Abstract
Background
There is emerging evidence that adjuvant treatments for breast cancer negatively impact cardiorespiratory fitness (CRF) or Vo2max, a key predictor of cardiovascular risk. Although a number of studies have measured CRF in breast cancer patients, there is currently limited data regarding expected CRF values in this patient population. Given that CRF is a poor prognostic sign and recently highlighted as a key measure to standardize by the American Heart Association, we sought to review the available literature on CRF among breast cancer patients.
Methods and Results
We identified 27 clinical trials and observational studies measuring Vo2max in the pre– and post–adjuvant treatment setting for breast cancer. We compared Vo2max before to Vo2max after adjuvant therapy and compared Vo2max in female breast cancer patients with Vo2max in healthy controls.
Conclusions
We found that CRF was substantially lower in women with a history of breast cancer compared with healthy women and this was most pronounced among breast cancer patients in the post‐adjuvant setting. We conclude that knowledge of normative CRF values is critical to tailor appropriately timed exercise interventions in breast cancer patients susceptible to low CRF and subsequent cardiovascular risk.
Keywords: breast cancer, cardiorespiratory fitness, women
Introduction
Breast cancer is the most commonly diagnosed malignancy of women in the United States, with 207 090 new cases in 2010, accounting for 28% of all cancer diagnoses.1 Due to significant improvements in screening protocols, diagnosis, and treatment over the past few decades, breast cancer mortality continues to decrease.2–3 As a result, more than 2.9 million American women are living with a prior history of breast cancer.4 Importantly, women diagnosed with early‐stage breast cancer are susceptible to the late occurring toxic effects of conventional treatment regimens,5–6 and are at greater risk for death from cardiovascular disease than from breast cancer after 65 years of age.7
Cardiac‐related effects of adjuvant therapy—namely, cardiomyocyte injury and decreases in left ventricular ejection fraction (LVEF)—are well documented.8–9 Both left‐sided radiotherapy and chemotherapy are associated with short‐ and long‐term cardiotoxicity.10–11 Anthracycline‐containing regimens are known to cause dose‐dependent, cumulative, progressive cardiac dysfunction manifested as decreased LVEF and congestive heart failure.9 The addition of adjuvant trastuzumab to the management of human epidermal growth factor receptor (HER)‐2–positive early breast cancer has further increased clinical and subclinical cardiotoxicity rates.12–13
There is emerging evidence that adjuvant therapy for breast cancer can also significantly affect cardiorespiratory fitness (CRF). CRF as measured by Vo2max assesses global cardiovascular function, cardiopulmonary reserve, and efficiency of oxygen transport and utilization and can unmask compensatory mechanisms of abnormal cardiac function as well as defects in other vital organs.14–15 A number of studies have shown that Vo2max is impaired in breast cancer patients compared with healthy controls.5–6,5–17 This finding is of major concern given that low Vo2max is associated with higher mortality among more–advanced stage breast cancer patients.17
Despite evidence that low Vo2max is a poor prognostic sign, there is currently little known regarding Vo2max levels among breast cancer patients. Knowledge of Vo2max values in breast cancer is of timely importance as the American Heart Association is calling for the development of a national adult database of CRF, given the critical impact of CRF on cardiovascular morbidity, all‐cause mortality,18 and cancer survival.17,19 Moreover, Vo2max values would enable healthcare providers to define levels of CRF associated with poor health outcomes in the breast cancer population, elucidate therapy‐related decrements in CRF, and help target appropriately timed interventions.
To address this gap in the literature, we identified 27 clinical trials and observational studies measuring Vo2max in the pre– or post–adjuvant treatment setting for breast cancer. We compared mean Vo2max values of female breast cancer patients in 6 studies that measured Vo2max before adjuvant therapy and 21 studies that measured Vo2max after adjuvant therapy was completed. We then compared mean Vo2max values in female breast cancer patients with the Vo2max values of healthy, sedentary, and active, endurance‐trained controls. Secondary variables of interest included patients' age, body mass index (BMI), and study type and period.
Methods
We performed a MEDLINE search for citations of peer reviewed clinical studies measuring CRF in breast cancer patients. We used the following search terms: cardiorespiratory fitness or fitness or exercise capacity and breast cancer. We excluded studies not printed in the English language. We included observational studies and clinical trials involving exercise interventions in which CRF was measured in female breast cancer patients. Clinical trials consisted of both randomized controlled trials (RCTs) and exercise intervention studies, each of which examined the effects of exercise interventions on CRF as well as other variables in breast cancer patients. RCTs also measured outcomes in control groups consisting of women without a history of breast cancer, whereas exercise intervention studies did not contain control groups. Studies were only included if CRF was measured using Vo2max [mL/(kg min) or L/min] and if CRF was measured within 5 years of completion of adjuvant therapy. For RCTs and exercise intervention studies, we reported mean baseline Vo2max values and associated SDs before the initiation of exercise training.
Twenty‐six clinical trials and observational studies that measured Vo2max in female breast cancer patients pre– or post–adjuvant therapy were identified. Because Schneider et al20 measured Vo2max in pre– and post–adjuvant therapy settings, the 2 subsets were treated as independent populations, making the effective sample size 27 studies. A control population of healthy women was identified from a previously performed meta‐analysis by Fitzgerald et al,21 which accounted for physical activity level and age‐related decline in Vo2max. The equations to calculate Vo2max were as follows: sedentary [43.82−0.35 (age)], active [54.02−0.44 (age)], and endurance‐trained [72.41−0.62 (age)].21 Estimated rates of decline for sedentary, active, and endurance‐trained women were −3.5 mL/(kg min), −4.4 mL/(kg min), and −6.2 mL/(kg min) per decade, respectively.21 The algorithms in the Fitzgerald report have been previously used and compared in other breast cancer populations.17
The majority of studies (16 of 27) measured CRF using a treadmill walking test and either a Bruce, Balke, or Naughton protocol. Of the remaining studies, 8 measured CRF with an electronic cycle ergometer, 1 measured CRF with a stepping ergometer, and 2 did not report the exercise equipment used to measure CRF. Vo2max was measured directly from expired gas analysis during maximal effort in 14 studies and estimated using previously derived equations in 13 studies. A majority of studies reported mean Vo2max in mL/(kg min), but for studies that reported mean Vo2max in L/min and also reported mean weight in kg, a conversion was done using the standard method of division of random variables for both the mean and SD.22 Of the 1856 patients studied in this review, 709 participated in studies in the United States and 1147 participated in studies performed outside the United States. The studies performed outside the United States and Canada included Brdareski et al23 (Serbia), Daley et al24 (United Kingdom), Herrero et al25–26 (Spain), Rahnama et al27 (Iran), Scott et al28 (United Kingdom), Turner et al29 (Australia), and Vincent et al30 (France).
