Introduction
Cardio‐oncology, a new clinical discipline focused on the cardiovascular care of cancer patients, represents a new avenue for interdisciplinary collaboration between clinical oncologists and cardiologists as well as between basic scientists and clinical investigators.(2013) Many of these collaborations have been driven by the emergence of novel targeted therapies that have revolutionized cancer treatment but also have the potential for cardiovascular toxicity.
The recent history of treatment for HER2 positive breast cancer is an early example of how unanticipated cardiotoxicity has led to collaborative efforts to address the problem. HER2, a proto‐oncogene encoding the HER2 receptor tyrosine kinase, is amplified in >20% of patients with breast cancer, resulting in HER2 receptor overexpression and an aggressive phenotype associated with high metastatic potential and short survival. The development of trastuzumab (Herceptin), a humanized monoclonal antibody targeting HER2, over the past 15 years has led to significant improvements in the survival of patients with HER2‐positive breast cancer. A pivotal study by Slamon et al demonstrated a clear benefit of trastuzumab in metastatic HER2+ breast cancer. However, there was an alarming rate of cardiotoxicity with 27% of patients who received trastuzumab concurrently with traditional chemotherapy (doxorubicin, cyclophosphamide) developing either symptomatic heart failure or asymptomatic cardiac dysfunction.(2001) As a result of these data, initial regulatory approval of trastuzumab for metastatic breast cancer was accompanied by caveats with respect to cardiac safety. Several requirements were instituted for therapy in practice. First, all patients would undergo frequent periodic monitoring of cardiac function with determination of left ventricular ejection fraction (EF) during the course of trastuzumab treatment. Second, trastuzumab would be administered sequentially after treatment with anthracycline‐based chemotherapy under the assumption that synergistic toxicity could result if the 2 agents were given concomitantly. These stipulations were not only applied to patient care but also to subsequent clinical trials involving trastuzumab, especially as this agent was being tested in the adjuvant setting, to increase the chance of disease‐free survival. In the oncology community, these initial observations with cardiotoxicity also led to a debate regarding the added value of anthracyclines for patients with curable, early‐stage HER2 positive breast cancer being treated with trastuzumab.(2012)
Perhaps as a result of close cardiac monitoring, subsequent clinical trials of trastuzumab in the adjuvant setting have demonstrated a lower incidence of cardiotoxicity, typically defined as either symptomatic congestive heart failure (CHF) or asymptomatic decline in left ventricular systolic function (cardiomyopathy). In large, randomized clinical trials, the incidence of symptomatic CHF ranged from 0.6% to 4.1% while the incidence of cardiomyopathy is higher at 3% to 19%.(2011)–(2007) The differences in the reported incidence may be related to several factors including differences in treatment regimens, differences in definitions of cardiomyopathy, and variation in the imaging modalities used to assess EF. Despite these sources of heterogeneity, it is clear that trastuzumab is associated with a small increased risk of clinical congestive heart failure and a larger increased risk of cardiomyopathy, especially on the background of anthracycline therapy. However, these risks are juxtaposed against the consistent finding in clinical trials that trastuzumab significantly decreases the risk of cancer recurrence and increases survival in women with HER2‐positive disease.
