Abstract
Background
Age‐related endothelial dysfunction and vascular stiffening are associated with increased cardiovascular (CV) risk. Many groups have encouraged goals of ≥10 000 steps/day or ≥30 min/day of moderate intensity physical activity (MPA) to reduce age‐related CV risk. The impact of MPA on the vasculature of older adults remains unclear.
Methods and Results
We randomized 114 sedentary older adults ages ≥50 to 12 weeks of either no intervention (group 1), a pedometer‐only intervention (group 2), or a pedometer with an interactive website employing strategies to increase the adoption of habitual physical activity (PA, group 3). Endothelial function by brachial flow‐mediated dilation (FMD%), vascular stiffness by tonometry, step‐count by pedometer, and PA intensity/distribution by accelerometer were measured. Step‐count increased in groups 2 (5136±1554 to 9596±3907, P<0.001) and 3 (5474±1512 to 8167±3111, P<0.001) but not in group 1 (4931±1667 to 5410±2410). Both groups 2 and 3 increased MPA ≥30 min/day. Only group 3 increased MPA in continuous bouts of ≥10 minutes (P<0.001) and improved FMD% (P=0.001). Neither achievement of ≥10 000 steps/day nor ≥30 min/day of MPA resulted in improved FMD%. However, achieving ≥20 min/day in MPA bouts resulted in improved FMD%. No changes in vascular stiffness were observed.
Conclusions
MPA reverses age‐related endothelial dysfunction, but may require MPA to be performed in bouts of ≥10 minutes duration for ≥20 min/day to be effective. Commonly encouraged PA goals do not guarantee improved endothelial function and may not be as effective in reducing CV risk.
Clinical Trial Registration
URL: Clinicaltrials.gov. Unique identifier: NCT‐01212978.
Keywords: elderly, endothelial function, physical exercise
Introduction
Aging is associated with reduced physical activity (PA) levels.(1994) Only ≈¼ of adults ≥50 years old meet physical activity goals as outlined by the Department of Health and Human Services (HHS), a disproportionately high rate of failure relative to younger adults.(2009) Reduced PA in older adults has a significant adverse effect on their overall cardiovascular (CV) health.(2004)–(2010)
The beneficial impact of PA on CV risk appears in significant part independent of its effects on traditional CV risk factors and related to repeated bouts of increased laminar shear stress that act favorably on the vascular endothelial structure and function.(2003)–(2011) Sedentary aging is associated with pathological remodeling of muscular arteries, resulting in larger vessel diameters, lower shear stress, and impaired endothelium‐dependent vasodilation.(2011)–(2012) In addition, sedentary aging also results in reduced compliance of large elastic arteries.(2004) Structured, habitual exercise performed at vigorous intensities (≈6 to 7 metabolic equivalents [METs]) and frequent intervals (5 to 6 days/week) protects against and reverses age‐associated vascular dysfunction in older adult men.(2011),(2000)–(2000)
These data supply key conceptual information about the vascular benefits of exercise. However, the duration and intensity of exercise in these studies exceeds current PA recommendations. Whether MPA (3 to 6 METs), achievable by brisk walking, the most common PA engaged in by older adults, also reverses age‐related vascular endothelial dysfunction and stiffening remains unclear.(2008) We recently piloted a study that combined pedometer use with motivational messaging, and showed we could significantly increase MPA in previously sedentary older adults with a high rate of adherence through increased walking.(2011) In the context of a randomized trial that included quantification of step count by pedometer measurement and PA intensity by accelerometry as recommended by the American Heart Association's most recent PA guidelines,(2013) we hypothesized that an intervention that combines pedometer guidance with internet‐based motivational messaging designed to guide sedentary older adults to increase their average daily step count to ≥10 000 steps, a widely accepted equivalent to the currently promulgated PA goal for older adults,(2008)–(2001) would reverse age‐related endothelial dysfunction and vascular stiffening.
Methods
Subjects
One hundred thirteen sedentary older adults (ages ≥50 and ≤80 years of age) were recruited for this study based at the Medical College of Wisconsin (Milwaukee, WI) between 2010 and 2012. Participants were recruited from the local Milwaukee metropolitan area by posted and distributed flyers, newspaper ads, and internet‐based advertisements. The study protocol was approved by the Medical College of Wisconsin's Institutional Research Board, and all participants provided written informed consent.
Screening prior to randomization included a detailed medical history and a focused cardiac and vascular physical exam by a study physician to screen for occult disease. Blood pressure measurement was also performed in triplicate. Individuals with uncontrolled hypertension (≥160/100), recent myocardial infarction (within 1 month of enrollment), angina, clinical evidence of heart failure or documented left ventricular ejection fraction of ≤45%, renal insufficiency, liver dysfunction, active malignancy, or cognitive impairment were excluded. All potential participants were screened for walking ability using either a 2‐stage treadmill test (2 and 3 miles/hour stages for 5 minutes) or asked to take 650 walking steps over 10 minutes in the study center if the subject was not comfortable walking on a treadmill. Individuals who could not complete one of these tasks were excluded from participation. At the end of the screening visit, those potential participants not yet excluded were provided a pedometer (Omron HJ‐720ITC; Omron), an accelerometer (ActiGraph GTX3; ActiGraph), a 7‐day log to record daily step‐count, and asked to return in 1 week. Participants were asked to wear the pedometer and accelerometer during all waking hours. Potential participants who averaged ≤8000 steps/day and met all other inclusion criteria were subsequently randomized. Individuals were blinded to the step count limit for study inclusion.
Randomization
Subjects who passed screening were randomized into 1 of 3 groups: a control group (group 1), a pedometer‐only intervention group (group 2), and a pedometer combined with interactive website intervention group (group 3). Due to technical issues with the website server, individuals enrolled in the first 4 months of the protocol were randomly selected to be in either group 1 or 2 if their designation by the initial randomization scheme was group 3. This accounts for the imbalance in numbers among groups. The control group was asked not to change their behavior during the study period. The pedometer‐only intervention group received a pedometer to wear on their belt during walking hours with a goal to increase their PA by 10% each week above baseline levels in order to reach an average of 10 000 steps/day. They received pedometer logs and 12 self‐addressed, stamped envelopes in which to return a 7‐day log each week to the study investigators. Group 3 received a pedometer and was additionally asked to log onto a secure website through the University of Wisconsin‐Milwaukee on at least a weekly basis throughout the intervention period. The interactive website employed key strategies proven to increase the adoption and retention of lifestyle‐integrated habitual physical activity in older adults.(1998)–(2007) These strategies included the use of frequent feedback,(1998) self‐regulation of activity,(1988)–(2000) education and practice in realistic behavioral change strategies and goal setting,(2000) and rewarding.(2010) At the end of each week, upon uploading pedometer information through a USB, graphical representations were provided of daily steps and how such corresponded with intrinsically set goals. At this stage of the program, each individual was either in compliance with set goals (defined as meeting walking step targets 5 out of 7 days), or they were not in compliance. If a participant was in compliance the user was guided through a series of congratulatory screens and a directive for setting the upcoming week's step goal. If the participant did not attain compliance, the user was guided through a series of interactive screens that were designed to collect barriers identified and then deliver motivational messages tagged and retrieved from a database library. The motivational messages were designed to offer strategies and tips for overcoming identified barriers to set goal attainment. Each week of the interactive program was also guided by an ongoing discussion forum, posing questions and solutions to increase PA, and access to “ask the expert” for points of clarification. Once a participant reached the 10 000 steps/day goal, the software encouraged the user to maintain this level of activity through continued tailored messaging.
