Abstract
Background
Systemic hypertension is a common characteristic in acute heart failure (HF). This increasingly recognized phenotype is commonly associated with renal dysfunction and there is an unmet need for renal enhancing therapies. In a canine model of HF and acute vasoconstrictive hypertension we characterized and compared the cardiorenal actions of M‐atrial natriuretic peptide (M‐ANP), a novel particulate guanylyl cyclase (pGC) activator, and nitroglycerin, a soluble guanylyl cyclase (sGC) activator.
Methods and Results
HF was induced by rapid RV pacing (180 beats per minute) for 10 days. On day 11, hypertension was induced by continuous angiotensin II infusion. We characterized the cardiorenal and humoral actions prior to, during, and following intravenous M‐ANP (n=7), nitroglycerin (n=7), and vehicle (n=7) infusion. Mean arterial pressure (MAP) was reduced by M‐ANP (139±4 to 118±3 mm Hg, P<0.05) and nitroglycerin (137±3 to 116±4 mm Hg, P<0.05); similar findings were recorded for pulmonary wedge pressure (PCWP) with M‐ANP (12±2 to 6±2 mm Hg, P<0.05) and nitroglycerin (12±1 to 6±1 mm Hg, P<0.05). M‐ANP enhanced renal function with significant increases (P<0.05) in glomerular filtration rate (38±4 to 53±5 mL/min), renal blood flow (132±18 to 236±23 mL/min), and natriuresis (11±4 to 689±37 mEq/min) and also inhibited aldosterone activation (32±3 to 23±2 ng/dL, P<0.05), whereas nitroglycerin had no significant (P>0.05) effects on these renal parameters or aldosterone activation.
Conclusions
Our results advance the differential cardiorenal actions of pGC (M‐ANP) and sGC (nitroglycerin) mediated cGMP activation. These distinct renal and aldosterone modulating actions make M‐ANP an attractive therapeutic for HF with concomitant hypertension, where renal protection is a key therapeutic goal.
Keywords: cyclic GMP, heart failure, hypertension, natriuretic peptide, nitroglycerin
Introduction
Cyclic guanosine monophosphate (cGMP) is an important second messenger molecule which targets protein kinase G resulting in beneficial, adaptive actions in the heart and kidney, particularly in response to stress.§ Cyclic GMP is activated by 2 distinct enzymatic pathways: nitric oxide activated soluble (cytosolic) guanylyl cyclase (sGC) and natriuretic peptide receptor activated particulate (membrane‐bound) guanylyl cyclase (pGC).4 Soluble GC and pGC are differentially expressed and are compartmentalized within the cell.5–6 Furthermore, while both sGC and pGC activation results in the accumulation of cGMP within cells, pGC activation (in contrast to sGC) also results in significant release of cGMP into the extracellular space and circulation.7–11 Therefore, while both sGC and pGC increase intracellular cGMP, the resulting biological actions are quite different.4,12–13
Cyclic GMP therapies are currently employed in the treatment of heart failure (HF). Specifically, the sGC activator nitroglycerin (NTG) is a potent vasodilator and is commonly used in HF especially in the presence of hypertension. Recombinant atrial natriuretic peptide (ANP; carperitide) is a potent pGC activator via the GC‐A receptor and is widely used in acute HF in Japan.14–15 ANP possesses pluripotent cardiorenal actions including vasodilatation, natriuresis, and aldosterone suppression.16 Furthermore, ANP plays a central role in blood pressure (BP) homeostasis17 and Newton‐Cheh et al18 have established that an ANP genetic variant (rs5068) resulting in increased circulating ANP protects again hypertension. M‐ANP is a designer natriuretic peptide, which is a 40‐amino acid (AA), consisting of the 28 AAs of native ANP with a 12 AA C‐terminus extension. These 12 AAs render M‐ANP highly resistant to degradation by neprilysin resulting in greater and more sustained reductions in BP, increases in natriuresis and glomerular filtration rate (GFR), and inhibition of aldosterone as compared to ANP.19–20 In a model of acute hypertension, M‐ANP was more natriuretic than B‐type NP (nesiritide).21
It is now recognized that systemic hypertension is a common characteristic, as high as 50% among subjects hospitalized with acute HF.22–24 Beyond the association of systemic hypertension and HF, it is speculated that in stable HF, an acute increase in BP due to excessive sodium intake or medication noncompliance may be one mechanism of acute decompensated HF. Importantly, acute hypertensive episodes are commonly associated with renal impairment,25 which in the presence of HF is a mediator and marker for increased mortality and morbidity.26 Indeed, the Studying the Treatment of Acute Hypertension registry25 underscores the need for BP‐lowering therapies, which enhance renal function so as to improve outcomes.
The current study was designed to characterize for the first time the acute cardiorenal actions of the innovative designer natriuretic peptide, M‐ANP, in a large animal model of hypertensive HF and to compare it to NTG, the most commonly used vasodilator in HF, thus defining the actions of 2 cGMP‐activating drugs, one which targets pGC and the other sGC. We hypothesized that both NTG and M‐ANP would lower cardiac filling pressures and BP in this model of LV dysfunction and acute hypertension. We further hypothesized that due to the greater and more widespread distribution of pGC compared to sGC (particularly in the kidney),16 M‐ANP would also have renal‐enhancing properties and aldosterone‐suppressing actions in contrast to NTG.
Methods
M‐ANP and Nitroglycerin
M‐ANP was synthesized by Phoenix Pharmaceuticals. Structure was confirmed by mass spectrometry and high‐performance liquid chromatography analysis confirmed purity to be ≥95%. NTG was obtained from American Regent, Inc.
Study Protocol
We investigated the cardiorenal actions of intravenous M‐ANP, NTG, and vehicle (0.9% normal saline; n=7 for each group) in a canine model of mild systolic HF with angiotensin II (Ang II)‐induced acute hypertension. Studies were performed in accordance with the Animal Welfare Act and with approval of the Mayo Clinic Institutional Animal Care and Use Committee.
