Introduction
With advances in technology, a new era in troponin assays is approaching. Previous‐generation troponin assays have been used as diagnostic and prognostic markers in acute coronary syndrome patients and for risk stratification to guide triage decisions and aid in treatment selection.1–2 New, high‐sensitivity troponin assays represent an important advance with added sensitivity for cardiac myocyte necrosis,3–5 but there remains a need for judicious interpretation with these tests. These new troponin assays could have several distinct roles in clinical practice: (1) facilitation of earlier diagnosis and rule out of myocardial infarction (MI); (2) risk stratification in acute cardiac conditions and prognostic information in stable disease states; and (3) therapeutic monitoring and drug toxicity evaluation. However, because they are not specific for the etiology of cardiac cell death, the clinician has an increasing responsibility to interpret each test in clinical context.6 When commercially available in the United States (US), these high‐sensitivity analyses are expected to offer both advantages and disadvantages to practicing clinicians and to enhance current roles of the troponin assay as well as to open doors to new uses for troponin testing. However, they will also create new challenges in clinical applications, epidemiology, and research. The aim of this review is to discuss the potential clinical and research roles of high‐sensitivity cardiac troponin assays and the challenges they may create.
Assay Development
High‐sensitivity troponin assays detect concentrations of the same proteins that conventional sensitivity assays are aimed at detecting, just in much lower concentrations.4 Before 2009, there was no agreed‐on definition of what constituted a high‐sensitivity assay or a consensus on nomenclature for these much more sensitive tests, but that seems to have been resolved in 2012. High‐sensitivity assays, by expert consensus,4 should have a coefficient of variance (CV) of <10% at the 99th percentile value in the population of interest. To be classified as high‐sensitivity assays, concentrations below the 99th percentile should be detectable above the assay's limit of detection for >50% of healthy individuals in the population of interest. Using these criteria, there are several contemporary assays that are available on an experimental basis in the US but presumably would soon be available clinically as well. The assays and their characteristics are shown in Table 1. Just as with the conventional‐sensitivity assays, there are a growing number of companies that are producing high‐sensitivity assays (most are producing troponin I assays; however, there is a single troponin T assay, both conventional and high sensitivity, allowing for standardization across centers). As shown in Table 1 there is wide variability in assay characteristics between manufacturers. This prevents comparison between assays and often between clinical centers and hospital systems for conventional troponin assays4 and will be no less a challenge with the advent of high‐sensitivity troponin testing. This problem is just one of several that face the high‐sensitivity assays.
Table 1.
Analytic Comparisons of Contemporary High‐Sensitivity Cardiac Troponin Assays*
| Limit of Detection (ng/L) | 99% (CV) (ng/L) | 10% CV (ng/L) | |
|---|---|---|---|
| Hs‐cTn‐T | |||
| Roche Elecsys | 5.0 | 14 (13%) | 13 |
| Hs‐cTn‐I | |||
| Abbot ARCHITECT | 1.2 | 16 (5.6%) | 3.0 |
| Beckman ACCESS | 2 to 3 | 8.6 (10%) | 8.6 |
| Mitsubishi Pathfast | 8.0 | 29 (5%) | 14 |
| Nanosphere | 0.2 | 2.8 (9.5%) | 0.5 |
| Radiometer AQT90 | 9.5 | 23 (17.7%) | 39 |
| Singulex Erenna | 0.09 | 10.1 (9.0%) | 0.88 |
| Siemens Vista | 0.5 | 9 (5.0%) | 3 |
| Siemens Centaur | 6.0 | 40 (10%) | 30 |
Importantly, just as with the earlier troponin assays, interpretation of high‐sensitivity troponin values needs to have basis in the 99th percentile value in the general population. Using this value in clinical practice, for each assay, has previously been shown to optimize the sensitivity and specificity of troponin and serves to minimize false‐positive testing.3,7 However, determination of this value and, more specifically, the population in which to derive this value consistently, is not universally agreed on. Previous research to determine the 99th percentile for high‐sensitivity troponin assays has yielded different values for the same assay based on different sample populations.8 Nonetheless, there should be collaboration at clinical centers among physicians and laboratory managers to identify the 99th percentile value for the preferred assay, from either the assay manufacturer's guidelines or laboratory reference literature. This will aid in consistent interpretation of the test.
Use of High‐Sensitivity Assays and Potential Challenges
Based on their analytic capabilities, the high‐sensitivity assays offer several advantages over their conventional assay counterparts. As previously mentioned, they are extremely sensitive, perhaps allowing for earlier and faster recognition of MI patients9 and giving clinicians an avenue to more quickly diagnose and treat patients appropriately. The new assays are also precise, having small CV levels even at the 99% in reference populations.4–5 They are specific for myocardial necrosis, although this topic is controversial. There are conflicting opinions on the specificity of troponin for myocyte infarction versus ischemia. In brief, there are limited data in the setting of stress tests, during which standard‐sensitivity troponin assays are static, while high‐sensitivity assays detect a small rise in troponin.10 In addition, experimental models of ischemia, achieved by pacing patients from the coronary sinus, have shown a low‐level elevation in troponin appreciated only with high‐sensitivity troponin assays.11 Some authors have interpreted similar findings as consistent with ischemia12–13; however, it has also been argued that the high‐sensitivity assays are detecting minute levels of myocyte necrosis (thus, miniscule MIs).14 In addition, there are several proposed mechanisms of troponin release that are not related to necrosis, namely apoptosis, cellular release of proteolytic products, increased cell wall permeability with stress or stretch, and the production of membranous blebs that contain troponin.15 Despite the controversy, conventional‐sensitivity assays are specific for myocyte necrosis, and we believe high‐sensitivity troponin assays will also have high specificity once appropriate threshold values have been determined. Finally, there is a considerable amount of data that indicate that increasing values (and perhaps changes in values over time) correlate with risk of adverse cardiac events.16–17 However, most of these advantages also bring disadvantages. With higher sensitivity comes the responsibility of understanding and interpreting very low levels of high‐sensitivity troponin elevation. Despite the high specificity of these assays, no troponin assay alone allows a clinician to determine the etiology of myocyte necrosis. While high precision is quite valuable in these tests, especially at low levels, biological variability again makes interpretation of high‐sensitivity troponin elevations difficult, and new thresholds will have to be defined for clinical use. Finally, as with current troponin I assays, there is no industry standard to the high‐sensitivity troponin assays, and they have varying assay characteristics that will make comparisons between hospitals and medical systems difficult at present (Table 2).