To accurately compare Vo2max pre– and post–adjuvant therapy with that of controls, the inverse‐variance method of weighting assuming a random‐effects model was used.31 Fitzgerald et al21 reported that the weighted and unweighted means for the control group were not different; thus, the mean Vo2max used for the control group was un‐weighted. The standard error and 95% confidence interval were also calculated according to the guidelines of Borenstein and Higgins.31
Raw mean and SD values for Vo2max (mL/(kg min)], age, and BMI were summarized for each study. Fisher's exact tests were used to compare the distribution of study type and therapy class, and Student t tests were used to compare the mean age and BMI between pre– and post–adjuvant therapy breast cancer studies. Meta‐regression was used to explore the linear relationship between the weighted mean Vo2max of each study, age, BMI, study type, and subgroup (pre– or post–adjuvant therapy). BMI was not reported for 5 studies. A sensitivity analysis was done to determine if imputation with the mean BMI would change the results of the linear regression. Imputation did not change the results, and as such, missing BMI values were replaced with the mean BMI for all other studies so that all 27 studies could be included in the meta‐regression.
Tests for heterogeneity were conducted to verify the appropriateness of meta‐analysis for mean values of Vo2max for all breast cancer studies and for pre–adjuvant therapy studies and post–adjuvant therapy studies separately. Methods described in Higgins and Thompson32 were used to carry out this analysis. The H statistic, a modified Cochran's χ2 test (Q test) statistic that does not intrinsically depend on the number of studies, was calculated, with H=1 indicating homogeneity.32
Results
Fitness in the Preadjuvant and Postadjuvant Setting
Twenty‐seven studies measuring Vo2max in breast cancer patients were identified between 2001 and 2013, involving a total of 1856 female participants with mean ages ranging from 47 to 59 years. The 6 studies that reported Vo2max prior to adjuvant therapy as well as the 21 studies that reported Vo2max after completion of adjuvant therapy are summarized in Table 1. Weight percent by subgroup (pretherapy and posttherapy) and weight percent overall of each of the 27 studies are also shown in Table 1.
Table 1.
Clinical Studies of Cardiorespiratory Fitness in Breast Cancer Patients
| Time Period | Study | Sample Size | Vo2max | Age | BMI | Study Type | Weight % by Subgroup* | Weight % Overall* |
|---|---|---|---|---|---|---|---|---|
| Pretherapy | Courneya et al33 | 242 | 25.17±6.49 | 49.2±— | 26.6±5.5 | RCT | 8.73 | 1.59 |
| Kolden et al34 | 40 | 30.60±4.30 | 55.0±8.4 | —±— | Exercise intervention | 19.88 | 3.61 | |
| Ligibel et al35 | 41 | 22.30±3.40 | 47.0±7.3 | 27.0±— | Exercise intervention | 31.80 | 5.77 | |
| Schneider et al20 | 13 | 22.50±6.20 | 54.9±10.6 | 25.6±— | Exercise intervention | 9.56 | 1.74 | |
| Segal et al36 | 123 | 25.50±5.57 | 50.9±8.7 | —±— | RCT | 11.86 | 2.15 | |
| Vincent et al30 | 34 | 22.10±4.50 | 49.0±8.4 | 24.0±7.4 | Exercise intervention | 18.16 | 3.30 | |
| Posttherapy | Brdareski et al23 | 18 | 21.00±3.22 | 52.1±7.5 | 26.5±3.4 | Exercise intervention | 7.88 | 6.45 |
| Burnett et al37 | 30 | 25.40±5.30 | 50.5±5.6 | 29.2±5.3 | Observational | 2.90 | 2.38 | |
| Campbell et al38 | 14 | 24.11 ±5.02 | 54.6±8.3 | 30.1±3.6 | Exercise intervention | 3.23 | 2.65 | |
| Courneya et al39 | 50 | 18.70±3.85 | 59.0±6.0 | 29.2±6.6 | RCT | 5.51 | 4.51 | |
| Daley et al24 | 108 | 29.82±5.08 | 51.0±8.5 | 28.6±5.0 | RCT | 3.16 | 2.59 | |
| Dolan et al16 | 242 | 24.50±6.40 | 49.2±— | 26.6±5.5 | RCT | 1.99 | 1.63 | |
| Fillion et al40 | 87 | 25.55±5.36 | 52.4±10.0 | —±— | RCT | 2.84 | 2.32 | |
| Herrero et al25 | 16 | 24.60±5.80 | 50.0±9.0 | 26.2±4.5 | Observational | 2.42 | 1.98 | |
| Herrero et al26 | 11 | 26.70±5.60 | 47.0±7.0 | 25.2±3.2 | Exercise intervention | 2.60 | 2.13 | |
| Hsieh et al41 | 96 | 20.60±6.23 | 57.9±10.5 | 28.6±— | Exercise intervention | 2.10 | 1.72 | |
| Hutnick et al42 | 47 | 19.49±5.21 | 50.1±10.0 | 26.7±4.8 | Exercise intervention | 3.01 | 2.46 | |
| Jones et al5 | 47 | 17.90±4.30 | 59.0±7.0 | 28.0±5.0 | Observational | 4.41 | 3.61 | |
| Jones et al6 | 26 | 19.20±4.60 | 48.0±8.5 | 29.0±6.0 | Observational | 3.85 | 3.15 | |
| Musanti et al43 | 55 | 23.19±4.96 | 50.5±7.5 | —±— | Exercise Intervention | 3.31 | 2.71 | |
| Rahnama et al27 | 29 | 15.75±5.52 | 57.5±— | 27.7±4.0 | Exercise intervention | 2.68 | 2.19 | |
| Rogers et al44 | 41 | 25.04±6.10 | 53.0±9.0 | 30.9±8.6 | RCT | 2.19 | 1.80 | |
| Schneider et al20 | 82 | 20.80±6.10 | 56.9±9.4 | 28.3±— | Exercise intervention | 2.19 | 1.79 | |
| Scott et al28 | 90 | 23.70±4.53 | 55.7±9.6 | 30.3±4.5 | RCT | 3.98 | 3.26 | |
| Taylor et al3 | 257 | 25.50±6.50 | 55.0±9.4 | 31.3±4.9 | Observational | 1.93 | 1.58 | |
| Tosti et al45 | 7 | 22.00±1.50 | 50.6±3.3 | 29.4±1.1 | Observational | 36.24 | 29.66 | |
| Turner et al29 | 10 | 23.00±7.20 | 47.0±8.0 | —±— | Exercise intervention | 1.57 | 1.29 |
Data presented as mean±SD. —, Missing or unreported data. Each study was weighted based on the inverse of the variance of that study's mean Vo2max so that studies with smaller variances had a greater influence on the mean across studies. BMI indicates body mass index; RCT, randomized controlled trial.
