive correlation; red hue indicates negative correlation. Darker shading indicates more extreme correlations. Ea indicates effective arterial elastance; EDV, end‐diastolic volume; Ees, end‐systolic elastance; ESV, end‐systolic volume; LV, left ventricular.
Strain and Strain Rate as Predictors of Adverse Clinical Outcomes
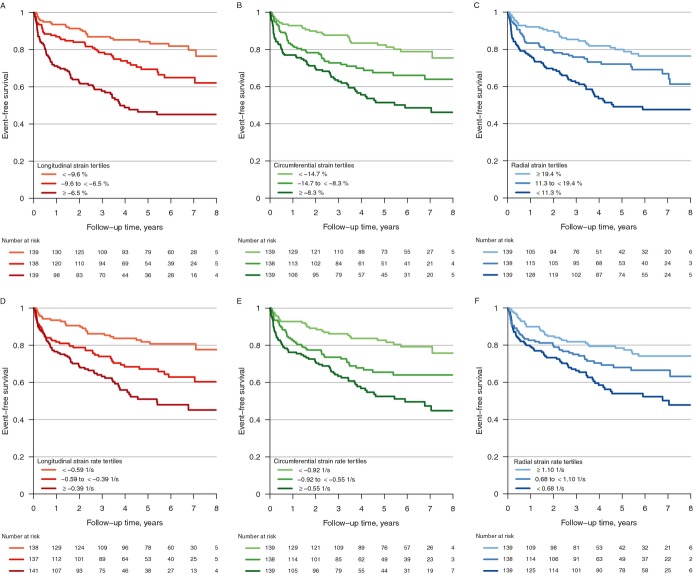
Over a maximum follow‐up of 8.9 years and median follow‐up of 4 years, there were 138 adverse events: 80 deaths, 46 transplants, and 12 VAD placements. In unadjusted models, all strain and strain rate parameters were associated with adverse clinical outcomes (Table 3). The likelihood of event‐free survival decreased steadily from the first to the third tertile for each parameter (Figure 2). In particular, patients in the lowest, or worst, tertile of longitudinal strain had a markedly increased risk of adverse outcomes compared with the patients in the highest tertile (HR=3.9, 95% CI 2.5 to 6.1, P<0.001). Minimally adjusted models considering age, gender, and race had no substantive impact on the significance of associations for all strain and strain rate parameters. In fully adjusted models, all parameters with the exception of radial strain rate (P=0.11) remained significantly associated with adverse outcomes. Patients in the lowest tertile of each strain or strain rate parameter had a ≈2‐fold increased risk of adverse outcomes compared with the reference group (P<0.05 for all). This was similar to the hazard ratio seen for the lowest tertile of EF when compared with the reference group. Consideration for degree of mitral regurgitation did not affect this association. Inclusion of EF in our models resulted in risk estimates that were attenuated, but tended in the same direction (Table 3).
Table 3.
Associations Between Echocardiography‐Derived Parameters and Risk of All‐Cause Mortality, Cardiac Transplantation, or Ventricular Assist Device Placement; Cut‐Points for Each Parameter Defined by Tertiles of its Distribution
| Echocardiographic Parameter | Unadjusted | Minimally Adjusted | Fully Adjusted | Fully Adjusted+LVEF | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Longitudinal Strain | <0.001 | <0.001 | 0.009 | 0.14 | ||||
| Tertile 1 | Referent | Referent | Referent | Referent | ||||
| <−9.6% | ||||||||
| Tertile 2 | 1.9 (1.2, 3.1) | 1.6 (0.94, 2.6) | 1.1 (0.64, 1.9) | 0.97 (0.55, 1.7) | ||||
| ≥−9.6% to <−6.5% | ||||||||
| Tertile 3 | 3.9 (2.5, 6.1) | 3.1 (1.9, 5.0) | 1.9 (1.1, 3.2) | 1.5 (0.79, 2.7) | ||||
| ≥−6.5% | ||||||||
| Circumferential Strain | <0.001 | <0.001 | 0.007 | 0.19 | ||||
