Abstract
Background
Onset of postoperative atrial fibrillation (POAF) is a common and costly complication of heart surgery despite major improvements in surgical technique and quality of patient care. The etiology of POAF, and the ability of clinicians to identify and therapeutically target high‐risk patients, remains elusive.
Methods and Results
Myocardial tissue dissected from right atrial appendage (RAA) was obtained from 244 patients undergoing cardiac surgery. Reactive oxygen species (ROS) generation from multiple sources was assessed in this tissue, along with total glutathione (GSHt) and its related enzymes GSH‐peroxidase (GPx) and GSH‐reductase (GR). Monoamine oxidase (MAO) and NADPH oxidase were observed to generate ROS at rates 10‐fold greater than intact, coupled mitochondria. POAF risk was significantly associated with MAO activity (Quartile 1 [Q1]: adjusted relative risk [ARR]=1.0; Q2: ARR=1.8, 95% confidence interval [CI]=0.84 to 4.0; Q3: ARR=2.1, 95% CI=0.99 to 4.3; Q4: ARR=3.8, 95% CI=1.9 to 7.5; adjusted Ptrend=0.009). In contrast, myocardial GSHt was inversely associated with POAF (Quartile 1 [Q1]: adjusted relative risk [ARR]=1.0; Q2: ARR=0.93, 95% confidence interval [CI]=0.60 to 1.4; Q3: ARR=0.62, 95% CI=0.36 to 1.1; Q4: ARR=0.56, 95% CI=0.34 to 0.93; adjusted Ptrend=0.014). GPx also was significantly associated with POAF; however, a linear trend for risk was not observed across increasing levels of the enzyme. GR was not associated with POAF risk.
Conclusions
Our results show that MAO is an important determinant of redox balance in human atrial myocardium, and that this enzyme, in addition to GSHt and GPx, is associated with an increased risk for POAF. Further investigation is needed to validate MAO as a predictive biomarker for POAF, and to explore this enzyme's potential role in arrhythmogenesis.
Keywords: biomarkers, cardiopulmonary bypass, catecholamines, oxidative stress, post‐operative atrial fibrillation, redox, tachyarrhythmias
Introduction
Substantial improvements in patient outcomes following cardiac surgery have occurred over the past decade due to developments in technology and quality of care. However, there are a number of significant and costly post‐operative complications associated with cardiac surgery, including post‐operative atrial fibrillation (POAF). Even with anti‐arrhythmic therapy and improvements in myocardial protection, the incidence of POAF remains at 25% to 40%.(2004) POAF typically occurs within the first 2 to 3 days after surgery and results in prolonged hospital length of stay. Patients with POAF have a doubled risk of cardiovascular mortality and a greater incidence of symptomatic hypotension, stroke, and other arrhythmias than patients without POAF.(2010)–(2010) Findings from the Virginia Cardiac Surgery Quality Initiative, a state‐wide cost analysis of all cardiac surgeries from 2004 to 2007, estimated that POAF increased total treatment costs by $12 000/patient.
Important gaps remain in understanding the etiology of POAF and why certain patients are more likely to have this complication. Systemic inflammation (generated primarily from extracorporeal circulation) and increased atrial reactive oxygen species (ROS) are believed to be causal factors. Both of these stressors potentially impair atrial contraction, disrupt myofibrillar energetics, and reduce the atrial effective refractory period.(2003)–(2005) ROS‐producing enzyme NADPH oxidase is up‐regulated(2008)–(2012) and oxidative stress is more persistent(2007) in patients with POAF than those that remain in sinus rhythm. The results of these studies support an association between ROS in human atrium and POAF.
Another factor contributing to POAF is the increased sympathetic tone and levels of circulating catecholamines following surgery.(1996)–(1996) The importance of the association between circulating catecholamines and POAF is compounded by the intra‐ and post‐operative use of high‐dose catecholamines (eg, dopamine, dobutamine) as inotropic agents, a standard‐of‐care practice which continues despite the known association between inotropic support and POAF.(2004),(2001) The 2 primary enzymes responsible for metabolizing catecholamines are monoamine oxidase (MAO) and catechol O‐methyltransferase (COMT). COMT is highly expressed in kidney and liver tissue, but expressed at low levels in the heart.(2001) MAO, which is present in the outer mitochondrial membrane, is responsible for oxidative deamination of catecholamines (eg, epinephrines, dopamine, serotonin) and the generation of H2O2, NH4+, and reactive aldehydes.(1991)–(2009) Furthermore, this enzyme is involved in mood disorders and is the target of several pharmaceutical agents (MAO inhibitors) acting on this pathway. Recently, increased MAO activity has been observed to play a causal role in cardiac dysfunction during pressure overload due to oxidative stress.(2010)–(2014)
In the current study, we sought to determine the overall contribution of MAO as a source of ROS in human myocardium. ROS generation by MAO, NADPH oxidase, and mitochondrial electron transport system (mito‐ETS) was assessed in myocardial tissue dissected from right atrial appendage (RAA) obtained from patients undergoing cardiac surgery. Given the putative association between catecholamine overload, oxidative stress, and POAF, we hypothesized that MAO activity in the atrium and ROS produced by this enzyme may be associated with POAF. Additionally, we postulated that an association exists between POAF, myocardial glutathione (GSHt), and related enzymes GSH‐peroxidase (GPx) and GSH‐reductase (GR), since this is the primary antioxidant system present in mammalian cells and tissues.
Methods
Patient Enrollment and Inclusion/Exclusion Criteria
Approval for this study was granted by the Institutional Review Board of the Brody School of Medicine at East Carolina University. A total of 244 patients undergoing primary, non‐emergent coronary artery bypass graft (CABG) or CABG/valve surgery between January 2009 and December 2012 were enrolled. Patients with severely enlarged atria (>4.0 cm diameter), history of arrhythmia, prior cardiac surgery, left ventricular ejection fraction (LVEF) <30%, and history of anti‐arrhythmic medication were excluded from this study.
Atrial Tissue Collection and Processing
Following median sternotomy, but prior to institution of cardiopulmonary bypass, a sample of the right atrial appendage (RAA) was resected and immediately rinsed in ice‐cold Buffer X.(2007) The sample was then blotted on gauze to remove excess buffer, trimmed of the epicardial layer, and frozen in liquid N2. This method ensured that all samples obtained were predominantly myocardium and rapidly processed and frozen (<90 seconds from time of removal) to minimize protein and mRNA degradation. In some cases viable atrial myocardium was transferred to the laboratory and used for preparation of permeabilized myofibers (PmFBs) and analysis of mitochondrial function.