Table 2.
Potential Utility and Challenges of the High‐Sensitivity Troponin Assay
| Potential Utility | Potential Challenges |
|---|---|
| More rapid diagnosis in ACS | Nonspecific to etiology |
| Population screening | False‐positive interpretation |
| Prognostic information in stable patients | Biological variability |
| Drug development and cardiotoxicity | Lack of assay standardization |
ACS indicates acute coronary syndrome.
With the increased sensitivity of these new assays and the evaluation of their precision and sensitivity in healthy populations (although not completely agreed on), investigators have discovered biological variability in the low detectable levels of healthy individuals. Biological variability is a type of preanalytic variation due to changes over time in normal individuals. These changes may be secondary to circadian rhythm, monthly changes, or seasonal changes inherent to a species. In addition there is random biological fluctuation around an inherent set point that may be specific to an individual. This certainly affects the utility of these new assays and should inform the use of threshold values and change values that might be used for diagnostic and prognostic clinical evaluation. Wu et al demonstrated a mean biological variability in healthy individuals using the Singulex cTnI assay in the range of ≈10% over 4 hours and ≈15% over 8 weeks.18 With this level of variability, the authors calculated a reference change value of 81% for increasing serial assays and −45% for decreasing serial assays to define abnormality. Vasile et al,19 using the Roche hs‐Troponin T assay, showed that mean delta values within 24‐hour studies were ±58%, while values over multiple days could vary by ±90%. Given this biological variability, the authors determined that a short‐term (eg, 4 hours) change of 85% or long‐term (eg, 8 weeks) change of 315% would be necessary to be reasonably certain that abnormality was present. Sharnhorst et al20 and Frankenstein et al21 also evaluated healthy individuals and found similar values for variability and reference change values.
This variation raises numerous questions about the clinical use of these tests. Biological variability would alter how high‐sensitivity troponin assays are interpreted in nearly all clinical applications in which >1 value is measured. Due to variability with time in an individual and detectable levels at healthy state, further study and statistical testing are needed to determine what levels of change are necessary and sufficient to signal an abnormality, rather than either biological variability or imprecision. Also, European guidelines for the diagnosis of MI indicate that appropriate thresholds, reference values, and change values should be determined for each high‐sensitivity assay.22
Diagnosis of Acute Coronary Syndrome
The primary role for the troponin assay since its inception has been to diagnose patients with acute MI (AMI). While the improved sensitivity and specificity of troponin (over creatine kinase–MB) may have improved diagnostic accuracy for AMI in the emergency department and hospital, its shortcoming may be that, depending on the time between symptom onset and presentations, there is a delay of several hours in elevation of the biomarker.23–24 This delay causes a delay in diagnosis and, thus, longer emergency department and observation stays both for patients with and those without AMI. New high‐sensitivity assays have overcome this weakness with their improved sensitivity, making the first low‐level elevations of troponin detectable within 90 to 180 minutes of the event.9,16,25 Two large prospective studies performed in the emergency department setting showed that the high‐sensitivity troponin assays are more accurate than previous assays in the successful diagnosis of AMI within 3 hours of the onset of symptoms.26–27 The increase in accuracy and speed could be beneficial in multiple ways. Patients are diagnosed more quickly and, as a result, may be treated more rapidly, including earlier invasive therapy. For the majority of patients, the improved assays will lead to a quicker rule‐out of MI.28 This may allow for more prompt subsequent evaluation and perhaps to earlier discharge of appropriate patients, decreasing further costs. However, there has not been adequate prospective evaluation of the use of these new assays in a diagnostic algorithm, and these assays may perform in a less‐specific manner in the general chest pain population outside of the strict criteria required to enter a randomized clinical trial. Also, the cost‐effectiveness of these new algorithms and strategies has not yet been established.
Despite increases in accuracy and earlier detection with new troponin assays, there are new problems for the diagnosis of MI with these assays. Previously, the use of troponin for the diagnosis of AMI was more straightforward in that an elevated value was deemed an AMI, whereas a value below a set threshold was not. Research with high‐sensitivity assays shows both a nominal level of high‐sensitivity troponin at baseline in apparently healthy individuals and the presence of biological variability over time. These features affect how clinicians use the high‐sensitivity assays (currently commercially available in Europe but not yet in the US). Some have proposed that an absolute change value over time should be used to diagnose AMI. The threshold is based on testing of reference populations, which vary widely among industry producers. There is also variation in how reference populations are defined, thus adding more questions to what threshold values might be for these new assays. Others have suggested using a percent change in troponin, a delta troponin. Keller and colleagues29 compared the use of a relative change in troponin within the first 3 hours of presentation for chest pain, with a baseline absolute value, and found that using the relative change in troponin at 3 hours significantly increased the positive and negative predictive values for these high‐sensitivity assays. Reichlin et al,30 in a prospective observational study, evaluated the utility of an absolute change versus a relative change in high‐sensitivity troponin from baseline to 2 hours after presentation. In their analysis, an absolute change of high‐sensitivity troponin was more accurate than relative change for diagnosis of MI. Mueller et al31 also found that absolute change (within 3 to 6 hours) in high‐sensitivity troponin had superior diagnostic value (c‐statistic 0.898 versus 0.752, P<0.0001) compared with relative changes in the same period. However, for either strategy, establishment of thresholds will be difficult, as will be standardization across assays.
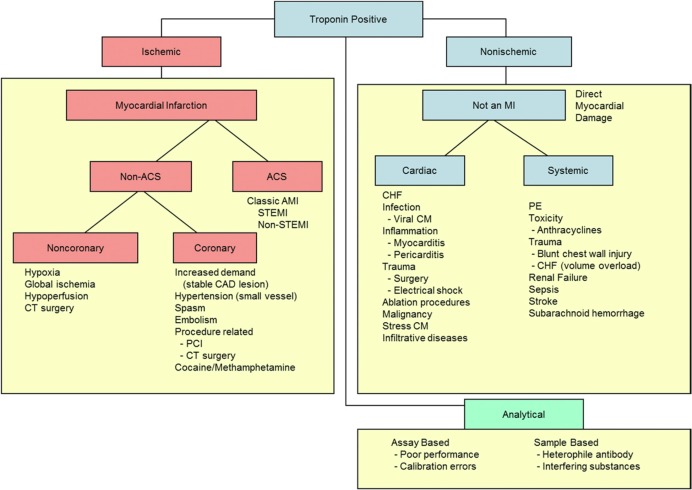
While the new assays can provide more accurate, rapid detection of myocardial necrosis, they do not indicate the cause of cell death. Improved assay performance will have to be accompanied by improved clinical evaluation of patients to effectively interpret the results and apply them in clinical practice. The universal definition of MI7 (Table 3) was a broad consensus developed by representatives from the American College of Cardiology Foundation, European Society of Cardiology, American Heart Association, and World Heart Federation that provided increased structure and clarity around the diagnosis of MI in the era of a troponin gold standard. Importantly, it firmly established the importance of clinical symptoms in conjunction with elevated troponin levels occurring with a characteristic rise and/or fall to establish type 1 MI. It also allowed for diagnosis in the setting of sudden cardiac death, as well as enhanced categorization for periprocedural MIs. As sensitivity and precision of troponin assays have improved, false‐positive results due to assay imprecision are becoming less of a problem. However, the pressure on clinical perspective in interpretation of positive troponin results has increased, given that there is a large differential diagnosis generated by a positive troponin assay (Figure).