An important, unanswered question is the clinical significance of the cardiotoxicity associated with HER2‐targeted therapies. In clinical trials involving trastuzumab, the majority of patients who developed heart failure or cardiomyopathy improved clinically, and most experienced an improvement in EF although approximately one‐third of patients had some degree of persistent LV systolic dysfunction.(2011),(2007)–(2012) In two trials testing trastuzumab in the adjuvant setting, there were no cardiac deaths in the trastuzumab arms at a median follow‐up of 3.6 and 5.4 years, respectively.(2011),(2010) In addition, new‐onset cardiomyopathy can interrupt and influence the course of cancer therapy. In clinical trials, 2% to 8% of HER2‐positive breast cancer patients were not candidates for trastuzumab due to LV dysfunction or cardiac symptoms after completing an anthracycline‐containing regimen.(2011),(2012) Similarly, the development of cardiac events, many of them asymptomatic, during treatment with trastuzumab can lead to interruption or cessation of treatment in up to 16% of women with a proven survival benefit.(2007)–(2012)
Defining the exact short‐term and long‐term consequences of trastuzumab‐associated cardiomyopathy will be important as clinicians are forced to weigh the risks versus benefits of trastuzumab therapy in the breast cancer population. Moreover, accurate risk prediction tools are needed to assist clinicians in identifying patients at high risk of cardiotoxicity. Answering these questions in the context of a “real world” population is essential given that most oncology clinical trials enroll younger patients with no history of cardiovascular disease. In practice, over 40% of breast cancer patients are >65 years of age, many of whom carry the burden of traditional cardiovascular risk factors or have a history of cardiovascular disease.(2012)
In this issue of JAHA, Ezaz et al provide a risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer.(2014) The authors build on their previous work using the Surveillance, Epidemiology, and End Results (SEER) Medicare‐linked database where they identified 45 537 women who were 67 to 94 years of age (mean age 76) with early‐stage breast cancer. The adjusted 3‐year incidence of heart failure or cardiomyopathy was as high as 42% for patients receiving anthracycline and trastuzumab, 32% for patients receiving trastuzumab alone, compared to 18% with no adjuvant therapy.(2012) There are obvious limitations with such a retrospective approach, most of which are acknowledged by the authors. Outcomes data were based on administrative codes rather than a rigorous adjudication of events, which limits the discrimination between symptomatic and asymptomatic systolic dysfunction. The lack of data on ejection fraction (EF) also limits the distinction between systolic and diastolic heart failure, which may be important in an older population. The degree of surveillance for asymptomatic LV dysfunction was also not available given the nature of the dataset. The strength of this type of registry analysis is its large sample size and the fact that it is embedded within a “real world” population. Combined with the work of others, these data highlight a clear signal of increased cardiotoxicity in an older population with rates significantly higher than those reported in clinical trials.(2013) The long‐term prognosis of these patients remains unknown.
The current paper by Ezaz et al represents an important contribution to the understanding of risk factors for the development of heart failure or cardiomyopathy in this population. Using a subset of patients from the SEER Medicare database, the authors developed a clinical risk score based on 7 factors: age, adjuvant chemotherapy, coronary artery disease, atrial fibrillation or flutter, diabetes, hypertension, and renal failure. In a split sample design, the authors developed the risk score in half the cohort and then demonstrated internal validity with the other half. Using this risk score allowed stratification of patients into low‐, medium‐, and high‐risk groups. Validating this risk score in other cohorts or registries will be critical for demonstrating its generalizability. Nevertheless, the finding that pre‐existing cardiovascular disease and the presence of traditional risk factors incrementally increase the risk of trastuzumab‐associated heart failure is an important finding and contribution to the field. Although these main results are not surprising (based on clinical experience and lessons from studying other forms of heart failure), formally reporting these findings in the cardio‐oncology population is important for confirming observations made in practice. All efforts to expand the evidence base in cardio‐oncology will not only guide future practice, but will also help to inform the design of future clinical trials. This is especially important because, at this time, patients with HER2+ breast cancer are treated with combinations of HER2‐targeted therapies, which, in turn, may add to the risk of cardiotoxicity.