Study Visit Procedures (Prior to and Following the 12‐Week Intervention Period)
General procedures
All subjects fasted overnight prior to their study visit. Height and weight were measured in metric units. Waist circumference was measured at the level of the umbilicus while standing. Heart rate and blood pressure (BP) were measured in triplicate and averaged. Peripheral venous blood was drawn from an upper extremity vein for biomarker analyses, including plasma glucose, insulin, and lipid profiles. We estimated insulin resistance by calculating the homeostatic model of assessment‐insulin resistance (HOMA‐IR=fasting glucose [mg/dL]×fasting insulin [μU/mL]/405) and the quantitative insulin sensitivity check test [QUICKI=1/[log (fasting insulin μU/mL)+log (fasting glucose mg/dL)]].
Measurement of endothelial function by brachial artery reactivity
In vivo measurement of endothelial function using high‐frequency vascular ultrasound (Logiq 500Pro; GE) of the brachial artery in the dominant arm were obtained with the arm supinated and abducted ≈80° 1 to 3 cm proximal to the cubital fossa. Brachial artery diameters were measured throughout the cardiac cycle at rest (baseline) and during reactive hyperemia produced by 5 minutes of forearm cuff inflation above suprasystolic levels (50 mm Hg above systolic pressure or 200 mm Hg, whichever is greater) was performed as previously described.(2010) Endothelial function is reported as percent flow‐mediated dilation (FMD%). Baseline and peak hyperemic flow velocity were also recorded to calculate resting and hyperemic shear stress in the brachial artery as previously described.(2010) Nitroglycerin‐mediated dilatation (NMD%) was assessed as a measure of endothelium‐independent vasodilation in all subjects without contraindication. Individuals who did not receive nitroglycerin were statistically more likely to be female (60% versus 85%, P=0.02) and had a higher average heart rate (67±10 versus 63±8 beats/min, P=0.02) but were otherwise similar to those who received nitroglycerin. All analyses were performed blinded to the subject's randomization assignment by trained technicians. Our laboratory intra‐ and inter‐observer variation for FMD% based on 10 to 15 subjects selected at random from this study showed correlation coefficients ranging between 0.76 and 0.87 and intra‐class correlation coefficients ranging from 0.72 to 0.87. The average intra‐observer variation was 1.1±0.7% and 1.3±1.1% for inter‐observer variation.
Measurement vascular compliance by peripheral tonometry
Carotid‐femoral pulse wave velocity (cfPWV) and augmentation index (AIx) were measured using commercially available digital tonometry equipment and software (Sphygmocor Mx; Atcor Medical). cfPWV was calculated by dividing the measured distance between the carotid and femoral sites of pulse wave capture by the time between the foot (initial sharp upstroke) of the carotid pulse wave to the foot of the femoral pulse wave. Aortic AIx was measured by sampling the radial arterial pulse. A validated transfer function was then employed by the Sphygmocor Mx to generate the corresponding central aortic waveform.(1993)–(2001) AIx, which is inversely proportional to systemic arterial stiffness, was calculated automatically by the device as the difference between the second and first systolic peaks divided by the central pulse pressure and normalized to a heart rate of 75 beats/min. A minimum of 10 pulse waves were obtained and averaged for each measurement of AIx and cfPWV. Internal quality controls employed by the Sphygmocor Mx were used to assure the quality of the waveforms obtained. Our laboratory intra‐ and inter‐observer variation for cfPWV based on 10 subjects selected at random from this study showed correlation coefficients ranging between 0.89 and 0.92. The average intra‐observer variation was 0.3±0.2 m/s and 0.6±0.2 m/s for inter‐observer variation. For AIx, intra‐ and inter‐observer variation based on 10 subjects selected at random showed correlation coefficients ranging between 0.95 and 0.96. The average intra‐observer variation was 0.9±1.2 m/s and 2.5±1.6 m/s for inter‐observer variation. Individuals missing AIx or cfPWV data were secondary to inadequate quality of waveforms for those individuals at either the week 1 or week 12 visit. Other than a borderline lower percentage of current smokers those with missing data (0% versus 8.8%, P=0.05), there were no significant differences in baseline characteristics between individuals with and without full sets of AIx or cfPWV measurements (data not shown).
Accelerometry data
Accelerometry data was collected using the ActiGraph GT3X device for 1 week at the time of enrollment and during the final week of the 12‐week intervention period for all groups. We used standardized data quality procedures to assess validity of the accelerometer data and create categories of activity intensity based on accelerometer counts.(2001) A bout of MPA was defined as at least 10 consecutive minutes of MPA.
Analysis of Accelerometer Data
Data equal to or >60 minutes where the accelerometer activity count data was zero was considered time when the monitor was not worn. This data was removed for analysis purposes. Valid days of accelerometer wear were deemed when the accelerometer was worn for a minimum of 600 minutes per day. Although all participants were asked to wear the device for 7 consecutive days, some did not wear it for the full time, or had days when they had <600 minutes of usable data. Only participants who had at least 4 days of valid accelerometer data were included in analysis.
A minute of accelerometer data was coded as sedentary activities (0 to 100 counts, <1.5 METs intensity), light activity (101 to 1951 counts, 1.5 to 3 METs), moderate intensity activity (1952 to 5924 counts, 3 to 6 METs), or vigorous activity (>5925 counts, >6 METs).(1998) Further, a bout of PA was defined as at least 10 consecutive minutes of MPA.
Statistical Analysis
Statistical analyses were performed using SigmaStat 12.0 and SPSS 21.0. The baseline characteristics were compared by 1‐way ANOVA or χ2 as appropriate. Correlations between step count and minutes of PA were calculated using Pearson's r. Anthropomorphic measurements, measurements of endothelial function and vascular stiffness, step count, and time spent in differing levels of PA intensity were compared using general linear models with time (measurements pre‐ and post‐12 week intervention period) as the within‐subjects factor and randomization assignment as the between‐subjects factor. Group by time interactions were analyzed for all outcomes. Post hoc testing was performed using Tukey HSD as appropriate. The primary outcome for this study was brachial artery FMD%. Our ad hoc power analysis suggested that enrollment of 114 subjects would give us 80% power to detect a 25% increase in FMD% from baseline assuming a 20% drop‐out rate at α=0.05. P values of <0.05 are considered statistically significant.
Results
Baseline Characteristics
Figure 1 delineates the study subject enrollment and randomization data. There were no significant differences between groups with respect to age, sex, history of hypertension, history of diabetes, and smoking status. There were no baseline differences in body mass index, waist circumference, blood pressure, heart rate, lipid profile, insulin sensitivity by both HOMA‐IR and QUICKI, fasting plasma glucose level, or high sensitivity CRP (Table 1).
Figure 1.