Permeabilized Fiber Preparation
Portions of this technique have been described elsewhere,(2007) but have been adapted for application in human cardiac muscle and for specific measurements made in this study. After RAA tissue harvest, myocardium was removed and placed in ice‐cold Buffer X, containing (in mmol/L: 7.23 K2EGTA, 2.77 CaK2EGTA, 20 Imidazole, 20 Taurine, 5.7 ATP, 14.3 PCr, 6.56 MgCl2·6H2O, 50 MES; pH 7.1). Muscle was then cut into strips ≈4 to 6 mm L×2 to 3 mm wide and placed in a solution of Buffer X containing 3 mg/mL collagenase Type I (Sigma‐Aldrich), and incubated for 30 to 45 minutes at 4°C. Fiber bundles were then carefully trimmed of vascular and connective tissue, separated along their longitudinal axis, and permeabilized for 30 minutes in Buffer X+50 μg/mL saponin at 4°C. We used 30 μg/mL saponin if patient was female, for reasons described elsewhere.(2011) Following permeabilization, myofiber bundles (PmFBs) were washed in ice‐cold Buffer Z containing (in mmol/L): 110 K‐MES, 35 KCl, 1 EGTA, 5 K2HPO2, 3 MgCl2·6H2O, and 5 mg/mL BSA (pH 7.4, 295 mOsm) and remained in Buffer Z on a rotator at 4°C until analysis (<2 hours). We have observed that PmFBs exhibit a very strong Ca2+‐independent contraction that is temperature sensitive and can occur even at 4°C,(2011) therefore, 20 μmol/L Blebbistatin (Sigma‐Aldrich) was added to the wash buffer, in addition to the respiration medium during experiments, to prevent contraction as previously described.
Measurement of Mitochondrial H2O2 Emission in Cardiac PmFBs
All mitochondrial H2O2 measurements were performed at 37°C. H2O2 coming from mito‐ETS as a result of palmitoyl‐l‐carnitine, glutamate, and succinate oxidation was determined in PmFB's with 100 μmol/L ADP, 5 mmol/L glucose, and 1 U/mL hexokinase present to keep the mitochondria in a permanent, submaximal phosphorylating state (ie, most physiological).(2009)–(2011) H2O2 emission rate was determined in real time by continuous monitoring of Amplex Red oxidation in presence of horseradish peroxidase (1 U/mL) and superoxide dismutase (25 U/mL) using a spectrofluorometer (Photon Technology Instruments, Birmingham, NJ,) equipped with a thermo‐jacketed cuvette chamber.
MAO and NADPH Oxidase Activity
Myocardial samples frozen in liquid N2 were homogenized in 10X (wt./vol) TEE buffer containing (in mmol/L: 10 Tris base, 1 EDTA, 1 EGTA, and 0.5% Tween‐20), using a glass grinder (Kimble Chase). All enzyme activity and glutathione assays were performed on the same day as the protein extraction. We have empirically determined that glutathione and enzyme activity must be assessed immediately in protein extractions to obtain accurate results, and that freezing samples or keeping them at 4°C overnight will cause dramatic loss of content and activity. H2O2 generation from MAO and NADPH oxidase was determined in real time by continuous monitoring of Amplex Red oxidation in presence of horseradish peroxidase (1 U/mL) and superoxide dismutase (25 U/mL) using a spectrofluorometer (Horiba Jobin Yvon) equipped with a thermo‐jacketed cuvette chamber maintained at 37°C. MAO activity was determined by continuous monitoring of clorgyline‐sensitive H2O2 production supported by 1 mmol/L Tyramine or 2 μmol/L Norepinephrine, as previously described.(1996) NADPH oxidase activity was determined by continuous monitoring of apocynin‐sensitive H2O2 production supported by 0.5 mmol/L NADPH.(2013)
GSHt, GPx, and GR Activity
All enzyme activity and glutathione assays were performed on the same day as the protein extraction. Total glutathione measurements were performed as described previously(2012),(2012) using a modified Tietze method.(1969) GR activity in myocardial tissue was measured in TEE buffer containing 1 mmol/L GSSG and 0.5 mmol/L NADPH, where activity was calculated from the linear decrease in NADPH absorbance with time.(1985) Glutathione peroxidase (GPx) activity was determined in TEE buffer containing 1 mmol/L GSH, 100 mU/mL glutathione reductase enzyme, 0.5 mmol/L NADPH. The reaction is initiated with a nominal amount of tert‐Butyl‐Hydroperoxide and the activity of GPx was calculated from the linear decrease in NADPH absorbance with time.(1967)
Determination of POAF
Postoperatively, patients' heart rate and rhythm were continuously monitored with telemetry until discharge. POAF was defined by a sustained episode of atrial fibrillation lasting ≥1 minute, or for any length of time requiring intervention for hemodynamic compromise.
Statistical Analysis
Categorical variables were reported as frequency and percentage while continuous variables were reported as mean±standard deviation, median, and interquartile range. Variables not previously categorized were divided into quartiles prior to statistical analysis. Quartile categorization is advantageous because it limits the influence of outliers and allows for the assessment of trend across categories.
Statistical significance of group comparisons for categorical variables was determined using Fisher exact and chi‐square (χ2) procedures and for continuous variables was determined using the Deuchler‐Wilcoxon method. Relative risk and 95% confidence intervals were computed using log‐binomial or robust Poisson regression. P values for trend were computed using a likelihood ratio test (or score test when convergence was not achieved). Assays were performed using a missing by design sampling strategy. The iterative expectation‐maximization (EM) algorithm was used to impute missing values.(1977)–(2012) The relative imputation efficiency ranged from 96% to 99% (variance inflation: MAO=0.38, GSHt=0.02, GPx=0.15, GR=0.54; fraction missing information: MAO=0.29, GSHt=0.02, GPx=0.14, GR=0.37). Patients with and without missing data did not differ by key demographic characteristics (ie, age, sex, race; Hochberg adjusted P>0.05).(1988) Furthermore, a complete‐case analysis was performed and it did not substantively change the results of the study. The multivariable models included variables that have been previously reported to be associated with POAF, regardless of their statistical significance in our dataset. These included age, sex, race, diabetes, hypertension, ACEI use, ARB use, statin use, and CPBT.(2012),(2012)–(2011) Statistical significance was defined as P<0.05. SAS Version 9.3 was used for all analyses.
Results
Analysis of Major ROS Sources in Human Atrial Myocardium
An assessment of 3 major ROS sources in atrial myocardium was performed from RAA biopsies of 12 individual patients (demographic and clinical characteristics of these 12 patients is provided in Table 1). Rates of H2O2 production in the myocardial tissue homogenate was confirmed to be derived from MAO and NADPH oxidase based on the sensitivity to their inhibitors clorgyline and apocynin, respectively (Figure 1A). H2O2 production derived from the mito‐ETS was driven by oxidation of substrates as they were individually titrated into the respiration medium containing the PmFBs (Figure 1A). Total rates of H2O2 production from these 3 sources were individually quantified and combined within each of the 12 patients (Figure 1B). The rate of H2O2 originating from mito‐ETS was determined to be at least 10‐fold lower than either MAO or NADPH oxidase alone. As previously reported by our group, diabetic patients had significantly higher rates of H2O2 from mito‐ETS compared with non‐diabetic patients.(2009)–(2011)
Table 1.