Several groups have previously reported risk factors for developing trastuzumab‐associated cardiomyopathy based on data from clinical trials testing the efficacy of trastuzumab in the adjuvant setting. In the Herceptin Adjuvant (HERA) trial, higher cumulative dose of doxorubicin, lower screening EF, and greater body mass index were associated with increased risk of clinical heart failure or cardiomyopathy.(2007) There was a nonsignificant trend towards higher risk with hypertension, smoking, and diabetes. Notably, this analysis involved a small number of patients with these risk factors, and the mean age of patients was 49 years. Similarly in another trial (NSABP‐31), age and EF were the only predictors of cardiotoxicity while the prevalence of co‐morbidities was also relatively low compared to that in the current study.(2012)
The current study should lead to further attempts by the cardio‐oncology community to improve cardiac risk stratification in patients with breast cancer. An increasing number of studies have indicated the utility of novel imaging parameters (eg, myocardial strain by echocardiography) and serum biomarkers (eg, troponin) on top of clinical risk factors in the prediction of cardiomyopathy in breast cancer patients prior to the onset of a reduction in EF.(2012)–(2013) Integrating clinical risk factors with novel imaging and serum biomarkers may ultimately provide the clinician with a comprehensive, robust risk prediction tool for all ages. A personalized risk score could influence a variety of decisions related to cancer therapy, such as whether to treat with an anthracycline, how frequently to conduct cardiac surveillance, and in which patients to initiate cardioprotective strategies (eg, beta blockers, ACE inhibitors) while continuing trastuzumab therapy (Figure). In an era of limited economic resources, such a risk tool may also have a high enough negative predictive value to identify a truly low‐risk population for whom no further intervention or surveillance is necessary. A carefully constructed comparative effectiveness study designed to capture both cardiovascular and oncologic outcomes would be necessary to validate such a tool. The current work by Ezaz et al is an important initial step towards a more patient‐centered approach in the assessment of cardiac risk during breast cancer therapy.
Figure 1.
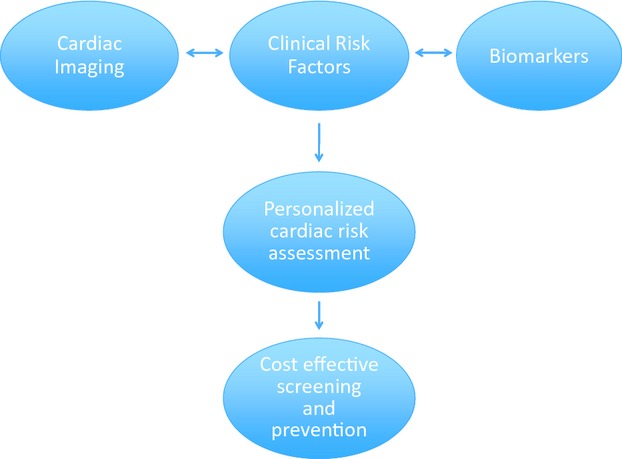
Proposed integrated cardiac risk assessment for patients undergoing breast cancer treatment.
Establishing risk prediction models for heart failure and cardiomyopathy becomes even more important when one considers the evolving landscape of treatment options for HER2 positive breast cancer. HER2 directed therapy now includes a diverse class of therapies including small molecular tyrosine kinase inhibitors targeting HER2 (such as lapatinib, afatinib, neratinib) and HER2 receptor dimerization inhibitors (such as pertuzumab). In addition, trastuzumab has been covalently linked to cytotoxic agents allowing more effective tumor cell targeting such as in the case of Trastuzumab emtansine (TDM‐1), which was recently approved for treatment of HER2+ metastatic breast cancer. Increasingly, these HER2‐targeted therapies are used in combination for the treatment of breast cancer, potentially increasing the risk of cardiotoxicity.(2010)–(2012) HER2 signaling promotes breast cancer proliferation though the RAS‐MAPK pathway and inhibits cell death through the phosphatidylinositol 3′‐kinase‐AKT‐mammalian target of rapamycin (mTOR) pathway. Inhibitors targeting these downstream pathways are being developed to both more potently inhibit HER2 signaling and/or develop ways of addressing resistance to trastuzumab.(2012) At the present time, the cardiac implications of these novel HER2‐targeted therapies are unknown.
The evolving narrative on cardiomyopathy in breast cancer patients treated with trastuzumab highlights the importance of a continued, close collaboration between oncologists and cardiologists. Further research into the mechanisms, risk factors, optimal methods of detection, and ultimately strategies for prevention will help to achieve the goal of optimizing both the cardiovascular and oncologic health in all of our patients.
Disclosures
None.