Table 3.
Universal Definition of Myocardial Infarction
| Type | Clinical Situation | Definition |
|---|---|---|
| 1 | Spontaneous | MI related to ischemia from primary coronary event such as plaque rupture, erosion, fissuring, or dissection |
| 2 | Demand/supply imbalance | MI related to secondary ischemia due to myocardial oxygen supply/demand imbalance such as spasm, anemia, hypotension, arrhythmia |
| 3 | Sudden death | Unexpected cardiac death, perhaps suggestive of MI, but occurring before blood samples can be obtained |
| 4a | PCI | MI associated with PCI procedure |
| 4b | Stent thrombosis | MI associated with stent thrombosis as seen on angiography or autopsy |
| 5 | CABG | MI associated with CABG |
CABG indicates coronary artery bypass graft surgery; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Adapted with permission from Thygesen et al.7
Figure 1.
Diagnostic algorithm for troponin positivity. ACS indicates acute coronary syndrome; AMI, acute myocardial infarction; CAD, coronary artery disease; CHF, congestive heart failure; CM, cardiomyopathy; CT, computed tomography; PCI, percutaneous coronary intervention; PE, pulmonary embolism; STEMI, ST‐segment elevation myocardial infarction. Reproduced with permission from Elsevier publications from Newby et al JACC Consensus Statement.6
Risk Stratification
Acute Coronary Syndrome Population
In AMI, troponin not only serves a diagnostic purpose but also can be useful for prognosis. The earlier‐generation assays showed troponin is a strong prognostic marker for cardiovascular death and recurrent ischemic events in both non–ST‐segment elevation myocardial infarction (STEMI) and STEMI.1–2 The new high‐sensitivity assays share this prognostic ability. In a single‐center study of nearly 1000 patients presenting to the emergency department with chest pain, troponin measured with a high‐sensitivity assay was a more robust predictor of cardiovascular death at 1‐year follow‐up than the conventional troponin assay.32 The same authors also evaluated whether a delta high‐sensitivity troponin level between presentation and 2 hours later would provide prognostic information but found that the absolute value was superior to the delta value at 2 hours for prognostication of cardiovascular death at 1 year.33
The prognostic information gained from troponin not only can give clinicians information on risk but also may help to guide therapy, although these management strategies have only been studied with standard‐sensitivity troponin assays. Troponin can be a useful measure for assigning patients to higher‐level monitoring, such as the cardiac care unit or telemetry. Previous research used standard sensitivity troponin as one of several factors that might direct triage to an invasive strategy for NSTEMI.34 Indeed, its use has also been suggested as part of clinical risk scores to influence timing of cardiac catheterization for NSTEMI patients.35 Troponin levels also have been associated with a benefit of more aggressive medical antiplatelet therapy, specifically for glycoprotein IIb/IIIa inhibitors.36 Further investigation is needed to provide thresholds by which high‐sensitivity assays might also be used to guide therapeutic strategies.
Acute Pulmonary Embolism
Troponin has also been recognized as a strong predictive biomarker in other acute clinical situations, such as acute pulmonary embolism (PE). While echocardiographic and chest computed tomography parameters have been used as signals of worsening cardiac function, troponin is a routine laboratory biomarker that is drawn in the vast majority of patients with suspected PE. A recent meta‐analysis of 20 studies with 1985 patients showed that troponin was independently associated with greater risk for short‐term death and morbidity after PE.37 Troponin measured with high‐sensitivity assays has also been studied. Lankeit et al performed a prospective study of 526 normotensive patients admitted with acute PE.38 They found that high‐sensitivity troponin results, alone and as part of a clinical risk score, were independently associated with cardiovascular death and in‐hospital complications. In the specific population of patients studied, the authors suggested that high‐sensitivity troponin might aid in selection of a low‐risk subgroup that could be treated as outpatients. This finding has led to further research on the combination of laboratory and echocardiographic biomarkers to risk stratify patients with PE.39 Acute coronary syndrome and acute PE represent the advances in clinical risk stratification that could be accomplished with high‐sensitivity troponin assays.
Asymptomatic Population
Stable Coronary Artery Disease and Congestive Heart Failure
High‐sensitivity troponin has also expanded the possibilities for risk assessment in asymptomatic patients. In a large observational cohort of patients with known coronary artery disease (CAD), but without congestive heart failure (CHF) or acute coronary syndrome (PEACE [Prevention of Events with Angiotensin Converting Enzyme Inhibition] trial population40), and follow‐up for a median of 5.2 years, high‐sensitivity troponin was independently associated with cardiovascular mortality and the development of CHF, although it was not associated with MI.41 In a similar study of patients at high risk for CAD events (HOPE [Heart Outcomes Prevention Evaluation] study population42), high‐sensitivity troponin levels were independently associated with the composite outcome of cardiovascular death and future MI over a mean follow‐up of 4.5 years.43
Troponin is also a prognostic marker in stable CHF. Previous investigation showed that troponin was often present in the blood of both acute and chronic heart failure patients and has been hypothesized to be due to an imbalance in myocardial oxygen demand or extreme cardiac stress in these states.44 The use of high‐sensitivity assays to measure serial levels of troponin in stable CHF patients has been examined in several analyses.45–47 In a large retrospective study of 2 clinical trial populations (Val‐HeFT [Valsartan Heart Failure Trial] and GISSI‐HF [Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico–Heart Failure]), even low levels of troponin using high‐sensitivity assays, detected at baseline and at an initial visit 4 months later, were independently associated with CHF‐related mortality and all‐cause mortality at 2 and 4 years of follow‐up.48 In smaller populations of stable CHF patients, high‐sensitivity troponin was consistently associated with cardiovascular deaths and CHF admissions during long‐term follow‐up, regardless of ischemic versus nonischemic etiology.49–50 Given that in each of these studies patients were known to have CAD or CHF and were at risk for future adverse cardiac events, the additional risk stratification imparted by high‐sensitivity troponin may not result in expansion or intensification of treatment and, thus, may have limited utility. However, high‐sensitivity troponin may provide a sensitive, noninvasive window into cardiac structure and function.