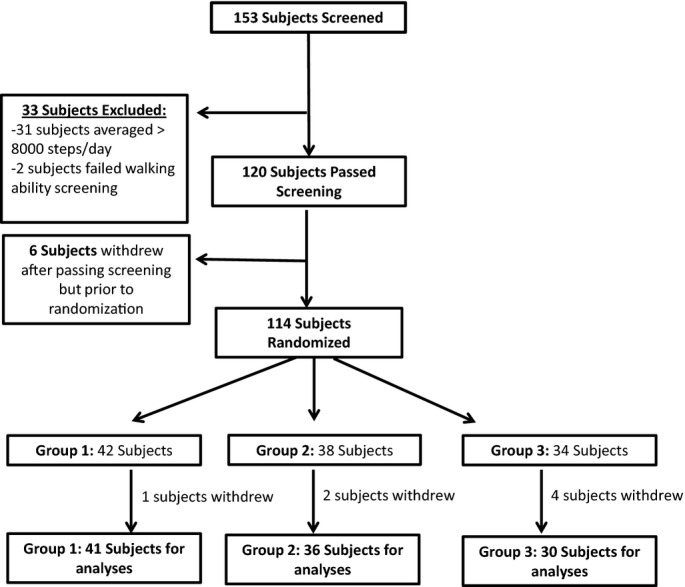
Study enrollment flow diagram.
Table 1.
Demographics and Characteristics by Study Group
| Control (N=41) | Pedometer Only (N=36) | Pedometer+Website (N=30) | |
|---|---|---|---|
| Age, y | 62±7 | 64±7 | 63±8 |
| Sex, % female | 24.4 | 38.9 | 40.0 |
| History of diabetes, % | 0 | 0 | 2.9 |
| History of hypertension, % | 29.3 | 30.6 | 36.7 |
| Smoking status, % current | 9.8 | 5.6 | 6.7 |
| Smoking status, % past | 26.8 | 33.3 | 40.0 |
| Weight, kg | 79.1±16.6 | 83.0±17.2 | 87.3±18.1 |
| Body mass index, kg/m2 | 29.4±6.4 | 28.8±4.9 | 29.7±5.5 |
| Waist circumference, cm | 98.6±14.3 | 99.7±12.8 | 102.4±13.9 |
| Glucose, mg/dL | 89±13 | 89±9 | 96±24 |
| Insulin, μU/L | 14.2±7.7 | 14.3±8.2 | 13.2±5.1 |
| QUICKI | 0.33±0.02 | 0.33±0.03 | 0.33±0.02 |
| HOMA‐IR | 3.2±1.9 | 3.2±2.0 | 3.2±2.1 |
| Total cholesterol, mg/dL | 134±35 | 130±31 | 134±34 |
| LDL cholesterol, mg/dL | 76±35 | 69±31 | 76±36 |
| HDL cholesterol, mg/dL | 41±16 | 45±17 | 41±12 |
| Triglycerides | 79±23 | 80±32 | 86±39 |
| hsCRP, mg/dL | 3.2±3.2 | 3.2±2.4 | 2.9±3.3 |
| Systolic blood pressure | 129±13 | 129±15 | 130±14 |
| Diastolic blood pressure | 70±8 | 68±8 | 69±6 |
| Heart rate, bpm | 64±9 | 63±9 | 63±9 |
HDL indicates high‐density lipoprotein; HOMA‐IR, homeostatic model of assessment‐insulin resistance; hsCRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; QUICKI, quantitative insulin sensitivity check test.
Changes in Baseline Characteristics by Intervention Group
Over the study period, weight (P=0.01), BMI (P=0.003) and waist circumference (P=0.009) decreased for the entire cohort. However, there were no significant differences over time based on randomization assignment. No changes in heart rate, blood pressure, fasting glucose, insulin sensitivity, lipid profile, or CRP were seen over the 12‐week period within or between study groups (Table 2).
Table 2.
Changes in Baseline Characteristics by Study Group
| Control (N=41) | Pedometer Only (N=36) | Pedometer+Website (N=30) | P Values (Time Effect) | P Values (Time×Group Interaction) | |
|---|---|---|---|---|---|
| Weight, kg | −0.5±3.0 | −1.0±2.1 | −1.2±5.2 | 0.01 | 0.72 |
| Body mass index, kg/m2 | −0.7±2.0 | −0.4±1.4 | −0.6±1.8 | 0.003 | 0.69 |
| Waist circumference, cm | −0.6±4.8 | −1.0±3.9 | −2.0±4.8 | 0.009 | 0.40 |
| Glucose, mg/dL | 1.4±6.9 | 1.9±6.6 | −1.2±8.7 | 0.33 | 0.21 |
| Insulin, μU/L | −0.2±4.2 | −0.6±4.9 | −0.4±3.3 | 0.38 | 0.94 |
| QUICKI | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.49 | 0.39 |
| HOMA‐IR | 0.0±1.1 | 0.0±1.2 | −0.1±0.8 | 0.78 | 0.86 |
| Total cholesterol, mg/dL | 4.9±26.0 | 4.3±40.4 | −6.3±36.9 | 0.79 | 0.39 |
| LDL cholesterol, mg/dL | 1.7±28.7 | 0.9±41.7 | −10.4±36.8 | 0.48 | 0.36 |
| HDL cholesterol, mg/dL | 2.1±16.3 | 3.8±17.4 | 4.14±10.9 | 0.03 | 0.84 |
| Triglycerides | 3.9±25.7 | 0.7±26.9 | 2.7±25.4 | 0.36 | 0.87 |
| hsCRP, mg/dL | 0.7±3.5 | 0.2±2.7 | 0.1±1.6 | 0.20 | 0.62 |
| Systolic Blood Pressure | 0±12 | −3±10 | −1±12 | 0.21 | 0.66 |
| Diastolic blood pressure | −1±7 | 1±5 | 0±5 | 0.79 | 0.66 |
| Heart rate, bpm | 1±5 | −1±7 | −2±5 | 0.19 | 0.07 |
HDL indicates high‐density lipoprotein; HOMA‐IR, homeostatic model of assessment‐insulin resistance; hsCRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; QUICKI, quantitative insulin sensitivity check test.
Changes in Step Count and Physical Activity by Intervention Group
The full set of step count and accelerometer measurements are reported in Table 3. One subject in group 3 had inadequate pedometer data for one visit and was excluded from the step count averages. Due to having <4 days of valid accelerometer measurements, (8 subjects [6 in group 1, 4 in group 2]) did not have adequate accelerometer data for analysis at one of the measurement time points. There were no significant differences in baseline step count between activity groups at baseline (P=0.71). Average step count significantly increased in groups 2 and 3 (5136±1554 to 9596±3907 and 5474±1512 to 8167±3111 steps in groups 2 and 3, respectively, P<0.001 for time×group interaction, P<0.001 within groups 2 and 3) with no change in step count for group 1 (4931±1667 to 5410±2410 steps, P=0.12). There was no significant difference between the 12‐week step counts of groups 2 and 3 (P=0.16). Overall, 5%, 50%, and 31% of subjects achieved ≥10 000 steps/day by week 12 in group 1, 2, and 3, respectively.
Table 3.
