Abstract
Background
Inflammation is fundamental to the development of atherosclerosis. We examined the effect of anti‐inflammatory doses of salicylate on endothelium‐dependent vasodilation, a biomarker of cardiovascular risk, in a broad range of subjects.
Methods and Results
We performed a randomized, double‐blind, placebo‐controlled crossover trial evaluating the effects of 4 weeks of high‐dose salsalate (disalicylate) therapy on endothelium‐dependent flow‐mediated and endothelium‐independent vasodilation. Fifty‐eight subjects, including 17 with metabolic syndrome, 13 with atherosclerosis, and 28 healthy controls, were studied. Among all subjects, endothelium‐dependent flow‐mediated vasodilation decreased after salsalate compared with placebo therapy (P=0.01), whereas nitroglycerin‐mediated, endothelium‐independent vasodilation was unchanged (P=0.97). Endothelium‐dependent flow‐mediated vasodilation after salsalate therapy was impaired compared with placebo therapy in subjects with therapeutic salicylate levels (n=31, P<0.02) but not in subjects with subtherapeutic levels (P>0.2).
Conclusions
Salsalate therapy, particularly when therapeutic salicylate levels are achieved, impairs endothelium‐dependent vasodilation in a broad range of subjects. These data raise concern about the possible deleterious effects of anti‐inflammatory doses of salsalate on cardiovascular risk.
Clinical Trial Registration
URL: www.clinicaltrials.gov. Unique Identifiers: NCT00760019 and NCT00762827.
Keywords: atherosclerosis, endothelium, glucose, inflammation, vasodilation
Introduction
Abundant evidence implicates inflammation in the pathogenesis of atherosclerosis.1 The recent Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) study, in which intensive statin therapy reduced cardiovascular events compared with placebo in patients with normal LDL cholesterol but high C‐reactive protein (CRP) levels, has intensified interest in the therapeutic potential of anti‐inflammatory therapy in cardiovascular disease.2 Clinical trials are currently under way testing the efficacy of proven anti‐inflammatory drugs as cardiovascular therapeutic agents.3–4
The proinflammatory transcription mediator nuclear factor (NF)‐κB has emerged as a potential therapeutic target in cardiovascular disease.5 Both systemic pharmacological and endothelium‐specific targeting of NF‐κB reduced plaque burden in experimental atherosclerosis.6–7 Recently, endothelium‐specific transgenic blockade of the intracellular NF‐κB pathway reduced vascular inflammation and oxidative stress and prevented vascular senescence in aged and obese mice.8
Low‐dose salicylates (eg, aspirin 81 mg/day) are broadly used in the prevention and treatment of atherosclerotic vascular disease. At a low dose, suppression of platelet thromboxane synthesis by aspirin dominates suppression of endothelial prostacyclin synthesis, and a net antithrombotic effect is achieved. A higher dose of salicylates (≥2 g/day) is required to exert a systemic anti‐inflammatory effect. The underlying mechanism is not well understood but is proposed to be in large part due to direct suppression of inhibitor of κB kinase (IKK) activity, thereby limiting upregulation of NF‐κB activity.9
Salicylates may also improve insulin sensitivity by inhibiting IKK‐induced serine phosphorylation of insulin receptor substrate‐1.10 Indeed, high‐dose salicylate therapy suppresses obesity‐ and diet‐induced inflammation and insulin resistance in experimental models.11–12 In clinical studies, high‐dose salicylate therapy improves glycemia in diabetes mellitus.13–14 The Targeting INflammation using SALsalate in CardioVascular Disease (TINSAL‐CVD) study (NCT00624923) is currently testing the potential for high‐dose salicylate treatment to improve glycemia and reduce coronary artery atherosclerotic plaque volume in diabetes.
Endothelial insulin signaling results in activation of endothelial nitric oxide synthase (eNOS) and endothelium‐dependent, nitric oxide (NO)‐mediated vasodilation. High‐dose salicylate therapy, by restoring endothelial insulin signaling, may improve endothelial function in states of inflammation and insulin resistance.15–16 We therefore hypothesized that chronic high‐dose salicylate therapy would improve endothelial function in persons with insulin resistance, identified by the presence of the metabolic syndrome, and in persons with atherosclerosis. The results of our clinical translational study suggest that high‐dose salicylate therapy may not have the intended effect on endothelial function.
Methods
Subject Selection
This report presents the combined results of 2 methodologically identical, simultaneously conducted studies (NCT00760019 and NCT00762827). One study involved subjects with the metabolic syndrome and the other, those with atherosclerosis. Each study also recruited a group of healthy controls that are combined for the purpose of this analysis.
Subjects were recruited by advertisement and from the clinical practices at Brigham and Women's Hospital. The presence of atherosclerosis was defined as a prior myocardial infarction, previous coronary lesion >70%, previous stroke or transient ischemic attack, internal carotid artery stenosis >70%, ankle‐brachial index <0.90, or previous lower extremity revascularization or amputation. The metabolic syndrome was defined as the presence of at least 3 of the following 5 symptoms as defined by the National Cholesterol Education Program (NCEP): abdominal obesity (waist circumference ≥88.9 cm for women, and ≥101.6 cm for men), impaired fasting glucose (5.55 mmol/L [100 mg/dL] ≤ glucose< 6.99 mmol/L [126 mg/dL]), elevated fasting triglycerides (≥1.69 mmol/L [150 mg/dL]), hypertension (blood pressure ≥135/85 mm Hg), and low HDL cholesterol (<1.30 mmol/L [50 mg/dL] for women, or <1.04 mmol/L [40 mg/dL] for men). For both groups, exclusion criteria included uncontrolled hypertension (≥160/100 mm Hg), untreated hyperlipidemia (LDL ≥4.14 mmol/L [160 mg/dL]), diabetes mellitus, current tobacco use, hypersensitivity to salicylates, increased bleeding risk (eg, thrombocytopenia, hemorrhagic stroke within 1 year, or use of warfarin), ingestion of >5 alcoholic beverages/week, elevation of liver enzymes (ALT ≥2× upper limit of normal [ULN]), and serum creatinine ≥106.76 mmol/L (1.4 mg/dL). Healthy subjects did not have a prior history of cardiovascular disease or any symptoms, physical findings, or electrocardiographic findings indicative of atherosclerosis. Additional exclusion criteria included a chronic inflammatory disorder (eg, rheumatoid arthritis), active malignancy, fasting glucose ≥5.55 mmol/L (100 mg/dL), LDL ≥4.92 mmol/L (190 mg/dL), blood pressure >140/90 mm Hg, smoking within 1 year, serum creatinine ≥106.76 mmol/L (1.4 mg/dL), and ALT ≥2× ULN.
