Abstract
Background
New‐onset atrial fibrillation (AF) is reported to increase the risk of death in myocardial infarction (MI) patients. However, previous studies have reported conflicting results and no data exist to explain the underlying cause of higher death rates in these patients.
Methods and Results
All patients with first acute MI between 1997 and 2009 in Denmark, without prior AF, were identified from Danish nationwide administrative registers. The impact of new‐onset AF on all‐cause mortality, cardiovascular death, fatal/nonfatal stroke, fatal/nonfatal re‐infarction and noncardiovascular death, were analyzed by multiple time‐dependent Cox models and additionally in propensity score matched analysis. In 89 703 patients with an average follow‐up of 5.0±3.5 years event rates were higher in patients developing AF (n=10 708) versus those staying in sinus‐rhythm (n=78 992): all‐cause mortality 173.9 versus 69.4 per 1000 person‐years, cardiovascular death 137.2 versus 50.0 per 1000 person‐years, fatal/nonfatal stroke 19.6/19.9 versus 6.2/5.6 per 1000 person‐years, fatal/nonfatal re‐infarction 29.0/60.7 versus 14.2/37.9 per 1000 person‐years. In time‐dependent multiple Cox analyses, new‐onset AF remained predictive of increased all‐cause mortality (HR: 1.9 [95% CI: 1.8 to 2.0]), cardiovascular death (HR: 2.1 [2.0 to 2.2]), fatal/nonfatal stroke (HR: 2.3 [2.1 to 2.6]/HR: 2.5 [2.2 to 2.7]), fatal/nonfatal re‐infarction (HR: 1.7 [1.6 to 1.8]/HR: 1.8 [1.7 to 1.9]), and non‐ cardiovascular death (HR: 1.4 [1.3 to 1.5]) all P<0.001). Propensity‐score matched analyses yielded nearly identical results (all P<0.001).
Conclusions
New‐onset AF after first‐time MI is associated with increased mortality, which is largely explained by more cardiovascular deaths. Focus on the prognostic impact of post‐infarct AF is warranted.
Keywords: cardiovascular mortality, mortality, myocardial infarction, new‐onset atrial fibrillation, re‐myocardial infarction, stroke
Introduction
New‐onset atrial fibrillation (AF) is a common complication in post‐myocardial infarction (MI) patients, which even in its paroxysmal stage independently predicts adverse outcome.1–4 This fact indicates that AF in post‐MI patients is an ominous sign, which should be prevented and promptly treated if detected. However, the prediction of new‐onset AF is difficult, because the mechanisms that promote the development of AF in the MI setting are complex and multifactorial, and our understanding of the pathophysiology remains incomplete.5–7 Similarly, and despite its frequent occurrence, very limited data exist on whether there are differences in competing causes of death in MI patients with AF versus those without AF,5–7 and very few have performed analysis with AF as a time‐dependent variable on long‐time follow‐up.3 Moreover, only 1 trial has investigated the impact of AF on re‐MI in post‐MI patients.8
Therefore, the aims of this study were first to examine whether new‐onset AF was predictive of all‐cause mortality in first‐time MI patients and whether this could be explained by an increase in cardiovascular mortality; and second, to study the general causes of new‐onset AF‐related death, including risks of dying from stroke or re‐infarction and noncardiovascular death during long‐time follow‐up in a large nationwide cohort of patients discharged after first‐time MI in Denmark.
Methods
Since 1978, the Danish National Patient Registry has registered all hospital admissions as well as diagnoses obtained in outpatient clinics in Denmark. Each admission is registered with 1 primary diagnosis and 1 or more secondary diagnoses according to the International Classification of Diseases (ICD), until 1994 the ICD‐8 and from 1994 the ICD‐10. Since 1995, the Danish Registry of Medicinal Product Statistics (national prescription registry) has kept records of every drug prescription dispensed from pharmacies in Denmark. Each medication is classified by the international Anatomical Therapeutical Chemical system, and the registry also includes information about the date of dispensing, formulation, strength, and quantity dispensed. Information on patients' vital status (dead or alive) was obtained from the civil registration system through Statistics Denmark. In Denmark, every resident is provided with a permanent and unique civil registration number that enables linkage between these administrative registries.
Population
From the National Patient Registry we identified all patients, aged ≥30 years, hospitalized with acute MI (ICD‐10 code I21 to I22) for the first time between 1997 and 2009. The diagnosis of acute MI and endpoints has been validated in the National Patient Registry, with a sensitivity of 91% and predictive value of 93%.9 A detailed description of patient selection has been published previously.10 All patients who were alive at discharge were included in the present study. The primary outcome was fatal and nonfatal stroke, fatal and nonfatal re‐infarction or cardiovascular death, noncardiovascular death and finally all‐cause mortality in respect to new‐onset AF following first‐time MI. Patients with a history of AF were excluded.