An experimental model of mild LV dysfunction (MLVD) was produced in male mongrel dogs by rapid RV pacing at 180 beats per minute for 10 days. Experimental procedures of this model have been extensively described previously.27 Importantly, this model produces a 38% reduction in cardiac output (CO), 52% increase in pulmonary wedge pressure (PCWP), and 55% increase in systemic vascular resistance (SVR). On day 11, an acute study was performed in which acute hypertension was induced and assessment of cardiorenal and neurohumoral parameters was performed during the acute study described below.
The night before the acute study (Day 10) dogs were fasted. On the day of the acute study (Day 11), RV pacing was terminated and dogs were anesthetized with pentobarbital sodium (15 mg/kg intravenous), intubated, and mechanically ventilated with supplemental oxygen (Harvard respirator) at 12 cycles per minute. A balloon‐tipped thermodilution catheter was advanced to the pulmonary artery via the external jugular vein for measurement of cardiac filling pressures (PCWP, pulmonary artery pressure [PAP], and right atrial pressure [RAP]) and CO. The right femoral artery was cannulated and a line inserted to the infrarenal aorta for mean arterial pressure (MAP) monitoring and arterial blood sampling. The right femoral vein was cannulated for inulin and Ang II infusion. Via a left lateral flank incision the left kidney was exposed and the left ureter was cannulated for urine sampling. An electromagnetic flow probe (Carolina Medical Electronics) was placed around the left renal artery to measure renal blood flow (RBF). Supplemental nonhypotensive doses of pentobarbital were administered as needed during the experiment. After completion of the above procedural set up, a weight‐adjusted inulin bolus was administered followed immediately by continuous inulin infusion to maintain plasma inulin levels between 40 and 60 mg/dL for determination of GFR. Renal vascular resistance (RVR) and SVR were calculated as previously described.21
After completion of the above procedural set‐up and 60 minutes of equilibration, a 30‐minute baseline clearance of the pacing model (MLVD) was performed. This and all clearances lasted 30 minutes and consisted of arterial blood sampling, hemodynamic measurements, and urine collection over 30 minutes. Immediately after the MLVD clearance, the saline infusion was replaced by a continuous infusion of Ang II infusion (40 ng kg−1 min−1, 1 mL/min; Phoenix Pharmaceuticals), which was continued throughout the experimental protocol to produce acute hypertension in the model of MLVD. After a 15‐minute lead‐in period of the Ang II infusion, a 30‐minute Ang II clearance was performed. This clearance is referred to as MLVD+HTN. Importantly, Ang II was continuously infused for the remainder of the study protocol. After the MLVD+HTN clearance, M‐ANP (30 pmol kg−1 min−1), NTG (10 μg/kg), or vehicle (0.9% normal saline) was infused at a rate of 1 mL/min. The dose of M‐ANP was based on previous reports which demonstrated significant MAP‐lowering actions.20–21 The dose of NTG was chosen to produce equivalent MAP lowering compared to M‐ANP (NTG 10 μg/kg was chosen based on dose findings studies—data not shown). M‐ANP, NTG, or vehicle was infused for a total of 45 minutes, which included a 15‐minute lead‐in period followed by a 30‐minute clearance. M‐ANP, NTG, or vehicle infusion was then discontinued, and four 30‐minute clearances were performed that were 0 to 30, 31 to 60, 61 to 90, and 91 to 120 minutes after M‐ANP, NTG, or vehicle infusions.
Neurohormonal and Electrolyte Analysis
Plasma and urine ANP28 (Phoenix Pharmaceuticals), plasma and urine cGMP29 (PerkinElmer), Ang II30 (Phoenix Pharmaceuticals), and aldosterone31 (Siemens Healthcare Diagnostics) were determined by radioimmunoassay as described previously. Inulin concentrations were measured using the anthrone method as previously described.32 Electrolytes including lithium were measured by flame photometry (IL943, Instrumentation Laboratory). GFR was measured by inulin clearance.
Statistical Analysis
Descriptive statistics are reported as mean±standard error (SE). Comparisons within groups were made by 2‐way analysis of variance (ANOVA) for repeated measures using time and animal as the main effects. Pairwise comparison of the individual time points within groups was done using the Tukey HSD method to control the overall error rate. Two‐way ANOVA for repeated measures was used to compare M‐ANP and NTG (the P value for interaction is reported) followed by Bonferroni posttests for specific time points. For nonparametric parameters (urine volume, urinary sodium excretion, plasma cGMP, urinary cGMP excretion, plasma ANP, urinary ANP, and aldosterone), the data were log transformed prior to analysis; the raw data are presented in the figures and tables. GraphPad Prism 5 (GraphPad Software) and JMP 10 was used for the above calculations. Statistical significance was accepted as P<0.05.
Results
Systemic Hemodynamics
Following assessment of the baseline characteristics of the MLVD pacing model, a continuous intravenous infusion of Ang II was initiated in all 3 groups. Ang II infusion (MLVD+HTN) resulted in significant increases in MAP and PCWP (Figure 1) and SVR (Table 1) compared to baseline MLVD assessment. CO (Table 1) was reduced with MLVD+HTN compared to baseline MLVD assessment. Hemodynamic parameters with Ang II infusion (MLVD+HTN) were similar (P>0.05) among all 3 groups.
Figure 1.