Study Design
The study design was a randomized, placebo‐controlled, double‐blind crossover trial. Treatment periods were 4 weeks each, with a 4‐week washout interval between treatments. Four weeks was chosen based on a demonstrated effect of salsalate in that time frame17 and to ensure repeat evaluation at the same phase of the female reproductive cycle. The Investigational Drug Service at Brigham and Women's Hospital performed randomization at the time of first study drug allocation. Study visits occurred at the end of each treatment period. All tests were conducted in the morning after an overnight fast.
Interventions
Treatment arms were salsalate 4.5 g/day or matching placebo. Three 500‐mg Disalcid tablets (Caraco Pharmaceutical Laboratories) were taken 3 times daily. Subjects who experienced a treatment‐associated side effect were asked to reduce the total dose to 4 and, if necessary, 3 g/day. Treatment adherence was assessed by pill count. Ranitidine 150 mg twice daily (atherosclerosis study) (Apotex Corporation) or omeprazole 20 mg once daily (metabolic study) (Sandoz, Inc) was administered during each treatment period to minimize gastrointestinal intolerance.
A total of 126 subjects provided informed consent, and 56 completed the study. Fifty‐seven subjects were excluded from participation as a result of not meeting inclusion criteria. Of the 69 subjects who met inclusion criteria and were offered continued participation, 56 subjects completed the protocol and 13 discontinued the study due to 1 or more adverse events: tinnitus, 5; rash, 3; gastrointestinal distress, 3; dizziness, 1; constipation, 2; and shortness of breath, 1. These adverse events occurred at rates similar to those of published clinical trials.13,18 Post hoc unblinding revealed that 1 constipation event and the shortness of breath event occurred with placebo, while the other adverse events occurred during salsalate treatment. Among those who completed the study, 6 had study drug dose reduced to 3 g/day due to tinnitus, which resolved after dose reduction.
Assessment of Vascular Function: Flow‐Mediated Vasodilation
Brachial artery ultrasonography was performed in a temperature‐controlled, quiet environment to evaluate vascular function after each treatment period. An ultrasound scanner (General Electric Vivid 7) equipped with a high‐resolution broadband linear array transducer (7.5 to 12.5 MHz) was used to image the brachial artery. Endothelium‐dependent and ‐independent dilations of the brachial artery were determined as we have done previously and according to established guidelines.19–20
Two independent reviewers performed acquisition and analysis of the digitized images in a blinded manner using software from Medical Imaging Applications, LLC. The vessel wall–lumen interface was determined using a derivative‐based edge detection algorithm after identification of the region of interest by the investigator. The maximum diameter of the vessel was then determined. In our laboratory, this method is associated with an interobserver variability of 0.05±0.16% and intraobserver variability of 0.01±0.15%. The same arm and site were used for all measurements.
Laboratory Measurements
Blood for laboratory assessment was collected after each treatment period and was either processed immediately or centrifuged and stored in aliquots in a −80°C freezer for future analyses. The clinical laboratory personnel at Brigham and Women's Hospital performed routine laboratory tests. Insulin levels were measured using the Beckman Access II immunoassay platform (GMI, Inc). Salicylate levels were measured using the Olympus AU640 analyzer (Beckman Coulter Ltd). The concentration of nonesterified free fatty acids (FFAs) was determined using an in vitro enzymatic colorimetric assay on the Hitachi 917 analyzer (Roche Diagnostics) with reagents from Wako Chemicals USA. High‐sensitivity CRP was measured using an immunoturbidimetric assay on the Hitachi 917 analyzer (Roche Diagnostics) using reagents and calibrators from DiaSorin. Tumor necrosis factor (TNF)‐α receptor 2 (TNF‐αR2) was measured using an ELISA from R&D systems. Myeloperoxidase concentrations were determined using an ELISA from Alpco Diagnostics.
Statistical Analysis
Baseline characteristics are described as mean and SD or median and interquartile range (IQR), as appropriate. Comparison of baseline characteristics between groups was performed using ANOVA, Kruskal–Wallis equality‐of‐populations test, or χ2 test, as appropriate. The primary outcome assessment was to compare brachial artery FMD response after placebo with that after salsalate therapy. The distribution of FMD at both time points was not normally distributed (P=0.036 by Shapiro–Wilk test). Consequently, a Wilcoxon signed‐rank analysis was performed to assess the difference in median FMD between salsalate and placebo (the primary endpoint). We then used repeated‐measures ANOVA to assess carryover effect with terms for treatment (salsalate versus placebo), period (salsalate given first or second), and their interaction. Separate models assessed treatment effects on FMD and interactions between treatment and disease (atherosclerosis, metabolic syndrome, or controls) and the effect of salsalate on endothelium‐independent vasodilation, glucose homeostasis, fatty acids and lipids, and biomarkers of inflammation. Other subgroup descriptive analyses were by the paired Student t test or the Wilcoxon signed‐rank test, as appropriate. STATA/IC version 11.2 for MAC (StataCorp) was used for all analyses.
Results
Subject Characteristics
The subjects had a mean age of 56 years (Table 1). The mean age varied between subject groups, with the oldest being those with atherosclerosis. The mean body mass index was 30 kg/m2, with the highest body mass index among those with the metabolic syndrome. The distribution of sex and race did not vary significantly between groups.
Table 1.