Atrial Fibrillation Ascertainment
Patients with atrial flutter were considered to have AF. From the National Patient Registry we identified all patients with new‐onset AF and atrial flutter (ICD‐10 code 427.93, 427.94, I48) between 1997 and 2009. The AF diagnosis in the registry was based on admission for AF or if AF was recorded during hospital admission for other disease. All AF episodes, whether permanent or paroxysmal, were verified on a 12‐lead electrocardiogram. The diagnosis AF has previous been validated, with a specificity of 99%.11
Drug Use
Since 1995, the Danish Registry of Medicinal Product Statistics has registered all prescriptions dispensed from Danish pharmacies. In Denmark the national health security system covers all inhabitants and partially reimburses drug expenses (with a maximum copayment for each individual of ≈US$ 500 a year). Therefore, pharmacies are required to register all prescriptions dispensed, which ensure complete registration, and the reimbursement results in minimal incentive for patients to obtain medication through other sources. Coding is done according to the Anatomic Therapeutical Chemical (ATC) system. The registry includes information about date of dispensing, strength and formulation, quantity dispensed, and the affiliation of the doctor who issues the prescription.
Comorbidity
Comorbidity was defined from diagnoses at discharge from index MI as specified in the Ontario acute MI mortality prediction rule.12 The comorbidity index was further enhanced by adding diagnoses from 1 year before the event, as done by Rasmussen et al13 Diagnoses used in the comorbidity index are shown in Table 1.
Table 1.
Baseline Characteristics
| Characteristic | n=89 412 |
|---|---|
| Age, y | 67.6±13 |
| Male, n (%) | 57 499 (64.1) |
| Medical Condition | |
| Cerebral vascular disease, n (%) | 3737 (4.2) |
| Pulmonary vascular disease, n (%) | 1293 (1.4) |
| Cancer, n (%) | 1650 (1.8) |
| Cardiac arrhythmias, n (%) | 2854 (3.2) |
| Acute renal failure, n (%) | 684 (0.8) |
| Chronic renal failure, n (%) | 1079 (1.5) |
| Diabetes with complications, n (%) | 3905 (4.4) |
| Pulmonary oedema, n (%) | 863 (1.0) |
| Shock, n (%) | 809 (0.9) |
| Peptic ulcer, n (%) | 1258 (1.4) |
| Prescribed Drugs | |
| Beta blocker, n (%) | 66 734 (74.4) |
| Ace‐inhibitor, n (%) | 40 065 (44.7) |
| Acetylsalicylacid, n (%) | 51 653 (57.6) |
| Clopidogrel, n (%) | 40 383 (45.0) |
| Antidiabetic drugs, n (%) | 10 769 (12.0) |
| Loop diuretics, n (%) | 32 906 (36.7) |
| Digitalis, n (%) | 6220 (6.9) |
| Statins, n (%) | 56.044 (62.5) |
Statistical Analysis
Descriptive data are reported as mean±standard deviation (SD) or frequencies expressed as percentages. The predictive value of new‐onset AF was assessed using the Cox regression model with delayed entry (ie, counting the time from new‐onset AF to censoring or event, provided that the analyzed end point had not occurred before new‐onset AF) with adjustment for age, sex, calendar year, re‐infarction, concomitant pharmacotherapy, and comorbidity.14 To deal with the time‐to‐event bias in the new‐onset AF patients we made Landmark analysis with yearly landmarks including Cox multiple analysis on all the fatal end points.15–16 To analyze the impact of age on the outcome we made 2 additional multiple Cox models with yearly landmarks; first a with fixed age adjustment, and second with increasing age adjustment. Proportional‐hazard assumptions, linearity of continuous variables and lack of interactions were tested for each model. We calculated a propensity score for the likelihood of developing new‐onset AF following first‐time MI by a multiple Cox regression model using baseline covariates. Using the Greedy matching macro (http://mayoresearch.mayo.edu/mayo/research/biostat/upload/gmatch.sas), we matched each new‐onset AF case to 1 AF‐free control from the risk set at the time of AF on the basis of the linear predictor from the propensity score Cox model and year of AF‐onset. Matched pairs were then analyzed in a Cox regression model with delayed entry and with AF as the only covariate. Cumulative incidence of all‐cause mortality as a function of incident new‐onset AF from the Landmark analysis was plotted. The hazard ratios and confidence interval from each Landmark were plotted in a forest plot. Interaction was tested among the Landmarks to test whether there was a difference in outcome in subsequent years after discharge from MI. Finally, the cumulative incidence of AF was calculated accounting for the competing risk of death. A 2‐tailed P value <0.05 was regarded as statistically significant in all calculations. SAS statistical software package version 9.1 for UNIX servers (SAS Institute Inc) was used for statistical analysis.
Ethics
The Danish Data Protection Agency has approved this study (ref. 2007‐58‐0015, int. ref: GEH‐2010‐001), and data at the individual level were made available in a manner by which specific individuals could not be identified. Retrospective register studies do not require ethical approval in Denmark. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
In all, 89 703 post‐MI patients with average follow‐up of 5.0±3.5 years were identified. During the course of the study, 10 708 (11.9% or 23.9 per 1000 person‐years) patients developed AF after discharge from the first‐MI hospitalization and 78 992 (88.1%) remained in sinus rhythm (SR). The incidence of new‐onset AF decreased each year after discharge from first time MI (Figure 1). A total of 29 695 (33.1%) patients received percutaneous coronary intervention. A detailed description of baseline characteristics of the study population and distributions are given in Table 1. Table 2 shows how the covariables affected the rate of AF.
Figure 1.