Stable Chronic Kidney Disease/End‐Stage Renal Disease
Chronic kidney disease is an important clinical condition that is associated with worse outcomes in coronary artery disease, and end stage renal disease patients are at high risk for adverse cardiovascular events. Troponin elevation in these patients has been shown to be a prognostic factor, though the mechanism of this relationship is unclear. Khan et al performed a meta‐analysis of 28 studies with nearly 4000 patients with stable end‐stage renal disease, and without acute coronary syndrome.51 They evaluated troponin elevation at baseline and determined that there was a significant, independent, association of troponin with cardiovascular death and all‐cause mortality. With new‐generation high‐sensitivity assays, mild troponin elevations are present in a large proportion of asymptomatic end‐stage renal disease patients.52–53 Additional study has found that troponin measured with these high‐sensitivity assays is more elevated in patients with a history of CAD,53–54 but no prognostic studies have yet shown a relationship with future events. Further research is necessary to determine how high‐sensitivity troponin assays might be used in patients with end‐stage renal disease on dialysis given that the majority of patients have elevated values at baseline.
Screening
While elevated troponin might signal worse prognosis among patients with known CAD or CHF or who are otherwise at significant risk, high‐sensitivity assays may provide a means of screening a general population of patients for cardiac dysfunction. In a prospective observational cohort of general patients randomly enrolled from a large metropolitan area (Dallas Heart Study), elevated levels of high‐sensitivity troponin were associated with greater rates of structural heart disease, including left ventricular hypertrophy and systolic dysfunction, as well as all‐cause mortality within a median follow‐up of 6.4 years.17 In a nationwide prospective cohort study of ambulatory older adults (≥65 years) without heart failure, high‐sensitivity troponin values (both baseline and absolute change over 2 years) were associated with incident heart failure and cardiovascular death over a median follow‐up of 11.8 years55(Table 4). Thus, in a general population, high‐sensitivity troponin might play a role in screening for structural and coronary heart disease, potentially prompting further evaluation and more aggressive primary prevention measures. However, there are several obstacles to overcome before such a clinical strategy could be used. Biological variability will have to be further explored and quantified to establish reasonable threshold change values. Standardization of high‐sensitivity troponin assays would be extremely important to allow for appropriate interpretation of troponin values with time and across different medical systems.
Table 4.
Summary of Selected Literature for High‐Sensitivity Troponin
| Study Authors | Populations Study Size | Change Units (Assay) | Outcomes | Adjusted Hazard Ratios |
|---|---|---|---|---|
| de Fillipi et al55 | Cardiovascular Health Study (mixed stable) (n=4221) | >12.94 pg/mL (hsTnT) | CV mortality | 2.91 (2.37 to 3.58) |
| de Lemos et al17 | Dallas Heart Study (mixed stable) (n=3546) | ≥0.0014 ηg/mL (hsTnT) | Mortality | 6.0 (4.7 to 11.3) |
| Omland et al41 | PEACE (stable CAD) (n=3679) | ≥0.0096 μg/L (men) ≥0.0074 μg/L (women) (hsTnT) |
CV mortality | 2.39 (1.85 to 3.09) (for hsTnT as a cont. variable) |
| Masson et al48 | Val‐HEFT GISSI‐HF (stable CHF for both) (n=5284) |
≥13.5 ηg/L for both (hsTnT) | Mortality for both | 1.60 (1.41 to 1.82) 1.97 (1.58 to 2.46) |
| Kavsak et al43 | HOPE (stable at risk for CAD/CHF) (n=2572) | ≥10 ηg/L (hs‐cTnI Beckman Coulter) | CV mortality | 2.2 (1.3 to 3.5) |
CAD indicates coronary artery disease; CHF, congestive heart failure; CV, cardiovascular; HsTnT, high‐sensitivity troponin T; Hs‐cTnI, high‐sensitivity cardiac troponin I.
Future Directions
The new high‐sensitivity troponin assays may allow clinicians to better stratify risk in other potentially high‐risk populations. However, the utility of troponin testing in these settings is unclear and awaits further investigation. A recent example is atrial fibrillation, which represents an important population of patients with few, if any, useful biomarkers currently available to aid clinical risk stratification. Traditionally, clinical risk scores are used to estimate risk for thromboembolic events in atrial fibrillation; then, anticoagulant medication is considered depending on embolic risk and bleeding risk, medication adherence, etc. Roldan et al56 found that in a large cohort of chronic atrial fibrillation patients on oral anticoagulation for atrial fibrillation, high‐sensitivity troponin levels were independently associated with an increased risk for thrombotic and vascular events as well as cardiovascular death. This relationship was maintained even after CHADS2 (which estimates risk based on the presence of congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, and prior stroke or transient ischemic attack) risk factors and HAS‐BLED (defined as hemorrhage involving a critical anatomic site, for example, intracranial, or a bleed requiring hospitalization, transfusion of ≥2 units of packed cells, or associated with a decrease in hemoglobin level of ≥2 g/L) risk factors were added to the model.
In addition, there are few clinical tools to identify which patients will have recurrent atrial fibrillation. A prospective cohort substudy of GISSI AF (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico–Atrial Fibrillation), a trial to determine the effects of valsartan on recurrence of atrial fibrillation, studied the utility of several biomarkers to predict recurrent of AF.57 Over 2‐year follow‐up, high‐sensitivity troponin was independently associated with the recurrence of AF, as were 2 other biomarkers. While high‐sensitivity troponin may add to prognostic information and elevated troponin levels may warrant increased surveillance of high‐risk patients, it remains uncertain whether any therapies might be triggered by this type of risk stratification (eg, prolonged antiarrhythmic medications, atrial fibrillation ablation, or atrial occluder devices).