Weight percent by subgroup indicates the influence of the study on the mean across all pretherapy or posttherapy studies.
Weight percent overall indicates the influence of that study on the mean across all pretherapy and posttherapy studies.
Table 2 shows descriptive variables from all 27 studies as well as a comparison among the overall and pre– and post–adjuvant therapy breast cancer groups. Overall, 20% were RCTs, 22% were observational studies, and 48% were exercise intervention studies. In the preadjuvant setting, 33% were RCTs, and 67% were exercise interventions. Of the studies performed in the postadjuvant setting, 29% were RCTs, 29% were observational studies, and 43% were exercise interventions. Adjuvant therapy included chemotherapy in 78% (predominantly anthracycline‐based), radiotherapy in 56%, and hormonal therapy in 33% of patients.
Table 2.
Comparison of Descriptive Variables for Pre– and Post–Adjuvant Therapy Groups
| Variable | All Breast Cancer Studies (N=27) | Pre–Adjuvant Therapy (N=6) | Post–Adjuvant Therapy (N=21) | P Value* |
|---|---|---|---|---|
| Age, y | 52.3±3.7 | 51.0±3.3 | 52.7±3.8 | 0.332 |
| BMI, kg/m2 | 28.0±1.9 | 25.8±1.3 | 28.4±1.7 | 0.009 |
| Study type | 0.407 | |||
| RCT | 8 (30%) | 2 (33%) | 6 (29%) | |
| Observational | 6 (22%) | 0 (0%) | 6 (29%) | |
| Exercise intervention | 13 (48%) | 4 (67%) | 9 (43%) | |
| Therapy class | ||||
| Surgery+adjuvant therapy | 27 (100%) | 6 (100%) | 21 (100%) | |
BMI indicates body mass index; RCT, randomized controlled trial.
Fisher's exact P‐value for categorical variables; t test P‐value for continuous P‐values.
The mean age and BMI for all breast cancer studies were 52±4 years and 28±2 kg/m2. The mean age was similar in the pre– and post–adjuvant therapy settings (P=0.33, Table 2). The weighted mean Vo2max before adjuvant therapy was 24.6 mL/(kg min). For the 21 studies reporting Vo2max after adjuvant therapy, the weighted mean Vo2max was 22.2 mL/(kg min). The Vo2max in the postadjuvant setting was 10% lower [−2.4 mL/(kg min)] than the mean Vo2max value assessed at the beginning of adjuvant therapy. The mean BMI measured after adjuvant therapy was completed was 2.6 kg/m2 higher than the BMI prior to adjuvant therapy (28.4±2 kg/m2 versus 25.8±1 kg/m2, P=0.009).
Table 3 contains the results of the linear meta‐regression examining the relationship between age, BMI, study type and period, and weighted mean Vo2max levels. There was no evidence for an association between weighted mean Vo2max and the study variables of interest such as age and BMI (P>0.05 for all). We also performed a test for heterogeneity for both preadjuvant and postadjuvant studies. The H value was 1 (95% CI 1 to 1.99, P=0.72) for the preadjuvant breast cancer studies and 1 (95% CI 1 to 1.37, P=0.98) for the postadjuvant breast cancer studies, respectively.
Table 3.