Step Count and Physical Activity Data by Study Group
| Control N=41 (Step Count) N=35 (Accelerometry) | Pedometer Only N=36 (Step Count) N=32 (Accelerometry) | Pedometer+Website N=29 (Step Count) N=29 (Accelerometry) | P Values (Time Effect) | P Values (Time×Group Interaction) | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Week 12 | Baseline | Week 12 | Baseline | Week 12 | |||
| Average step count | 4931±1667 | 5410±2410 | 5136±1554 | 9596±3907 | 5474±1512 | 8167±3111 | <0.001 | <0.001 |
| Average minutes observed/day | 909±98 | 889±75 | 934±112 | 930±121 | 944±93 | 944±133 | 0.48 | 0.75 |
| Average minutes‐sedentary, ≤1.5 METS | 654±109 | 636±109 | 659±115 | 624±120 | 657±90 | 641±113 | 0.04 | 0.77 |
| Average light activity minutes, 1.5 to 3 METS | 237±62 | 234±71 | 256±62 | 257±67 | 266±74 | 267±74 | 0.98 | 0.94 |
| Average moderate intensity activity minutes, 3 to 6 METs | 16±10 | 17±14 | 19±11 | 48±31 | 19±14 | 35±11 | <0.001 | <0.001 |
| Average vigorous intensity minutes, ≥6 METs | 1±5 | 1±4 | 0±2 | 1±3 | 1±2 | 0±1 | 0.94 | 0.22 |
| Average moderate intensity in bouts | 4±8 | 4±8 | 7±8 | 14±10 | 7±9 | 27±21 | <0.001 | <0.001 |
| Subjects achieving ≥10 000 steps | 0 | 2 | 0 | 18 | 0 | 9 | — | <0.001 |
| Subjects achieving ≥20 min/day of MPA in bouts | 2 | 3 | 2 | 12 | 2 | 18 | — | <0.001 |
| Subjects achieving ≥30 min total of MPA | 5 | 8 | 4 | 21 | 6 | 17 | — | 0.001 |
METs indicates metabolic equivalents; MPA, moderate intensity physical activity.
Accelerometer data revealed a significant decrease in overall sedentary time for the entire cohort over the study period (P=0.04) but this decrease was not significantly different between groups (P=0.77). We observed no differences in the total time observed, the amount of light activity time, and the amount of vigorous activity time between or within groups over the 12‐week period (Table 3). However, average daily MPA increased between weeks 1 and 12 (P<0.001). There were no differences in MPA at baseline between activity groups (P=0.60). MPA significantly increased in groups 2 (19±11 to 48±31 minutes, P<0.001) and 3 (19±4 to 35±11, P=0.001) but not in group 1 (16±10 to 17±14 minutes, P=0.86). There was no difference in the amount of MPA between groups 2 and 3 at the conclusion of the intervention period (P=0.08). There were no significant differences in MPA performed in bouts at baseline (P=0.38). MPA bout activity significantly increased within both group 2 (7±8 to 14±10 minutes, P<0.001) and group 3 (7±9 to 27±21 min, P<0.001), but not group 1 (4±8 to 4±8, P=0.71). MPA bout activity was significantly higher in groups 2 and 3 at week 12 compared with group 1 (P=0.01 and <0.001 for group 1 versus group 2 and group 3, respectively), and MPA bout activity was significantly higher in group 3 than group 2 at week 12 (P=0.005).
Changes in Brachial Artery Endothelial Function and Vascular Stiffness by Intervention Group
Full data on the vascular changes over the study period are presented in Table 4 and Figure 2. Brachial FMD% significantly increased overall during the study period for the entire cohort and this increase differed by activity group (Figure 2, P<0.001 for time, P=0.004 for time×group interaction). FMD% did not significantly change in group 1 (5.6±2.5% to 6.3±2.7%, P=0.10) or group 2 (6.5±3.0% to 6.7±3.9%, P=0.85), but significantly increased in group 3 (4.7±2.5% to 8.0±4.3%, P=0.001).
Table 4.
Endothelial Function and Vascular Compliance Data by Study Group
| Control (N=41) | Pedometer Only (N=36) | Pedometer+Website (N=29) | P Values (Time Effect) | P Values (Time×Group Interaction) | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Week 12 | Baseline | Week 12 | Baseline | Week 12 | |||
| Baseline brachial diameter, mm | 3.49±0.57 | 3.60±0.58 | 3.84±0.70 | 3.81±0.70 | 4.03±0.87 | 3.93±0.91 | 0.77 | 0.02 |
| Baseline peak shear, dynes/cm2 | 44±13 | 43±14 | 45±16 | 40±13 | 40±13 | 40±14 | 0.08 | 0.19 |
| Hyperemic peak shear, dynes/cm2 | 80±22 | 77±26 | 74±23 | 72±29 | 71±22 | 70±23 | 0.41 | 0.93 |
| Nitroglycerin mediated dilation, %* | 22.4±7.8 | 19.9±6.6 | 18.7±5.5 | 17.2±6.4 | 21.0±6.2 | 20.5±6.7 | 0.14 | 0.70 |
| Carotid‐femoral pulse wave velocity, cm/s* | 9.6±2.2 | 9.2±2.3 | 9.8±2.0 | 9.6±1.7 | 9.7±2.0 | 9.8±2.7 | 0.44 | 0.61 |
| Augmentation index* | 29.2±9.8 | 27.1±9.7 | 26.1±7.3 | 26.6±15.1 | 25.4±8.6 | 24.4±8.0 | 0.39 | 0.52 |
| Aortic systolic blood pressure* | 120±12 | 121±16 | 119±14 | 117±14 | 120±12 | 121±14 | 0.87 | 0.31 |
| Aortic diastolic blood pressure* | 72±9 | 71±20 | 68±8 | 70±8 | 71±7 | 71±8 | 0.88 | 0.16 |
N=30, 23, and 18 for groups 1, 2, and 3, respectively.
N=35, 31, and 24 for groups 1, 2, and 3, respectively.
N=40, 34, and 29 for groups 1, 2, and 3, respectively.
Figure 2.
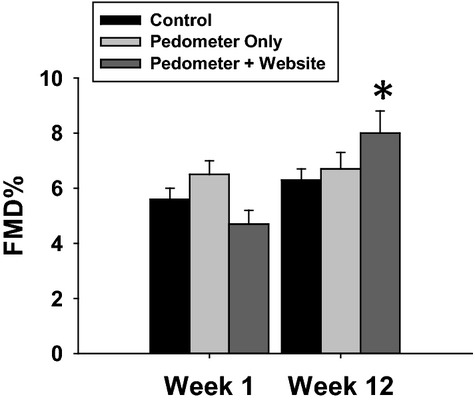
Brachial flow‐mediated dilation (FMD) improved in the group randomized to access to the pedometer and website, but not in either of the other groups. (P<0.001 for time, P=0.004 for time×group interaction by ANOVA). FMD% did not significantly change in group 1 (5.6±2.5% to 6.3±2.7%, P=0.10) or group 2 (6.5±3.0% to 6.7±3.9%, P=0.85), but significantly increased in group 3 (4.7±2.5% to 8.0±4.3%, *P=0.001 within the group).
There was a significant interaction between time of brachial artery diameter measurement and activity group (P=0.02). Brachial artery diameter was significantly smaller at baseline in group 1 compared with both group 2 (P=0.03) and group 3 (P=0.01), but was not significantly different at the week 12 visit (P=0.46 and 0.18 comparing group 1 to groups 2 and 3, respectively). Brachial artery diameter was larger at week 12 compared with week 1 in group 1 (3.49±0.57 to 3.60±0.58 mm, P=0.01) and trended toward a smaller size at week 12 in group 3 (4.03±0.87 to 3.93±0.91, P=0.08). There was no change in brachial diameter in group 2 (3.84±0.70 to 3.81±0.70, P=0.58). There were no significant changes in baseline and peak hyperemic shear, cfPWV, Aix, or nitroglycerin‐mediated vasodilation over the study period (Table 4).