Troponin measured with high‐sensitivity assays is also being evaluated for use in cardiac transplant patients. In this population, low levels of troponin are present in the majority of patients shortly after transplantation. Higher levels of troponin, measured with high‐sensitivity assays, have been associated with acute allograft rejection58 and cardiovascular death.59 Troponin in conjunction with optical coherence tomography has also been studied in the diagnosis of cardiac allograft vasculopathy. Specifically, high‐sensitivity troponin was directly associated with measurements of maximal intimal thickness in posttransplant patients,60 suggesting a potential new use for troponin to rule out vasculopathy in cardiac transplant patients, although this seems premature given how little evidence exists.
Many drugs, both currently used and newly developed, have the potential for cardiac toxicity. These effects are sometimes discovered in preclinical work with animal models and sometimes are not discovered until human studies are undertaken. Given troponin's high specificity for myocardial necrosis, there would seem to be a biological plausibility for its use in monitoring for cardiotoxicity. However, this does not imply that in practical implementation it would provide similar information in detecting drug‐induced cardiotoxic states. First, by definition, high‐sensitivity troponin must be detectable below the 99th percentile and above the assay limit of detection in at least 50% of the reference population. Second, the precision of the high‐sensitivity assays is limited less by analytical issues than by biological variability at these low levels that may be particularly relevant in serial testing to monitor for early detection of myonecrosis that may reflect cardiotoxicity. Further, it is important to remember that high‐sensitivity troponin provides no information on the etiology of cardiac myonecrosis and that there are many nonischemic causes of troponin release (Table 5).
Table 5.
Selected Nonthrombotic Causes of Troponin Elevation
| Clinical Syndrome |
|---|
| Congestive heart failure |
| Drug toxicity |
| Hypertension |
| Hypotension |
| Renal failure |
| Sepsis |
| Hypothyroidism |
| Stress‐induced cardiomyopathy |
| Myocarditis/pericarditis |
| Post PCI without complication |
| Post CABG without complication |
| Pulmonary hypertension (severe) |
| Stroke or intracerebral hemorrhage |
| Rhabdomyolysis |
| Strenuous exercise |
| Transplant vasculopathy |
| Traumatic injury |
CABG indicates coronary artery bypass graft surgery; PCI, percuatneous coronary intervention.
Adapted from Newby et al.62
Almost all data available on measuring cardiotoxicity with troponin assays have been produced in laboratory animals and mammalian models of disease. There are few data from human subjects, and much of it is in pediatric subjects undergoing treatment for acute leukemia. In the past 5 years, there has been an increase in research on biochemical and echocardiographic biomarkers of myocardial function in adult patients receiving chemotherapy. Two studies of patients treated for breast cancer with anthracyclines and trastuzumab showed that both longitudinal strain and troponin (measured with high‐sensitivity assays) were independently associated with cardiac dysfunction as measured by traditional echo parameters.61 These findings might allow for the development of protocols and standards by which new therapeutics could be measured, including new antidiabetic agents and chemotherapeutic agents. However, additional work is needed to confirm the utility of high‐sensitivity troponin testing for cardiotoxicity monitoring, including a better understanding of biological variability and whether patient‐specific curves would be needed before treatment. At present, there is no unified approach to monitoring drug safety with troponin, but there has been a call for further investigation and standardization in this area.62
Conclusions
Troponin presently has several important diagnostic and prognostic roles in clinical medicine. While high‐sensitivity troponin assays are not yet available in the US, it is imperative that clinicians, researchers, and laboratorians begin to understand and consider the strengths, limitations, and unique characteristics of these assays to develop the evidence that will both enable informed implementation into clinical practice and guide future research aimed at harnessing their potential. With the advent of high‐sensitivity troponin assays, the roles for troponin testing may expand, potentially allowing for detection of very low levels of troponin in healthy individuals that are meaningful in a variety of clinical scenarios. However, with greater sensitivity comes the responsibility to integrate laboratory biomarker data with clinical data. While troponin is highly specific for myocardial necrosis, it provides no information on the etiology of myocyte death. Thus, more research and cross‐disciplinary collaboration are necessary to determine new thresholds and to establish parameters for high‐sensitivity troponin testing in practice. This will allow for clinicians to appropriately use these new and more powerful troponin assays.
Sources of Funding
Dr Sherwood was funded by National Institutes of Health T‐32 training grant 5 T32 HL 7101‐37.
Disclosures
Dr Sherwood reports no conflict of interest. Dr Newby has received consulting honoraria from Roche Diagnostics and Medscape related to troponin testing. A full report of Dr Newby's relationships with industry is available at https://www.dcri.org/about‐us/conflict‐of‐interest.