| Tertile 1 | Referent | Referent | Referent | Referent | ||||
| <−14.7% | ||||||||
| Tertile 2 | 1.9 (1.2, 3.1) | 1.7 (1.1, 2.8) | 1.7 (1.0, 2.8) | 1.4 (0.81, 2.6) | ||||
| ≥−14.7% to <−8.3% | ||||||||
| Tertile 3 | 3.1 (2.0, 4.8) | 2.8 (1.8, 4.4) | 2.2 (1.4, 3.7) | 1.8 (0.95, 3.4) | ||||
| ≥−8.3% | ||||||||
| Radial Strain | <0.001 | <0.001 | 0.014 | 0.18 | ||||
| Tertile 1 | Referent | Referent | Referent | Referent | ||||
| ≥19.4% | ||||||||
| Tertile 2 | 1.6 (1.0, 2.6) | 1.7 (1.0, 2.8) | 1.5 (0.93, 2.5) | 1.3 (0.79, 2.3) | ||||
| ≥11.3% to <19.4% | ||||||||
| Tertile 3 | 2.9 (1.9, 4.4) | 2.7 (1.7, 4.2) | 2.0 (1.3, 3.3) | 1.6 (0.97, 2.8) | ||||
| <11.3% | ||||||||
| Longitudinal SR | <0.001 | <0.001 | 0.022 | 0.27 | ||||
| Tertile 1 | Referent | Referent | Referent | Referent | ||||
| <−0.59 1/s | ||||||||
| Tertile 2 | 2.1 (1.3, 3.4) | 1.9 (1.2, 3.1) | 1.8 (1.0, 3.0) | 1.5 (0.85, 2.6) | ||||
| ≥−0.59 to <−0.39 1/s | ||||||||
| Tertile 3 | 3.3 (2.1, 5.2) | 2.6 (1.6, 4.2) | 2.0 (1.2, 3.4) | 1.6 (0.90, 2.9) | ||||
| ≥−0.39 1/s | ||||||||
| Circumferential SR | <0.001 | <0.001 | 0.006 | 0.14 | ||||
| Tertile 1 | Referent | Referent | Referent | Referent | ||||
| <−0.92 1/s | ||||||||
| Tertile 2 | 1.9 (1.2, 3.1) | 1.8 (1.1, 2.9) | 1.9 (1.1, 3.2) | 1.6 (0.92, 2.9) | ||||
| ≥−0.92 to <−0.55 1/s | ||||||||
| Tertile 3 | 3.1 (1.9, 4.8) | 2.8 (1.8, 4.4) | 2.3 (1.4, 3.8) | 1.9 (1.0, 3.6) | ||||
| ≥−0.55 1/s | ||||||||
| Radial SR | <0.001 | 0.003 | 0.11 | 0.53 | ||||
| Tertile 1 | Referent | Referent | Referent | Referent | ||||
| ≥1.10 1/s | ||||||||
| Tertile 2 | 1.5 (0.95, 2.4) | 1.3 (0.82, 2.1) | 1.1 (0.69, 1.8) | 0.97 (0.58, 1.6) | ||||
| ≥0.68 to <1.10 1/s | ||||||||
| Tertile 3 | 2.3 (1.5, 3.5) | 2.1 (1.3, 3.2) | 1.6 (0.98, 2.5) | 1.2 (0.73, 2.0) | ||||
| <0.68 1/s | ||||||||
| Ejection Fraction | <0.001 | 0.003 | 0.031 | |||||
| Tertile 1 | Referent | Referent | Referent | |||||
| ≥32% | ||||||||
| Tertile 2 | 2.2 (1.4, 3.6) | 1.8 (1.1, 2.9) | 1.8 (1.0, 3.1) | |||||
| ≥22% to <32% | ||||||||
| Tertile 3 | 2.8 (1.7, 4.3) | 2.3 (1.4, 3.7) | 2.0 (1.2, 3.3) | |||||
| <22% | ||||||||
Minimally adjusted: Age (time‐varying), gender, race, heart failure etiology. Fully adjusted: Age (time‐varying), gender, race, heart failure etiology, height, weight, heart rate, estimated GFR, ACE inhibitor or ARB use, aldosterone antagonist use, beta‐blocker use. ACE indicates angiotensin converting enzyme; ARB, angiotensin receptor blockers; CI, confidence interval; GFR, glomerular filtration rate; HR, hazard ratio; LVEF, left ventricular ejection fraction.
Figure 2.