Changes in Endothelial Function Based on (1) 10 000 Steps/Day Threshold and (2) 30 Min/Day MPA Average
Twenty‐nine subjects reached ≥10 000 steps/day by the end of the study protocol. As shown in Figure 3A¸ step count increased to a significantly greater extent in the group that achieved a ≥10 000 steps/day average compared with those who did not meet this goal (4803±1531 to 5741±2111 steps versus 6069±1371 to 12486±1947 steps, P<0.001 overall, P<0.001 time×group interaction, P<0.001 between groups at week 12). Similarly, the 46 subjects who achieved ≥30 min/day of MPA by week 12 also significantly increased their step count over the study period while those who did not achieve this goal did not reach 10 000 steps (4825±1604 to 5327±2671 steps versus 5570±1517 to 10196±3149, P<0.001 overall, P<0.001 time×group interaction, P<0.001 between groups at week 12, Figure 3B). There was no interaction between FMD% changes over time and achieving either ≥10 000 steps/day or ≥30 min/day of MPA (P=0.40 and 0.93 for time×group interaction terms for ≥10 000 steps/day and ≥30 min/day, respectively Figures 3C and 3D).
Figure 3.
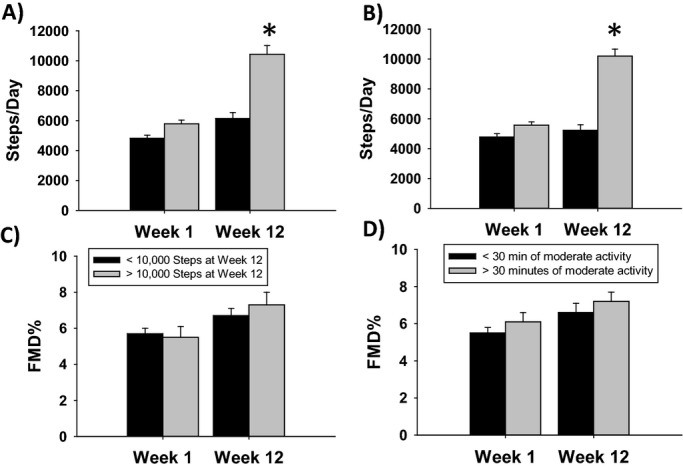
Step count and FMD% based on analysis by achievement of ≥10 000 steps/day (A and C) or ≥30 min/day of MPA (B and D). A, Step count increased to a greater extent in the group that achieved ≥10 000 steps/day compared to those who did not achieve this goal (4803±1531 to 5741±2111 steps vs 6069±1371 to 12486±1947, *P<0.001 between groups at week 12). B, The 46 subjects who achieved ≥30 min/day of MPA also increased their step count over the study period (4825±1604 to 5327±2671 steps vs 5570±1517 to 10196±314, *P<0.001 between groups at week 12). There was no interaction between FMD% changes over time and achieving either ≥10 000 steps/day (C), 5.5±3.1 to 7.4±3.6 for <10 000 group vs 5.7±2.6 to 6.7±3.5 for ≥10 000 step group, P=0.40 for interaction) or ≥30 min/day of MPA (d, 5.5±2.2 to 6.6±3.5 for <30 min/day vs 6.1±3.3 to 7.2±3.6 for ≥30 min/day, P=0.93 for interaction). FMD indicates flow‐mediated dilation; MPA, moderate intensity physical activity.
Changes in Endothelial Function Based on ≥20 Min/Day Average of MPA in Bouts ≥10 Minutes in Length
A total of 33 subjects averaged ≥20 min/day of MPA in bouts of ≥10 minutes in length by the end of the study protocol. Those who achieved ≥20 min/day in MPA bouts significantly increased their step count to a greater extent compared those who did not achieve this goal (4866±1599 to 6204±3172 steps versus 5785±1437 to 10439±3313 steps, P<0.001 for both time and time×group interaction, P<0.001 between groups at week 12, Figure 4A). Further, those who achieved ≥20 min/day in MPA bouts significantly increased their FMD% over the study period, while those who were not performing ≥20 min/day of MPA bouts showed no increase (5.4±3.2% to 8.1±3.7% versus 6.0±2.6% to 6.3±3.4%, P=0.001 for time, P=0.008 for time×group interaction, P<0.001 for ≥20 min/day of MPA in bouts at week 12 versus baseline and versus both FMD measurements for those achieving ≤20 min/day of MPA, Figure 4B). Table 5 presents more detail on the impact of increased MPA bout activity of vascular structure and function. While brachial artery diameter did not change in those achieving ≤20 min/day (3.66±0.61 to 3.71± 0.70 mm, P=0.25), brachial diameter trended toward a decrease in those achieving ≥20 min/day (3.98±0.91 to 3.88±0.88 mm, P=0.066). Similar to the intention to treat analysis, we found no differences within or between groups with respect to baseline and hyperemic peak shear, nitroglycerin mediated dilation, cfPWV, AIx, aortic or brachial pressures, fasting glucose levels, insulin levels, measures of insulin sensitivity, or serum lipids. While both BMI and weight decreased over the study period, there was no interaction between activity grouping and either of these parameters. However, an interaction between activity group and time was seen with waist circumference (P=0.048), with waist circumference significantly decreasing in those who achieved ≥20 min/day in MPA bouts (98.7±13.6 to 96.2±13.2 cm, P=0.003) but not for those who did not achieve this landmark (100.5±12.1 to 100.0±12.2, P=0.38). Resting heart rate was significantly lowered in those who achieved this goal (64±9 to 61±8 beats/min, P=0.002).
Figure 4.
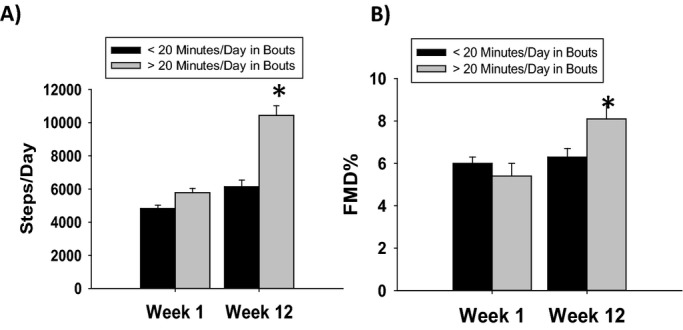
Step count and FMD% based on those who achieved ≥20 min/day. (A) Those who achieved ≥20 min/day of in MPA in bouts significantly increase their step count to a greater extent than those who did not (4866±1599 to 6204±3172 steps vs 5785±1437 to 10439±3313 steps, *P<0.001 for both time and time×group interaction, P<0.001 between groups at week 12). (B) Those who achieved ≥20 min/day in moderate intensity in bouts significantly also increased their FMD% (5.4±3.2% to 8.1±3.7% vs 6.0±2.6% to 6.3±3.4%, *P=0.001 for time, P=0.008 for time×group interaction, P<0.001 for ≥20 min/day of MPA in bouts at week 12 vs baseline and vs both FMD measurements for those achieving ≤20 min/day of MPA). FMD indicates flow‐mediated dilation; MPA, moderate intensity physical activity.
Table 5.