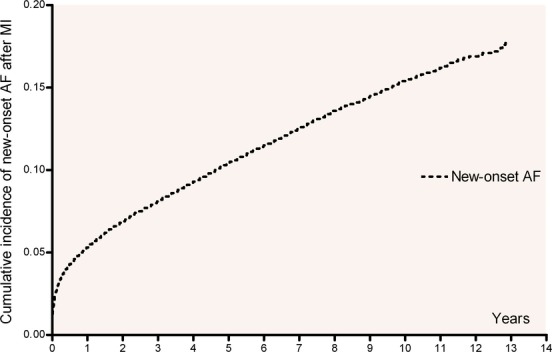
Cumulative incidence of new‐onset AF after myocardial infarction with death as competing event. AF indicates atrial fibrillation; MI, myocardial infarction.
Table 2.
Co‐Variables Contribution to Atrial Fibrillation*
| Characteristic | Hazard Ratio | Confidence Interval | P Value |
|---|---|---|---|
| Age | 1.041 | 1.039 to 1.043 | <0.001 |
| Male gender | 0.949 | 0.906 to 0.983 | <0.001 |
| Medical Condition | |||
| Cerebral vascular disease | 0.930 | 0.846 to 1.022 | 0.196 |
| Pulmonary vascular disease | 1.065 | 0.914 to 1.240 | 0.184 |
| Cancer | 0.975 | 0.836 to 1.137 | 0.855 |
| Cardiac arrhythmias | 1.532 | 1.408 to 1.667 | <0.001 |
| Acute renal failure | 1.291 | 1.054 to 1.582 | 0.006 |
| Chronic renal failure | 1.282 | 1.027 to 1.512 | 0.002 |
| Diabetes with complications | 0.971 | 0.873 to 1.080 | 0.759 |
| Pulmonary oedema | 1.054 | 0.890 to 1.249 | 0.585 |
| Shock | 1.003 | 0.828 to 1.216 | 0.995 |
| Peptic ulcer | 1.102 | 0.949 to 1.280 | 0.199 |
| Prescribed Drugs | |||
| Beta blocker | 0.943 | 0.902 to 0.985 | 0.038 |
| Ace‐inhibitor | 1.027 | 0.987 to 1.070 | 0.011 |
| Acetylsalicylacid | 0.925 | 0.888 to 0.964 | <0.001 |
| Clopidogrel | 0.889 | 0.844 to 0.936 | 0.001 |
| Antidiabetic drugs | 1.082 | 1.015 to 1.154 | 0.003 |
| Loop diuretics | 1.470 | 1.408 to 1.535 | <0.001 |
| Digitalis | 2.867 | 0.851 to 0.935 | <0.001 |
| Statins | 0.892 | 0.851 to 0.935 | 0.002 |
Based on results from propensity score from Cox multiple regression analysis.
All‐Cause Mortality After New‐Onset Atrial Fibrillation
All‐cause mortality occurred in 5423 (50.6% or 173.9 per 1000 person‐years) versus 26 885 (34.0% or 69.4 per 1000 person‐years) among patients with and without new‐onset AF, respectively (Figure 2). In a multiple Cox model, with new‐onset AF inserted as a time‐dependent variable, adjusted for age, gender, year, concomitant medical treatment, and comorbidity there was a significant increased all‐cause mortality (HR: 1.89 [95% CI: 1.84 to 1.95], P<0.001) in patients with new‐onset AF. The propensity‐score matched analysis yielded nearly identical results (HR: 1.22 [95% CI: 1.17 to 1.27], P<0.001).
Figure 2.
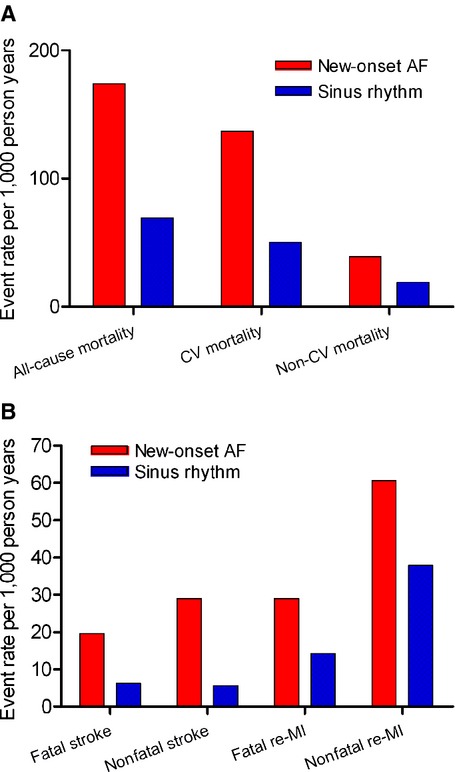
Incidence of end points among patients with and without new‐onset atrial fibrillation. A, Fatal end points. B, Nonfatal end points. AF indicates atrial fibrillation; CV, cardiovascular; MI, myocardial infarction.
To address survival bias, Landmark analysis with yearly Landmarks was performed. This showed persistent results (Figures 3 and 4A). In addition, the Landmark analysis revealed an increasing risk of mortality during subsequent Landmarks (P for interaction <0.001, Figure 4A). However, this association was no longer seen when adjusting each Landmark to increasing age (Figure 4B).
Figure 3.