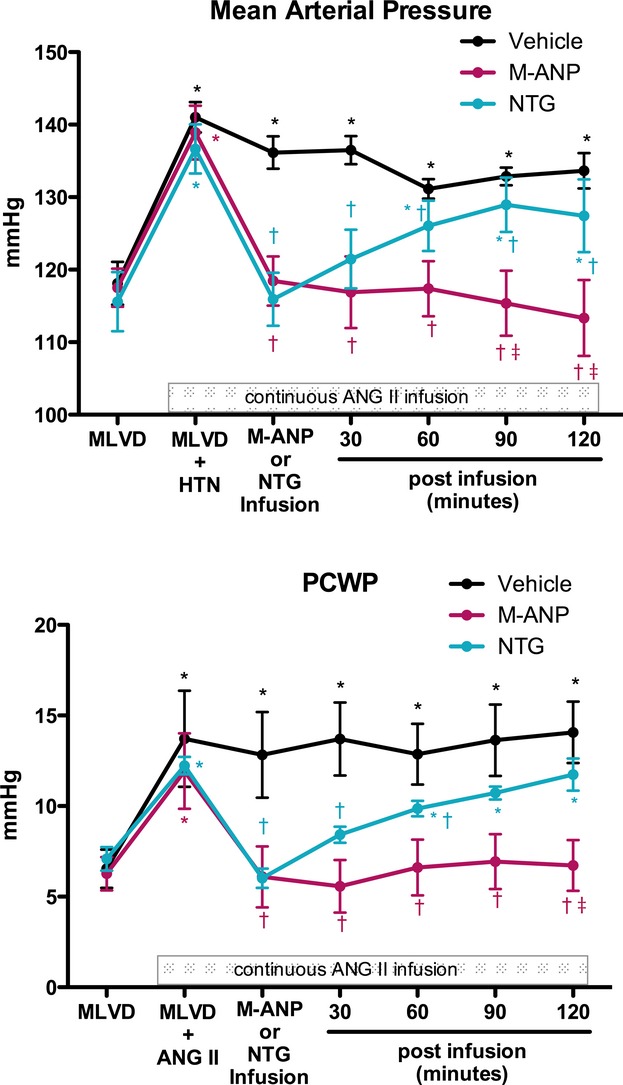
Mean arterial pressure (MAP) and mean pulmonary capillary wedge pressure (PCWP) during mild LV dysfunction (MLVD) clearance; MLVD and angiotensin II‐induced hypertension (MLVD+HTN); M‐ANP, NTG, or vehicle infusion; and 30, 60, 90, and 120 minutes after M‐ANP, NTG, or vehicle infusion. *P<0.05 vs MLVD and †P<0.05 vs MLVD+HTN, 2‐way ANOVA with pairwise comparison of individual timepoints within groups using the Tukey HSD method. ‡P<0.05 for M‐ANP vs vehicle at a specific time point, 2‐way ANOVA with Bonferroni posttests. P interaction <0.05 for the main effects of treatment and time between M‐ANP vs NTG for MAP and PCWP, 2‐way ANOVA. ANG II indicates angiotensin II; ANOVA, analysis of variance; HSD, honestly significant difference; M‐ANP, M‐atrial natriuretic peptide; NTG, nitroglycerin.
Table 1.
Hemodynamic Data Following M‐ANP, Nitroglycerin, or Vehicle Administration
| Treatment | MLVD | MLVD+HTN | Vehicle, M‐ANP, or NTG Infusion | Post Vehicle, M‐ANP, or NTG Infusion | ||
|---|---|---|---|---|---|---|
| 30 Minutes | 60 Minutes | 120 Minutes | ||||
| CO, L/min | ||||||
| Vehicle | 3.2±0.1 | 2.9±0.2* | 2.7±0.1* | 2.7±0.1* | 2.6±0.1* | 2.6±0.1* |
| M‐ANP | 3.4±0.2 | 2.7±0.2* | 2.9±0.2 | 2.6±0.2* | 2.5±0.1* | 2.8±0.2* |
| NTG | 3.4±0.1 | 2.8±0.1* | 3.1±0.1 | 3.1±0.1 | 3.0±0.1 | 2.7±0.1* |
| SVR, mm Hg L−1 min−1 | ||||||
| Vehicle | 39.2±1.2 | 52.9±3.1* | 52.6±2.6* | 52.3±2.8* | 50.9±3.2* | 53.4±3.9* |
| M‐ANP | 34.2±2.4 | 48.8±3.2* | 39.5±2.5† | 42.8±2.3* | 44.6±2.3* | 39.9±1.9*† |
| NTG | 35.9±1.8 | 50.3±2.3* | 39.0±1.6† | 41.6±2.2† | 45.2±2.1* | 48.1±1.3* |
| PAP§, mm Hg | ||||||
| Vehicle | 14.8±0.7 | 22.2±2.8* | 20.8±1.9* | 21.7±1.5* | 21.3±1.5* | 23.4±1.7* |
| M‐ANP | 15.0±0.7 | 20.2±2.5* | 14.3±1.4† | 13.5±1.3† | 15.8±2.7† | 15.5±1.4† |
| NTG | 15.1±0.7 | 19.2±0.9* | 12.9±0.6† | 15.1±0.7† | 16.2±0.7† | 19.1±1.0* |
| RAP§, mm Hg | ||||||
| Vehicle | 2.9±0.7 | 3.7±0.7* | 3.2±0.9 | 3.2±0.8 | 3.1±0.7 | 3.3±0.6 |
| M‐ANP | 2.7±0.5 | 3.8±0.9* | 1.7±0.7*† | 1.6±0.6*#x2020; | 2.7±0.9† | 2.8±0.6† |
| NTG | 3.1±0.3 | 3.8±0.5* | 1.9±0.2*† | 3.1±0.3 | 3.6±0.2 | 4.1±0.3* |
| RVR§, mm Hg L−1 min−1 | ||||||
| Vehicle | 672±52 | 967±80* | 909±87* | 865±93* | 795±94 | 746±80 |
| M‐ANP | 747±96 | 1121±145* | 530±53† | 578±47† | 648±65† | 604±45† |
| NTG | 665±40 | 986±79* | 713±51† | 765±46 | 804±62 | 812±87 |
| Heart rate§, beats/min | ||||||
| Vehicle | 130±6 | 153±7* | 158±7* | 160±6* | 160±6* | 162±4* |
| M‐ANP | 137±5 | 152±6* | 151±5* | 146±5 | 143±7 | 143±5 |
| NTG | 138±3 | 160±3* | 167±1* | 158±4* | 159±4* | 158±4* |
ANOVA indicates analysis of variance; CO, cardiac output; M‐ANP, M‐atrial natriuretic peptide; MLVD, mild left ventricular dysfunction; MLVD+HTN, MLVD and angiotensin II induced hypertension; NTG, nitroglycerin; PAP, pulmonary artery pressure; RAP, right atrial pressure; RVR, renal vascular resistance; SVR, systemic vascular resistance.