Association Between Weighted Mean Vo2max and Clinical Study Variables
| Model # | Dependent Variable | Independent Variables | Parameter Estimate (SE) | P Value | Model P‐Value | R‐Squared |
|---|---|---|---|---|---|---|
| 1 | Weighted mean Vo2max | Intercept | 1.031 (7.289) | 0.889 | 0.641 | 0.140 |
| Age | −0.073 (0.106) | 0.497 | ||||
| BMI* | 0.191 (0.275) | 0.495 | ||||
| Study type (RCT) | −1.424 (1.004) | 0.171 | ||||
| Study type (exercise) | −1.125 (0.998) | 0.272 | ||||
| Period (posttherapy) | −0.380 (0.959) | 0.696 | ||||
| 2 | Weighted mean Vo2max | Intercept | −0.460 (6.811) | 0.947 | 0.732 | 0.053 |
| Age | −0.093 (0.104) | 0.382 | ||||
| BMI* | 0.238 (0.249) | 0.349 | ||||
| Period (posttherapy) | −0.099 (0.941) | 0.917 | ||||
| 3 | Weighted mean Vo2max | Intercept | 4.709 (4.944) | 0.351 | 0.567 | 0.120 |
| Age | −0.043 (0.096) | 0.656 | ||||
| Study type (RCT) | −1.440 (0.992) | 0.161 | ||||
| Study type (exercise) | −1.370 (0.923) | 0.152 | ||||
| Period (posttherapy) | −0.140 (0.883) | 0.876 | ||||
| 4 | Weighted mean Vo2max | Intercept | −0.534 (6.846) | 0.939 | 0.567 | 0.120 |
| BMI* | 0.113 (0.247) | 0.654 | ||||
| Study type (RCT) | −1.482 (0.988) | 0.148 | ||||
| Study type (exercise) | −1.266 (0.965) | 0.203 | ||||
| Period (posttherapy) | −0.399 (0.947) | 0.678 | ||||
| 5 | Weighted mean Vo2max | Intercept | 3.973 (4.982) | 0.433 | 0.827 | 0.016 |
| Age | −0.056 (0.097) | 0.567 | ||||
| Period (posttherapy) | 0.277 (0.853) | 0.749 | ||||
| 6 | Weighted mean Vo2max | Intercept | −3.026 (6.146) | 0.627 | 0.778 | 0.021 |
| BMI* | 0.156 (0.230) | 0.504 | ||||
| Period (posttherapy) | −0.107 (0.937) | 0.910 | ||||
| 7 | Weighted mean Vo2max | Intercept | 2.538 (1.111) | 0.032 | 0.425 | 0.112 |
| Study type (RCT) | −1.476 (0.971) | 0.142 | ||||
| Study type (exercise) | −1.399 (0.904) | 0.136 | ||||
| Period (posttherapy) | −0.223 (0.849) | 0.795 | ||||
| 8 | Weighted mean Vo2max | Intercept | 3.870 (4.882) | 0.436 | 0.596 | 0.011 |
| Age | −0.050 (0.093) | 0.596 | ||||
| 9 | Weighted mean Vo2max | Intercept | −2.776 (5.630) | 0.626 | 0.480 | 0.020 |
| BMI* | 0.144 (0.201) | 0.480 | ||||
| 10 | Weighted mean Vo2max | Intercept | 2.315 (0.702) | 0.003 | 0.249 | 0.110 |
| Study type (RCT) | −1.421 (0.929) | 0.139 | ||||
| Study type (exercise) | −1.330 (0.849) | 0.130 | ||||
| 11 | Weighted mean Vo2max | Intercept | 1.113 (0.728) | 0.139 | 0.829 | 0.002 |
| Period (posttherapy) | 0.181 (0.826) | 0.829 |
BMI indicates body mass index; RCT, randomized controlled trial.
Missing BMI values were imputed with the mean BMI.
Fitness Compared With Healthy, Sedentary, and Endurance Trained Women
The weighted mean Vo2max measured in breast cancer patients prior to adjuvant therapy [24.6 mL/(kg min)] was 17% lower than the Vo2max of healthy, sedentary women [29.7 mL/(kg min)], or 83% of predicted (P=0.007) (Figure 1). After completion of adjuvant therapy, weighted mean Vo2max in breast cancer patients [22.2 mL/(kg min)] was 25% lower than the Vo2max of healthy, sedentary women (75% of predicted, P<0.001). Figure 2 illustrates Vo2max by age and physical activity level for healthy women based on Fitzgerald et al21 and weighted Vo2max among breast cancer patients in the current study. Vo2max varied with age and physical activity level. The weighted mean Vo2max of a 50‐year‐old breast cancer patient [22.6 mL/(kg min)] was most similar to that of sedentary, 60‐year‐old women [≈22.7 mL/(kg min)].
Figure 1.
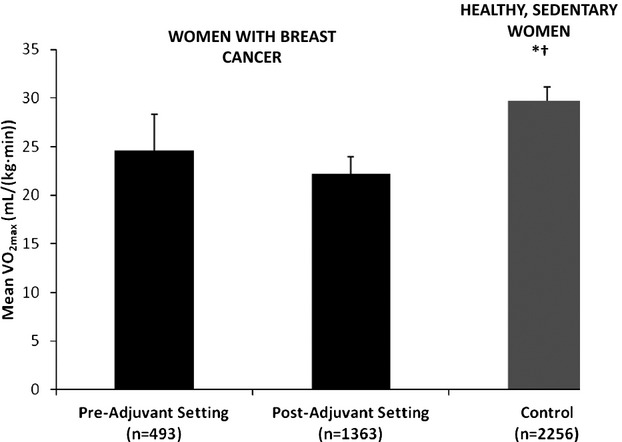
Mean Vo2max [mL/(kg min)] in breast cancer patients before and after adjuvant therapy and in healthy, sedentary controls.20 *P=0.007 comparing breast cancer patient before adjuvant therapy to control. †P<0.001 comparing breast cancer patients after adjuvant therapy to control.
Figure 2.
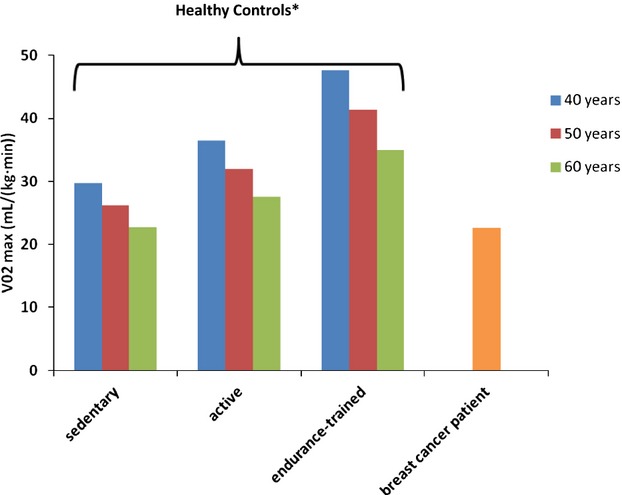
Mean Vo2max among sedentary, active, and endurance‐trained healthy women by age category* compared with mean Vo2max among breast cancer patients. *The equations to calculate Vo2max were as follows: sedentary [43.82−0.35 (age)], active [54.02−0.44 (age)], endurance‐trained [72.41−0.62 (age)]. Estimated rates of decline per decade for sedentary, active, and endurance‐trained women were −3.5 mL/(kg min), −4.4 mL/(kg min), and −6.2 mL/(kg min) per decade, respectively.21
Discussion
The current review demonstrates impairment in Vo2max among female breast cancer patients compared with healthy controls, as well as lower Vo2max depending on the timing of measurement in relation to breast cancer treatment status. These data suggest existing normative CRF values in healthy women may not be representative of breast cancer populations. Based on our previous work, the decline in CRF appears sustained in breast cancer patients even 7 years after treatment compared with age‐matched controls.46 These findings are of key importance given even small differences in CRF [eg, 1 MET or 3.5 mL/(kg min)] are associated with a significantly higher risk for cardiovascular mortality (≈18%).47–48
The reason for impairment in CRF is likely multifactorial, involving multiple organ components of oxygen transport. Of the major components of oxygen transport—namely, pulmonary, cardiac, vascular, and skeletal muscle function, a limitation in cardiac function is the most well studied in breast cancer patients. Chemotherapeutic agents used in breast cancer management are associated with both short‐ and long‐term cardiac complications, which can ultimately lead to congestive heart failure.49 Anthracycline‐based adjuvant chemotherapy in particular carries a substantial long‐term risk of heart failure. The mechanism of action of anthracyclines involves intercalation between DNA base pairs, inhibition of DNA topoisomerase II with subsequent blocking of replication and transcription, and the generation of iron‐mediated oxygen free radicals that damage DNA as well as proteins and cell membranes.50 These oxygen free radicals are thought to play a central role in the evolution of the cardiotoxic effects of anthracyclines.8 Moreover, several lines of evidence suggest that cytotoxic damage caused by anthracyclines leads to compensatory alterations in autonomic tone, which may have important implications for heart rate reserve and CRF.51–52 Radiotherapy used to treat breast cancer can cause cardiac perfusion defects that are associated with abnormalities in regional wall motion.53 Furthermore, radiotherapy‐induced cardiotoxicity is amplified by the use of adjuvant systemic chemotherapy—particularly anthracycline‐based regimens54 and newer agents such as trastuzumab.55