Endothelial Function, Vascular Stiffness, Blood Chemistries, and Vital Signs by Achievement of Bout Activity
| Bout <20 minutes (N=63) | Bout ≥20 minutes (N=33) | P Values (Time Effect) | P Values (Time×Group Interaction) | |||
|---|---|---|---|---|---|---|
| Baseline | Week 12 | Baseline | Week 12 | |||
| Baseline brachial diameter, cm | 3.67±0.61 | 3.71±0.70 | 3.98±0.92 | 3.88±0.89 | 0.43 | 0.03 |
| Baseline peak shear, dynes/cm2 | 44±13 | 41±15 | 43±14 | 38±13 | 0.07 | 0.47 |
| Hyperemic peak shear, dynes/cm2 | 78±21 | 76±29 | 71±23 | 69±22 | 0.44 | 0.99 |
| Nitroglycerin mediated dilation, %* | 21.1±7.0 | 18.3±6.7 | 20.0±5.6 | 20.0±5.9 | 0.17 | 0.20 |
| Carotid‐femoral pulse wave velocity, cm/s* | 9.6±2.2 | 9.4±2.1 | 9.6±1.6 | 9.6±2.1 | 0.60 | 0.48 |
| Augmentation index* | 27.5±9.3 | 26.2±13.3 | 26.6±8.8 | 24.8±7.6 | 0.19 | 0.85 |
| Aortic systolic blood pressure* | 119±13 | 118±14 | 120±11 | 121±15 | 0.93 | 0.33 |
| Aortic diastolic blood pressure* | 69±8 | 69±8 | 71±8 | 71±9 | 0.85 | 0.79 |
| Weight, kg | 82.0±16.1 | 81.4±16.4 | 83.9±16.7 | 82.0±17.6 | 0.002 | 0.08 |
| Body mass index, kg/m2 | 29.2±4.9 | 28.9±5.3 | 29.1±5.8 | 28.3±6.1 | 0.001 | 0.09 |
| Waist circumference, cm | 100.5±12.1 | 100.0±12.2 | 98.7±13.6 | 96.2±13.2 | 0.003 | 0.048 |
| Glucose, mg/dL | 92±20 | 93±23 | 90±8 | 89±11 | 0.88 | 0.18 |
| Insulin, μU/L | 14.2±7.5 | 13.9±7.1 | 13.3±6.9 | 12.6±7.6 | 0.34 | 0.62 |
| QUICKI | 0.33±0.03 | 0.33±0.03 | 0.33±0.02 | 0.34±0.03 | 0.27 | 0.11 |
| HOMA‐IR | 3.3±2.1 | 3.3±2.1 | 3.0±1.7 | 2.9±.2.1 | 0.56 | 0.60 |
| Total cholesterol, mg/dL | 132±35 | 132±29 | 134±31 | 141±30 | 0.42 | 0.44 |
| LDL cholesterol, mg/dL | 73±36 | 71±31 | 75±32 | 78±29 | 0.96 | 0.52 |
| HDL cholesterol, mg/dL | 42±15 | 44±16 | 42±16 | 45±13 | 0.11 | 0.71 |
| Triglycerides | 79±26 | 82±31 | 82±31 | 85±40 | 0.31 | 0.94 |
| hsCRP, mg/dL | 3.23±3.00 | 3.90±3.95 | 2.69±2.95 | 2.83±3.21 | 0.22 | 0.42 |
| Brachial systolic blood pressure | 129±14 | 127±15 | 130±13 | 129±14 | 0.14 | 0.40 |
| Brachial diastolic blood pressure | 69±8 | 68±8 | 69±7 | 70±9 | 0.85 | 0.51 |
| Heart rate, bpm | 63±9 | 63±10 | 64±9 | 61±8 | 0.01 | 0.003 |
HDL indicates high‐density lipoprotein; HOMA‐IR, homeostatic model of assessment‐insulin resistance; hsCRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; QUICKI, quantitative insulin sensitivity check test.
N=45 and 21 for <20 min/day in bouts and ≥20 min/day in bouts.
N=53 and 29 for <20 min/day in bouts and ≥20 min/day in bouts.
N=61 and 32 for <20 min/day in bouts and ≥20 min/day in bouts.
A subgroup analysis of the 46 subjects achieving ≥30 min/day of MPA was performed, stratifying these subjects into 2 groups: those who concomitantly achieved ≥20 min/day of MPA bouts versus and those who did not to determine how MPA bout activity might impact the 30 min/day MPA threshold's effect on FMD%. While FMD% for the combined group did not significantly change with intervention (P=0.69), there was a significant interaction for time by bout achievement for FMD%. FMD% did not significantly increase over the study period in those who did not also reach ≥20 min/day average (N=15, 7.7±2.9 versus 5.7±2.7, P=0.12) while FMD% significantly increased in those who achieved both goals (N=31, 5.4±3.3 versus 7.9±3.8, P=0.002).
Correlations Between Step Counts, Bout Minutes, and Total Moderate Intensity Minutes
The step count at the end of the study correlated strongly with total MPA minutes (r=0.82, P<0.001), but less strongly with the total MPA done in bouts (r=0.62, P<0.001).
Discussion
The present study reports several important findings. First, while both pedometers alone and pedometers combined with our internet‐based tailored messaging significantly increased MPA in our study, only the group that received the pedometers with our internet‐based tailored messaging and feedback concomitantly improved vascular endothelial function. Second, improvements in endothelial function seen with our combined intervention occurred in the absence of significant changes in traditional cardiovascular risk factors or systemic inflammation measured by hsCRP. Third, commonly promulgated goals for MPA, including 10 000 steps/day and ≥30 min/day of MPA, do not appear sufficient to reverse age‐related vascular endothelial dysfunction in older adults. However, reaching these goals in the context of a self‐regulated PA regimen where individuals engage in ≥20 min/day of MPA in continuous bouts of ≥10 minutes does reverse age‐related endothelial dysfunction. Taken together, these data provide important and novel dosing dimensions to current PA recommendations for older adults. The lack of dependence of the favorable impact of this dose of PA on changes to traditional cardiovascular risk factors and systemic inflammation suggests bout‐centered PA with its sustained increases in shear stress may be responsible for the favorable effects of PA on CV risk that are independent of known CV risk factors.
Previous work in healthy older adults establishes that aerobic exercise training reverses both age‐related endothelial dysfunction and arterial stiffening.(2011),(2000)–(2000) The exercise regimens in these studies were 8 to 12 weeks in duration and involved 40 to 50 minutes of continuous activity at 70% to 75% of maximal predicted heart rate. While not reported, based on age, sex, and the intensity of work reported, the PA intensity performed by most of the participants in these studies likely exceeded 6 METs and the overall duration of activity significantly exceeds current PA guidelines. Our work significantly extends these prior data by establishing that self‐regulated PA at more moderate intensities (3 to 6 METs) that are more sustainable and attainable by many older adults also improves endothelial function.
We did not identify any improvements in age‐related vascular stiffness in our study protocol regardless of the thresholds achieved. MPA may either require a longer duration to impact vascular remodeling to alter vascular stiffness. However, we did observe a significant decrease in brachial artery diameter in individuals who achieved ≥20 min/day average of MPA bouts with no change in those who did not achieve this goal. Increased brachial diameter size strongly correlates with increased CV risk.(2002)–(2007) With sedentary aging, the brachial artery pathologically outwardly remodels resulting in lower overall shear stress in a vessel with a larger luminal diameter.(2009) Our data, from a group of older adults with an overall modest CV risk factor burden outside of age, suggest this duration, intensity, and architecture of PA may result in favorable reverse remodeling of muscular conduit arteries.