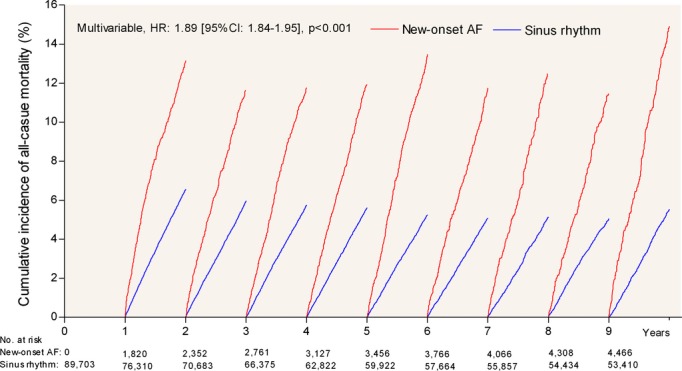
Landmark analysis freedom from all‐cause mortality with new‐onset atrial fibrillation after myocardial infarction compared with remaining in sinus rhythm. AF indicates atrial fibrillation; CI, confidence interval; HR, hazard ratio.
Figure 4.
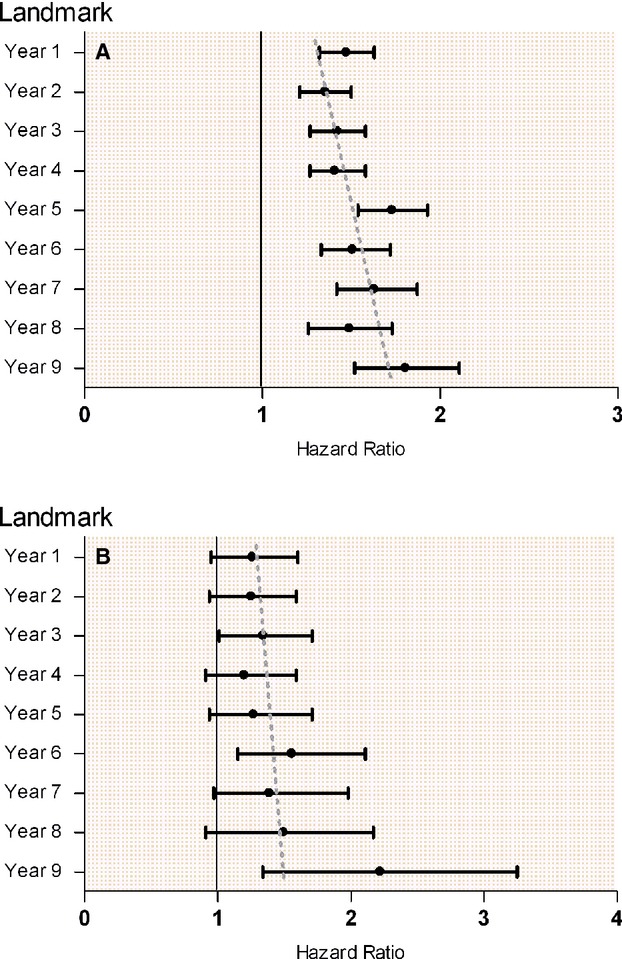
Subsequently increased all‐cause mortality using yearly landmarks and multiple Cox. A, With fixed age adjustment. B, With increasing age adjustment.
Cardiovascular Mortality After New‐Onset Atrial Fibrillation
Cardiovascular mortality was registered in 4277 (39.9% or 137.2 per 1000 person‐years) versus 19 383 (24.5% or 50.0 per 1000 person‐years) patients with and without new‐onset AF, respectively (Figure 2). In multiple Cox models, with new‐onset AF inserted as a time‐dependent variable, adjusted for age, gender, year, concomitant medical treatment, and comorbidity there was a significant increased cardiovascular mortality (HR: 2.09 [95% CI: 2.01 to 2.16], P<0.001) in patients with new‐onset AF. The propensity‐score matched analysis yielded nearly identical results (HR: 1.24 [95% CI: 1.24 to 1.36], P<0.001).
Fatal Stroke After New‐Onset of Atrial Fibrillation
Fatal stroke was registered in 610 (5.7% or 19.6 per 1000 person‐years) versus 2414 (3.1% or 6.2 per 1000 person‐years) among patients with and without new‐onset AF, respectively (Figure 2). Nonfatal stroke was registered in 606 (5.6% or 19.9 per 1000 person‐years) versus 2172 (0.3% or 5.6 per 1000 person‐years) among patients with and without new‐onset AF, respectively (Figure 2). In multiple Cox model, with new‐onset AF inserted as a time‐dependent variable, adjusted for age, gender, year, concomitant medical treatment and comorbidity; there was a significant increased risk of both fatal and nonfatal stroke after new‐onset AF (HR: 2.34 [95% CI: 2.12 to 2.57], P<0.001 and HR: 2.47 [95% CI: 2.24 to 2.73], P<0.001 respectively). Propensity‐score matched analysis on fatal‐stroke yielded nearly identical results.
Fatal Re‐Infarction After New‐Onset Atrial Fibrillation
Fatal re‐infarction was registered in 905 (8.5% or 29.0 per 1000 person‐years) versus 5502 (7.0% or 14.2 per 1000 person‐years) among patients with and without new‐onset AF, respectively (Figure 2). Nonfatal re‐infarction was registered in 1521 (14.2% or 60.7 per 1000 person‐years) versus 12 444 (15.8% or 37.9 per 1000 person‐years) among patients with and without new‐onset AF, respectively (Figure 2). In multiple Cox models, with new‐onset AF inserted as a time‐dependent variable, adjusted for age, gender, year, concomitant medical treatment, and comorbidity there was a significant increase of both fatal and nonfatal re‐infarction after new‐onset AF (HR: 1.67 [95% CI: 1.55 to 1.80], P<0.001 and HR: 1.79 [95% CI: 1.69 to 1.89], P<0.001 respectively).