Jones et al17 has previously shown that mean Vo2max is markedly impaired in a population of breast cancer patients despite normal cardiac function (as indicated by LVEF ≥50%). This important finding suggests that injury to other components of oxygen transport (ie, pulmonary, hematologic, vascular, and skeletal muscle function) must contribute to CRF decline.17 In particular, incidental radiation to the lungs during radiotherapy for breast cancer causes fibrosis and a subsequent impairment in pulmonary gas exchange.56 Anemia, a frequent complication of treatment while undergoing therapy,57 reduces oxygen delivery to muscle cells.58 Dolan et al16 found a significant correlation between hemoglobin levels and percent change in Vo2max in women receiving adjuvant therapy for breast cancer. Both radiotherapy and chemotherapy for breast cancer, especially anthracyline‐containing chemotherapy, cause increases in reactive oxygen species generation, which can lead to endothelial injury, endothelial dysfunction, vascular remodeling, and increased arterial stiffness,59 further affecting oxygen delivery. Finally, preclinical studies have shown that anthracyclines impair both maximal twitch force and muscle relaxation.60–61 Such impairments in skeletal muscle function decrease oxygen utilization and thereby contribute to reduced CRF. To compound the effects of chemotherapy, numerous studies have also shown that chemotherapy negatively affects skeletal muscle mass in cancer patients. Although chemotherapy is associated with weight gain among breast cancer patients,62–67 chemotherapy‐induced weight gain tends to occur in the absence of gains in muscle mass or in the presence of muscle loss and can lead to the development of sarcopenic obesity.66
Importantly, the summation of these insults decreases cardiopulmonary reserve and increases the susceptibility of female breast cancer patients to late‐occurring adverse cardiovascular effects and premature mortality. This phenomenon has been labeled the “multiple hit hypothesis” by Jones et al.49 In keeping with this hypothesis, women with breast cancer in the current review at 50 years of age had a similar CRF to sedentary, 60‐year‐old women without a history of breast cancer (Figure 2). This finding points to an accelerated aging process among female breast cancer patients that can negatively affect CRF and potentially prognosis.
Recent evidence suggests that exercise training is an effective intervention to improve CRF as well as quality of life, physical functioning/strength, and symptoms of fatigue in breast cancer patients.2 These findings are supported in several of the studies presented in this review. For example, Courneya et al39 reported a 14.5% increase in Vo2max after 15 weeks of aerobic exercise training at an intensity of 70% to 75% of maximal oxygen consumption. Daley et al24 also demonstrated significant increases in Vo2max with both light (<40% of maximum heart rate) and moderate (65% to 85% of maximum heart rate) intensity exercise training for 8 weeks. Scott et al28 showed a 32% increase in Vo2max after a 24‐week lifestyle intervention combining exercise and a hypocaloric healthy eating program. Exercise interventions that resulted in significant improvements in Vo2max varied widely, ranging from home‐based30 and telephone‐based35 interventions to individual20,41 and group34,23,38–39,24,26–28 sessions with certified trainers. Interventions also varied with respect to type of exercise, intensity of exercise, and study length. Further study is needed to understand the propensity of newer exercise strategies as well as combinations of treatments (ie, statins, angiotensin‐converting enzyme inhibitors, β‐blockers) to protect against cardiotoxicity leading to lower CRF.
We analyzed data presented in 27 selected clinical studies with patient populations ranging from 7 to 257 women. Although we were able to draw several conclusions about CRF in breast cancer patients based on these studies, further work is required to assess CRF in ethnically diverse populations at multiple different time points in breast cancer treatment. Importantly, the timing of these decrements and potential recovery can be determined with prospective studies evaluating sequential changes in CRF across patient populations receiving adjuvant therapy regimens.46 For exercise training interventions to maximally decrease cardiovascular risk in the breast cancer population, interventions should be used before, during, and after treatment, as well as specifically at the time of greatest CRF decline. Other therapeutic modalities, such as statin therapy, angiotensin‐converting enzyme inhibitors, and β‐blockers, should also be considered in the setting of cardiac dysfunction.
In summary, breast cancer patients are subjected to sequential cardiovascular insults throughout their treatment regimens that decrease their CRF and increase their susceptibility to premature cardiovascular mortality compared with the general population.17,49 CRF has been shown to be a strong predictor of the adverse health outcomes that can result from breast cancer treatment regimens. Normative Vo2max data for this patient population will allow healthcare providers to predict which patients are at particular risk for late‐occurring cardiotoxicity that may be amenable to exercise interventions. This is of key importance given that exercise training can ameliorate impairments in Vo2max and may thereby have the potential to enhance prognosis in breast cancer patients and in other cancer patients as well.2,68
Disclosures
None.