While the “some PA is better than none” and “more PA is better than less” statements are well supported, more precise dosing of PA by frequency, intensity, and duration has been an elusive and limiting knowledge gap with respect to PA interventions.(2013),(2010)–(2014) Current recommendations are based largely on epidemiological studies with primarily self‐reported PA levels.(2008),(2001)–(2003) Only a minority of the studies focused on older adults.(2011) The data are dominated by self‐reported activity levels and are conflicting with respect to the intensity of activity required to earn PA's CV benefits.(2010) While current recommendations state that PA can be done in separate bouts of ≥10 minutes duration,(2007) there is limited quantitative data behind this recommendation and recent data suggested short bout lengths could be equally effective.(2009)–(2013) We significantly extend these findings by adding specificity to the dosing and duration (≥20 min/day of MPA performed in bouts of ≥10 minutes in length) of PA required for older adults to earn its CV benefits through our quantitative approach to PA measurement and our randomized trial design.
The use of step count, particularly the goal of 10 000 steps/day, to help guide individuals to meet promulgated PA guidelines has risen in popularity with the increased penetration of low‐cost, high‐quality pedometers.(2003)–(2004) The 10 000‐step threshold has been adopted by multiple prominent groups, been popularized in the lay press and internet, and is included as a recommended way to meet the Department of Health and Human Services’ Physical Activity Guidelines and the American Heart Association's literature.(2013) The health impact of pedometer‐based interventions on traditional CV risk factors appears to vary based on the comorbidities of the population being followed, and a recent meta‐analysis suggests improvements in blood pressure, BMI, and glycemic control may be attained through pedometer‐based interventions in hypertensive and insulin resistant populations.(2006) However, recent data suggest that 10 000 steps may not well approximate current PA goals, particularly in older adults who may have musculoskeletal or other issues that limit their walking speed.(2003),(2013) Our data provide important new evidence that PA interventions focusing solely on the number of steps without emphasis on appropriate PA architecture are likely to be less effective at reducing CV risk. Specifically, our study results suggest that in older adults, the commonly used 10 000 steps/day goal is not sufficient to reverse age‐related endothelial function unless it occurs in the context of ≥20 min/day of MPA in bouts.
While the mechanisms behind all of PA's CV benefits remain to be elucidated, PA's protective effects on vascular physiology derive from its intermittent bouts of increased laminar shear stress. Shear stress has recently been shown to be the primary stimulus for improvements in endothelial function related to PA, largely independent of PA's effect on other CV risk factors.(2003)–(2010) Sedentary aging leads to increased endothelial inflammation, reduced endothelium‐derived NO synthase expression, and increased oxidative stress leading to phenotypical endothelial dysfunction.(2008)–(2011) Episodes of increased laminar shear stress activate shear responsive elements inducing profound epigenetic changes and changes in genomic expression leading to favorable changes in the vascular endothelial phenotype.(2003) This study largely supports the concept that age‐related vascular endothelial dysfunction can be reversed by PA independent of its influence of CV risk factors. Further work will be necessary to more precisely determine the dose‐response relationship between different “doses” of shear stress and the responses of the vasculature.
Our study has some limitations. Our cohort represents a relatively healthy group of adults aged ≥50 years. It is possible that different intensities and durations of PA may have different effects in patients with different comorbidity profiles or at younger ages. MPA could also have greater impact on vascular structure in a longer duration study. Balanced against these limitations are the important new quantitative PA dosing data this study suggests that may enhance the clinical impact of current PA recommendations for older adults.
Our randomized trial demonstrates that self‐regulated PA at moderate intensities can reverse age‐related vascular endothelial dysfunction. Our data suggests the most important aspect of PA is that it be done at moderate intensity in continuous bouts of ≥10 minutes for ≥20 min/day. Future work further delineating the ideal bout length for CV risk reduction and the mechanisms by which bout activity reverse age‐related endothelial dysfunction hold promise for maximizing PA's CV risk reduction in older adults as well as unlocking mechanisms behind pathological vascular aging.
Sources of Funding
This study was supported by a T. Franklin Williams Scholars Award provided by Atlantic Philanthropies, the American Heart Association (10GRNT3880044), the John A. Hartford Foundation, and the Association of Specialty Physicians (PI: Dr Widlansky). Dr Widlansky also receives grant support from the NIH (K23HL089326 and HL081587), the Diabetes Complication Consortium (subaward 25732‐1), the Doris Duke Foundation, and Merck, Sharp, & Dohme Corporation. Dr Strath receives funding from the NIH (HL091019) and from a Veterans Administration Merit Award (1/01RX000555). Drs Suboc and Wang have been funded by T32HL007792.
Disclosures
None.
References
- Caspersen CJ, Merritt RK, Stephens T. In: Dishman RK. (ed.). International activity patterns: a methodological perspective. Advances in Exercise Adherence. 1994Champaign: Human Kinetics; 1994. 73-110 [Google Scholar]
- U.S. Department of Health and Human Services Centers for Disease Control and Prevention National Center for Health Statistics Summary Health Statistics for U.S. Adults: National Health Interview Survey, 2008. 2009. Report No.: 242.