*P<0.05 vs MLVD and †P<0.05 vs MLVD+HTN, 2‐way ANOVA with pairwise comparison of individual time points within groups using the Tukey HSD method.
§P interaction <0.05 for the main effects of treatment and time between M‐ANP vs NTG, 2‐way ANOVA.
After assessment of the MLVD+HTN model, intravenous M‐ANP, NTG, or vehicle was administered over 45 minutes. Importantly, the Ang II infusion was continued throughout the experimental protocol. MAP (Figure 1) significantly decreased during M‐ANP and NTG infusion compared with MLVD+HTN measurements as well as the vehicle group. Per the experimental protocol, there was no difference in the absolute MAP reduction between M‐ANP and NTG during the treatment infusion period. However, the sustained MAP lowering actions were significantly greater for M‐ANP compared to NTG (P<0.05 by 2‐way ANOVA). Vehicle administration did not significantly alter MAP, which remained elevated throughout the study. In a similar manner, both M‐ANP and NTG significantly lowered PCWP (Figure 1). The effects of M‐ANP and NTG on PAP and RAP (Table 1) were similar to MAP.
There was a trend for higher CO following NTG infusion when compared to the MLVD+HTN measurements; however, this did not achieve statistical significance (Table 1). There was no difference in CO following M‐ANP administration. Heart rate significantly increased in the MLVD+HTN compared to MLVD in all 3 groups. Although both GC activators reduced SVR, neither M‐ANP nor NTG had a significant effect on heart rate during or following the treatment infusion compared to MLVD+HYT. However, there were lower heart rates (P<0.05) following M‐ANP infusion compared to NTG and vehicle (Table 1).
Renal Hemodynamics
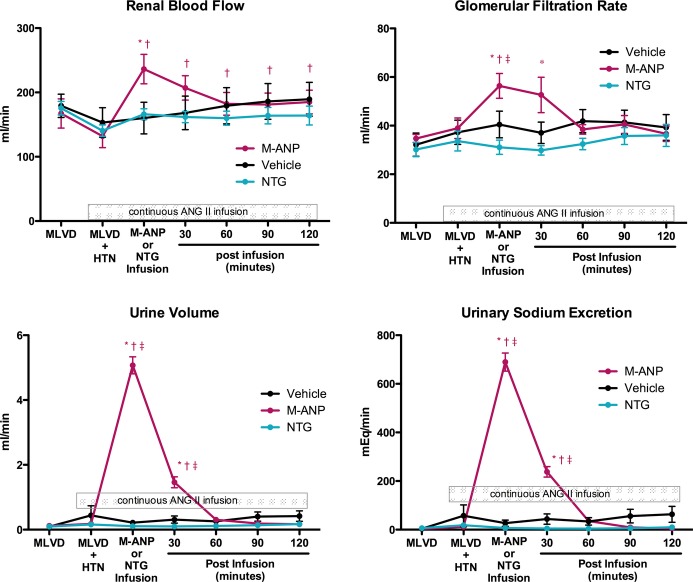
Ang II infusion resulted in a nonsignificant decrease in RBF and nonsignificant increases in GFR, urine volume, and urinary sodium excretion compared to baseline assessment of the MLVD model (Figure 2) in all 3 groups. Importantly, M‐ANP infusion resulted in significant increases in RBF and GFR while also inducing marked diuresis and natriuresis. For RBF this increase was sustained for 120 minutes following the completion of the M‐ANP infusion despite the concomitant ANG II infusion. Concurrent with the sustained increase in RBF, there were sustained reductions in RVR (Table 1) for 120 minutes following M‐ANP infusion. The increases in GFR, diuresis, and natriuresis following M‐ANP infusion were sustained for 60 minutes. In contrast to M‐ANP, NTG infusion lowered RVR but did not significantly alter renal hemodynamics, and had similar actions compared to vehicle with no significant changes in RBF, GFR, diuresis, or natriuresis. Renal blood flow, GFR, urine flow, and urinary sodium excretion were significantly greater following M‐ANP infusion compared to NTG (P<0.05 by 2‐way ANOVA).
Figure 2.
Renal blood flow, glomerular filtration rate, urine volume, and urinary sodium excretion during mild LV dysfunction (MLVD) clearance; MLVD and angiotensin II‐induced hypertension (MLVD+HTN); M‐ANP, NTG, or vehicle infusion; and 30, 60, 90, and 120 minutes after M‐ANP, NTG, or vehicle infusion. *P<0.05 vs MLVD and †P<0.05 vs MLVD+HTN, 2‐way ANOVA with pairwise comparison of individual timepoints within groups using the Tukey HSD method. ‡P<0.05 vs NTG at a specific time point, 2‐way ANOVA with Bonferroni posttests. P interaction <0.05 for the main effects of treatment and time between M‐ANP vs NTG for renal blood flow, glomerular filtration rate, urine volume, and urinary sodium, 2‐way ANOVA. ANG II indicates angiotensin II; ANOVA, analysis of variance; M‐ANP, M‐atrial natriuretic peptide; NTG, nitroglycerin.