Acknowledgments
Dr Lakoski is supported in part by the National Institute of General Medical Sciences/National Institutes of Health (P20GM103644‐01A1).
References
- 1.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010; 19:1893-1907 [DOI] [PubMed] [Google Scholar]
- 2.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta‐analysis. Can Med Assoc J. 2006; 175:34-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor DL, Nichols JF, Pakiz B, Bardwell WA, Flatt SW, Rock CL. Relationships between cardiorespiratory fitness, physical activity, and psychosocial variables in overweight and obese breast cancer survivors. Int J Behav Med. 2010; 17:264-270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R, Desantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012; 62:220-241 [DOI] [PubMed] [Google Scholar]
- 5.Jones LW, Haykowsky M, Pituskin EN, Jendzjowsky NG, Tomczak CR, Haennel RG, Mackey JR. Cardiovascular reserve and risk profile of postmenopausal women after chemoendocrine therapy for hormone receptor‐positive operable breast cancer. Oncologist. 2007; 12:1156-1164 [DOI] [PubMed] [Google Scholar]
- 6.Jones LW, Haykowsky M, Peddle CJ, Joy AA, Pituskin EN, Tkachuck LM, Courneya KS, Slamon DJ, Mackey JR. Cardiovascular risk profile of patients with HER2/neu‐positive breast cancer treated with anthracycline‐taxane‐containing adjuvant chemotherapy and/or trastuzumab. Cancer Epidemiol Biomarkers Prev. 2007; 16:1026-1031 [DOI] [PubMed] [Google Scholar]
- 7.Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011; 13:1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shan K, Lincoff AM, Young JB. Anthracycline‐induced cardiotoxicity. Ann Intern Med. 1996; 125:47-58 [DOI] [PubMed] [Google Scholar]
- 9.Van Hoff DD, Rozencweig M, Layard M. Daunomycin‐induced cardiotoxicity in children and adults: review of 110 cases. Am J Med. 1977; 62:200-208 [DOI] [PubMed] [Google Scholar]
- 10.Cuzick J, Stewart H, Rutqvist L, Houghton J, Edwards R, Redmond C, Peto R, Baum M, Fisher B, Host H. Cause specific mortality in long term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 1994; 12:447-453 [DOI] [PubMed] [Google Scholar]
- 11.Floyd JD, Nguyen DT, Lobins RL, Bashir Q, Doll DC, Perry MC. Cardiotoxicity of cancer therapy. J Clin Oncol. 2005; 23:7685-7696 [DOI] [PubMed] [Google Scholar]
- 12.Piccart‐Gebhart MJ, Procter M, Leyland‐Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD. Trastuzumab after adjuvant chemotherapy in HER2‐positive breast cancer. N Engl J Med. 2005; 353:1659-1672 [DOI] [PubMed] [Google Scholar]
- 13.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan‐Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2‐positive breast cancer. N Engl J Med. 2005; 353:1673-1684 [DOI] [PubMed] [Google Scholar]
- 14.Wasserman K, Hansen JE, Sue DY, Stringer WW, Sietsema KE, Sun XG, Whipp B. Chapter 5: Principles of Exercise Testing and Interpretation. 20125th edPhiladelphia: Lippincott Williams & Wilkins [Google Scholar]
- 15.Weisman IM, Zeballos RJ. An integrated approach to the interpretation of cardiopulmonary exercise testing. Clin Chest Med. 1994; 15:421-445 [PubMed] [Google Scholar]
- 16.Dolan LB, Gelmon K, Courneya KS, Mackey JR, Segal RJ, Lane K, Reid RD, McKenzie DC. Hemoglobin and aerobic fitness changes with supervised exercise training in breast cancer patients receiving chemotherapy. Cancer Epidemiol Biomarkers Prev. 2010; 19:2826-2832 [DOI] [PubMed] [Google Scholar]
- 17.Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, Hornsby WE, Coan AD, Herndon JE, II, Douglas PS, Haykowsky M. Cardiopulmonary function and age‐related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012; 30:2530-2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaminsky LA, Arena R, Beckie TM, Brubaker PH, Church TS, Forman DE, Franklin BA, Gulati M, Lavie CJ, Myers J, Patel MJ, Piña IL, Weintraub WS, Williams MA. The importance of cardiorespiratory fitness in the United States: the need for a national registry. A policy statement from the American Heart Association. Circulation. 2013; 127:652-662 [DOI] [PubMed] [Google Scholar]
- 19.Jones LW, Hornsby WE, Goetzinger A, Forbes LM, Sherrard EL, Quist M, Lane AT, West M, Eves ND, Gradison M, Coan A, Herndon JE, Abernethy AP. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non‐small cell lung cancer. Lung Cancer. 2012; 76:248-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider CM, Hsieh CC, Sprod LK, Carter SD, Hayward R. Effects of supervised exercise training on cardiopulmonary function and fatigue in breast cancer survivors during and after treatment. Cancer. 2007; 110:918-925 [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald MD, Tanaka H, Tran ZV, Seals DR. Age‐related declines in maximal aerobic capacity in regularly exercising vs sedentary women: a meta‐analysis. J Appl Physiol. 1997; 83:160-165 [DOI] [PubMed] [Google Scholar]
- 22.Holmes DA, Buhr KA. Error propagation in calculated ratios. Clin Biochem. 2007; 40:728-734 [DOI] [PubMed] [Google Scholar]
- 23.Brdareski Z, Djurovic A, Susnjar S, Zivotic‐Vanovic M, Ristic A, Konstantinovic L, Vuckovic‐Dekic L, Tankosic M. Effects of a short‐term differently dosed aerobic exercise on maximum aerobic capacity in breast cancer survivors: a pilot study. Vojnosanit Pregl. 2012; 69:237-242 [PubMed] [Google Scholar]