- Garrett NA, Brasure M, Schmitz KH, Schultz MM, Huber MR. Physical inactivity: direct cost to a health plan. Am J Prev Med. 2004; 27:304-309 [DOI] [PubMed] [Google Scholar]
- National Health Expenditure Projections 2009‐2019 US Department of Health and Human Services. 2010
- Green DJ, Walsh JH, Maiorana A, Best MJ, Taylor RR, O'Driscoll JG. Exercise‐induced improvement in endothelial dysfunction is not mediated by changes in CV risk factors: pooled analysis of diverse patient populations. Am J Physiol Heart Circ Physiol. 2003; 285:H2679-H2687 [DOI] [PubMed] [Google Scholar]
- Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension. 2010; 55:312-318 [DOI] [PubMed] [Google Scholar]
- Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex‐specific effects of habitual aerobic exercise on brachial artery flow‐mediated dilation in middle‐aged and older adults. Clin Sci (Lond). 2011; 120:13-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Walker AE, Pierce GL, Lesniewski LA. Habitual exercise and vascular ageing. J Physiol. 2009; 587:5541-5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age‐related decline in women. J Am Coll Cardiol. 1994; 24:471-476 [DOI] [PubMed] [Google Scholar]
- Thijssen DH, Cable NT, Green DJ. Impact of exercise training on arterial wall thickness in humans. Clin Sci (Lond). 2012; 122:311-322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004; 43:1239-1245 [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age‐related declines in endothelium‐dependent vasodilation in healthy men. Circulation. 2000; 102:1351-1357 [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000; 102:1270-1275 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Behavioral Risk Factor Surveillance System Survey Data. 2008Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2008 [Google Scholar]
- Strath SJ, Swartz AM, Parker SJ, Miller NE, Grimm EK, Cashin SE. A pilot randomized controlled trial evaluating motivationally matched pedometer feedback to increase physical activity behavior in older adults. J Phys Act Health. 2011; 8suppl 2:S267-S274 [DOI] [PubMed] [Google Scholar]
- Strath SJ, Kaminsky LA, Ainsworth BE, Ekelund U, Freedson PS, Gary RA, Richardson CR, Smith DT, Swartz AM. Guide to the assessment of physical activity: clinical and research applications: a scientific statement from the American Heart Association. Circulation. 2013; 128.20:2259-2279 [DOI] [PubMed] [Google Scholar]
- Physical Activity Guidelines for Americans: Office of Disease Prevention and Health Promotion. 2008Washington, DC: US Department of Health and Human Services; 2008 [Google Scholar]
- Welk GJ, Differding JA, Thompson RW, Blair SN, Dziura J, Hart P. The utility of the Digi‐walker step counter to assess daily physical activity patterns. Med Sci Sports Exerc. 2000; 32:S481-S488 [DOI] [PubMed] [Google Scholar]
- Tudor‐Locke CE, Myers AM. Methodological considerations for researchers and practitioners using pedometers to measure physical (ambulatory) activity. Res Q Exerc Sport. 2001; 72:1-12 [DOI] [PubMed] [Google Scholar]
- Wilde BE, Sidman CL, Corbin CB. A 10,000‐step count as a physical activity target for sedentary women. Res Q Exerc Sport. 2001; 72:411-414 [DOI] [PubMed] [Google Scholar]
- Oman RF, King AC. Predicting the adoption and maintenance of exercise participation using self‐efficacy and previous exercise participation rates. Am J Health Promot. 1998; 12:154-161 [DOI] [PubMed] [Google Scholar]
- McAuley E, Morris KS, Motl RW, Hu L, Konopack JF, Elavsky S. Long‐term follow‐up of physical activity behavior in older adults. Health Psychol. 2007; 26:375-380 [DOI] [PubMed] [Google Scholar]
- King AC, Taylor CB, Haskell WL, DeBusk RF. Strategies for increasing early adherence to and long‐term maintenance of home‐based exercise training in healthy middle‐aged men and women. Am J Cardiol. 1988; 61:628-632 [DOI] [PubMed] [Google Scholar]
- Chao D, Foy CG, Farmer D. Exercise adherence among older adults: challenges and strategies. Control Clin Trials. 2000; 21:212S-217S [DOI] [PubMed] [Google Scholar]
- Artinian NT, Fletcher GF, Mozaffarian D, Kris‐Etherton P, Van HL, Lichtenstein AH, Kumanyika S, Kraus WE, Fleg JL, Redeker NS, Meininger JC, Banks J, Stuart‐Shor EM, Fletcher BJ, Miller TD, Hughes S, Braun LT, Kopin LA, Berra K, Hayman LL, Ewing LJ, Ades PA, Durstine JL, Houston‐Miller N, Burke LE. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: a scientific statement from the American Heart Association. Circulation. 2010; 122:406-441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizhakekuttu TJ, Gutterman DD, Phillips SA, Jurva JW, Arthur EI, Das E, Widlansky ME. Measuring FMD in the brachial artery: how important is QRS gating? J Appl Physiol. 2010; 109:959-965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamanoglu M, O'Rourke MF, Avolio AP, Kelly RP. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur Heart J. 1993; 14:160-167 [DOI] [PubMed] [Google Scholar]
- Pauca AL, O'Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001; 38:932-937 [DOI] [PubMed] [Google Scholar]
- Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998; 30:777-781 [DOI] [PubMed] [Google Scholar]
- Holubkov R, Karas RH, Pepine CJ, Rickens CR, Reichek N, Rogers WJ, Sharaf BL, Sopko G, Merz CN, Kelsey SF, McGorray SP, Reis SE. Large brachial artery diameter is associated with angiographic coronary artery disease in women. Am Heart J. 2002; 143:802-807 [DOI] [PubMed] [Google Scholar]
- Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow‐mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007; 115:2390-2397 [DOI] [PubMed] [Google Scholar]
- Chung WB, Hamburg NM, Holbrook M, Shenouda SM, Dohadwala MM, Terry DF, Gokce N, Vita JA. Brachial artery remodels to maintain local shear stress despite the presence of cardiovascular disease risk factors. Arterioscler Thromb Vasc Biol. 2009; 29:606-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroma EJ, Lee IM. Physical activity and cardiovascular health: lessons learned from epidemiological studies across age, gender, and race/ethnicity. Circulation. 2010; 122:743-752 [DOI] [PubMed] [Google Scholar]
- Zhao G, Li C, Ford ES, Fulton JE, Carlson SA, Okoro CA, Wen XJ, Balluz LS. Leisure‐time aerobic physical activity, muscle‐strengthening activity and mortality risks among US adults: the NHANES linked mortality study. Br J Sports Med. 2014; 48:244-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PT. Physical fitness and activity as separate heart disease risk factors: a meta‐analysis. Med Sci Sports Exerc. 2001; 33:754-761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattelmair J, Pertman J, Ding EL, Kohl HW, III, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease. Circulation. 2011; 124:789-795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IM, Sesso HD, Oguma Y, Paffenbarger RS., Jr Relative intensity of physical activity and risk of coronary heart disease. Circulation. 2003; 107:1110-1116 [DOI] [PubMed] [Google Scholar]
- Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA, Castaneda‐Sceppa C. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007; 116:1094-1105 [DOI] [PubMed] [Google Scholar]
- Murphy MH, Blair SN, Murtagh EM. Accumulated versus continuous exercise for health benefit: a review of empirical studies. Sports Med. 2009; 39:29-43 [DOI] [PubMed] [Google Scholar]
- Glazer NL, Lyass A, Esliger DW, Blease SJ, Freedson PS, Massaro JM, Murabito JM, Vasan RS. Sustained and shorter bouts of physical activity are related to cardiovascular health. Med Sci Sports Exerc. 2013; 45:109-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Masurier GC, Sidman CL, Corbin CB. Accumulating 10,000 steps: does this meet current physical activity guidelines? Res Q Exerc Sport. 2003; 74:389-394 [DOI] [PubMed] [Google Scholar]
- Tudor‐Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004; 34:1-8 [DOI] [PubMed] [Google Scholar]
- 10,000 Steps. Available at: http://www.shapeup.org/resources/10ksteps.html 2013. Accessed October 28, 2013.
- Araiza P, Hewes H, Gashetewa C, Vella CA, Burge MR. Efficacy of a pedometer‐based physical activity program on parameters of diabetes control in type 2 diabetes mellitus. Metabolism. 2006; 55:1382-1387 [DOI] [PubMed] [Google Scholar]
- White DK, Tudor‐Locke C, Felson DT, Gross KD, Niu J, Nevitt M, Lewis CE, Torner J, Neogi T. Walking to meet physical activity guidelines in knee osteoarthritis: is 10,000 steps enough? Arch Phys Med Rehabil. 2013; 94:711-717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NFkappaB, reduced IkappaBalpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell. 2008; 7:805-812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Donato AJ, LaRocca TJ, Eskurza I, Silver AE, Seals DR. Habitually exercising older men do not demonstrate age‐associated vascular endothelial oxidative stress. Aging Cell. 2011; 10:1032-1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone PH, Coskun AU, Kinlay S, Clark ME, Sonka M, Wahle A, Ilegbusi OJ, Yeghiazarians Y, Popma JJ, Orav J, Kuntz RE, Feldman CL. Effect of endothelial shear stress on the progression of coronary artery disease, vascular remodeling, and in‐stent restenosis in humans: in vivo 6‐month follow‐up study. Circulation. 2003; 108:438-444 [DOI] [PubMed] [Google Scholar]