Clinical and demographic information specific for patients in Figure 1.
| Pt # | Age | Sex | Race | Diabetes | HbA1c | HF | POAF | Tobacco | COPD | Prior MI | HTN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 79 | F | AA | Y | 6.9 | N | N | N | N | N | Y |
| 2 | 63 | F | C | Y | 10 | N | N | Y | Y | Y | Y |
| 3 | 67 | F | AA | Y | 7.2 | Y | Y | Y | N | N | Y |
| 4 | 62 | F | AA | N | – | N | N | N | N | N | Y |
| 5 | 60 | F | C | Y | 9.2 | N | N | N | N | N | Y |
| 6 | 69 | F | AA | N | – | N | N | Y | N | N | Y |
| 7 | 47 | M | C | N | – | N | N | N | N | N | N |
| 8 | 56 | M | C | N | – | N | N | N | N | N | Y |
| 9 | 52 | M | AA | N | – | N | N | Y | N | N | Y |
| 10 | 44 | M | C | N | – | N | N | Y | N | Y | Y |
| 11 | 58 | M | C | N | – | N | Y | N | N | N | Y |
| 12 | 52 | M | C | Y | 8.9 | N | Y | Y | N | Y | Y |
Absent values (–) for glycated hemoglobin (HbA1c) indicate that levels were within normal range (4.5% to 5.9%) for that particular patient (Pt). AA indicates African‐American; C, Caucasian; COPD, history of chronic obstructive pulmonary disease; HF, history of heart failure; HTN, history of hypertension; MI, myocardial infarction; POAF, post‐operative atrial fibrillation.
Figure 1.
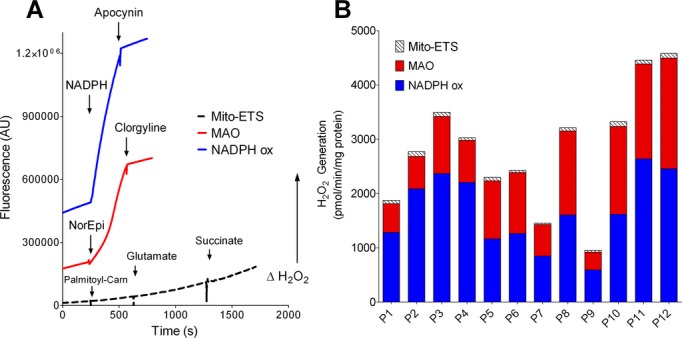
Comparative analysis of major ROS sources in human atrial myocardium. A, Representative H2O2 production traces from NADPH oxidase (blue), MAO (red), and mito‐ETS (black dash) in RAA tissue obtained from one individual patient. PmFBs were used for determining H2O2 from mito‐ETS, while homogenate was used for NADPH oxidase and MAO. Substrates were added to cuvette where indicated. Apocynin and Clorgyline are administered where indicated to confirm the source of H2O2 production to be NADPH oxidase and MAO, respectively. In (B) are the quantified rates from each of these 3 sources in RAA tissue obtained from 12 individual patients. MAO indicates monoamine oxidase; mito‐ETS, mitochondrial electron transport system; nadph, β‐Nicotinamide adenine dinucleotide phosphate hydrate; PmFBs, permeabilized myofibers; RAA, right atrial appendage; ROS, reactive oxygen species.
Patient Characteristics, Biochemical Markers, and Relationship to POAF
A total of 80 (33%) patients developed POAF. Patients with POAF were older and presented more frequently with hypertension than those without POAF (Table 2). Additionally, they experienced longer CPBT. Mean MAO levels were significantly higher among patients with POAF (P<0.0001) and a linear trend across quartile levels was observed (Ptrend<0.0001), with the incidence of POAF being the highest in quartile 4 compared with quartile 1 (Figure 2). POAF was not associated with GSHt, GPx, and GR (Figures 3 and 4B and 4C) in the univariable analysis. In multivariable analysis, MAO remained statistically significant after adjusting for age, sex, race, diabetes, hypertension, ACEI/ARB use, statin use, and CPBT (Ptrend=0.009, Table 3). A statistically significant linear trend also was observed for GSHt in multivariable analysis (Ptrend=0.014).
Table 2.
Patient and Operative Characteristics Stratified by Postoperative Rhythm Class and Univariable Relative Risk for POAF (N=244)
| Variables | POAF n (%) | POSR n (%) | P Value | Univariable RR (95% CI) |
|---|---|---|---|---|
| Overall | 80 (33) | 164 (67) | — | — |
| Demographics/comorbidities | ||||
| Age | ||||
| Mean±SD | 66±8.7 | 62±10 | 0.0019 | — |
| Median (IQR) | 67 (14) | 62 (16) | ||
| Q1 (≤56) | 10 (13) | 54 (33) | 0.0045 | Referent |
| Q2 (56 to 64) | 20 (25) | 39 (24) | 2.2 (1.1 to 4.2) | |
| Q3 (64 to 71) | 23 (29) | 37 (23) | 2.5 (1.3 to 4.7) | |
| Q4 (>71) | 27 (34) | 34 (21) | 2.8 (1.5 to 5.3) | |
| Ptrend=0.0008 | ||||
| Sex | ||||
| Female | 14 (18) | 41 (25) | 0.19 | Referent |
| Male | 66 (83) | 123 (75) | 1.4 (0.84 to 2.2) | |
| Race | ||||
| White | 69 (86) | 132 (80) | 0.27 | Referent |
| Black | 11 (14) | 32 (20) | 1.3 (0.78 to 2.3) | |
| Diabetes | ||||
| No | 50 (63) | 84 (51) | 0.096 | Referent |
| Yes | 30 (38) | 80 (49) | 0.73 (0.50 to 1.06) | |
| Hypertension | ||||
| No | 7 (9) | 34 (21) | 0.019 | Referent |
| Yes | 73 (91) | 130 (79) | 2.1 (1.05 to 4.2) | |
| BMI* | ||||
| Mean±SD | 30±5.7 | 30±6.2 | 0.74 | — |
| Median (IQR) | 30 (7.4) | 30 (7.2) | ||
| Q1 (≤26) | 23 (29) | 39 (24) | 0.69 | Referent |
| Q2 (26 to 30) | 17 (22) | 43 (26) | 0.76 (0.46 to 1.3) | |
| Q3 (30 to 33) | 22 (27) | 40 (25) | 0.96 (0.60 to 1.5) | |