Humoral
Increases in Ang II levels following the start of the continuous ANG II infusion were similar between the 3 treatment groups. Despite the continuous Ang II infusion, aldosterone activation was inhibited during the M‐ANP infusion whereas there was no significant inhibition of aldosterone activation with NTG infusion (Table 2).
Table 2.
Neurohumoral Data Following M‐ANP, Nitroglycerin, or Vehicle Administration
| Treatment | MLVD | MLVD+HTN | Vehicle, M‐ANP, or NTG Infusion | Post Vehicle, M‐ANP, or NTG Infusion | ||
|---|---|---|---|---|---|---|
| 30 Minutes | 60 Minutes | 120 Minutes | ||||
| ANG II, pg/mL | ||||||
| Vehicle | 43±9 | 181±31* | 245±29* | 191±25* | 174±37.1* | 171±27* |
| M‐ANP | 29±7 | 213±18* | 222±39* | 201±37* | 273±43* | 185±15* |
| NTG | 39±11 | 183±20* | 209±52* | 249±51* | 165±11* | 184±23* |
| Plasma ANP§, pg/mL | ||||||
| Vehicle | 193±76 | 402±185* | 435±125* | 428±107* | 431±103* | 547±136* |
| M‐ANP | 173±53 | 404±182* | 1863±464*†‡ | 735±180*‡ | 408±165 | 230±91 |
| NTG | 204±41 | 299±22* | 190±35‡ | 200±27‡ | 247±36 | 264±43 |
| Plasma cGMP§, pg/mL | ||||||
| Vehicle | 11.3±1.1 | 13.9±1.0* | 14.4±1.3* | 14.1±1.3* | 14.4±1.7* | 14.9±1.9* |
| M‐ANP | 9.9±0.9 | 11.7±1.4* | 44.7±2.1*†‡ | 33.3±1.9*†‡ | 20.0±1.3*†‡ | 11.9±1.3 |
| NTG | 9.5±0.7 | 12.8±0.5* | 11.3±0.6 | 10.2±0.5† | 10.1±0.4† | 11.4±0.7 |
| Urine ANP§, pg/min | ||||||
| Vehicle | 22.5±2.9 | 44.3±12.0* | 39.1±6.6* | 44.6±7.8* | 47.3±8.0* | 44.2±8.0* |
| M‐ANP | 30.9±11.5 | 64.8±15.8* | 195.2±60.7*†‡ | 155.1±50.0*†‡ | 57.5±17.2 | 59.9±28.4 |
| NTG | 33.6±9.7 | 49.6±7.5* | 44.1±13.4 | 41.8±17.9 | 43.1±18.4 | 64.4±26.2 |
| Renal cGMP generation§, pmol/min | ||||||
| Vehicle | 361±38 | 574±105 | 631±104 | 590±131 | 641±109 | 587±98 |
| M‐ANP | 288±37 | 384±55 | 2514±234*†‡ | 1760±268*†‡ | 769±62†‡ | 430±56 |
| NTG | 278±18 | 435±63 | 346±28 | 303±20 | 332±35 | 418±65* |
| Filtered cGMP§, pmol/min | ||||||
| Vehicle | 492±42 | 540±205 | 454±66 | 630±126 | 593±76 | 756±136 |
| M‐ANP | 499±77 | 570±124 | 5451±1098*†‡ | 5156±1120*†‡ | 1762±181*†‡ | 914±147 |
| NTG | 507±55 | 433±110 | 524±64 | 509±56 | 441±37 | 739±75* |
| Plasma aldosterone, ng/dL | ||||||
| Vehicle | 13.1±3.2 | 30.9±3.7* | 38.1±2.0*† | 40.9±2.6*† | 39.4±3.1*† | 45.3±4.5*† |
| M‐ANP | 9.8±1.4 | 31.5±3.0* | 23.2±2.0*† | 26.3±2.5* | 37.9±3.4* | 46.8±3.6*† |
| NTG | 11.1±3.8 | 27.0±5.5* | 34.7±6.0* | 37.4±6.0*† | 39.5±5.7*† | 41.8±4.2*† |
ANG II indicates angiotensin II; ANOVA, analysis of variance; cGMP, cyclic guanosine monophosphate; M‐ANP, M‐atrial natriuretic peptide; MLVD, mild left ventricular dysfunction; MLVD+HTN, MLVD and angiotensin II induced hypertension; NTG, nitroglycerin.
*P<0.05 vs MLVD and †P<0.05 vs MLVD+HTN, 2‐way ANOVA with pairwise comparison of individual time points within groups using the Tukey HSD methods.
‡P<0.05 vs NTG at a specific time point, 2‐way ANOVA with Bonferroni posttests.
§P interaction <0.05 for the main effects of treatment and time between M‐ANP vs NTG, 2‐way ANOVA.
M‐ANP infusion resulted in significant and sustained increases in plasma cGMP (Table 2) for up to 60 minutes whereas there was no effect on plasma cGMP following NTG infusion. In a similar manner M‐ANP infusion resulted in significant increases in urinary filtered as well as urinary cGMP generation for up to 60 minutes whereas there was no increase in urinary cGMP following NTG or vehicle infusion (Table 2).
Discussion
The current study is the first to define the cardiorenal and humoral actions of a novel designer natriuretic peptide and pGC activator (M‐ANP) and compare to NTG (a sGC activator) in a model of HF and acute systemic hypertension. Our results demonstrate that both M‐ANP and NTG promote vasodilatation, lower LV filling pressures, and reduce BP. In contrast to NTG, M‐ANP demonstrated significant renal enhancing actions exhibited by increases in GFR, RBF, and natriuresis and aldosterone inhibition. These results underscore the differential cardiorenal actions of sGC and pGC cGMP activation as well as the promising therapeutic potential of M‐ANP in HF with systemic hypertension.