- 24.Daley AJ, Crank H, Saxton JM, Mutrie N, Coleman R, Roalfe A. Randomized trial of exercise therapy in women treated for breast cancer. J Clin Oncol. 2007; 25:1713-1721 [DOI] [PubMed] [Google Scholar]
- 25.Herrero F, San Juan AF, Fleck SJ, Balmer J, Pérez M, Cañete S, Earnest CP, Foster C, Lucía C. Combined aerobic and resistance training in breast cancer survivors: a randomized, controlled pilot trial. Int J Sports Med. 2006; 27:573-580 [DOI] [PubMed] [Google Scholar]
- 26.Herrero F, San Juan AF, Fleck SJ, Foster C, Lucía A. Effects of detraining on the functional capacity of previously trained breast cancer survivors. Int J Sports Med. 2007; 28:257-264 [DOI] [PubMed] [Google Scholar]
- 27.Rahnama N, Nouri R, Rahmaninia F, Damirchi A, Emami H. The effects of exercise training on maximum aerobic capacity, resting heart rate, blood pressure, and anthropometric variables of postmenopausal women with breast cancer. J Res Med Sci. 2010; 15:78-83 [PMC free article] [PubMed] [Google Scholar]
- 28.Scott E, Daley AJ, Doll H, Woodroofe N, Coleman RE, Mutrie N, Crank H, Powers HJ, Saxton JM. Effects of an exercise and hypocaloric healthy eating program on biomarkers associated with long‐term prognosis after early‐stage breast cancer: a randomized controlled trial. Cancer Causes Control. 2013; 24:181-191 [DOI] [PubMed] [Google Scholar]
- 29.Turner J, Hayes S, Reul‐Hirche H. Improving the physical status and quality of life of women treated for breast cancer: a pilot study of a structured exercise intervention. J Surg Oncol. 2004; 86:141-146 [DOI] [PubMed] [Google Scholar]
- 30.Vincent F, Labourey JL, Leobon S, Antonini MT, Lavau‐Denes S, Tubiana‐Mathieu N. Effects of a home‐based walking training program on cardiorespiratory fitness in breast cancer patients receiving adjuvant chemotherapy: a pilot study. Eur J Phys Rehabil Med. 2013; 49:319-329 [PubMed] [Google Scholar]
- 31.Borenstein M, Higgins JPT. Meta‐analysis and subgroups. Prev Sci. 2013; 14:134-143 [DOI] [PubMed] [Google Scholar]
- 32.Higgins JPT, Thompson SG. Quantifying heterogeneity in meta‐analysis. Stat Med. 2002; 21:1539-1558 [DOI] [PubMed] [Google Scholar]
- 33.Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, Ladha AB, Proulx C, Vallance JKH, Lane K, Yasui Y, McKenzie DC. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007; 25:4396-4404 [DOI] [PubMed] [Google Scholar]
- 34.Kolden GG, Strauman TJ, Ward A, Kuta J, Woods TE, Schneider KL, Heerey E, Sanborn L, Burt C, Millbrandt L, Kalin NH, Stewart JA, Mullen B. A pilot study of group exercise training (GET) for women with primary breast cancer: feasibility and health benefits. Psycho‐oncology. 2002; 11:447-456 [DOI] [PubMed] [Google Scholar]
- 35.Ligibel JA, Patridge A, Giobbie‐Hurder A, Campbell N, Shockro L, Salinardi T, Winer EP. Physical and psychological outcomes among women in a telephone‐based exercise intervention during adjuvant therapy for early stage breast cancer. J Women's Health. 2010; 19:1553-1559 [DOI] [PubMed] [Google Scholar]
- 36.Segal R, Evans W, Johnson D, Smith J, Colletta S, Gayton J, Woodard S, Wells G, Reid R. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001; 19:657-665 [DOI] [PubMed] [Google Scholar]
- 37.Burnett D, Kluding P, Porter C, Fabian C, Klemp J. Cardiorespiratory fitness in breast cancer survivors. Springerplus. 2013; 2:1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell KL, Van Patten CL, Neil SE, Kirkham AA, Gotay CC, Gelmon KA, McKenzie DC. Feasibility of a lifestyle intervention on body weight and serum biomarkers in breast cancer survivors with overweight and obesity. J Acad Nutr Diet. 2012; 112:559-567 [DOI] [PubMed] [Google Scholar]
- 39.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life measures. J Clin Oncol. 2003; 21:1660-1668 [DOI] [PubMed] [Google Scholar]
- 40.FiIllion L, Gagnon P, Leblond F, Gélinas C, Savard J, Dupuis R, Duval K, Larochelle M. A brief intervention for fatigue management in breast cancer survivors. Cancer Nurs. 2008; 31:145-159 [DOI] [PubMed] [Google Scholar]
- 41.Hsieh CC, Sprod LK, Hydock DS, Carter SD, Hayward R, Schneider CM. Effects of a supervised exercise intervention on recovery from treatment regimens in breast cancer survivors. Oncol Nurs Forum. 2008; 35:909-915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hutnick NA, Williams NI, Kraemer WJ, Orsega‐Smith E, Dixon RH, Bleznak AD, Mastro AM. Exercise and lymphocyte activation following chemotherapy for breast cancer. Med Sci Sports Exerc. 2005; 37:1827-1835 [DOI] [PubMed] [Google Scholar]
- 43.Musanti R. A study of exercise modality and physical self‐esteem in breast cancer survivors. Med Sci Sports Exerc. 2012; 44:352-361 [DOI] [PubMed] [Google Scholar]
- 44.Rogers LQ, Hopkins‐Price P, Vicari S, Pamenter R, Courneya KS, Markwell S, Verhulst S, Hoelzer K, Naritoku C, Jones L, Dunnington J, Lanzotti V, Wynstra J, Shah L, Edson B, Graff A, Lowy M. A randomized trial to increase physical activity in breast cancer survivors. Med Sci Sports Exerc. 2009; 41:935-946 [DOI] [PubMed] [Google Scholar]
- 45.Tosti KP, Hackney AC, Battaglini CL, Evans ES, Groff D. Exercise in patients with breast cancer and healthy controls: energy substrate oxidation and blood lactate responses. Integr Cancer Ther. 2011; 10:6-15 [DOI] [PubMed] [Google Scholar]