| Q4 (>33) | 18 (23) | 42 (26) | 0.81 (0.49 to 1.3) | |
| Ptrend=0.60 | ||||
| Smoking | ||||
| No | 59 (74) | 107 (65) | 0.18 | Referent |
| Yes | 21 (26) | 57 (35) | 0.76 (0.50 to 1.2) | |
| COPD | ||||
| No | 60 (75) | 136 (83) | 0.14 | Referent |
| Yes | 20 (25) | 28 (17) | 1.4 (0.92 to 2.02) | |
| Prior stroke | ||||
| No | 74 (93) | 154 (94) | 0.68 | Referent |
| Yes | 6 (8) | 10 (6) | 1.2 (0.60 to 2.2) | |
| Prior MI | ||||
| No | 45 (56) | 75 (46) | 0.12 | Referent |
| Yes | 35 (44) | 89 (54) | 0.75 (0.52 to 1.08) | |
| HF | ||||
| No | 79 (99) | 155 (95) | 0.089* | Referent |
| Yes | 1 (1) | 9 (5) | 0.30 (0.046 to 1.9) | |
| Ejection fraction* | ||||
| Mean±SD | 54±11 | 53±14 | 0.30 | — |
| Median (IQR) | 58 (10) | 54 (15) | ||
| Q1 (≤48) | 17 (22) | 52 (32) | 0.066 | Referent |
| Q2 (48 to 55) | 19 (24) | 42 (26) | 1.3 (0.72 to 2.2) | |
| Q3 (55 to 62) | 25 (31) | 28 (17) | 1.9 (1.2 to 3.2) | |
| Q4 (>62) | 19 (24) | 42 (26) | 1.3 (0.72 to 2.2) | |
| Ptrend=0.15 | ||||
| CAD severity* | ||||
| 1‐vessel | 3 (4) | 11 (7) | 0.20 | Referent |
| 2‐vessel | 15 (19) | 44 (27) | 1.2 (0.40 to 3.5) | |
| 3‐vessel | 62 (77) | 109 (67) | 1.7 (0.61 to 4.7 | |
| Ptrend=0.079 | ||||
| Left main disease | ||||
| No | 65 (81) | 135 (82) | 0.84 | Referent |
| Yes | 15 (19) | 29 (18) | 1.0 (0.66 to 1.7) | |
| Preoperative Medications | ||||
| Beta‐blockers | ||||
| No | 12 (15) | 28 (17) | 0.68 | Referent |
| Yes | 68 (85) | 136 (83) | 1.1 (0.67 to 1.9) | |
| ACEI/ARBS | ||||
| No | 70 (88) | 128 (78) | 0.076 | Referent |
| Yes | 10 (13) | 36 (22) | 0.61 (0.34 to 1.1) | |
| Statins | ||||
| No | 18 (23) | 33 (20) | 0.67 | Referent |
| Yes | 62 (78) | 131 (80) | 0.91 (0.60 to 1.4) | |
| Intraoperative Characteristics | ||||
| CPB | ||||
| No | 3 (4) | 10 (6) | 0.44 | Referent |
| Yes | 77 (96) | 154 (94) | 1.4 (0.53 to 4.0) | |
| CPBT (min) | ||||
| Mean±SD | 120±33 | 110±37 | 0.012 | — |
| Median (IQR) | 115 (37) | 104 (48) | ||
| Q1 (≤87) | 9 (12) | 49 (32) | 0.0092 | Referent |
| Q2 (87 to 108) | 22 (29) | 37 (24) | 2.4 (1.2 to 4.8) | |
| Q3 (108 to 134) | 26 (34) | 35 (23) | 2.7 (1.4 to 5.4) | |
| Q4 (>134) | 20 (26) | 33 (21) | 2.4 (1.2 to 4.9) | |
| Biomarkers | ||||
| MAO* | ||||
| Mean±SD | 2843±1987 | 1725±1130 | <0.0001 | — |
| Median (IQR) | 2608 (2038) | 1691 (1286) | ||
| Q1 (≤1344) | 8 (10) | 54 (33) | <0.0001 | Referent |
| Q2 (1344 to 2035) | 15 (19) | 44 (27) | 2.0 (0.90 to 4.3) | |
| Q3 (2035 to 2820) | 19 (23) | 42 (25) | 2.4 (1.1 to 5.1) | |
| Q4 (>2820) | 38 (47) | 24 (15) | 4.8 (2.4 to 9.3) | |
| Ptrend<0.0001 | ||||
| GSHt* | ||||
| Mean±SD | 19±6.0 | 20±6.5 | 0.15 | — |
| Median (IQR) | 18 (7.6) | 20 (7.9) | ||
| Q1 (≤16) | 24 (30) | 36 (22) | 0.36 | Referent |
| Q2 (16 to 20) | 22 (28) | 39 (24) | 0.90 (0.57 to 1.4) | |
| Q3 (20 to 23) | 17 (21) | 45 (27) | 0.69 (0.41 to 1.1) | |
| Q4 (>23) | 17 (22) | 44 (27) | 0.70 (0.42 to 1.2) | |
| Ptrend=0.099 | ||||
| GPX* | ||||
| Mean±SD | 17±5.6 | 17±6.9 | 0.42 | — |
| Median (IQR) | 17 (6.6) | 16 (9.4) | ||
| Q1 (≤12) | 13 (16) | 48 (29) | 0.024 | Referent |
| Q2 (12 to 17) | 25 (31) | 37 (22) | 1.9 (1.1 to 3.3) | |
| Q3 (17 to 21) | 26 (32) | 34 (21) | 2.0 (1.2 to 3.6) | |
| Q4 (>21) | 16 (20) | 45 (27) | 1.2 (0.65 to 2.3) | |
| Ptrend=0.46 | ||||
| GR* | ||||
| Mean±SD | 4.7±2.5 | 4.7±2.6 | 0.42 | — |
| Median (IQR) | ||||
| Q1 (≤3.8) | 21 (26) | 40 (24) | 0.64 | Referent |
| Q2 (4.8) | 21 (26) | 41 (25) | 0.98 (0.60 to 1.6) | |
| Q3 (5.6) | 22 (28) | 38 (23) | 1.1 (0.66 to 1.7) | |
| Q4 (>5.6) | 16 (20) | 45 (27) | 0.76 (0.44 to 1.3) | |
| Ptrend=0.40 |
Tests of statistical significance (chi‐square for categorical variables, Deuchler‐Wilcoxon for continuous variables). ACEI indicates angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CAD, coronary artery disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CPBT, cardiopulmonary bypass time; GPX, glutathione peroxidase; GR, glutathione reductase; GSHt, total glutathione; HF, heart failure; IQR, interquartile range; MAO, monoamine oxidase; MI, myocardial infarction; POAF, postoperative atrial fibrillation; POSR, postoperative sinus rhythm; Q1, first quartile; Q2, second quartile; Q3, third quartile; Q4, fourth quartile; RR, relative risk; SD, standard deviation.
Missing values imputed using EM algorithm (n=10 simulations).
Statistical significance computed using Fisher's Exact. Ptrend computed using likelihood ratio trend test.
Figure 2.