References
- 1.Antman EM, Tanasijevic MJ, Thompson B, Schactman M, McCabe CH, Cannon CP, Fischer GA, Fung AY, Thompson C, Wybenga D, Braunwald E. Cardiac‐specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med. 1996; 335:1342-1349 [DOI] [PubMed] [Google Scholar]
- 2.Ohman EM, Armstrong PW, Christenson RH, Granger CB, Katus HA, Hamm CW, O'Hanesian MA, Wagner GS, Kleiman NS, Harrell FE, Jr, Califf RM, Topol EJ. Cardiac troponin T levels for risk stratification in acute myocardial ischemia. GUSTO IIA Investigators. N Engl J Med. 1996; 335:1333-1341 [DOI] [PubMed] [Google Scholar]
- 3.Jaffe AS. The 10 commandments of troponin, with special reference to high sensitivity assays. Heart. 2011; 97:940-946 [DOI] [PubMed] [Google Scholar]
- 4.Apple FS, Collinson PO. Analytical characteristics of high‐sensitivity cardiac troponin assays. Clin Chem. 2012; 58:54-61 [DOI] [PubMed] [Google Scholar]
- 5.Twerenbold R, Jaffe A, Reichlin T, Reiter M, Mueller C. High‐sensitive troponin T measurements: what do we gain and what are the challenges? Eur Heart J. 2012; 33:579-586 [DOI] [PubMed] [Google Scholar]
- 6.Newby LK, Jesse RL, Babb JD, Christenson RH, De Fer TM, Diamond GA, Fesmire FM, Geraci SA, Gersh BJ, Larsen GC, Kaul S, McKay CR, Philippides GJ, Weintraub WS. ACCF 2012 expert consensus document on practical clinical considerations in the interpretation of troponin elevations: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2012; 60:2427-2463 [DOI] [PubMed] [Google Scholar]
- 7.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction; Authors/Task Force Members Chairpersons. Thygesen K, Alpert JS, White HD, Biomarker Subcommittee. Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, ECG Subcommittee. Chaitman BR, Clemmensen PM, Johanson P, Hod H, Imaging Subcommittee. Underwood R, Bax JJ, Bonow JJ, Pinto F, Gibbons RJ, Classification Subcommittee. Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Intervention Subcommittee. Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Trials & Registries Subcommittee. Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Trials & Registries Subcommittee. Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Trials & Registries Subcommittee. Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Trials & Registries Subcommittee. Smith SC, Hu D, Lopez‐Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S, ESC Committee for Practice Guidelines (CPG) Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Document Reviewers. Morais J, Aguiar C, Almahmeed W, Arnar DO, Barili F, Bloch KD, Bolger AF, Botker HE, Bozkurt B, Bugiardini R, Cannon C, de Lemos J, Eberli FR, Escobar E, Hlatky M, James S, Kern KB, Moliterno DJ, Mueller C, Neskovic AN, Pieske BM, Schulman SP, Storey RF, Taubert KA, Vranckx P, Wagner DR. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012; 60:1581-159822958960 [Google Scholar]
- 8.Koerbin G, Abhayaratna WP, Potter JM, Apple FS, Jaffe AS, Ravalico TH, Hickman PE. Effect of population selection on 99th percentile values for a high sensitivity cardiac troponin I and T assays. Clin Biochem. 2013; 46:1636-1643 [DOI] [PubMed] [Google Scholar]
- 9.Than M, Cullen L, Aldous S, Parsonage WA, Reid CM, Greenslade J, Flaws D, Hammett CJ, Beam DM, Ardagh MW, Troughton R, Brown AF, George P, Florkowski CM, Kline JA, Peacock WF, Maisel AS, Lim SH, Lamanna A, Richards AM. Two‐hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. J Am Coll Cardiol. 2012; 59:2091-2098 [DOI] [PubMed] [Google Scholar]
- 10.Sabatine MS, Morrow DA, de Lemos JA, Jarolim P, Braunwald E. Detection of acute changes in circulating troponin in the setting of transient stress test‐induced myocardial ischaemia using an ultrasensitive assay: results from TIMI 35. Eur Heart J. 2009; 30:162-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turer AT, Addo TA, Martin JL, Sabatine MS, Lewis GD, Gerszten RE, Keeley EC, Cigarroa JE, Lange RA, Hillis LD, de Lemos JA. Myocardial ischemia induced by rapid atrial pacing causes troponin T release detectable by a highly sensitive assay: insights from a coronary sinus sampling study. J Am Coll Cardiol. 2011; 57:2398-2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu AH, Ford L. Release of cardiac troponin in acute coronary syndromes: ischemia or necrosis? Clin Chim Acta. 1999; 284:161-174 [DOI] [PubMed] [Google Scholar]
- 13.Hickman PE, Potter JM, Aroney C, Koerbin G, Southcott E, Wu AH, Roberts MS. Cardiac troponin may be released by ischemia alone, without necrosis. Clin Chim Acta. 2010; 411:318-323 [DOI] [PubMed] [Google Scholar]
- 14.Jaffe AS, Ravkilde J, Roberts R, Naslund U, Apple FS, Galvani M, Katus H. It's time for a change to a troponin standard. Circulation. 2000; 102:1216-1220 [DOI] [PubMed] [Google Scholar]
- 15.White HD. Pathobiology of troponin elevations: do elevations occur with myocardial ischemia as well as necrosis? J Am Coll Cardiol. 2011; 57:2406-2408 [DOI] [PubMed] [Google Scholar]
- 16.Cullen L, Muller C, Parsonage WA, Wildi K, Greenslade JH, Twerenbold R, Aldous S, Meller B, Tate JR, Reichlin T, Hammett CJ, Zellweger C, Ungerer Mmed JP, Gimenez MR, Troughton R, Murray K, Brown AF, Mueller M, George P, Mosimann T, Flaws DF, Reiter M, Lamanna A, Haaf P, Pemberton CJ, Richards AM, Chu K, Reid CM, Peacock WF, Jaffe AS, Florkowski C, Deely JM, Than M. Validation of high‐sensitivity troponin I in a 2‐h diagnostic strategy to assess 30‐day outcomes in emergency‐department patients with possible acute coronary syndrome. J Am Coll Cardiol. 2013; 62:1242-1249 [DOI] [PubMed] [Google Scholar]
- 17.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010; 304:2503-2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu AH, Lu QA, Todd J, Moecks J, Wians F. Short‐ and long‐term biological variation in cardiac troponin I measured with a high‐sensitivity assay: implications for clinical practice. Clin Chem. 2009; 55:52-58 [DOI] [PubMed] [Google Scholar]
- 19.Vasile VC, Saenger AK, Kroning JM, Jaffe AS. Biological and analytical variability of a novel high‐sensitivity cardiac troponin T assay. Clin Chem. 2010; 56:1086-1090 [DOI] [PubMed] [Google Scholar]