Baseline Characteristics of the Study Subjects
| All | Healthy Controls | Patients With Metabolic Syndrome | Patients With Atherosclerosis | P Value | |
|---|---|---|---|---|---|
| N | 58 | 28 | 17 | 13 | n/a |
| Age, y | 56 (11) | 52 (13) | 57 (9) | 65 (8) | 0.005 |
| Men, N (%) | 38 (65) | 16 (57) | 10 (59) | 12 (92) | 0.07 |
| White, N (%) | 44 (76) | 21 (75) | 11 (65) | 12 (92) | 0.21 |
| BMI, kg/m2 | 30 (7) | 26 (4) | 35 (6) | 28 (4) | <0.001 |
Data are presented as mean (SD) or number (%). BMI indicates body mass index.
The mean plasma salicylate concentration during salsalate treatment among all subjects (0.90±0.69 mmol/L [12.4±9.5 mg/dL]) was within the therapeutic range (0.72 to 2.17 mmol/L [10 to 30 mg/dL]). Mean salicylate levels during salsalate treatment varied by subject group (P=0.01). The mean level in those with atherosclerosis (1.36± 0.74 mmol/L [18.8±10.2 mg/dL]) was higher than healthy subjects (0.73±0.69 mmol/L [10.1±9.5 mg/dL]) and those with the metabolic syndrome (0.80±0.49 mmol/L [11.0± 6.7 mg/dL]). Overall, 54% (n=30) of subjects' had salicylate levels within the therapeutic range. The proportion of subjects with salicylate levels below the therapeutic range did not vary between groups (P=0.19).
Flow‐Mediated Vasodilation
Among all subjects, baseline brachial artery diameter and the postocclusion reactive hyperemia stimulus were not different after placebo compared with after salsalate therapy (Table 2). Median FMD was significantly less after salsalate treatment (6.8% [IQR=5.2, 12.0]) compared with placebo treatment (8.7% [IQR=5.4, 13.5]) (P=0.01) (Figure 1). Nitroglycerin‐mediated, endothelium‐independent vasodilation was not different after placebo (12.6% [IQR=8.6, 19.7]) and salsalate (13.0% [IQR=8.5, 17.0]) treatments (P=0.97) (Figure 2).
Table 2.
Vascular Function Parameters
| Treatment | Baseline Diameter, mm | RH Stimulus | FMD, % | NMD, % |
|---|---|---|---|---|
| Placebo | 3.8 (3.3 to 4.3) | 5.8 (3.9 to 8.1) | 8.7 (5.4 to 13.5) | 12.6 (8.6 to 19.7) |
| Salsalate | 3.9 (3.3 to 4.3) | 6.3 (4.8 to 9.5) | 6.8* (5.2 to 12.0) | 13.0 (8.5 to 17) |
Data are presented as median (IQR). FMD indicates flow‐mediated dilation; NMD, nitroglycerin‐mediated dilation; RH, reactive hyperemia.
P=0.027.
Figure 1.
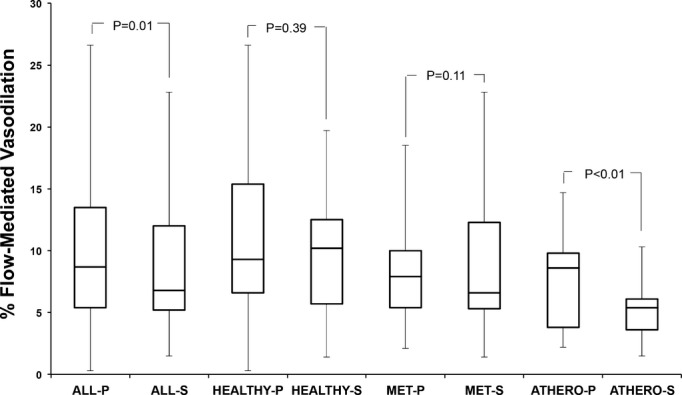
Flow‐mediated, endothelium‐dependent vasodilation of the brachial artery after placebo (P) and salsalate (S) therapy. Data shown are median (IQR). ATHERO indicates atherosclerosis; MET, metabolic syndrome.
Figure 2.
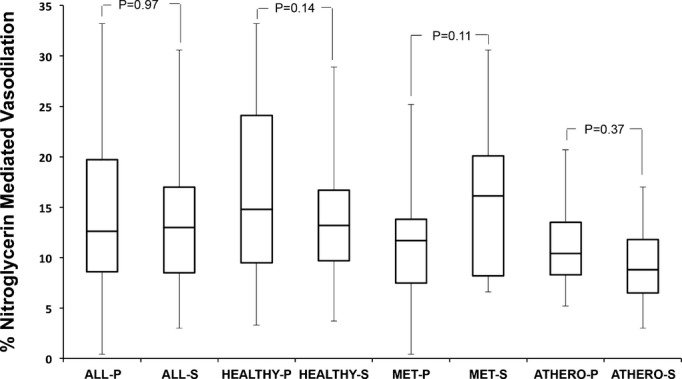
Nitroglycerin‐mediated, endothelium‐independent vasodilation of the brachial artery after placebo (P) and salsalate (S) therapy. Data shown are median (IQR). ATHERO indicates atherosclerosis; MET, metabolic syndrome.
In separate models, repeated‐measures ANOVA showed no significant carryover effect (treatment×period interaction P=0.57) and no significant difference in treatment effect between the subject groups with atherosclerosis or metabolic syndrome or healthy subjects (treatment×group interaction P=0.26). In post‐hoc analyses, within individual subject groups, FMD was lower in each group after salsalate compared with after placebo but reached statistical significance only in the subjects with atherosclerosis (P=0.009). Nitroglycerin‐mediated vasodilation did not change after salsalate treatment compared with placebo in any subject group.
Insulin Resistance and Inflammation
Fasting insulin levels trended higher after salsalate compared with after placebo (Table 3). Fasting glucose levels decreased significantly with salsalate treatment compared with placebo treatment, P=0.001). The rise in insulin and fall in glucose levels offset such that homeostatic model assessment insulin resistance (HOMAIR) did not differ after salsalate compared with placebo treatment.
Table 3.