Kaplan‐Meier survival estimates for all‐cause death, cardiac transplantation, or ventricular assist device placement. Estimates according to tertiles of longitudinal strain (A); circumferential strain (B); radial strain (C); longitudinal strain rate (D); circumferential strain rate (E); and radial strain rate (F); all P<0.001 by log‐rank test.
We then sought to evaluate the additive ability of strain or strain rate to identify those patients who subsequently experienced an adverse outcome (cardiac transplantation, VAD placement, or death). Alone, EF exhibited modest predictive accuracy (AUC 0.633 [95% CI 0.561 to 0.706] at 1 year and 0.638 [95% CI 0.578 to 0.698] at 5 years). Addition of strain parameters to EF led to a significantly improved AUC at both 1 year (0.697 [95% CI 0.622 to 0.771], P=0.032 versus EF alone) and 5 years (0.700 [95% CI 0.636 to 0.764], P=0.014) (Figure 3). In contrast, strain rate parameters did not provide incremental value to EF at 1 year (0.666 [95% CI 0.588 to 0.744], P=0.16 versus EF alone) or at 5 years (0.668 [95% CI 0.602 to 0.734], P=0.13).
Figure 3.
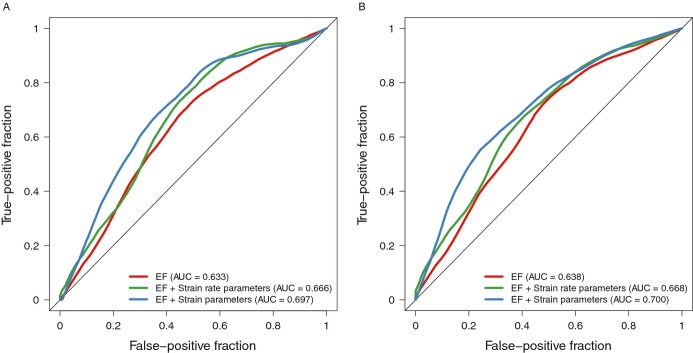
Receiver operating characteristic (ROC) curves. In comparison to EF alone, strain and EF demonstrated an improved AUC at both 1 year (0.697 versus 0.633, P=0.032) (A) and 5 years (0.700 versus 0.638, P=0.014) (B). In comparison to EF alone, strain rate and EF did not provide incremental value (0.666 versus 0.633, P=0.16) at 1 year (A) and 5 years (0.668 versus 0.638, P=0.13) (B). AUC indicates area under the ROC curve; EF, ejection fraction.
Discussion
Our objectives in this study were to define the relationships between strain and strain rate parameters and conventional echocardiographic measures of cardiac size, function, and ventricular‐arterial coupling, and to assess the prognostic utility of longitudinal, circumferential, and radial strain and strain rate. In a diverse cohort of 416 patients with chronic systolic heart failure, worse strain and strain rate correlated strongly with higher NYHA class, greater NT‐proBNP levels, and increased chamber size. Strain in all 3 dimensions (longitudinal, circumferential, and radial) and strain rate in 2 dimensions (longitudinal and circumferential) were independently associated with an increased risk of adverse cardiovascular outcomes, in our multivariable models adjusted for clinical variables. Furthermore, strain in combination with EF added incremental value in the prediction of adverse clinical outcomes while strain rate did not. These findings provide further clarity to the relationships between myocardial deformation parameters and clinical outcomes in chronic heart failure.
Prior studies in patients with acute heart failure or postmyocardial infarction have shown global longitudinal strain and strain rate and global circumferential strain and strain rate to be prognostic of adverse outcomes.10–11 The VALIANT study included 603 patients who were postmyocardial infarction with LV dysfunction or heart failure and demonstrated independent associations between longitudinal and circumferential strain and strain rate with adverse outcomes.10 However, only longitudinal strain rate provided incremental value to the prediction of all‐cause mortality. We postulate that the differences in study population (acute myocardial infarction), analytic approach (whereby the incremental value of each strain parameter was evaluated individually), and duration of follow‐up time (20 months) used in their study may account for the discrepancies with our findings. Motoki, et al studied a cohort of 194 outpatients with chronic systolic heart failure and found global longitudinal strain, but not global circumferential strain, to be prognostic of adverse events with incremental predictive value over the EF.9 Again, the inconsistencies with our findings may be explained by differences in study populations including cohort size (416 versus 194 patients), heart failure etiology (24% versus 41% ischemic), and duration of follow‐up time (maximum 8.9 versus 5 years).