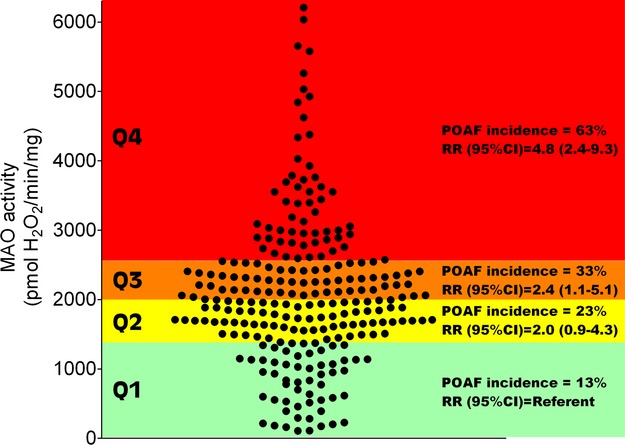
MAO activity in atrial myocardium and incidence of POAF. All rates of MAO activity from entire cohort of patients recruited for this study are shown (1 circle=1 patient). Quartiles of pooled data were generated, and univariable analysis performed with POAF as the outcome variable using Poisson regression. Each quartile is delineated with color shading to illustrate the risk of POAF within that particular quartile (Green=<15%, Yellow=15% to 30%, Orange=30% to 40%, Red=>60%). Within each quartile, POAF incidence=number of patients in that particular quartile experiencing POAF. RR=relative risk, with 95% confidence interval (CI). MAO indicates monoamine oxidase; POAF, post‐operative atrial fibrillation.
Figure 3.

Total GSH (GSHt) in atrial myocardium and incidence of POAF. Data shown in this figure is GSHt for the entire cohort of patients recruited for this study. Quartiles of pooled data were generated, and univariable analysis performed with POAF as the outcome variable using Poisson regression. Each quartile is delineated with color shading to illustrate the risk of POAF within that particular quartile (Red=>40%, Yellow=15% to 30%, Orange=30% to 40%). Within each quartile, POAF incidence=number of patients in that particular quartile experiencing POAF. RR=relative risk, with 95% confidence interval (CI). GSHt indicates total glutathione; POAF, post‐operative atrial fibrillation.
Figure 4.
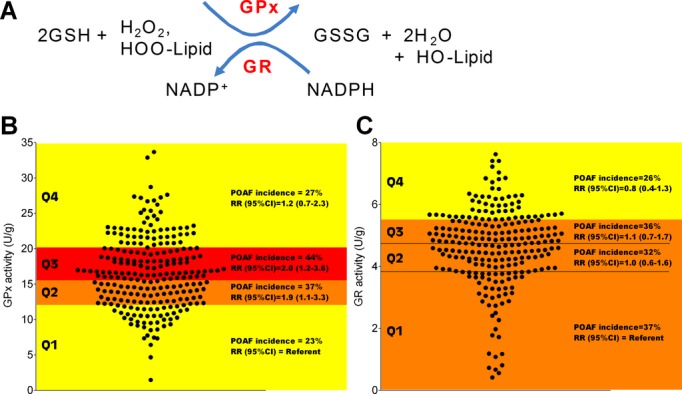
GPx‐GR activity in atrial myocardium and incidence of POAF. A, Simplified schematic of the redox cycle involving GSH and related enzymes GPx and GR (HOO‐Lipid=Lipid peroxide). Data shown in (B) is GPx activity and (C) GR activity for the entire cohort of patients recruited for this study. Quartiles of pooled data were generated, and univariable analysis performed with POAF as the outcome variable using Poisson regression. Each quartile is delineated with color shading to illustrate the risk of POAF within that particular quartile (Red=>40%, Yellow=15% to 30%, Orange=30% to 40%). Within each quartile, POAF incidence=number of patients in that particular quartile experiencing POAF. RR=relative risk, with 95% confidence interval (CI). GPx indicates GSH‐peroxidase; GR, GSH‐reductase; GSH, glutathione; GSSG, oxidized glutathione; POAF, post‐operative atrial fibrillation.
Table 3.
Multivariate Analysis of Independent Risk Factors Predictive of POAF*
| Models | ARR 95% CI |
|---|---|
| MAO* | |
| Q1 (≤1344) | Referent |
| Q2 (1344 to 2035) | 1.8 (0.83 to 4.0) |
| Q3 (2035 to 2820) | 2.1 (0.99 to 4.3) |
| Q4 (>2820) | 3.8 (1.9 to 7.5) |
| Ptrend=0.009 | |
| GSH* | |
| Q1 (≤16) | Referent |
| Q2 (16 to 20) | 0.93 (0.60 to 1.4) |
| Q3 (20 to 23) | 0.62 (0.36 to 1.1) |
| Q4 (>23) | 0.56 (0.34 to 0.93) |
| Ptrend=0.014 | |
| GPX* | |
| Q1 (≤12) | Referent |
| Q2 (12 to 17) | 1.9 (1.1 to 3.3) |
| Q3 (17 to 21) | 2.4 (1.4 to 4.2) |
| Q4 (>21) | 1.4 (0.75 to 2.7) |
| Ptrend=0.21 |
ARR indicates adjusted relative risk; CI, confidence interval; GPX, glutathione peroxidase; GSH, glutathione; MAO, monoamine oxidase; POAF, postoperative atrial fibrillation; Q1, first quartile; Q2, second quartile; Q3, third quartile; Q4, fourth quartile.
Models adjusted for age, sex, race, diabetes, angiotensin converting enzyme inhibitor and angiotensin receptor blocker use, statin use, and hypertension.
Missing values imputed using EM algorithm (n=10 simulations). Ptrend computed using score trend test.
Discussion
Several reports have documented the inverse association of POAF and β‐blocker use, illustrating the underlying etiologic role of catecholamines and excessive sympathetic discharge.(2004)–(2003) Others have used prophylactic amiodarone,(1993) sotalol,(1993) magnesium,(2005) and statins,(2006) all of which were successful at reducing the incidence of POAF to varying degrees; however, all patients were treated regardless of POAF status. These studies illustrate the importance of investigating biological factors that may predispose patients to POAF.
The findings of this study demonstrate for the first time that MAO is a major source of ROS in human atrial myocardium, and its activity varies across a 50‐fold range among patients. It also provides evidence that atrial MAO activity serves as an independent predictor of POAF and lends further support to the current theory that redox imbalance (ie, oxidative stress) in atrial myocardium is a significant factor in the etiology of POAF, particularly with respect to our myocardial GSHt‐related data. Furthermore, the results collectively integrate a number of perioperative factors known to contribute to the etiology of POAF (eg, catecholamine overload and oxidative stress).