Markers of Insulin Resistance and Inflammation After Placebo and Salsalate Treatment
| All | Healthy Controls | Patients With Metabolic Syndrome | Patients With Atherosclerosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Salsalate | P Value | Placebo | Salsalate | P Value | Placebo | Salsalate | P Value | Placebo | Salsalate | P Value | |
| Fasting glucose, mg/dL | 90 (80 to 99) | 82 (73 to 89) | <0.01 | 83 (78 to 97) | 79 (72 to 91) | 0.24 | 97 (92 to 104) | 88 (80 to 101) | 0.08 | 97 (89 to 102) | 82 (78 to 88) | 0.001 |
| Fasting insulin, pmol/L | 39 (21 to 60) | 48 (26 to 66) | 0.07 | 26 (22 to 65) | 30 (25 to 70) | 0.07 | 69 (51 to 89) | 69 (56 to 81) | 0.54 | 40 (21 to 48) | 45 (26 to 55) | 0.64 |
| HOMAIR | 1.6 | 1.7 | 0.68 | 1.0 | 1.2 | 0.14 | 2.8 | 2.7 | 0.64 | 1.8 | 1.7 | 0.84 |
| C‐reactive protein, mg/L | 1.18 (1.04 to 3.39) | 1.32 (1.11 to 2.92 | 0.64 | 1.34 (0.98 to 3.65) | 1.32 (0.9 to 3.52) | 0.81 | 1.85 (1.23 to 3.11) | 17.1 (11.4 to 29.5) | 0.07 | 0.39 (0.3 to 1.18) | 0.44 (0.18 to 3.06) | 0.42 |
| TNF‐α receptor 2, pg/mL | 2147 (1676 to 2716) | 1960 (1646 to 2554) | 0.17 | 1902 (1618 to 2689) | 1987 (1581 to 2720) | 0.48 | 2067 (1847 to 2299) | 1939 (1756 to 2241) | 0.69 | 2278 (2142 to 2716) | 1976 (1847 to 2541) | 0.22 |
| Myeloperoxidase, ng/mL | 13.1 (7.7 to 20.7) | 10.8 (7.2 to 17.2) | 0.16 | 15.0 (8.0 to 15.0) | 10.7 (7.3 to 18.0) | 0.63 | 16.0 (12.3 to 21.9) | 16.0 (12.0 to 16.0) | 0.22 | 7.6 (6.4 to 12.4) | 6.9 (5.2 to 9.4) | 0.38 |
Data presented as median (IQR). HOMAIR=[fasting insulin (mU/L)×fasting glucose (mmol/L)]/405. HOMAIR indicates homeostatic model assessment insulin resistance; TNF, tumor necrosis factor.
We measured plasma CRP, TNF‐αR2, and myeloperoxidase as markers of systemic inflammation. Levels of these markers were not significantly different after salsalate compared with placebo among all studied subjects or within any subject group (Table 3).
Other Parameters
Salsalate therapy did not change total or LDL cholesterol levels (Table 4). Triglycerides and FFAs were lower after salsalate therapy compared with placebo in all subjects combined, with a consistent trend in each individual subject group. HDL cholesterol was significantly higher in control subjects after salsalate treatment compared with placebo, with a numerically higher but not significantly different HDL level in the other subject groups. Of all the parameters measured, only the change in triglycerides associated with the change in FMD. Salsalate‐mediated reductions in triglyceride level correlated inversely with changes in FMD (Spearman's ρ −0.32, P=0.014). The inclusion of triglycerides as a covariate in a repeated‐measures ANOVA model with treatment indicated a significant positive effect of a fall in triglycerides and improved FMD (P=0.002) and strengthened the significance of the adverse treatment effect of salsalate on FMD (treatment P=0.004).
Table 4.
Lipid Levels, Blood Pressures, and Serum Creatinine After Placebo and Salsalate Therapy
| All Participants | Healthy Controls | Patients With Metabolic Syndrome | Patients With Atherosclerosis | |||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Salsalate | Placebo | Salsalate | Placebo | Salsalate | Placebo | Salsalate | |
| LDL‐C, mmol/L | 2.39 (1.88 to 3.26) | 2.59 (2.03 to 3.33) | 3.00 (2.16 to 3.37) | 3.00 (2.40 to 3.33) | 3.0 (2.03 to 3.37) | 2.9 (1.88 to 3.33) | 1.89 (1.78 to 2.51) | 2.20 (1.94 to 2.79) |
| HDL‐C, mmol/L, | 1.16 (1.04 to 1.36) | 1.20 (0.96 to 1.34)* | 1.20 (0.96 to 1.34) | 1.34 (1.03 to 1.40)* | 1.11 (0.83 to 1.29) | 1.14 (1.06 to 1.29) | 1.14 (1.06 to 1.29) | 1.19 (1.14 to 1.29) |
| Triglycerides, mmol/L | 1.4 (0.8 to 1.8) | 1.1 (0.6 to 1.3)‡ | 0.9 (0.7 to 1.1) | 0.7 (0.5 to 0.9) | 1.8 (1.4 to 2.4) | 1.4 (1.0 to 2.2)* | 1.0 (0.8 to 1.8) | 0.6 (0.5 to 1.0)† |
| FFA, mmol/L | 0.48 (0.41 to 0.88) | 0.35 (0.30 to 0.66)† | 0.41 (0.40 to 0.74) | 0.32 (0.30 to 0.57) | 0.81 (0.53 to 0.96) | 0.57 (0.49 to 0.67)* | 0.37 (0.32 to 0.60) | 0.33 (0.27 to 0.39) |
| SBP, mm Hg | 126 (110 to 137) | 121 (114 to 137) | 115 (104 to 128) | 120 (109 to 134) | 133 (126 to 140) | 127 (118 to 140) | 132 (121 to 136) | 122 (118 to 134) |
| DBP, mm Hg | 72 (67 to 79) | 73 (69 to 83) | 69 (66 to 75) | 74 (69 to 83) | 76 (68 to 82) | 76 (72 to 84) | 73 (70 to 77) | 71 (67 to 81) |
| SCr, mmol/L | 0.87 (0.17) | 0.94 (0.21)‡ | 0.84 (0.19) | 0.89 (0.22) | 0.87 (0.17) | 0.91 (0.19) | 0.94 (0.12) | 1.06 (0.17) |
Data are presented as median (IQR). DBP indicates diastolic blood pressure; FFA, free fatty acids; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; SCr, serum creatinine.