To our knowledge, this is the first study to evaluate radial, in conjunction with longitudinal and circumferential, strain and strain rate in patients with systolic heart failure. Our results indicate that parameters in the radial dimension had greater CVs and were least robustly associated with outcomes. This is consistent with reports in animal and human models, which have shown measures in the radial dimension to have greater CVs24 and be less sensitive than those in the longitudinal or circumferential dimension for the detection of myocardial ischemia.25–26 The poor reliability of radial speckle tracking has been postulated to be secondary to: (1) a smaller area of analyses; (2) poor tracking of the endocardial border secondary to respiratory artifacts; (3) larger deformation in the radial direction which results in a greater degree of variability. It may be that radial thickening, secondary to cross fiber shortening of muscle fibers oriented in orthogonal direction at the inner and outer surfaces of the myocardium, is more directly assessed by longitudinal or circumferential strain.27
Interestingly, we found longitudinal and circumferential strain and strain rate to be highly correlated with end systolic elastance (Ees,sb), suggesting that these measures reflect chamber elastance and contractility. We also found that strain and strain rate correlated poorly with Ea, an index of total arterial load. This finding provides human data to support the hypothesis that strain may be a less load‐dependent metric, in comparison to other measures of cardiac function such as EF,28 confirming the potential additive value of strain to routine EF assessment. EF correlated weakly with Ees,sb, and strongly with arterial load (Ea) providing additional data to support our understanding of EF to be a highly load‐dependent measure that provides limited insight into contractile function. However, we note that Ea evaluates largely resistive load (ie, systemic vascular resistance) rather than pulsatile arterial load,29 and these findings represent cross‐sectional analyses rather than intervention experiments. Prior to concluding that strain is truly load independent, additional measures of pulsatile load should be evaluated and acute‐loading experiments should be performed.
Our study raises important points regarding the utility of strain in the management of chronic heart failure. Strain is an easily derived and reproducible measure that provides added value to existing tests, such as EF, and may also provide insight into the pathophysiology and mechanics of heart failure. We do note that in our multivariable association models, addition of EF to strain or strain rate yielded risk estimates that tended in the same direction, but the significance of these relationships was attenuated. We note that the lack of association between strain and strain rate, when adjusted for EF, does not detract from our findings and overall conclusions regarding the incremental value of these parameters. Indeed, in our discrimination/prediction models, inclusion of strain to EF yielded a statistically significant increase in the AUC. They may suggest that the association between strain and strain rate and clinical outcomes is not independent of EF. However, this lack of significance may also be related statistically to colinearity.
As such, this present study has broad and highly relevant implications. Clinicians and researchers should be aware that strain can be used to identify high‐risk patients and, with additional study, could be used to also guide chronic heart failure management. Strain could also be used as part of the selection criteria for specific advanced therapies or as a sensitive cardiac endpoint in future heart failure clinical studies.
We acknowledge several limitations to our study. Of note, the LV was foreshortened in the apical 2‐chamber view, limiting the accuracy of longitudinal strain from this view in many of our studies. However, we observed close correlation between apical 4‐chamber and apical 2‐chamber measurements of longitudinal strain and strain rate for those images that could be traced (R=0.74, P<0.001). All images analyzed were archived at 30 frames per second, potentially limiting the assessment of strain and in particular, strain rate. It is possible that the predictive value of strain rate could have been improved with analyses of images archived at higher temporal resolution. Furthermore, we did not have measures of diastolic strain or strain rate, but detailed characterization of sensitive measures of diastolic function are additional measures that will be explored in future studies. As the goal of our study was to compare the incremental value of strain and strain rate to EF alone, and not to create a risk prediction model based upon multiple echocardiography parameters, we did not evaluate the utility of strain and strain rate in comparison to additional echocardiography measures that may also have had prognostic significance. Although we used a jackknife approach to avoid the potential for bias when applying a prognostic score to the same dataset from which it was derived, we were unable to perform independent validation of our findings on a prospective sample. Finally, interventional and invasive studies are needed to truly determine the load dependence or independence of these measures as well as the correlation between strain and strain rate and contractility.