HF with systemic hypertension is a common but underappreciated clinical entity.24 While associated with a lower short‐term mortality risk compared to HF and low systolic BP (<120 mm Hg), there remains significant morbidity associated with systemic hypertension, particularly related to hospital readmission.23–24 Commonly used therapeutic agents in HF and systemic hypertension are aimed at vasodilatation and diuresis, which while effective in lowering filling pressure, have limited beneficial and potentially negative effects on renal function.33–34 This is an important therapeutic consideration as renal function is a powerful prognostic marker in HF and deterioration in renal function related to acute treatment is associated with poor outcomes.25 Thus, there is a significant unmet need for renal‐enhancing therapeutics for HF and the clinical development of the natriuretic peptides and urodilatin,35 which are cardiac unloading but still preserve renal function, aim to address this unmet need.
The current study employs a canine model of MLVD+HTN which is characterized by acutely decompensated HF associated with increased SVR, LV filling pressures, and activation of aldosterone and ANP. Its hemodynamic and humoral phenotype, in part, recapitulates the phenotype of human stable HF and acute hypertension which is increasingly recognized among hospitalized HF subjects.22–24 Importantly, large animal models of HF to date have been limited to models of systolic dysfunction often with hypotension.27,36 Thus, testing of therapeutic agents in such traditional models of HF may not be entirely relevant to the setting of HF with hypertension. The goal of the MLVD+HTN model was therefore to create a large animal HF model which may be more appropriate for assessment of conventional and novel drugs in the increasingly common clinical setting of HF and hypertension. There are, however, important limitations to the current model. First, this particular pacing model (180 beats per minute for 10 days) results in compensated LV dysfunction. Only with the addition of angiotensin II and acute hypertension does the model exhibit overt HF. Second, acute hypertension via angiotensin II‐induced vasoconstriction may not represent the mechanism of hypertension in the majority HF subjects, which may be predominately mediated by hypervolemia.
M‐ANP is a novel, designer ANP‐based pGC activator comprised of the 28 AAs of native ANP with an additional 12 AAs linked to the C‐terminus. The extended C‐terminus renders resistance to enzymatic degradation by neprilysin and results in greater and more sustained diuretic, cardiac unloading, renal enhancing, aldosterone inhibiting, and vasodilatory properties actions compared to native ANP.19–21 These properties make M‐ANP an attractive therapeutic for disease states associated with volume overload and high filling pressures, particularly in the presence of concomitant hypertension.
In the current study, our goal was to define the actions of M‐ANP (a pGC cGMP activator) compared to a conventional vasodilator, NTG (a sGC cGMP activator). By study design the acute BP lowering actions during M‐ANP and NTG infusion were similar. However, the sustained BP lowering effects of M‐ANP were greater than NTG. The initial reduction in LV filling pressures and BP following M‐ANP and NTG were primarily driven by vasodilatation as SVR was similarly reduced in both groups. The greater sustained BP lowering actions of M‐ANP, despite concomitant ANG II infusion, were likely secondary to the significant diuresis and natriuresis following M‐ANP infusion as observed in previous studies.20–21 Importantly, despite a significant diuretic effect, M‐ANP did not lower CO or adversely affect HR when compared to NTG or vehicle. Future studies are required to assess the cardiorenal actions of chronic M‐ANP administration in cardiovascular disease models.
The renal actions of M‐ANP were significantly enhanced compared to NTG and occurred despite a significant reduction in BP. NTG did decrease RVR and this response is consistent with the elegant work of Elkayam who has demonstrated the potent renal vasodilating action of NTG in human HF.37–38 Unlike M‐ANP, the renovascular dilatation with NTG is unassociated with an increase in natriuresis, diuresis, or GFR.37,39–40 The renal actions of M‐ANP most likely reflect GC‐A receptor localization to the glomerulus and the renal tubule.16,41–42 These results underscore the physiological compartmentalization of the cGMP linked to either sGC or pGC cGMP signaling with differing cellular actions.5–10,5–13 An additional finding was that M‐ANP antagonized aldosterone activation despite continuous Ang II infusion. Most recently in a model of aldosterone‐mediated glomerular injury, the GC‐A receptor at the level of the glomerulus protected the glomerulus from structural remodeling43 underscoring an important potential action of M‐ANP if given long‐term.
In summary, we report the cardiorenal actions of the pGC activator M‐ANP and the sGC activator NTG in a model of pacing induced MLVD and acute hypertension in order to further define therapeutic strategies in the important clinical entity of acute HF with systolic hypertension. The current study advances the differential cardiorenal actions of sGC and pGC cGMP activation. While both sGC and pGC cGMP activators have vasodilating actions especially in the peripheral circulation, they have distinct renal and aldosterone modulating actions as demonstrated in the current study. Our findings suggest that M‐ANP has sustained LV unloading properties compared to NTG and possesses renal enhancing and aldosterone inhibiting properties. These properties make M‐ANP an attractive therapeutic candidate for HF, particularly in the presence of acute hypertension, which is an increasingly common phenotype among hospitalized patients and in which renal protection is emerging as a key therapeutic goal.
Disclosures
None.