Non‐Cardiovascular Mortality After New‐Onset Atrial Fibrillation
Non‐cardiovascular mortality was registered in 1146 (10.7% or 38.8 per 1000 person‐years) versus 7502 (9.5% or 19.4 per 1000 person‐years) among patients with and without new‐onset AF, respectively (Figure 2). In multiple Cox models, with new‐onset AF inserted as a time‐dependent variable, adjusted for age, gender, year, concomitant medical treatment, and comorbidity there was an increased risk of noncardiovascular mortality (HR: 1.43 [95% CI: 1.34 to 1.53], P<0.001) in patients with new‐onset AF. To address the cause of increased risk of noncardiovascular mortality, an interaction analysis with PCI was made. This showed a significant lower risk of noncardiovascular mortality in non‐PCI treated patients with new‐onset AF compared to PCI treated patients (HR: 1.42 [95% CI: 1.33 to 1.53]; (HR: 1.55 [95% CI: 1.30 to 1.86], P for interaction <0.001).
Discussion
This study is, to the best of our knowledge, the first study to examine the associations of new‐onset AF with specific causes of death in a large contemporary post‐MI population. Second, this study adds information on the impact of new‐onset AF on nonfatal cerebral stroke and nonfatal re‐MI in a large cohort of first‐time MI patients with long‐term follow‐up. The main results of the study were: (1) that new‐onset AF is associated with an increased risk of all‐cause mortality, fatal and nonfatal stroke, fatal and nonfatal re‐infarction, cardiovascular mortality and non‐cardiovascular mortality; and (2) rate of new‐onset AF decreased each year after discharge from first‐time MI, however the mortality increased with subsequent years.
New‐Onset AF and Myocardial Infarction
In accordance with previous studies, incidence rate of patients admitted for AF was higher after MI discharge (23.9 per 1000 person‐years) compared to the background population in Denmark of similar age (5 to 7 per 1000 person‐years).11 Studies have suggested that increases in atrial pressure can occur in relation to MI either caused by atrial ischemia,5–6 increased LV filling pressure or LV pumping become affected. 7,17 Moreover, AF in MI patients has been associated with a variety of clinical risk factors including increased age and history of hypertension and diabetes, kidney dysfunction, and, in particular, the presence of heart failure symptoms and left ventricular dysfunction.17–19 However, there is also evidence to suggest that AF in MI setting is seemingly independent of age, heart failure, and ventricular dysfunction.20
All‐Cause Mortality After New‐Onset AF
The observed rate of all‐cause mortality among patients who developed new‐onset AF in this study (173.9 per 1000 person‐years) is higher than that reported in patients who developed new‐onset AF in the Danish background population (2.84 to 3.54 per 1000 patient‐years).21 Nevertheless, even after adjustment for several risk factors, new‐onset AF was independently predictive of all‐cause mortality in the present study. This is in accordance with the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) I study22 that showed higher 1‐year mortality in post‐MI patients with AF compared to patients without AF. Increased long‐term all‐cause mortality in patients with AF in the setting of MI has also been verified in the Optimal Trial in Myocardial Infarction with the Angiotensin II Antagonist Losartan (OPTIMAAL),23 Trandolapril Cardiac Evaluation (TRACE)20 and VALsartan In Acute myocardial iNfarcTion (VALIANT)24 studies.
The Cause of Death in MI Patients With and Without New‐Onset AF
It has been demonstrated that AF can facilitate ventricular arrhythmias,25–27 which could explain the increased risk of all‐cause mortality after new‐onset AF in MI patients. However, to the best of our knowledge, no data exist on the cause of death in MI patients with and without new‐onset AF. Pedersen et al28 reported increased risk of all‐cause and cardiovascular mortality including sudden cardiac death in MI patients with AF. Unfortunately, they did not explore specific causes of death, did not exclude patients with previous AF and did not analyze AF as a time‐dependent variable. Thus, the present study demonstrated for the first time that MI patients with new‐onset AF have an increased risk of dying from stroke and re‐infarction. Consistent with our data, the OPTIMAAL study23 showed an increased risk of stroke associated with AF in patients with MI and left ventricular dysfunction. Also the GUSTO I study22 showed an increased risk of stroke in patients with AF after MI. The observed increases in risks of fatal and nonfatal stroke are probably to a large extent explained by the well‐established association of increased thromboembol in AF. This raises the question whether post‐AMI patients with transient AF should be prescribed anticoagulation similar to patients with primary AF and for how long?
Some studies have argued that AF is merely a risk marker of death, and not a causal mediator of MI.29–30 However, there is evidence to suggest AF is associated with impairment of endothelial function even in the absence of heart failure or hypertension which could explain the increased incidence of AF associated with ischemia‐reperfusion injury after MI.31 Interestingly, inflammation has been demonstrated to be important not only in AF but also in MI pathophysiology.32–33 C‐reactive protein (CRP) has been shown to reduce nitric oxide production in endothelial cells and increase endothelial expression of adhesion molecules.33 It also plays a crucial role in chemotaxis of monocytes and foam cell formation in atherosclerotic plaques and enhances vasoreactivity of unstable plaques.33 This could partly explain the associations among MI, new‐onset AF, and re‐MI, and support the idea that AF reflects an underlying disease in the heart.