In conclusion, longitudinal and circumferential strain and strain rate, and radial strain are significantly associated with prognosis in chronic systolic heart failure. Strain, but not strain rate, adds incremental value to EF in the prediction of adverse outcomes. These data suggest that comprehensive evaluation of 2D strain in HF patients provides additive prognostic information and may have a role in improving risk stratification in chronic systolic heart failure.
Sources of Funding
Dr Ky was supported by the NIH/Clinical and Translational Science Award KL1 RR024132, NIH K23 HL095661‐01, and the Heart Failure Society of America Research Fellowship Award. This work was also supported by NIH HL088577 (Dr Cappola).
Disclosures
None.
References
- 1.McDermott MM, Feinglass J, Lee PI, Mehta S, Schmitt B, Lefevre F, Gheorghiade M. Systolic function, readmission rates, and survival among consecutively hospitalized patients with congestive heart failure. Am Heart J. 1997; 134:728-736 [DOI] [PubMed] [Google Scholar]
- 2.St John Sutton M, Pfeffer MA, Moye L, Plappert T, Rouleau JL, Lamas G, Rouleau J, Parker JO, Arnold MO, Sussex B, Braunwald E. Cardiovascular death and left ventricular remodeling two years after myocardial infarction: baseline predictors and impact of long‐term use of captopril: information from the survival and ventricular enlargement (SAVE) trial. Circulation. 1997; 96:3294-3299 [DOI] [PubMed] [Google Scholar]
- 3.Curtis JP, Sokol SI, Wang Y, Rathore SS, Ko DT, Jadbabaie F, Portnay EL, Marshalko SJ, Radford MJ, Krumholz HM. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol. 2003; 42:736-742 [DOI] [PubMed] [Google Scholar]
- 4.Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, Cushman M, Polak J, Gardin JM, Gersh BJ, Aurigemma GP, Manolio TA. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002; 137:631-639 [DOI] [PubMed] [Google Scholar]
- 5.Kelly TL, Cremo R, Nielsen C, Shabetai R. Prediction of outcome in late‐stage cardiomyopathy. Am Heart J. 1990; 119:1111-1121 [DOI] [PubMed] [Google Scholar]
- 6.Marwick TH. Measurement of strain and strain rate by echocardiography: ready for prime time? J Am Coll Cardiol. 2006; 47:1313-1327 [DOI] [PubMed] [Google Scholar]
- 7.Mor‐Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU, Zamorano JL. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr. 2011; 12:167-205 [DOI] [PubMed] [Google Scholar]
- 8.Abraham TP, Dimaano VL, Liang HY. Role of tissue Doppler and strain echocardiography in current clinical practice. Circulation. 2007; 116:2597-2609 [DOI] [PubMed] [Google Scholar]
- 9.Motoki H, Borowski AG, Shrestha K, Troughton RW, Tang WH, Thomas JD, Klein AL. Incremental prognostic value of assessing left ventricular myocardial mechanics in patients with chronic systolic heart failure. J Am Coll Cardiol. 2012; 60:2074-2081 [DOI] [PubMed] [Google Scholar]
- 10.Hung CL, Verma A, Uno H, Shin SH, Bourgoun M, Hassanein AH, McMurray JJ, Velazquez EJ, Kober L, Pfeffer MA, Solomon SDVALIANT investigators Longitudinal and circumferential strain rate, left ventricular remodeling, and prognosis after myocardial infarction. J Am Coll Cardiol. 2010; 56:1812-1822 [DOI] [PubMed] [Google Scholar]
- 11.Cho GY, Marwick TH, Kim HS, Kim MK, Hong KS, Oh DJ. Global 2‐dimensional strain as a new prognosticator in patients with heart failure. J Am Coll Cardiol. 2009; 54:618-624 [DOI] [PubMed] [Google Scholar]
- 12.Bertini M, Ng AC, Antoni ML, Nucifora G, Ewe SH, Auger D, Marsan NA, Schalij MJ, Bax JJ, Delgado V. Global longitudinal strain predicts long‐term survival in patients with chronic ischemic cardiomyopathy. Circ Cardiovasc Imaging. 2012; 5:383-391 [DOI] [PubMed] [Google Scholar]