*P<0.05; †P<0.01; ‡P<0.001.
Salsalate therapy did not influence systolic or diastolic blood pressure. Serum creatinine was significantly higher after salsalate treatment compared with placebo, consistent with the previously described observation that salicylate decreases creatinine clearance independent of a change in the glomerular filtration rate.21
Results Stratified by Therapeutic Versus Nontherapeutic Salicylate Level
A salicylate level of ≥0.72 mmol/L (10 mg/dL) is considered necessary to achieve a systemic anti‐inflammatory effect.22 We therefore repeated our analysis of the effects of salsalate on FMD with subjects stratified by subtherapeutic (<0.72 mmol/L, n=27) or therapeutic (≥0.72 mmol/L, n=30) salicylate levels (Figure 3). We observed a significant decrement in FMD after salsalate compared with placebo therapy when therapeutic salicylate levels were achieved. In contrast, salsalate therapy did not alter endothelial function when salicylate levels were subtherapeutic (P=0.95). The brachial artery response to nitroglycerin was not influenced by salicylate level, nor was the brachial artery baseline diameter or the reactive hyperemia stimulus.
Figure 3.

Flow‐mediated, endothelium‐dependent vasodilation of the brachial artery after placebo (P) and salsalate (S) therapy in subjects with a therapeutic salicylate level and those with a subtherapeutic level. Data shown are median (IQR).
Analyses of CRP, TNF‐αR2, and myeloperoxidase stratified by therapeutic versus subtherapeutic salicylate level revealed lower myeloperoxidase levels (P=0.009) and a trend toward lowers TNF‐αR2 levels (P=0.07) when therapeutic salicylate levels were achieved. Despite a significant reduction in oxidant markers and a favorable trend in inflammatory markers, there was no association between the change in either oxidant or inflammatory markers and FMD. In contrast, there was no treatment effect on these inflammatory markers in subjects with subtherapeutic salicylate levels.
Discussion
The anti‐inflammatory effects of salicylates prompted us to examine the effect of salsalate on endothelial function. Contrary to our expectations, salsalate therapy was associated with impaired endothelium‐dependent vasodilation. This was particularly true when therapeutic salicylate levels were achieved at the time of vascular function testing. Salsalate did not alter endothelium‐independent vasodilation, indicating that the observed attenuation in vascular function was specific to the endothelium. This occurred in a broad range of subjects including healthy individuals, subjects with the metabolic syndrome, and those with atherosclerosis, suggesting that salsalate‐induced impairment in endothelial function is largely independent of the underlying health of the endothelium. Given that endothelium‐dependent vasodilation is a biomarker for cardiovascular risk, these results suggest caution against pursuing anti‐inflammatory high‐dose salicylate therapy as a strategy to prevent or treat cardiovascular disease.
The Effect of Salicylate on Endothelium‐Dependent Vasodilation
Contrary to our hypothesis, salsalate therapy attenuated endothelium‐dependent vasodilation. While our study cannot determine the mechanism by which salicylate impaired endothelium‐dependent vasodilation, we speculate that salicylate reduced the bioavailability of 1 or more of the endothelium‐derived vasodilators: NO, prostacyclin, and the endothelium‐derived hyperpolarizing factors. The mean reduction in FMD after salsalate therapy in healthy controls and in subjects with atherosclerosis was 16% and 44%, respectively. In our laboratory, complete inhibition of NO by NG‐monomethyl‐l‐arginine reduced FMD by 75% in healthy subjects and by 25% in subjects with atherosclerosis.23 This would suggest that salsalate impairs a vasodilator other than NO or a combination of NO and another endothelium‐derived vasodilator. Salsalate does not directly inhibit cyclooxygenase but can limit the up‐regulation of cyclooxygenase‐2 in the setting of inflammation.24 Clinical studies have shown that selective cyclooxygenase‐2 inhibition does not alter endothelium‐dependent vasodilation in healthy vessels,25 and either no change or an improvement in endothelium‐dependent vasodilation with cyclooxygenase‐2 inhibition has been reported in atherosclerosis.26–27 We therefore suspect that impairment in 1 or more of the endothelium‐derived hyperpolarizing factors may in part account for our findings. In addition to arachidonic acid metabolites derived from the cyclooxygenases, endothelium‐derived hyperpolarizing factors include arachidonic acid metabolites derived from lipoxygenases and cytochrome P450 pathways, hydrogen peroxide, carbon monoxide, and hydrogen sulfide. Additional experiments using inhibitors of each of these vasodilators are necessary to discern which, if any, of the endothelium‐derived vasodilators play a role in salsalate‐mediated reduction in endothelium‐dependent vasodilation.
We are not aware of a study examining the effect of high‐dose salicylate therapy on endothelial function in a preclinical model. Pierce et al found that 13 of 14 overweight/obese middle‐aged and older adults demonstrated improvement in brachial artery FMD after 4 days of high‐dose salicylate compared with placebo therapy.28 In addition, they found that subjects with the most impaired FMD at baseline experienced the greatest improvement after salsalate therapy. These results are the opposite of those observed in our study. Shorter duration of therapy and occlusive cuff location are the major differences between these studies' design, though it is unclear how either of these would account for the discordant results. Mean salicylate levels were lower overall in our study; however, higher therapeutic salicylate levels correlated with the most severe impairment of FMD (r=−0.36, P=0.05 in patients with therapeutic salsalate levels).
Few other clinical studies examining the effect of high‐dose salicylate therapy on endothelial function are available. Salsalate therapy has had conflicting results on endothelial function in patients with HIV.29–30 Tabit and colleagues found no effect of sulfasalazine therapy on endothelial function in patients with coronary artery disease.31 Similarly, Goldfine et al found no difference in FMD in diabetic subjects treated with high‐dose salsalate therapy.32 Thus, it appears that a definitive conclusion about the effect of salicylate therapy on endothelial function cannot be drawn from the available data.