Non‐Cardiovascular Mortality After New‐Onset AF
In our study we found that new‐onset AF was associated with noncardiovascular mortality. To a large extent, this could be explained by the therapeutic challenge with the need of both vitamin K antagonists (VKAs) and 2 antiplatelet therapy to prevent stroke and further coronary events in the patients with new‐onset AF, which we have shown to be associated with increased risk of fatal and nonfatal bleeding.34 This is supported by the interaction and lower risk of noncardiovascular mortality in non‐PCI treated patients with new‐onset AF in the present study. These results imply that new‐onset AF in MI patients is a cardiovascular disease and the independent relation to adverse cardiovascular prognosis disproves that new‐onset AF only reflects age, gender, an increased or general disease throughout the body such as cancer, chronic kidney, and/or lung diseases. In addition, you could imagine that AF makes physiologic stresses from other conditions harder to tolerate due to poor heart rate regulation and decrease in cardiac output in some patients, which would make new‐onset AF patients more vulnerable to other diseases.
Increasing Mortality Despite Decreasing New‐Onset AF After Discharge
This study revealed that there is a subsequently increasing risk of mortality in the later years after discharge from first‐time MI, which was mainly due to increasing age. This is supported by earlier observations including addition of age in CHA2DS2‐VASc score (Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke/TIA, Vascular disease, Aged 65 to 74 years and female Sex [1 point for presence of each, except for 2 points for age ≥75 years and stroke/TIA]; score range from 0 to 6).35–36 The decreasing rate of new‐onset AF after discharge could be caused by patients' immediate atrial ischemia5–6 or backward failure from an affected LV pump,7,17 or simply that the sickest patient dies earlier, leaving increasingly healthy patients alive with lower risk of new‐onset AF.
Strengths and Limitations
Our study suffers the limitations with any analysis of longitudinal registry data. However, the completeness of data, which enabled comprehensive follow‐up for 10 years, adds strength to this study. The Danish National Patient Registry keeps records on discharge diagnosis from all hospitals in Denmark, and the present study thus avoids selection bias, which may be present in surveys that are based on selected centers. Furthermore, the Danish National Patient Registry as well as the national prescription registry has been shown to be accurate.9,11,37 The Danish healthcare system is government financed, which secures equal access to health care for all inhabitants free of charge, regardless of socioeconomic status. The present study based on registries did not include clinical data and some comorbid status, most importantly, hypertension, ejection fraction, MI severity, and heart failure, which are known to be associated with risk of developing AF, stroke, MI, and death. This, of course, was a limitation. In addition, we were not able to subdivide AF patients into groups according to the severity or persistence of the arrhythmia that would have allowed us to distinguish between patients with low risk and patients with even worse prognosis. We have addressed issues of potential bias and baseline imbalances between the groups, by performing 3 different sensitivity analyses; (1) a multiple model adjusting for potential confounders; (2) time‐dependent variable analysis; and (3) a propensity score‐matched analysis. New‐onset AF, whether paroxysmal or persistent, was identified from the National Patient Registry. AF diagnosis is in this registry based on admission for AF and AF recorded in outpatient clinics or during hospital admission for other reasons. This method leaves episodes of paroxysmal AF without need of hospital admission undetected. Thus, our method may have underestimated AF occurrence and primarily detected symptomatic AF.
Conclusions
This study shows that MI patients with new‐onset AF have an increased risk of all‐cause mortality, which to a large extent is caused by cardiovascular mortality. This implies that new‐onset AF after MI should be seen as an “adverse prognostic sign” which should remind the physician to monitor and treat underlying disease; in particular cardiovascular, as soon as possible after new‐onset AF has occurred.
Sources of Funding
This work was supported by The Danish Heart Association. Grant number: [10‐04‐R78‐A2962‐22582], the A.P. Møller Foundation and Interreg Iva—European Regional Fund; and had no relationships with the industry. Dr Gislason is supported by an unrestricted research scholarship from the Novo Nordisk Foundation.
Disclosures
Drs Devereux and Wachtell received grant support from Merck & Co, Inc. Dr Torp‐Pedersen is a consultant to Sanofi‐Aventis, Merck, and Cardiome, and receives consulting fees from all 3 companies. Dr Devereux consults for Merck & Co, Inc and General Electric Medical Systems. The remaining authors report no conflicts.