References
- 1.Kukreja RC, Salloum FN, Das A. Cyclic guanosine monophosphate signaling and phosphodiesterase‐5 inhibitors in cardioprotection. J Am Coll Cardiol. 2012; 59:1921-1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005; 11:214-222 [DOI] [PubMed] [Google Scholar]
- 3.Kim KH, Kim YJ, Ohn JH, Yang J, Lee SE, Lee SW, Kim HK, Seo JW, Sohn DW. Long‐term effects of sildenafil in a rat model of chronic mitral regurgitation: benefits of ventricular remodeling and exercise capacity. Circulation. 2012; 125:1390-1401 [DOI] [PubMed] [Google Scholar]
- 4.Lucas KA, Pitari GM, Kazerounian S, Ruiz‐Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000; 52:375-414 [PubMed] [Google Scholar]
- 5.Su J, Scholz PM, Weiss HR. Differential effects of cGMP produced by soluble and particulate guanylyl cyclase on mouse ventricular myocytes. Exp Biol Med (Maywood). 2005; 230:242-250 [DOI] [PubMed] [Google Scholar]
- 6.Castro LR, Schittl J, Fischmeister R. Feedback control through cGMP‐dependent protein kinase contributes to differential regulation and compartmentation of cGMP in rat cardiac myocytes. Circ Res. 2010; 107:1232-1240 [DOI] [PubMed] [Google Scholar]
- 7.Tsutamoto T, Kinoshita M, Ohbayashi Y, Wada A, Maeda Y, Adachi T. Plasma arteriovenous cGMP difference as a useful indicator of nitrate tolerance in patients with heart failure. Circulation. 1994; 90:823-829 [DOI] [PubMed] [Google Scholar]
- 8.Vorderwinkler KP, Artner‐Dworzak E, Jakob G, Mair J, Diensti F, Pichler M, Puschendorf B. Release of cyclic guanosine monophosphate evaluated as a diagnostic tool in cardiac diseases. Clin Chem. 1991; 37:186-190 [PubMed] [Google Scholar]
- 9.Heim JM, Gottmann K, Weil J, Schiffl H, Lauster F, Loeschke K, Gerzer R. Effects of a small bolus dose of ANF in healthy volunteers and in patients with volume retaining disorders. Klin Wochenschr. 1990; 68:709-717 [DOI] [PubMed] [Google Scholar]
- 10.Jakob G, Mair J, Puschendorf B. On the relation of atrial natriuretic peptide and cyclic guanosine 3′,5′‐monophosphate plasma concentrations during ergometric exercise in healthy individuals. Horm Metab Res. 1994; 26:121-122 [DOI] [PubMed] [Google Scholar]
- 11.Jakob G, Mair J, Vorderwinkler KP, Judmaier G, Konig P, Zwierzina H, Pichler M, Puschendorf B. Clinical significance of urinary cyclic guanosine monophosphate in diagnosis of heart failure. Clin Chem. 1994; 40:96-100 [PubMed] [Google Scholar]
- 12.Tsai EJ, Kass DA. Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol Ther. 2009; 122:216-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen JF, Cui X, Jin JY, Kim SM, Kim SZ, Kim SH, Lee HS, Cho KW. High and low gain switches for regulation of cAMP efflux concentration: distinct roles for particulate GC‐ and soluble GC‐cGMP‐PDE3 signaling in rabbit atria. Circ Res. 2004; 94:936-943 [DOI] [PubMed] [Google Scholar]
- 14.Hata N, Seino Y, Tsutamoto T, Hiramitsu S, Kaneko N, Yoshikawa T, Yokoyama H, Tanaka K, Mizuno K, Nejima J, Kinoshita M. Effects of carperitide on the long‐term prognosis of patients with acute decompensated chronic heart failure: the PROTECT multicenter randomized controlled study. Circ J. 2008; 72:1787-1793 [DOI] [PubMed] [Google Scholar]
- 15.Kitakaze M, Asakura M, Kim J, Shintani Y, Asanuma H, Hamasaki T, Seguchi O, Myoishi M, Minamino T, Ohara T, Nagai Y, Nanto S, Watanabe K, Fukuzawa S, Hirayama A, Nakamura N, Kimura K, Fujii K, Ishihara M, Saito Y, Tomoike H, Kitamura S. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J‐WIND): two randomised trials. Lancet. 2007; 370:1483-1493 [DOI] [PubMed] [Google Scholar]
- 16.Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. 2009; 191:341-366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macheret F, Heublein D, Costello‐Boerrigter LC, Boerrigter G, McKie P, Bellavia D, Mangiafico S, Ikeda Y, Bailey K, Scott CG, Sandberg S, Chen HH, Malatino L, Redfield MM, Rodeheffer R, Burnett J, Jr, Cataliotti A. Human hypertension is characterized by a lack of activation of the antihypertensive cardiac hormones ANP and BNP. J Am Coll Cardiol. 2012; 60:1558-1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newton‐Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, Guiducci C, Kathiresan S, Benjamin EJ, Struck J, Morgenthaler NG, Bergmann A, Blankenberg S, Kee F, Nilsson P, Yin X, Peltonen L, Vartiainen E, Salomaa V, Hirschhorn JN, Melander O, Wang TJ. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009; 41:348-353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickey DM, Yoder AR, Potter LR. A familial mutation renders atrial natriuretic peptide resistant to proteolytic degradation. J Biol Chem. 2009; 284:19196-19202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKie PM, Cataliotti A, Huntley BK, Martin FL, Olson TM, Burnett JC., Jr A human atrial natriuretic peptide gene mutation reveals a novel peptide with enhanced blood pressure‐lowering, renal‐enhancing, and aldosterone‐suppressing actions. J Am Coll Cardiol. 2009; 54:1024-1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKie PM, Cataliotti A, Boerrigter G, Chen HH, Sangaralingham SJ, Martin FL, Ichiki T, Burnett JC., Jr A novel atrial natriuretic peptide based therapeutic in experimental angiotensin II mediated acute hypertension. Hypertension. 2010; 56:1152-1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milo‐Cotter O, Adams KF, O'Connor CM, Uriel N, Kaluski E, Felker GM, Weatherley B, Vered Z, Cotter G. Acute heart failure associated with high admission blood pressure—a distinct vascular disorder? Eur J Heart Fail. 2007; 9:178-183 [DOI] [PubMed] [Google Scholar]