Our results raise the possibility of an adverse cardiovascular effect of salsalate when present in therapeutic concentrations. Medications beneficial for cardiovascular disease have been shown to increase flow‐mediated vasodilation, such as statins19 or angiotensin‐converting enzyme inhibitors.33 However, there is no stimulus that impairs endothelial function that is associated with improved cardiovascular outcomes. Even medications that improve outcomes in cancer but worsen endothelial function increase adverse cardiovascular events.34–35 These findings suggest that salsalate may require a clinical trial to ensure its safety in patients with cardiovascular disease.
Salicylate and Insulin Resistance
Salicylate improves insulin sensitivity in experimental models, in part due to inhibition of IKK and prevention of serine phosphorylation of insulin receptor substrate‐1.16 Clinical studies, however, have had mixed results. Two groups of investigators found that acetylated salicylate decreased insulin‐stimulated glucose disposal in healthy subjects.36–37 In contrast, high‐dose aspirin increased insulin‐stimulated glucose disposal by ≈25% in subjects with type 2 diabetes.38 Goldfine and colleagues reported that high‐dose salsalate improved insulin‐stimulated glucose disposal by 43% in diabetes, and they subsequently showed that long‐term salsalate therapy achieved a 0.5% improvement in HbA1c.13–14 More recently, Goldfine and colleagues have published a large randomized trial of salsalate showing reductions in fasting glucose and an increase in fasting insulin.32 HOMAIR, however, remained similar at baseline and after salsalate therapy. Other groups, as well, did not find that salsalate improved insulin resistance.3940–42 In total, the available data do not provide a clear view of the effect of high‐dose salsalate therapy on insulin resistance.
Effect of Salicylate on Lipid Metabolism
Salsalate therapy significantly reduced FFA levels, as first reported by Carlson and Osiman.43 This is likely in part due to salicylate directly inhibiting release of FFAs from adipocytes, improving insulin signaling, and preventing TNF‐α–induced lipolysis.44–45 Reduction of FFA levels might be expected to improve endothelial function and/or insulin sensitivity in states of insulin resistance,16,46 but despite FFA levels falling ≈30% after salsalate therapy, no such improvements were noted in our study.16,46 We also observed a fall in triglycerides, possibly because of a direct relation between IKK‐β activation and triglyceride production within the hepatocyte.47 The decrease in triglyceride production provided a modest compensatory support for flow‐mediated vasodilation, but its effect was inadequate to offset the deleterious effect of therapeutic salicylate levels.
The Anti‐inflammatory Effect of Salicylate
The potential benefit of salicylate therapy on endothelial function is rooted in the anti‐inflammatory effects of salicylate and the adverse effects of inflammation on NO bioavailability.48 However, the mechanisms underlying the anti‐inflammatory effect of salicylates are not well understood. Several potential mechanisms by which salicylates are anti‐inflammatory have been proposed, and much of the focus has been on the ability of salicylate to inhibit the proinflammatory kinase IKK.49 IKK activates the proinflammatory and proatherosclerotic transcription mediator NF‐κB. Pharmacological or genetic inhibition of NF‐κB reduces aortic atherosclerotic plaque burden in atherosclerotic mice.6–7 Thus, NF‐κB suppression is a potential way in which salicylates are both anti‐inflammatory and antiatherosclerotic. However, it has also been proposed that salicylate exerts its anti‐inflammatory effects independent of NF‐κB.24,50–52 Goldfine and colleagues have reported that adipose tissue NF‐κB activity declines with salsalate treatment, but it did not correlate with metabolic improvements.32 Similarly, Tabit and colleagues have shown sulfasalazine‐mediated reductions in NF‐κB–regulated gene expression in peripheral blood mononuclear cells, but this treatment did not restore endothelial function in patients with coronary artery disease.53 Despite multiple postulated mechanisms, we found only minor changes in circulating markers of inflammation after salsalate therapy and cannot establish a role for anti‐inflammatory effects of this medication.
Conclusion
In this randomized, placebo‐controlled crossover trial of 4 weeks of high‐dose salsalate therapy in healthy control subjects, subjects with the metabolic syndrome, and subjects with atherosclerosis, we observed that high‐dose salsalate therapy worsened endothelial function and did not improve insulin sensitivity. Though favorable effects on FFA and triglyceride levels were observed, in total these data raise concern about the potential effects of long‐term high‐dose salicylate therapy on cardiovascular risk.
Sources of Funding
This research was supported by the American Diabetes Association (ADA 1‐06‐CD‐01). Dr Nohria was supported by a Clinical Scientist Development Award from the Doris Duke Charitable Foundation.
Disclosures
None.