References
- 1.Jons C, Jacobsen UG, Joergensen RM, Olsen NT, Dixen U, Johannessen A, Huikuri H, Messier M, McNitt S, Thomsen PE. The incidence and prognostic significance of new‐onset atrial fibrillation in patients with acute myocardial infarction and left ventricular systolic dysfunction: a CARISMA substudy. Heart Rhythm. 2011; 8:342-348 [DOI] [PubMed] [Google Scholar]
- 2.Mehta RH, Dabbous OH, Granger CB, Kuznetsova P, Kline‐Rogers EM, Anderson FA, Jr, Fox KA, Gore JM, Goldberg RJ, Eagle KA. Comparison of outcomes of patients with acute coronary syndromes with and without atrial fibrillation. Am J Cardiol. 2003; 92:1031-1036 [DOI] [PubMed] [Google Scholar]
- 3.Jabre P, Jouven X, Adnet F, Thabut G, Bielinski SJ, Weston SA, Roger VL. Atrial fibrillation and death after myocardial infarction: a community study. Circulation. 2011; 123:2094-2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zusman O, Amit G, Gilutz H, Zahger D. The significance of new onset atrial fibrillation complicating acute myocardial infarction. Clin Res Cardiol. 2012; 101:17-22 [DOI] [PubMed] [Google Scholar]
- 5.Hod H, Lew AS, Keltai M, Cercek B, Geft IL, Shah PK, Ganz W. Early atrial fibrillation during evolving myocardial infarction: a consequence of impaired left atrial perfusion. Circulation. 1987; 75:146-150 [DOI] [PubMed] [Google Scholar]
- 6.Tjandrawidjaja MC, Fu Y, Kim DH, Burton JR, Lindholm L, Armstrong PW. Compromised atrial coronary anatomy is associated with atrial arrhythmias and atrioventricular block complicating acute myocardial infarction. J Electrocardiol. 2005; 38:271-278 [DOI] [PubMed] [Google Scholar]
- 7.Lopes RD, Elliott LE, White HD, Hochman JS, Van de Werf F, Ardissino D, Nielsen TT, Weaver WD, Widimsky P, Armstrong PW, Granger CB. Antithrombotic therapy and outcomes of patients with atrial fibrillation following primary percutaneous coronary intervention: results from the APEX‐AMI trial. Eur Heart J. 2009; 30:2019-2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pizzetti F, Turazza FM, Franzosi MG, Barlera S, Ledda A, Maggioni AP, Santoro L, Tognoni G. Incidence and prognostic significance of atrial fibrillation in acute myocardial infarction: the GISSI‐3 data. Heart. 2001; 86:527-532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madsen M, Davidsen M, Rasmussen S, Abildstrom SZ, Osler M. The validity of the diagnosis of acute myocardial infarction in routine statistics: a comparison of mortality and hospital discharge data with the Danish MONICA registry. J Clin Epidemiol. 2003; 56:124-130 [DOI] [PubMed] [Google Scholar]
- 10.Gislason GH, Abildstrom SZ, Rasmussen JN, Rasmussen S, Buch P, Gustafsson I, Friberg J, Gadsboll N, Kober L, Stender S, Madsen M, Torp‐Pedersen C. Nationwide trends in the prescription of beta‐blockers and angiotensin‐converting enzyme inhibitors after myocardial infarction in Denmark, 1995–2002. Scand Cardiovasc J. 2005; 39:42-49 [DOI] [PubMed] [Google Scholar]
- 11.Frost L, Andersen LV, Vestergaard P, Husted S, Mortensen LS. Trend in mortality after stroke with atrial fibrillation. Am J Med. 2007; 120:47-53 [DOI] [PubMed] [Google Scholar]
- 12.Tu JV, Austin PC, Walld R, Roos L, Agras J, McDonald KM. Development and validation of the Ontario acute myocardial infarction mortality prediction rules. J Am Coll Cardiol. 2001; 37:992-997 [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen S, Zwisler AD, Abildstrom SZ, Madsen JK, Madsen M. Hospital variation in mortality after first acute myocardial infarction in Denmark from 1995 to 2002: lower short‐term and 1‐year mortality in high‐volume and specialized hospitals. Med Care. 2005; 43:970-978 [DOI] [PubMed] [Google Scholar]
- 14.Andersen PK, Gill RD. Cox's regression model for counting processes: a large sample study. Ann Stat. 1982; 10:1100-1120 [Google Scholar]
- 15.Dafni U. Landmark analysis at the 25‐year landmark point. Circ Cardiovasc Qual Outcomes. 2011; 4:363-371 [DOI] [PubMed] [Google Scholar]
- 16.Nicolaie MA, Van Houwelingen JC, De Witte TM, Putter H. Dynamic prediction by landmarking in competing risks. Stat Med. 2013; 32:2031-2047 [DOI] [PubMed] [Google Scholar]
- 17.Aronson D, Mutlak D, Bahouth F, Bishara R, Hammerman H, Lessick J, Carasso S, Dabbah S, Reisner S, Agmon Y. Restrictive left ventricular filling pattern and risk of new‐onset atrial fibrillation after acute myocardial infarction. Am J Cardiol. 2011; 107:1738-1743 [DOI] [PubMed] [Google Scholar]
- 18.Schmitt J, Duray G, Gersh BJ, Hohnloser SH. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J. 2009; 30:1038-1045 [DOI] [PubMed] [Google Scholar]