Investigation into the etiology of POAF has largely focused on systemic inflammation and oxidative stress in the post‐operative period. Redox modifications of ion channels and proteins have been observed to directly impact cardiomyocyte electrical(2005)–(2001) and mechanical(2001) function and has been implicated in the early stages of electrical remodeling which accompanies the onset of AF.(2002) Inflammation is interconnected with myocardial oxidative stress.(2003)–(2005) Circulating cytokines and electrophilic lipids increase strain on antioxidant mechanisms in cardiomyocytes, a system already burdened with buffering oxidants originating from endogenous sources (eg, MAO, NADPH oxidase, and mitochondria) (Figure 1). The most important buffer of ROS in mammalian cells and tissue is GSH, which is converted to its oxidized form (GSSG) by GPx in the presence of hydroperoxides, and recycled back to its reduced form by NADPH‐dependent GR. The GSH/GSSG (reduced/oxidized) redox couple is considered to be the key indicator of cellular redox environment.(2001) Also important to cellular/tissue redox environment is total amount of GSH (GSHt), defined as the additive amount of free GSH and GSSG. A decrease in GSHt potentially increases a cell's susceptibility to the adverse outcomes associated with oxidative stress (eg, oxidative modifications of proteins, lipids, and DNA). Our findings that GSHt and GPx are inversely correlated with POAF (Figures 3 and 4) suggests that a greater antioxidant capacity should lead to lower incidence of POAF because of a greater buffering of ROS during the postoperative period. Clinical trials have shown that anti‐inflammatory/antioxidant therapies lead to a decreased incidence of POAF.(2008)–(2008) For example, preoperative n‐3 polyunsaturated fatty acids (PUFAs) and concentrated antioxidant supplementation have been observed to enhance anti‐inflammatory/antioxidant capacity in atrial myocardium at the cellular level.(2011) A follow‐up clinical trial with this therapy led to a substantial decrease in POAF.(2013) Use of n‐3 PUFAs alone as prophylactic therapy to mitigate incidence of POAF has led to mixed results. For example, the omega‐3 fatty acids for prevention of postoperative atrial fibrillation (OPERA) trial showed that a very high dose (8 to 10 g/day) of n‐3 PUFAs for 2 to 3 days preoperatively did not reduce the incidence of POAF.(2012) Nevertheless, use of n‐3 PUFAs as prophylactic therapy for arrhythmia and other cardiovascular diseases remains a viable therapeutic option due to the pleiotropic, beneficial effects of these fatty acids in the heart.
Mitochondria, as a consequence of their intracellular volume and density, are considered the predominant source of intracellular ROS in myocardium.(2005) However, the total ROS that escapes (ie, ROS emission) from the mitochondria is minimized by the reducing environment within the matrix of this organelle in addition to its redox enzyme network.(1999)–(2013) This potentially explains our observation that H2O2 originating from mito‐ETS was markedly lower than from either MAO or NADPH oxidase alone (Figure 1). ROS derived from NADPH oxidase in atrial myocardium, and downstream ROS (eg, peroxynitrite and reactive aldehydes) have been shown to be significantly correlated with POAF.(2008)–(2012) Our findings further support the clinical importance of ROS‐generating enzymes in atrial tissue and provide novel evidence that ROS derived from MAO may be a key determinant of myocardial redox balance in the postoperative period.
While MAO is an enzyme physically tethered to the outer mitochondrial membrane, MAO‐derived ROS typically is not considered to be “mitochondrial ROS.” Our findings support a paradigm shift in the way this enzyme is viewed within the context of cellular redox balance. The wide range in MAO activity (≈50‐fold) across patients is a significant feature of our findings (Figure 2). Theoretically, the expression and activity of cardiac MAO should reflect sympathetic tone; however, there is considerable variation in promoter activity and transcriptional control of MAO genes in humans.(2000) This may explain the underlying variation in enzyme activity seen in our patient cohort. Conceivably, high levels of catecholamines in the postoperative period may lead to increased concentrations inside cardiomyocytes by neuronal monoamine transporters in the sarcolemmal membrane.(2001) Thus, in patients where MAO activity is high (Q3 and Q4, Figure 2), MAO‐derived ROS may in turn be increased, leading to oxidative stress and potentially triggering POAF. In the remodeled myocardium, where fibrosis and altered ion channel expression are present, oxidative stress and inflammation only comprise a portion of the arrhythmogenic substrate. Accordingly, therapeutic strategies to mitigate POAF need to account for all of these possibilities.
Our study is strengthened by its prospective and systematic data collection. Additionally, biomarkers were obtained from myocardial tissue and reflect local cardiac versus serum levels. However, several limitations should be noted. Only the right atrial myocardium was collected in this study, and this may not be the best anatomic site to represent cardiac remodeling and oxidative stress pathways in the heart, given the known importance of the left atrium as a site of arrhythmogenesis. Moreover, studies have shown that as the pathology of AF progresses, a gradient of cardiac remodeling occurs starting in the left atrium and ending in the right atrium.(2011) The temporality and spatial heterogeneity of this remodeling may be missed by capturing only the right atrium. However, because this patient cohort did not have any history of arrhythmia or cardiac surgery, it is unlikely that any remodeling that may exist is due to atrial arrhythmia.
Saturating concentrations of substrate (eg, Tyramine, NADPH, glutamate, etc) were used to measure enzyme activities in our assays. This rarely exists in vivo. However, the use of saturating substrate concentration was appropriate because the objective was to compare the maximal capacity for ROS generation and scavenging from myocardial enzymes. Biopsies were obtained at a single point in time; therefore it was not possible to determine the temporality of biomarker levels. Limited longitudinal information was available in our dataset. We were unable to determine the dose, duration, and frequency of β‐blocker use prior to surgery for each patient. Furthermore, our sample size was small and residual confounding may have been present.
In conclusion, our study suggests that MAO is a major ROS source in human atrial myocardium and is an important biomarker for POAF, providing clinicians with the ability to predict which patients are predisposed to this postoperative complication. Advanced knowledge of POAF risk will enable appropriate prophylactic treatment to be initiated at the time of surgery, potentially leading to reduced hospital stay and healthcare costs associated with this complication. Additional investigation is needed to elucidate the role of MAO in arrhythmogenesis and to validate our findings in other populations and disease processes.
Acknowledgments
The authors would like to specifically thank the research nurses and clinical staff at ECHI for their assistance with informed consent and study coordination.
Sources of Funding
This research was supported by grant R21HL098780 (Anderson, Kypson) from National Institutes of Health.
Disclosures
None.