References
- 1.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002; 105:1135-1143 [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. N Engl J Med. 2008; 359:2195-2207 [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the Cardiovascular Inflammation Reduction Trial (CIRT). J Thromb Haemost. 2009; 7suppl 1:332-339 [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin‐1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti‐inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J. 2011; 162:597-605 [DOI] [PubMed] [Google Scholar]
- 5.Liao F, Andalibi A, Qiao JH, Allayee H, Fogelman AM, Lusis AJ. Genetic evidence for a common pathway mediating oxidative stress, inflammatory gene induction, and aortic fatty streak formation in mice. J Clin Invest. 1994; 94:877-884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiba T, Kondo Y, Shinozaki S, Kaneko E, Ishigami A, Maruyama N, Umezawa K, Shimokado K. A selective NFkappaB inhibitor, DHMEQ, reduced atherosclerosis in apoE‐deficient mice. J Atheroscler Thromb. 2006; 13:308-313 [DOI] [PubMed] [Google Scholar]
- 7.Gareus R, Kotsaki E, Xanthoulea S, van der Made I, Gijbels MJ, Kardakaris R, Polykratis A, Kollias G, de Winther MP, Pasparakis M. Endothelial cell‐specific NF‐kappaB inhibition protects mice from atherosclerosis. Cell Metab. 2008; 8:372-383 [DOI] [PubMed] [Google Scholar]
- 8.Hasegawa Y, Saito T, Ogihara T, Ishigaki Y, Yamada T, Imai J, Uno K, Gao J, Kaneko K, Shimosawa T, Asano T, Fujita T, Oka Y, Katagiri H. Blockade of the nuclear factor‐kappaB pathway in the endothelium prevents insulin resistance and prolongs life spans. Circulation. 2012; 125:1122-1133 [DOI] [PubMed] [Google Scholar]
- 9.Frantz B, O'Neill EA. The effect of sodium salicylate and aspirin on NF‐kappaB. Science. 1995; 270:2017-2019 [DOI] [PubMed] [Google Scholar]
- 10.Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002; 277:48115-48121 [DOI] [PubMed] [Google Scholar]
- 11.Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, Yuan M, Li ZW, Karin M, Perret P, Shoelson SE, Shulman GI. Prevention of fat‐induced insulin resistance by salicylate. J Clin Invest. 2001; 108:437-446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK‐beta and NF‐kappaB. Nat Med. 2005; 11:183-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA, Shoelson SE. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2010; 152:346-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldfine AB, Silver R, Aldhahi W, Cai D, Tatro E, Lee J, Shoelson SE. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci. 2008; 1:36-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim F, Tysseling KA, Rice J, Gallis B, Haji L, Giachelli CM, Raines EW, Corson MA, Schwartz MW. Activation of IKKbeta by glucose is necessary and sufficient to impair insulin signaling and nitric oxide production in endothelial cells. J Mol Cell Cardiol. 2005; 39:327-334 [DOI] [PubMed] [Google Scholar]
- 16.Kim F, Tysseling KA, Rice J, Pham M, Haji L, Gallis BM, Baas AS, Paramsothy P, Giachelli CM, Corson MA, Raines EW. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler Thromb Vasc Biol. 2005; 25:989-994 [DOI] [PubMed] [Google Scholar]
- 17.Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008; 31:289-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldfine AB, Fonseca V, Jablonski KA, Chen YD, Tipton L, Staten MA, Shoelson SE. Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2013; 159:1-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beckman JA, Liao JK, Hurley S, Garrett LA, Chui D, Mitra D, Creager MA. Atorvastatin restores endothelial function in normocholesterolemic smokers independent of changes in low‐density lipoprotein. Circ Res. 2004; 95:217-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard‐Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial‐dependent flow‐mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002; 39:257-265 [DOI] [PubMed] [Google Scholar]
- 21.Burry HC, Dieppe PA. Apparent reduction of endogenous creatinine clearance by salicylate treatment. Br Med J. 1976; 2:16-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris HG, Sherman NA, McQuain C, Goldlust MB, Chang SF, Harrison LI. Effects of salsalate (nonacetylated salicylate) and aspirin on serum prostaglandins in humans. Ther Drug Monit. 1985; 7:435-438 [DOI] [PubMed] [Google Scholar]
- 23.Lieberman EH, Gerhard MD, Uehata A, Selwyn AP, Ganz P, Yeung AC, Creager MA. Flow‐induced vasodilation of the human brachial artery is impaired in patients <40 years of age with coronary artery disease. Am J Cardiol. 1996; 78:1210-1214 [DOI] [PubMed] [Google Scholar]
- 24.Mitchell JA, Saunders M, Barnes PJ, Newton R, Belvisi MG. Sodium salicylate inhibits cyclo‐oxygenase‐2 activity independently of transcription factor (nuclear factor kappaB) activation: role of arachidonic acid. Mol Pharmacol. 1997; 51:907-912 [DOI] [PubMed] [Google Scholar]
- 25.Verma S, Raj SR, Shewchuk L, Mather KJ, Anderson TJ. Cyclooxygenase‐2 blockade does not impair endothelial vasodilator function in healthy volunteers: randomized evaluation of rofecoxib versus naproxen on endothelium‐dependent vasodilatation. Circulation. 2001; 104:2879-2882 [DOI] [PubMed] [Google Scholar]
- 26.Bogaty P, Brophy JM, Noel M, Boyer L, Simard S, Bertrand F, Dagenais GR. Impact of prolonged cyclooxygenase‐2 inhibition on inflammatory markers and endothelial function in patients with ischemic heart disease and raised C‐reactive protein: a randomized placebo‐controlled study. Circulation. 2004; 110:934-939 [DOI] [PubMed] [Google Scholar]
- 27.Florez A, de Haro J, Martinez E, Varela C, Bleda S, Acin F. Selective cyclooxygenase‐2 inhibition reduces endothelial dysfunction and improves inflammatory status in patients with intermittent claudication. Rev Esp Cardiol. 2009; 62:851-857 [DOI] [PubMed] [Google Scholar]
- 28.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor‐{kappa}b activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle‐aged and older humans. Circulation. 2009; 119:1284-1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta SK, Johnson RM, Saha C, Mather KJ, Greenwald ML, Waltz JS, Rehman J, Dube MP. Improvement in HIV‐related endothelial dysfunction using the anti‐inflammatory agent salsalate: a pilot study. AIDS. 2008; 22:653-655 [DOI] [PubMed] [Google Scholar]