- 23.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005; 149:209-216 [DOI] [PubMed] [Google Scholar]
- 24.Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O'Connor CM, She L, Stough WG, Yancy CW, Young JB, Fonarow GC. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006; 296:2217-2226 [DOI] [PubMed] [Google Scholar]
- 25.Szczech LA, Granger CB, Dasta JF, Amin A, Peacock WF, McCullough PA, Devlin JW, Weir MR, Katz JN, Anderson FA, Jr, Wyman A, Varon J. Acute kidney injury and cardiovascular outcomes in acute severe hypertension. Circulation. 2010; 121:2183-2191 [DOI] [PubMed] [Google Scholar]
- 26.McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation. 2004; 109:1004-1009 [DOI] [PubMed] [Google Scholar]
- 27.Redfield MM, Aarhus LL, Wright RS, Burnett JC., Jr Cardiorenal and neurohumoral function in a canine model of early left ventricular dysfunction. Circulation. 1993; 87:2016-2022 [DOI] [PubMed] [Google Scholar]
- 28.Burnett JC, Jr, Kao PC, Hu DC, Heser DW, Heublein D, Granger JP, Opgenorth TJ, Reeder GS. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986; 231:1145-1147 [DOI] [PubMed] [Google Scholar]
- 29.Steiner AL, Parker CW, Kipnis DM. The measurement of cyclic nucleotides by radioimmunoassay. Adv Biochem Psychopharmacol. 1970; 3:89-111 [PubMed] [Google Scholar]
- 30.Lisy O, Redfield MM, Jovanovic S, Jougasaki M, Jovanovic A, Leskinen H, Terzic A, Burnett JC., Jr Mechanical unloading versus neurohumoral stimulation on myocardial structure and endocrine function in vivo. Circulation. 2000; 102:338-343 [DOI] [PubMed] [Google Scholar]
- 31.Sancho J, Haber E. A direct microassay for aldosterone in plasma extracts. J Clin Endocrinol Metab. 1978; 47:391-396 [DOI] [PubMed] [Google Scholar]
- 32.Davidson WD, Sackner MA. Simplification of the anthrone method for the determination of inulin in clearance studies. J Lab Clin Med. 1963; 62:351-356 [PubMed] [Google Scholar]
- 33.Gottlieb SS, Skettino SL, Wolff A, Beckman E, Fisher ML, Freudenberger R, Gladwell T, Marshall J, Cines M, Bennett D, Liittschwager EB. Effects of BG9719 (CVT‐124), an A1‐adenosine receptor antagonist, and furosemide on glomerular filtration rate and natriuresis in patients with congestive heart failure. J Am Coll Cardiol. 2000; 35:56-59 [DOI] [PubMed] [Google Scholar]
- 34.Fett DL, Cavero PG, Burnett JC., Jr Low‐dose atrial natriuretic factor and furosemide in experimental acute congestive heart failure. J Am Soc Nephrol. 1993; 4:162-167 [DOI] [PubMed] [Google Scholar]
- 35.Mitrovic V, Seferovic PM, Simeunovic D, Ristic AD, Miric M, Moiseyev VS, Kobalava Z, Nitsche K, Forssmann WG, Luss H, Meyer M. Haemodynamic and clinical effects of ularitide in decompensated heart failure. Eur Heart J. 2006; 27:2823-2832 [DOI] [PubMed] [Google Scholar]
- 36.Dixon JA, Spinale FG. Large animal models of heart failure: a critical link in the translation of basic science to clinical practice. Circ Heart Fail. 2009; 2:262-271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elkayam U, Cohen G, Gogia H, Mehra A, Johnson JV, Chandraratna PA. Renal vasodilatory effect of endothelial stimulation in patients with chronic congestive heart failure. J Am Coll Cardiol. 1996; 28:176-182 [DOI] [PubMed] [Google Scholar]
- 38.Ng TM, Ackerbauer KA, Hyderi AF, Hshieh S, Elkayam U. Comparative effects of nesiritide and nitroglycerin on renal function, and incidence of renal injury by traditional and RIFLE criteria in acute heart failure. J Cardiovasc Pharmacol Ther. 2012; 17:79-85 [DOI] [PubMed] [Google Scholar]
- 39.Elkayam U, Bitar F, Akhter MW, Khan S, Patrus S, Derakhshani M. Intravenous nitroglycerin in the treatment of decompensated heart failure: potential benefits and limitations. J Cardiovasc Pharmacol Ther. 2004; 9:227-241 [DOI] [PubMed] [Google Scholar]
- 40.Elkayam U, Akhter MW, Singh H, Khan S, Usman A. Comparison of effects on left ventricular filling pressure of intravenous nesiritide and high‐dose nitroglycerin in patients with decompensated heart failure. Am J Cardiol. 2004; 93:237-240 [DOI] [PubMed] [Google Scholar]
- 41.Edwards BS, Schwab TR, Zimmerman RS, Heublein DM, Jiang NS, Burnett JC., Jr Cardiovascular, renal, and endocrine response to atrial natriuretic peptide in angiotensin II mediated hypertension. Circ Res. 1986; 59:663-667 [DOI] [PubMed] [Google Scholar]
- 42.Kurtz A, Della Bruna R, Pfeilschifter J, Taugner R, Bauer C. Atrial natriuretic peptide inhibits renin release from juxtaglomerular cells by a cGMP‐mediated process. Proc Natl Acad Sci USA. 1986; 83:4769-4773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogawa Y, Mukoyama M, Yokoi H, Kasahara M, Mori K, Kato Y, Kuwabara T, Imamaki H, Kawanishi T, Koga K, Ishii A, Tokudome T, Kishimoto I, Sugawara A, Nakao K. Natriuretic peptide receptor guanylyl cyclase‐A protects podocytes from aldosterone‐induced glomerular injury. J Am Soc Nephrol. 2012; 23:1198-1209 [DOI] [PMC free article] [PubMed] [Google Scholar]