References
- Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, Barash PG, Hsu PH, Mangano DT. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004; 291:1720-1729 [DOI] [PubMed] [Google Scholar]
- Ahlsson A, Fengsrud E, Bodin L, Englund A. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur J Cardiothorac Surg. 2010; 37:1353-1359 [DOI] [PubMed] [Google Scholar]
- El‐Chami MF, Kilgo P, Thourani V, Lattouf OM, Delurgio DB, Guyton RA, Leon AR, Puskas JD. New‐onset atrial fibrillation predicts long‐term mortality after coronary artery bypass graft. J Am Coll Cardiol. 2010; 55:1370-1376 [DOI] [PubMed] [Google Scholar]
- Aviles RJ, Martin DO, Apperson‐Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003; 108:3006-3010 [DOI] [PubMed] [Google Scholar]
- Ishii Y, Schuessler RB, Gaynor SL, Yamada K, Fu AS, Boineau JP, Damiano RJ., Jr Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation. 2005; 111:2881-2888 [DOI] [PubMed] [Google Scholar]
- Kim YM, Kattach H, Ratnatunga C, Pillai R, Channon KM, Casadei B. Association of atrial nicotinamide adenine dinucleotide phosphate oxidase activity with the development of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008; 51:68-74 [DOI] [PubMed] [Google Scholar]
- Antoniades C, Demosthenous M, Reilly S, Margaritis M, Zhang MH, Antonopoulos A, Marinou K, Nahar K, Jayaram R, Tousoulis D, Bakogiannis C, Sayeed R, Triantafyllou C, Koumallos N, Psarros C, Miliou A, Stefanadis C, Channon KM, Casadei B. Myocardial redox state predicts in‐hospital clinical outcome after cardiac surgery effects of short‐term pre‐operative statin treatment. J Am Coll Cardiol. 2012; 59:60-70 [DOI] [PubMed] [Google Scholar]
- Ramlawi B, Otu H, Mieno S, Boodhwani M, Sodha NR, Clements RT, Bianchi C, Sellke FW. Oxidative stress and atrial fibrillation after cardiac surgery: a case‐control study. Ann Thorac Surg. 2007; 84:1166-1172‐ [DOI] [PubMed] [Google Scholar]
- Aranki SF, Shaw DP, Adams DH, Rizzo RJ, Couper GS, VanderVliet M, Collins JJ, Jr, Cohn LH, Burstin HR. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation. 1996; 94:390-397 [DOI] [PubMed] [Google Scholar]
- Mathew JP, Parks R, Savino JS, Friedman AS, Koch C, Mangano DT, Browner WS. Atrial fibrillation following coronary artery bypass graft surgery: predictors, outcomes, and resource utilization. MultiCenter Study of Perioperative Ischemia Research Group. JAMA. 1996; 276:300-306 [PubMed] [Google Scholar]
- Koletsis EN, Prokakis C, Crockett JR, Dedeilias P, Panagiotou M, Panagopoulos N, Anastasiou N, Dougenis D, Apostolakis E. Prognostic factors of atrial fibrillation following elective coronary artery bypass grafting: the impact of quantified intraoperative myocardial ischemia. J Cardiothorac Surg. 2011; 6:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhofer G. The role of neuronal and extraneuronal plasma membrane transporters in the inactivation of peripheral catecholamines. Pharmacol Ther. 2001; 91:35-62 [DOI] [PubMed] [Google Scholar]
- Youdim MB, Finberg JP. New directions in monoamine oxidase A and B selective inhibitors and substrates. Biochem Pharmacol. 1991; 41:155-162 [DOI] [PubMed] [Google Scholar]
- Edmondson DE, Binda C, Wang J, Upadhyay AK, Mattevi A. Molecular and mechanistic properties of the membrane‐bound mitochondrial monoamine oxidases. Biochemistry. 2009; 48:4220-4230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaludercic N, Takimoto E, Nagayama T, Feng N, Lai EW, Bedja D, Chen K, Gabrielson KL, Blakely RD, Shih JC, Pacak K, Kass DA, Di Lisa F, Paolocci N. Monoamine oxidase A‐mediated enhanced catabolism of norepinephrine contributes to adverse remodeling and pump failure in hearts with pressure overload. Circ Res. 2010; 106:193-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaludercic N, Carpi A, Nagayama T, Sivakumaran V, Zhu G, Lai EW, Bedja D, De Mario A, Chen K, Gabrielson KL, Lindsey ML, Pacak K, Takimoto E, Shih JC, Kass DA, Di Lisa F, Paolocci N. Monoamine oxidase B prompts mitochondrial and cardiac dysfunction in pressure overloaded hearts. Antioxid Redox Signal. 2014; 20:267-28010.1089/ars.2012.4616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Yamazaki H, Neufer PD. Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid‐supported respiration. J Biol Chem. 2007; 282:31257-31266 [DOI] [PubMed] [Google Scholar]
- Kane DA, Lin CT, Anderson EJ, Kwak HB, Cox JH, Brophy PM, Hickner RC, Neufer PD, Cortright RN. Progesterone increases skeletal muscle mitochondrial H2O2 emission in nonmenopausal women. Am J Physiol Endocrinol Metab. 2011; 300:E528-E535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CG, Kane DA, Lin CT, Kozy R, Cathey BL, Lark DS, Kane CL, Brophy PM, Gavin TP, Anderson EJ, Neufer PD. Inhibiting myosin‐ATPase reveals a dynamic range of mitochondrial respiratory control in skeletal muscle. Biochem J. 2011; 437:215-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD. Substrate‐specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol. 2009; 54:1891-1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Rodriguez E, Anderson CA, Thayne K, Chitwood WR, Kypson AP. Increased propensity for cell death in diabetic human heart is mediated by mitochondrial‐dependent pathways. Am J Physiol Heart Circ Physiol. 2011; 300:H118-H124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann N, Grimsby J, Shih JC, Cadenas E. The metabolism of tyramine by monoamine oxidase A/B causes oxidative damage to mitochondrial DNA. Arch Biochem Biophys. 1996; 335:295-304 [DOI] [PubMed] [Google Scholar]
- La Favor JD, Anderson EJ, Dawkins JT, Hickner RC, Wingard CJ. Exercise prevents Western‐diet associated erectile dysfunction and coronary artery endothelial dysfunction: response to acute apocynin and sepiapterin treatment. Am J Physiol Regul Integr Comp Physiol. 2013; 305:R423-R43410.1152/ajpregu.00049.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, III, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009; 119:573-58110.1172/JCI37048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Thayne K, Harris M, Carraway K, Shaikh SR. Aldehyde stress and up‐regulation of Nrf2‐mediated antioxidant systems accompany functional adaptations in cardiac mitochondria from mice fed n‐3 polyunsaturated fatty acids. Biochem J. 2012; 441:359-366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969; 27:502-522 [DOI] [PubMed] [Google Scholar]
- Carlberg I, Mannervik B. Glutathione reductase. Methods Enzymol. 1985; 113:484-490 [DOI] [PubMed] [Google Scholar]
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967; 70:158-169 [PubMed] [Google Scholar]
- Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm. J R Stat Soc Series B Stat Methodol. 1977; 39:1-38 [Google Scholar]