- 46.Lakoski SG, Barlow CE, Koelwyn GJ, Hornsby WE, Hernandez J, DeFina LF, Radford NB, Thomas SM, Herndon JE, II, Peppercorn J, Douglas PS, Jones LW. The influence of adjuvant therapy on cardiorespiratory fitness in early‐stage breast cancer seven years after diagnosis: the Cooper Center Longitudinal Study. Breast Cancer Res Treat. 2013; 183:909-916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002; 346:793-801 [DOI] [PubMed] [Google Scholar]
- 48.Barlow CE, Defina LF, Radford NB, Berry JD, Cooper KH, Haskell WL, Jones LW, Lakoski SG. Cardiorespiratory fitness and long‐term survival in “low‐risk” adults. J Am Heart Assoc. 2012; 1:1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007; 50:1435-1441 [DOI] [PubMed] [Google Scholar]
- 50.Carvalho FS, Burgeiro A, Garcia R, Moreno AJ, Carvalho RA, Oliveira PJ. Doxorubicin‐induced cardiotoxicity: from bioenergetic failure to cell death and cardiomyopathy. Med Res Rev. 2014; 34:106-135 [DOI] [PubMed] [Google Scholar]
- 51.Kenk M, Thackeray JT, Thorn SL, Dhami K, Chow BJ, Ascah KJ, DaSilva JN, Beanlands RS. Alterations of pre‐ and postsynaptic noradrenergic signaling in a rat model of adriamycin‐induced cardiotoxicity. J Nucl Cardiol. 2010; 17:254-263 [DOI] [PubMed] [Google Scholar]
- 52.Nousiainan T, Vanninen E, Jantunen E, Remes J, Ritanens E, Vuolteenaho O, Hartikainen J. Neuroendocrine changes during the evolution of doxorubicin‐induced left ventricular dysfunction in adult lymphoma patients. Clin Sci. 2001; 101:601-607 [PubMed] [Google Scholar]
- 53.Marks LB, Yu X, Prosnitz RG, Zhou S, Hardenbergh PH, Blazing M, Hollis D, Lind P, Tisch A, Wong TZ, Borges‐Neto S. The incidence and functional consequences of RT‐associated cardiac perfusion defects. Int J Radiat Oncol. 2005; 63:214-223 [DOI] [PubMed] [Google Scholar]
- 54.Billingham ME, Briston MR, Glatstein E, Mason JW, Masek MA, Daniels JR. Adriamycin cardiotoxicity: endomyocardial biopsy evidence of enhancement by irradiation. Am J Surg Pathol. 1977; 1:17-23 [PubMed] [Google Scholar]
- 55.Farolfi A, Melegari E, Aquilina M, Scarpi E, Ibrahim T, Maltoni R, Sarti S, Cecconetto L, Pietri E, Ferrario C, Fedeli A, Faedi M, Nanni O, Frassineti GL, Amadori D, Rocca A. Trastuzumab‐induced cardiotoxicity in early breast cancer patients: a retrospective study of possible risk and protective factors. Heart. 2013; 99:634-639 [DOI] [PubMed] [Google Scholar]
- 56.Marks LB, Munley MT, Bentel GC, Zhou SM, Hollis D, Scarfone C, Sibley GS, Kong FM, Jirtle R, Jaszczak R, Coleman RE, Tapson V, Anscher M. Physical and biological predictors of changes in whole‐lung function following thoracic irradiation. Int J Radiat Oncol Biol Phys. 1997; 39:563-570 [DOI] [PubMed] [Google Scholar]
- 57.Grotto HZ. Anaemia of cancer: an overview of mechanisms involved in its pathogenesis. Med Oncol. 2008; 25:12-21 [DOI] [PubMed] [Google Scholar]
- 58.Lakoski SG, Eves ND, Douglas PS, Jones LW. Exercise rehabilitation in patients with cancer. Nat Rev Clin Oncol. 2012; 9:288-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beckman JA, Thakore A, Kalinowski BH, Harris JR, Creager MA. Radiation therapy impairs endothelium‐dependent vasodilation in humans. J Am Coll Cardiol. 2001; 37:761-765 [DOI] [PubMed] [Google Scholar]
- 60.Hydock DS, Lien CY, Schneider CM, Hayward R. Effects of voluntary wheel running on cardiac function and myosin heavy chain in chemically gonadectomized rats. Am J Physiol Heart Circ Physiol. 2007; 293:254-264 [DOI] [PubMed] [Google Scholar]
- 61.van Norren K, van Helvoort A, Argilés JM, van Tuijl S, Arts K, Gorselink M, Laviano A, Kegler D, Haagsman HP, van der Beek EM. Direct effects of doxorubicin on skeletal muscle function contribute to fatigue. Br Cancer J. 2009; 100:311-314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aslani A, Smith RC, Allen BJ, Levi JA. Changes in body composition during adjuvant therapy for breast cancer. Appl Radiat Isot. 1998; 49:637-638 [DOI] [PubMed] [Google Scholar]
- 63.Aslani A, Smith RC, Allen BJ, Pavlakis N, Levi JA. Changes in body composition during breast cancer chemotherapy with the CMF regimen. Breast Cancer Res Treat. 1999; 57:285-290 [DOI] [PubMed] [Google Scholar]
- 64.Cheney CL, Mahloch J, Freeny P. Computerized tomography assessment of women with weight changes associated with adjuvant treatment for breast cancer. Am J Clin Nutr. 1997; 66:141-146 [DOI] [PubMed] [Google Scholar]
- 65.Demark‐Wahnefried W, Hars V, Conaway MR, Havlin K, Rimer BK, McElveen G, Winer EP. Reduced rates of metabolism and decreased physical activity in breast cancer patients receiving adjuvant therapy. Am J Clin Nutr. 1997; 65:1495-1501 [DOI] [PubMed] [Google Scholar]
- 66.Demark‐Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK, Blackwell K, Rimer BK. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001; 19:2381-2389 [DOI] [PubMed] [Google Scholar]
- 67.Kutynek CL, McCargar L, Barr SI, Hislop TG. Energy balance in women with breast cancer during adjuvant chemotherapy. J Am Diet Assoc. 1999; 99:1222-1227 [DOI] [PubMed] [Google Scholar]
- 68.Fairey AS, Courneya KS, Field CJ, Bell BJ, Jones LW, Martin BS, Mackey JR. Effect of exercise training on C‐reactive protein in postmenopausal breast cancer survivors: a randomized controlled trial. Brain Behav Immun. 2005; 19:381-388 [DOI] [PubMed] [Google Scholar]