- 13.Ky B, Kimmel SE, Safa RN, Putt ME, Sweitzer NK, Fang JC, Sawyer DB, Cappola TP. Neuregulin‐1 beta is associated with disease severity and adverse outcomes in chronic heart failure. Circulation. 2009; 120:310-317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cappola TP, Matkovich SJ, Wang W, van Booven D, Li M, Wang X, Qu L, Sweitzer NK, Fang JC, Reilly MP, Hakonarson H, Nerbonne JM, Dorn GW., II Loss‐of‐function DNA sequence variant in the CLCNKA chloride channel implicates the cardio‐renal axis in interindividual heart failure risk variation. Proc Natl Acad Sci USA. 2011; 108:2456-2461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJChamber Quantification Writing Group, American Society of Echocardiography's Guidelines and Standards Committee, European Association of Echocardiography Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18:1440-1463 [DOI] [PubMed] [Google Scholar]
- 16.Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single‐beat determination of left ventricular end‐systolic elastance in humans. J Am Coll Cardiol. 2001; 38:2028-2034 [DOI] [PubMed] [Google Scholar]
- 17.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular‐vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007; 115:1982-1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ky B, French B, McCloskey K, Rame JE, McIntosh E, Shahi P, Dries DL, Tang WH, Wu AH, Fang JC, Boxer R, Sweitzer NK, Levy WC, Goldberg LR, Jessup M, Cappola TP. High‐sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011; 4:180-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heagerty PJ, Lumley T, Pepe MS. Time‐dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000; 56:337-344 [DOI] [PubMed] [Google Scholar]
- 20.French B, Saha‐Chaudhuri P, Ky B, Cappola TP, Heagerty PJ. Development and evaluation of multi‐marker risk scores for clinical prognosis. Stat Methods Med Res. 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heagerty P, Saha‐Chaudhuri P. SurvivalROC: Time‐dependent ROC curve estimation from censored survival data. 2013; R package version 1.0.3.
- 22.Manovel A, Dawson D, Smith B, Nihoyannopoulos P. Assessment of left ventricular function by different speckle‐tracking software. Eur J Echocardiogr. 2010; 11:417-421 [DOI] [PubMed] [Google Scholar]
- 23.Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH. Normal ranges of left ventricular strain: a meta‐analysis. J Am Soc Echocardiogr. 2013; 26:185-191 [DOI] [PubMed] [Google Scholar]
- 24.Koopman LP, Slorach C, Manlhiot C, McCrindle BW, Jaeggi ET, Mertens L, Friedberg MK. Assessment of myocardial deformation in children using digital imaging and communications in medicine (DICOM) data and vendor independent speckle tracking software. J Am Soc Echocardiogr. 2011; 24:37-44 [DOI] [PubMed] [Google Scholar]
- 25.Reant P, Labrousse L, Lafitte S, Bordachar P, Pillois X, Tariosse L, Bonoron‐Adele S, Padois P, Deville C, Roudaut R, Dos Santos P. Experimental validation of circumferential, longitudinal, and radial 2‐dimensional strain during dobutamine stress echocardiography in ischemic conditions. J Am Coll Cardiol. 2008; 51:149-157 [DOI] [PubMed] [Google Scholar]
- 26.Chan J, Hanekom L, Wong C, Leano R, Cho GY, Marwick TH. Differentiation of subendocardial and transmural infarction using two‐dimensional strain rate imaging to assess short‐axis and long‐axis myocardial function. J Am Coll Cardiol. 2006; 48:2026-2033 [DOI] [PubMed] [Google Scholar]
- 27.Rademakers FE, Rogers WJ, Guier WH, Hutchins GM, Siu CO, Weisfeldt ML, Weiss JL, Shapiro EP. Relation of regional cross‐fiber shortening to wall thickening in the intact heart. Three‐dimensional strain analysis by NMR tagging. Circulation. 1994; 89:1174-1182 [DOI] [PubMed] [Google Scholar]
- 28.Greenberg NL, Firstenberg MS, Castro PL, Main M, Travaglini A, Odabashian JA, Drinko JK, Rodriguez LL, Thomas JD, Garcia MJ. Doppler‐derived myocardial systolic strain rate is a strong index of left ventricular contractility. Circulation. 2002; 105:99-105 [DOI] [PubMed] [Google Scholar]
- 29.Segers P, Stergiopulos N, Westerhof N. Relation of effective arterial elastance to arterial system properties. Am J Physiol Heart Circ Physiol. 2002; 282:H1041-H1046 [DOI] [PubMed] [Google Scholar]