- 20.Scharnhorst V, Krasznai K, van ‘t Veer M, Michels RH. Variation of cardiac troponin I and T measured with sensitive assays in emergency department patients with noncardiac chest pain. Clin Chem. 2012; 58:1208-1214 [DOI] [PubMed] [Google Scholar]
- 21.Frankenstein L, Wu AH, Hallermayer K, Wians FH, Jr, Giannitsis E, Katus HA. Biological variation and reference change value of high‐sensitivity troponin T in healthy individuals during short and intermediate follow‐up periods. Clin Chem. 2011; 57:1068-1071 [DOI] [PubMed] [Google Scholar]
- 22.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HDWriting Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction Third universal definition of myocardial infarction. Eur Heart J. 2012; 33:2551-2567 [DOI] [PubMed] [Google Scholar]
- 23.Adams JE, III, Bodor GS, Davila‐Roman VG, Delmez JA, Apple FS, Ladenson JH, Jaffe AS. Cardiac troponin I. A marker with high specificity for cardiac injury. Circulation. 1993; 88:101-106 [DOI] [PubMed] [Google Scholar]
- 24.Adams JE, III, Schechtman KB, Landt Y, Ladenson JH, Jaffe AS. Comparable detection of acute myocardial infarction by creatine kinase MB isoenzyme and cardiac troponin I. Clin Chem. 1994; 40:1291-1295 [PubMed] [Google Scholar]
- 25.Reichlin T, Schindler C, Drexler B, Twerenbold R, Reiter M, Zellweger C, Moehring B, Ziller R, Hoeller R, Rubini Gimenez M, Haaf P, Potocki M, Wildi K, Balmelli C, Freese M, Stelzig C, Freidank H, Osswald S, Mueller C. One‐hour rule‐out and rule‐in of acute myocardial infarction using high‐sensitivity cardiac troponin T. Arch Intern Med. 2012; 172:1211-1218 [DOI] [PubMed] [Google Scholar]
- 26.Keller T, Zeller T, Peetz D, Tzikas S, Roth A, Czyz E, Bickel C, Baldus S, Warnholtz A, Fröhlich M, Sinning CR, Eleftheriadis MS, Wild PS, Schnabel RB, Lubos E, Jachmann N, Genth‐Zotz S, Post F, Nicaud V, Tiret L, Lackner KJ, Münzel TF, Blankenberg S. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009; 361:868-877 [DOI] [PubMed] [Google Scholar]
- 27.Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, Biedert S, Schaub N, Buerge C, Potocki M, Noveanu M, Breidthardt T, Twerenbold R, Winkler K, Bingisser R, Mueller C. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009; 361:858-867 [DOI] [PubMed] [Google Scholar]
- 28.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high‐sensitivity cardiac troponin T assay. Clin Chem. 2010; 56:254-261 [DOI] [PubMed] [Google Scholar]
- 29.Keller T, Zeller T, Ojeda F, Tzikas S, Lillpopp L, Sinning C, Wild P, Genth‐Zotz S, Warnholtz A, Giannitsis E, Mockel M, Bickel C, Peetz D, Lackner K, Baldus S, Munzel T, Blankenberg S. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA. 2011; 306:2684-2693 [DOI] [PubMed] [Google Scholar]
- 30.Reichlin T, Irfan A, Twerenbold R, Reiter M, Hochholzer W, Burkhalter H, Bassetti S, Steuer S, Winkler K, Peter F, Meissner J, Haaf P, Potocki M, Drexler B, Osswald S, Mueller C. Utility of absolute and relative changes in cardiac troponin concentrations in the early diagnosis of acute myocardial infarction. Circulation. 2011; 124:136-145 [DOI] [PubMed] [Google Scholar]
- 31.Mueller M, Biener M, Vafaie M, Doerr S, Keller T, Blankenberg S, Katus HA, Giannitsis E. Absolute and relative kinetic changes of high‐sensitivity cardiac troponin T in acute coronary syndrome and in patients with increased troponin in the absence of acute coronary syndrome. Clin Chem. 2012; 58:209-218 [DOI] [PubMed] [Google Scholar]
- 32.Aldous SJ, Richards M, Cullen L, Troughton R, Than M. Diagnostic and prognostic utility of early measurement with high‐sensitivity troponin T assay in patients presenting with chest pain. CMAJ. 2012; 184:E260-E268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aldous SJ, Richards AM, Cullen L, Than MP. Early dynamic change in high‐sensitivity cardiac troponin T in the investigation of acute myocardial infarction. Clin Chem. 2011; 57:1154-1160 [DOI] [PubMed] [Google Scholar]
- 34.Cannon CP, Weintraub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N, Neumann FJ, Robertson DH, DeLucca PT, DiBattiste PM, Gibson CM, Braunwald ETACTICS (Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy)–Thrombolysis in Myocardial Infarction 18 Investigators. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001; 344:1879-1887 [DOI] [PubMed] [Google Scholar]
- 35.Mehta SR, Granger CB, Boden WE, Steg PG, Bassand JP, Faxon DP, Afzal R, Chrolavicius S, Jolly SS, Widimsky P, Avezum A, Rupprecht HJ, Zhu J, Col J, Natarajan MK, Horsman C, Fox KA, Yusuf S, Investigators T. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med. 2009; 360:2165-2175 [DOI] [PubMed] [Google Scholar]
- 36.Newby LK, Ohman EM, Christenson RH, Moliterno DJ, Harrington RA, White HD, Armstrong PW, Van De Werf F, Pfisterer M, Hasselblad V, Califf RM, Topol EJ. Benefit of glycoprotein IIb/IIIa inhibition in patients with acute coronary syndromes and troponin T‐positive status: the PARAGON‐B Troponin T substudy. Circulation. 2001; 103:2891-2896 [DOI] [PubMed] [Google Scholar]
- 37.Becattini C, Vedovati MC, Agnelli G. Prognostic value of troponins in acute pulmonary embolism: a meta‐analysis. Circulation. 2007; 116:427-433 [DOI] [PubMed] [Google Scholar]
- 38.Lankeit M, Jimenez D, Kostrubiec M, Dellas C, Hasenfuss G, Pruszczyk P, Konstantinides S. Predictive value of the high‐sensitivity troponin T assay and the simplified pulmonary embolism severity index in hemodynamically stable patients with acute pulmonary embolism: a prospective validation study. Circulation. 2011; 124:2716-2724 [DOI] [PubMed] [Google Scholar]
- 39.Lankeit M, Gomez V, Wagner C, Aujesky D, Recio M, Briongos S, Moores LK, Yusen RD, Konstantinides S, Jimenez DInstituto Ramon y Cajal de Investigacion Sanitaria Pulmonary Embolism Study G A strategy combining imaging and laboratory biomarkers in comparison with a simplified clinical score for risk stratification of patients with acute pulmonary embolism. Chest. 2012; 141:916-922 [DOI] [PubMed] [Google Scholar]