- 30.Hileman CO, Carman TL, Gripshover BM, O'Riordan M, Storer NJ, Harrill DE, White CA, McComsey GA. Salsalate is poorly tolerated and fails to improve endothelial function in virologically suppressed HIV‐infected adults. AIDS. 2010; 24:1958-1961 [DOI] [PubMed] [Google Scholar]
- 31.Tabit CE, Holbrook M, Shenouda SM, Dohadwala MM, Widlansky ME, Frame AA, Kim BH, Duess MA, Kluge MA, Levit A, Keaney JF, Jr, Vita JA, Hamburg NM. Effect of sulfasalazine on inflammation and endothelial function in patients with established coronary artery disease. Vasc Med. 2012; 17:101-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldfine AB, Conlin PR, Halperin F, Koska J, Permana P, Schwenke D, Shoelson SE, Reaven PD. A randomised trial of salsalate for insulin resistance and cardiovascular risk factors in persons with abnormal glucose tolerance. Diabetologia. 2013; 56:714-723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arcaro G, Zenere BM, Saggiani F, Zenti MG, Monauni T, Lechi A, Muggeo M, Bonadonna RC. Ace inhibitors improve endothelial function in type 1 diabetic patients with normal arterial pressure and microalbuminuria. Diabetes Care. 1999; 22:1536-1542 [DOI] [PubMed] [Google Scholar]
- 34.Mayer EL, Dallabrida SM, Rupnick MA, Redline WM, Hannagan K, Ismail NS, Burstein HJ, Beckman JA. Contrary effects of the receptor tyrosine kinase inhibitor vandetanib on constitutive and flow‐stimulated nitric oxide elaboration in humans. Hypertension. 2011; 58:85-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, Woulfe K, Pravda E, Cassiola F, Desai J, George S, Morgan JA, Harris DM, Ismail NS, Chen JH, Schoen FJ, Van den Abbeele AD, Demetri GD, Force T, Chen MH. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007; 370:2011-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giugliano D, Sacca L, Scognamiglio G, Ungaro B, Torella R. Influence of acetylsalicylic acid on glucose turnover in normal man. Diabetes Metab. 1982; 8:279-282 [PubMed] [Google Scholar]
- 37.Newman WP, Brodows RG. Aspirin causes tissue insensitivity to insulin in normal man. J Clin Endocrinol Metab. 1983; 57:1102-1106 [DOI] [PubMed] [Google Scholar]
- 38.Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, Shulman GI. Mechanism by which high‐dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002; 109:1321-1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faghihimani E, Aminorroaya A, Rezvanian H, Adibi P, Ismail‐Beigi F, Amini M. Salsalate reduces insulin resistance and plasma glucose level in persons with prediabetes. Endocr Pract. 2012:1-17 [DOI] [PubMed] [Google Scholar]
- 40.Meex RC, Phielix E, Moonen‐Kornips E, Schrauwen P, Hesselink MK. Stimulation of human whole‐body energy expenditure by salsalate is fueled by higher lipid oxidation under fasting conditions and by higher oxidative glucose disposal under insulin‐stimulated conditions. J Clin Endocrinol Metab. 2011; 96:1415-1423 [DOI] [PubMed] [Google Scholar]
- 41.Chai W, Liu J, Jahn LA, Fowler DE, Barrett EJ, Liu Z. Salsalate attenuates free fatty acid‐induced microvascular and metabolic insulin resistance in humans. Diabetes Care. 2011; 34:1634-1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koska J, Ortega E, Bunt JC, Gasser A, Impson J, Hanson RL, Forbes J, de Courten B, Krakoff J. The effect of salsalate on insulin action and glucose tolerance in obese non‐diabetic patients: results of a randomised double‐blind placebo‐controlled study. Diabetologia. 2009; 52:385-393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carlson LA, Osiman J. Effect of sancylates on plasmafree fatty acid in normal and diabetic subjects. Metabolism. 1961; 10:781-787 [PubMed] [Google Scholar]
- 44.Bizzi A, Codegoni AM, Garattini S. Salicylate, a powerful inhibitor of free fatty acid release. Nature. 1964; 204:1205. [DOI] [PubMed] [Google Scholar]
- 45.Zu L, Jiang H, He J, Xu C, Pu S, Liu M, Xu G. Salicylate blocks lipolytic actions of tumor necrosis factor‐alpha in primary rat adipocytes. Mol Pharmacol. 2008; 73:215-223 [DOI] [PubMed] [Google Scholar]
- 46.Bajaj M, Suraamornkul S, Romanelli A, Cline GW, Mandarino LJ, Shulman GI, DeFronzo RA. Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long‐chain fatty acyl‐CoAs and insulin action in type 2 diabetic patients. Diabetes. 2005; 54:3148-3153 [DOI] [PubMed] [Google Scholar]
- 47.van Diepen JA, Wong MC, Guigas B, Bos J, Stienstra R, Hodson L, Shoelson SE, Berbee JF, Rensen PC, Romijn JA, Havekes LM, Voshol PJ. Hepatocyte‐specific IKK‐beta activation enhances VLDL‐triglyceride production in ApoE*3‐Leiden mice. J Lipid Res. 2011; 52:942-950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clapp BR, Hingorani AD, Kharbanda RK, Mohamed‐Ali V, Stephens JW, Vallance P, MacAllister RJ. Inflammation‐induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc Res. 2004; 64:172-178 [DOI] [PubMed] [Google Scholar]
- 49.Yin MJ, Yamamoto Y, Gaynor RB. The anti‐inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)b kinase‐beta. Nature. 1998; 396:77-80 [DOI] [PubMed] [Google Scholar]
- 50.Schwenger P, Alpert D, Skolnik EY, Vilcek J. Activation of p38 mitogen‐activated protein kinase by sodium salicylate leads to inhibition of tumor necrosis factor‐induced IkappaB alpha phosphorylation and degradation. Mol Cell Biol. 1998; 18:78-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cronstein BN, Montesinos MC, Weissmann G. Salicylates and sulfasalazine, but not glucocorticoids, inhibit leukocyte accumulation by an adenosine‐dependent mechanism that is independent of inhibition of prostaglandin synthesis and p105 of NFkappaB. Proc Natl Acad Sci USA. 1999; 96:6377-6381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu XM, Sansores‐Garcia L, Chen XM, Matijevic‐Aleksic N, Du M, Wu KK. Suppression of inducible cyclooxygenase 2 gene transcription by aspirin and sodium salicylate. Proc Natl Acad Sci USA. 1999; 96:5292-5297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tabit CE, Holbrook M, Shenouda SM, Dohadwala MM, Widlansky ME, Frame AA, Kim BH, Duess MA, Kluge MA, Levit A, Keaney JF, Jr, Vita JA, Hamburg NM. Effect of sulfasalazine on inflammation and endothelial function in patients with established coronary artery disease. Vasc Med. 2012; 17:101-107 [DOI] [PMC free article] [PubMed] [Google Scholar]