- Ware JH, Harrington D, Hunter DJ, D'Agostino R. Missing data. N Engl J Med. 2012; 367:1353-1354 [Google Scholar]
- Little RJ, D'Agostino R, Cohen ML, Dickersin K, Emerson SS, Farrar JT, Frangakis C, Hogan JW, Molenberghs G, Murphy SA, Neaton JD, Rotnitzky A, Scharfstein D, Shih WJ, Siegel JP, Stern H. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012; 367:1355-1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988; 75:800-802 [Google Scholar]
- Rienstra M, McManus DD, Benjamin EJ. Novel risk factors for atrial fibrillation: useful for risk prediction and clinical decision making? Circulation. 2012; 125:e941-e946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosiak M, Dziuba M, Chudzik M, Cygankiewicz I, Bartczak K, Drozdz J, Wranicz JK. Risk factors for atrial fibrillation: not always severe heart disease, not always so ‘lonely’. Cardiol J. 2010; 17:437-442 [PubMed] [Google Scholar]
- Magnani JW, Rienstra M, Lin H, Sinner MF, Lubitz SA, McManus DD, Dupuis J, Ellinor PT, Benjamin EJ. Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation. 2011; 124:1982-1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbadebo TD, Okafor H, Darbar D. Differential impact of race and risk factors on incidence of atrial fibrillation. Am Heart J. 2011; 162:31-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CI, Perkerson KA, Gillespie EL, Kluger J, Gallagher R, Horowitz S, White CM. Impact of prophylactic postoperative beta‐blockade on post‐cardiothoracic surgery length of stay and atrial fibrillation. Ann Pharmacother. 2004; 38:2012-2016 [DOI] [PubMed] [Google Scholar]
- Connolly SJ, Cybulsky I, Lamy A, Roberts RS, O'Brien B, Carroll S, Crystal E, Thorpe KE, Gent M. Double‐blind, placebo‐controlled, randomized trial of prophylactic metoprolol for reduction of hospital length of stay after heart surgery: the beta‐Blocker Length Of Stay (BLOS) study. Am Heart J. 2003; 145:226-232 [DOI] [PubMed] [Google Scholar]
- Mitchell LB, Exner DV, Wyse DG, Connolly CJ, Prystai GD, Bayes AJ, Kidd WT, Kieser T, Burgess JJ, Ferland A, MacAdams CL, Maitland A. Prophylactic oral amiodarone for the prevention of arrhythmias that begin early after revascularization, valve replacement, or repair: PAPABEAR: a randomized controlled trial. JAMA. 2005; 294:3093-3100 [DOI] [PubMed] [Google Scholar]
- Nystrom U, Edvardsson N, Berggren H, Pizzarelli GP, Radegran K. Oral sotalol reduces the incidence of atrial fibrillation after coronary artery bypass surgery. Thorac Cardiovasc Surg. 1993; 41:34-37 [DOI] [PubMed] [Google Scholar]
- Miller S, Crystal E, Garfinkle M, Lau C, Lashevsky I, Connolly SJ. Effects of magnesium on atrial fibrillation after cardiac surgery: a meta‐analysis. Heart. 2005; 91:618-623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti G, Chello M, Candura D, Pasceri V, D'Ambrosio A, Covino E, Di Sciascio G. Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: results of the ARMYDA‐3 (Atorvastatin for Reduction of MYocardial Dysrhythmia After cardiac surgery) study. Circulation. 2006; 114:1455-1461 [DOI] [PubMed] [Google Scholar]
- Adamson PB, Barr RC, Callans DJ, Chen PS, Lathrop DA, Makielski JC, Nerbonne JM, Nuss HB, Olgin JE, Przywara DA, Rosen MR, Rozanski GJ, Spach MS, Yamada KA. The perplexing complexity of cardiac arrhythmias: beyond electrical remodeling. Heart Rhythm. 2005; 2:650-659 [DOI] [PubMed] [Google Scholar]
- Van Wagoner DR. Redox modulation of cardiac electrical activity. J Cardiovasc Electrophysiol. 2001; 12:183-184 [DOI] [PubMed] [Google Scholar]
- Ukai T, Cheng CP, Tachibana H, Igawa A, Zhang ZS, Cheng HJ, Little WC. Allopurinol enhances the contractile response to dobutamine and exercise in dogs with pacing‐induced heart failure. Circulation. 2001; 103:750-755 [DOI] [PubMed] [Google Scholar]
- Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002; 54:230-246 [DOI] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001; 30:1191-1212 [DOI] [PubMed] [Google Scholar]
- Pepe S, Leong JY, Van der Merwe J, Marasco SF, Hadj A, Lymbury R, Perkins A, Rosenfeldt FL. Targeting oxidative stress in surgery: effects of ageing and therapy. Exp Gerontol. 2008; 43:653-657 [DOI] [PubMed] [Google Scholar]
- Ozaydin M, Peker O, Erdogan D, Kapan S, Turker Y, Varol E, Ozguner F, Dogan A, Ibrisim E. N‐acetylcysteine for the prevention of postoperative atrial fibrillation: a prospective, randomized, placebo‐controlled pilot study. Eur Heart J. 2008; 29:625-631 [DOI] [PubMed] [Google Scholar]
- Castillo R, Rodrigo R, Perez F, Cereceda M, Asenjo R, Zamorano J, Navarrete R, Villalabeitia E, Sanz J, Baeza C, Aguayo R. Antioxidant therapy reduces oxidative and inflammatory tissue damage in patients subjected to cardiac surgery with extracorporeal circulation. Basic Clin Pharmacol Toxicol. 2011; 108:256-262 [DOI] [PubMed] [Google Scholar]
- Rodrigo R, Korantzopoulos P, Cereceda M, Asenjo R, Zamorano J, Villalabeitia E, Baeza C, Aguayo R, Castillo R, Carrasco R, Gormaz JG. A randomized controlled trial to prevent postoperative atrial fibrillation by antioxidant reinforcement. J Am Coll Cardiol. 2013; 62:1457-146510.1016/j.jacc.2013.07.014 [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Marchioli R, Macchia A, Silletta MG, Ferrazzi P, Gardner TJ, Latini R, Libby P, Lombardi F, O'Gara PT, Page RL, Tavazzi L, Tognoni G. Fish oil and postoperative atrial fibrillation: the Omega‐3 Fatty Acids for Prevention of Post‐operative Atrial Fibrillation (OPERA) randomized trial. JAMA. 2012; 308:2001–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezek P, Hlavata L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int J Biochem Cell Biol. 2005; 37:2478-2503 [DOI] [PubMed] [Google Scholar]
- Palace V, Kumar D, Hill MF, Khaper N, Singal PK. Regional differences in non‐enzymatic antioxidants in the heart under control and oxidative stress conditions. J Mol Cell Cardiol. 1999; 31:193-202 [DOI] [PubMed] [Google Scholar]
- Fisher‐Wellman KH, Mattox TA, Thayne K, Katunga LA, La Favor JD, Neufer PD, Hickner RC, Wingard CJ, Anderson EJ. Novel role for thioredoxin reductase‐2 in mitochondrial redox adaptations to obesogenic diet and exercise in heart and skeletal muscle. J Physiol. 2013; 591:3471-3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holschneider DPS, Shih JC. Monoamine Oxidase: Basic and Clinical Perspectives. 2000New York, NY: Raven Press; 2000 [Google Scholar]
- De Jong AM, Maass AH, Oberdorf‐Maass SU, Van Veldhuisen DJ, Van Gilst WH, Van Gelder IC. Mechanisms of atrial structural changes caused by stretch occurring before and during early atrial fibrillation. Cardiovasc Res. 2011; 89:754-765 [DOI] [PubMed] [Google Scholar]