- 40.Braunwald E, Domanski MJ, Fowler SE, Geller NL, Gersh BJ, Hsia J, Pfeffer MA, Rice MM, Rosenberg YD, Rouleau JL, Investigators PT. Angiotensin‐converting‐enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004; 351:2058-2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Gersh BJ, Rouleau JL, Pfeffer MA, Braunwald EPrevention of Events with Angiotensin Converting Enzyme Inhibition Trial I A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009; 361:2538-2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000; 342:145-153 [DOI] [PubMed] [Google Scholar]
- 43.Kavsak PA, Xu L, Yusuf S, McQueen MJ. High‐sensitivity cardiac troponin I measurement for risk stratification in a stable high‐risk population. Clin Chem. 2011; 57:1146-1153 [DOI] [PubMed] [Google Scholar]
- 44.Peacock WF, IV, De Marco T, Fonarow GC, Diercks D, Wynne J, Apple FS, Wu AHADHERE Investigators Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008; 358:2117-2126 [DOI] [PubMed] [Google Scholar]
- 45.Jungbauer CG, Riedlinger J, Buchner S, Birner C, Resch M, Lubnow M, Debl K, Buesing M, Huedig H, Riegger G, Luchner A. High‐sensitive troponin T in chronic heart failure correlates with severity of symptoms, left ventricular dysfunction and prognosis independently from N‐terminal pro‐B‐type natriuretic peptide. Clin Chem Lab Med. 2011; 49:1899-1906 [DOI] [PubMed] [Google Scholar]
- 46.Tentzeris I, Jarai R, Farhan S, Perkmann T, Schwarz MA, Jakl G, Wojta J, Huber K. Complementary role of copeptin and high‐sensitivity troponin in predicting outcome in patients with stable chronic heart failure. Eur J Heart Fail. 2011; 13:726-733 [DOI] [PubMed] [Google Scholar]
- 47.Tsutamoto T, Kawahara C, Nishiyama K, Yamaji M, Fujii M, Yamamoto T, Horie M. Prognostic role of highly sensitive cardiac troponin I in patients with systolic heart failure. Am Heart J. 2010; 159:63-67 [DOI] [PubMed] [Google Scholar]
- 48.Masson S, Anand I, Favero C, Barlera S, Vago T, Bertocchi F, Maggioni AP, Tavazzi L, Tognoni G, Cohn JN, Latini R. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: data from 2 large randomized clinical trials. Circulation. 2012; 125:280-288 [DOI] [PubMed] [Google Scholar]
- 49.Nagarajan V, Hernandez AV, Tang WH. Prognostic value of cardiac troponin in chronic stable heart failure: a systematic review. Heart. 2012; 98:1778-1786 [DOI] [PubMed] [Google Scholar]
- 50.Kawahara C, Tsutamoto T, Sakai H, Nishiyama K, Yamaji M, Fujii M, Yamamoto T, Horie M. Prognostic value of serial measurements of highly sensitive cardiac troponin I in stable outpatients with nonischemic chronic heart failure. Am Heart J. 2011; 162:639-645 [DOI] [PubMed] [Google Scholar]
- 51.Khan NA, Hemmelgarn BR, Tonelli M, Thompson CR, Levin A. Prognostic value of troponin T and I among asymptomatic patients with end‐stage renal disease: a meta‐analysis. Circulation. 2005; 112:3088-3096 [DOI] [PubMed] [Google Scholar]
- 52.Kumar N, Michelis MF, DeVita MV, Panagopoulos G, Rosenstock JL. Troponin I levels in asymptomatic patients on haemodialysis using a high‐sensitivity assay. Nephrol Dial Transplant. 2011; 26:665-670 [DOI] [PubMed] [Google Scholar]
- 53.Pianta TJ, Horvath AR, Ellis VM, Leonetti R, Moffat C, Josland EA, Brown MA. Cardiac high‐sensitivity troponin T measurement: a layer of complexity in managing haemodialysis patients. Nephrology. 2012; 17:636-641 [DOI] [PubMed] [Google Scholar]
- 54.Jacobs LH, van de Kerkhof J, Mingels AM, Kleijnen VW, van der Sande FM, Wodzig WK, Kooman JP, van Dieijen‐Visser MP. Haemodialysis patients longitudinally assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and cardiac troponin I assays. Ann Clin Biochem. 2009; 46:283-290 [DOI] [PubMed] [Google Scholar]
- 55.de Filippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010; 304:2494-2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roldan V, Marin F, Diaz J, Gallego P, Jover E, Romera M, Manzano‐Fernandez S, Casas T, Valdes M, Vicente V, Lip GY. High sensitivity cardiac troponin T and interleukin‐6 predict adverse cardiovascular events and mortality in anticoagulated patients with atrial fibrillation. J Thromb Haemost. 2012; 10:1500-1507 [DOI] [PubMed] [Google Scholar]
- 57.Latini R, Masson S, Pirelli S, Barlera S, Pulitano G, Carbonieri E, Gulizia M, Vago T, Favero C, Zdunek D, Struck J, Staszewsky L, Maggioni AP, Franzosi MG, Disertori M, Investigators G‐A. Circulating cardiovascular biomarkers in recurrent atrial fibrillation: data from the GISSI‐Atrial Fibrillation Trial. J Intern Med. 2011; 269:160-171 [DOI] [PubMed] [Google Scholar]
- 58.Munoz‐Esparza C, Garrido IP, Blanco R, Casas T, Gonzalez‐Canovas C, Pastor‐Perez F, Penafiel P, Minguela A, Valdes M, Pascual‐Figal DA. [Usefulness of high sensitivity troponin T assay in detecting acute allograft rejection after heart transplantation]. Rev Esp Cardiol. 2011; 64:1109-1113 [DOI] [PubMed] [Google Scholar]
- 59.Erbel C, Taskin R, Doesch A, Dengler TJ, Wangler S, Akhavanpoor M, Ruhparwar A, Giannitsis E, Katus HA, Gleissner CA. High‐sensitive troponin T measurements early after heart transplantation predict short‐ and long‐term survival. Transpl Int. 2013; 26:267-272 [DOI] [PubMed] [Google Scholar]
- 60.Garrido IP, Garcia‐Lara J, Pinar E, Pastor‐Perez F, Sanchez‐Mas J, Valdes‐Chavarri M, Pascual‐Figal DA. Optical coherence tomography and highly sensitivity troponin T for evaluating cardiac allograft vasculopathy. Am J Cardiol. 2012; 110:655-661 [DOI] [PubMed] [Google Scholar]
- 61.Cardinale D, Colombo A, Torrisi R, Sandri MT, Civelli M, Salvatici M, Lamantia G, Colombo N, Cortinovis S, Dessanai MA, Nole F, Veglia F, Cipolla CM. Trastuzumab‐induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010; 28:3910-3916 [DOI] [PubMed] [Google Scholar]
- 62.Newby LK, Rodriguez I, Finkle J, Becker RC, Hicks KA, Hausner E, Chesler R, Harper C, Targum S, Berridge BR, Lewis E, Walker DB, Dollery C, Turner JR, Krucoff MW. Troponin measurements during drug development—considerations for monitoring and management of potential cardiotoxicity: an educational collaboration among the Cardiac Safety Research Consortium, the Duke Clinical Research Institute, and the US Food and Drug Administration. Am Heart J. 2011; 162:64-73 [DOI] [PubMed] [Google Scholar]