References
- Moslehi J, Cheng S. Cardio‐oncology: it takes two to translate. Sci Transl Med. 2013; 5:187fs20. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Leyland‐Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001; 344:783-792 [DOI] [PubMed] [Google Scholar]
- Burstein HJ, Piccart‐Gebhart MJ, Perez EA, Hortobagyi GN, Wolmark N, Albain KS, Norton L, Winer EP, Hudis CA. Choosing the best trastuzumab‐based adjuvant chemotherapy regimen: should we abandon anthracyclines? J Clin Oncol. 2012; 30:2179-2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, Pinter T, Valero V, Liu M‐C, Sauter G, Minckwitz von G, Visco F, Bee V, Buyse M, Bendahmane B, Tabah‐Fisch I, Lindsay M‐A, Riva A, Crown JBreast Cancer International Research Group Adjuvant trastuzumab in HER2‐positive breast cancer. N Engl J Med. 2011; 365:1273-1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, Tan‐Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2‐positive breast cancer. N Engl J Med. 2005; 353:1673-1684 [DOI] [PubMed] [Google Scholar]
- Suter TM, Procter M, van Veldhuisen DJ, Muscholl M, Bergh J, Carlomagno C, Perren T, Passalacqua R, Bighin C, Klijn JGM, Ageev FT, Hitre E, Groetz J, Iwata H, Knap M, Gnant M, Muehlbauer S, Spence A, Gelber RD, Piccart‐Gebhart MJ. Trastuzumab‐associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol. 2007; 25:3859-3865 [DOI] [PubMed] [Google Scholar]
- Romond EH, Jeong J‐H, Rastogi P, Swain SM, Geyer CE, Ewer MS, Rathi V, Fehrenbacher L, Brufsky A, Azar CA, Flynn PJ, Zapas JL, Polikoff J, Gross HM, Biggs DD, Atkins JN, Tan‐Chiu E, Zheng P, Yothers G, Mamounas EP, Wolmark N. Seven‐year follow‐up assessment of cardiac function in NSABP B‐31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node‐positive, human epidermal growth factor receptor 2‐positive breast cancer. J Clin Oncol. 2012; 30:3792-3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procter M, Suter TM, de Azambuja E, Dafni U, van Dooren V, Muehlbauer S, Climent MA, Rechberger E, Liu WT‐W, Toi M, Coombes RC, Dodwell D, Pagani O, Madrid J, Hall M, Chen S‐C, Focan C, Muschol M, van Veldhuisen DJ, Piccart‐Gebhart MJ. Longer‐term assessment of trastuzumab‐related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol. 2010; 28:3422-3428 [DOI] [PubMed] [Google Scholar]
- Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. 2012; 60:2504-2512 [DOI] [PubMed] [Google Scholar]
- Ezaz G, Long JB, Gross CP, Chen J. A risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Heart Assoc. 2014; 3:e00047210.1161/JAHA.113.000472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez‐MacGregor M, Zhang N, Buchholz TA, Zhang Y, Niu J, Elting L, Smith BD, Hortobagyi GN, Giordano SH. Trastuzumab‐related cardiotoxicity among older patients with breast cancer. J Clin Oncol. 2013; 31:4222-4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer‐Crosbie M. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012; 5:596-603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ky B, Putt M, Sawaya H, French B, Januzzi JL, Sebag IA, Plana JC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer‐Crosbie M. Early increases in multiple biomarkers predict subsequent cardiotoxicity in breast cancer patients treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2013. 10.1016/j.jacc.2013.10.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, Ellis C, Casey M, Vukelja S, Bischoff J, Baselga J, O'Shaughnessy J. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2‐positive, trastuzumab‐refractory metastatic breast cancer. J Clin Oncol. 2010; 28:1124-1130 [DOI] [PubMed] [Google Scholar]
- Baselga J, Cortés J, Kim S‐B, Im S‐A, Hegg R, Im Y‐H, Roman L, Pedrini JL, Pienkowski T, Knott A, Clark E, Benyunes MC, Ross G, Swain SMCLEOPATRA Study Group Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012; 366:109-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2‐positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2012; 9:16-32 [DOI] [PubMed] [Google Scholar]