- 19.Bahouth F, Mutlak D, Furman M, Musallam A, Hammerman H, Lessick J, Dabbah S, Reisner S, Agmon Y, Aronson D. Relationship of functional mitral regurgitation to new‐onset atrial fibrillation in acute myocardial infarction. Heart. 2010; 96:683-688 [DOI] [PubMed] [Google Scholar]
- 20.Pedersen OD, Bagger H, Kober L, Torp‐Pedersen C. The occurrence and prognostic significance of atrial fibrillation/‐flutter following acute myocardial infarction. TRACE Study group. TRAndolapril Cardiac Evalution. Eur Heart J. 1999; 20:748-754 [DOI] [PubMed] [Google Scholar]
- 21.Frost L, Engholm G, Moller H, Husted S. Decrease in mortality in patients with a hospital diagnosis of atrial fibrillation in Denmark during the period 1980–1993. Eur Heart J. 1999; 20:1592-1599 [DOI] [PubMed] [Google Scholar]
- 22.Crenshaw BS, Ward SR, Granger CB, Stebbins AL, Topol EJ, Califf RM. Atrial fibrillation in the setting of acute myocardial infarction: the GUSTO‐I experience. Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries. J Am Coll Cardiol. 1997; 30:406-413 [DOI] [PubMed] [Google Scholar]
- 23.Lehto M, Snapinn S, Dickstein K, Swedberg K, Nieminen MS. Prognostic risk of atrial fibrillation in acute myocardial infarction complicated by left ventricular dysfunction: the OPTIMAAL experience. Eur Heart J. 2005; 26:350-356 [DOI] [PubMed] [Google Scholar]
- 24.Kober L, Swedberg K, McMurray JJ, Pfeffer MA, Velazquez EJ, Diaz R, Maggioni AP, Mareev V, Opolski G, Van de Werf F, Zannad F, Ertl G, Solomon SD, Zelenkofske S, Rouleau JL, Leimberger JD, Califf RM. Previously known and newly diagnosed atrial fibrillation: a major risk indicator after a myocardial infarction complicated by heart failure or left ventricular dysfunction. Eur J Heart Fail. 2006; 8:591-598 [DOI] [PubMed] [Google Scholar]
- 25.Somberg JC, Torres V, Keren G, Butler B, Tepper D, Kleinbaum H, Molnar J. Enhancement of myocardial vulnerability by atrial fibrillation. Am J Ther. 2004; 11:33-43 [DOI] [PubMed] [Google Scholar]
- 26.Stein KM, Euler DE, Mehra R, Seidl K, Slotwiner DJ, Mittal S, Markowitz SM, Lerman BB. Do atrial tachyarrhythmias beget ventricular tachyarrhythmias in defibrillator recipients? J Am Coll Cardiol. 2002; 40:335-340 [DOI] [PubMed] [Google Scholar]
- 27.Roy D, Brugada P, Wellens HJ. Atrial tachycardia facilitating initiation of ventricular tachycardia. Pacing Clin Electrophysiol. 1983; 6:47-52 [DOI] [PubMed] [Google Scholar]
- 28.Pedersen OD, Abildstrom SZ, Ottesen MM, Rask‐Madsen C, Bagger H, Kober L, Torp‐Pedersen C. Increased risk of sudden and non‐sudden cardiovascular death in patients with atrial fibrillation/flutter following acute myocardial infarction. Eur Heart J. 2006; 27:290-295 [DOI] [PubMed] [Google Scholar]
- 29.Klass M, Haywood LJ. Atrial fibrillation associated with acute myocardial infarction: a study of 34 cases. Am Heart J. 1970; 79:752-760 [DOI] [PubMed] [Google Scholar]
- 30.Cristal N, Peterburg I, Szwarcberg J. Atrial fibrillation developing in the acute phase of myocardial infarction. Prognostic implications. Chest. 1976; 70:8-11 [DOI] [PubMed] [Google Scholar]
- 31.Adam O, Neuberger HR, Bohm M, Laufs U. Prevention of atrial fibrillation with 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitors. Circulation. 2008; 118:1285-1293 [DOI] [PubMed] [Google Scholar]
- 32.Bang CN, Greve AM, Boman K, Egstrup K, Gohlke‐Baerwolf C, Kober L, Nienaber CA, Ray S, Rossebo AB, Wachtell K. Effect of lipid lowering on new‐onset atrial fibrillation in patients with asymptomatic aortic stenosis: the Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) study. Am Heart J. 2012; 163:690-696 [DOI] [PubMed] [Google Scholar]
- 33.Chan AW, Bhatt DL, Chew DP, Reginelli J, Schneider JP, Topol EJ, Ellis SG. Relation of inflammation and benefit of statins after percutaneous coronary interventions. Circulation. 2003; 107:1750-1756 [DOI] [PubMed] [Google Scholar]
- 34.Lamberts M, Olesen JB, Ruwald MH, Hansen CM, Karasoy D, Kristensen SL, Kober L, Torp‐Pedersen C, Gislason GH, Hansen ML. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention: a nationwide cohort study. Circulation. 2012; 126:1185-1193 [DOI] [PubMed] [Google Scholar]
- 35.Hughes M, Lip GY. Stroke and thromboembolism in atrial fibrillation: a systematic review of stroke risk factors, risk stratification schema and cost effectiveness data. Thromb Haemost. 2008; 99:295-304 [DOI] [PubMed] [Google Scholar]
- 36.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest. 2010; 137:263-272 [DOI] [PubMed] [Google Scholar]
- 37.Gaist D, Andersen M, Aarup AL, Hallas J, Gram LF. Use of sumatriptan in Denmark in 1994–5: an epidemiological analysis of nationwide prescription data. Br J Clin Pharmacol. 1997; 43:429-433 [DOI] [PMC free article] [PubMed] [Google Scholar]


