Abstract
Background
Electrocardiography has been considered an important tool in the management of Chagas disease (ChD) patients, although its value in elderly infected patients is unknown. This study was designed to investigate the prevalence and prognostic value of electrocardiographic abnormalities in Trypanosoma cruzi infected and noninfected older adults.
Methods and Results
We studied 1462 participants in Bambuí City, Brazil, with electrocardiogram (ECG) records classified by the Minnesota Code. Follow‐up time was 10 years; the endpoint was mortality. Adjustment for potential confounding variables included age, gender, conventional risk factors, and B‐type natriuretic peptide (BNP). The mean age was 69 years (60.9% women). The prevalence of ChD was 38.1% (n=557). ECG abnormalities were more frequent in ChD patients (87.6% versus 77.7%, P<0.001). Right bundle branch block (RBBB) with left anterior hemiblock (LAH) was strongly related to ChD (OR: 11.99 [5.60 to 25.69]). During the mean follow‐up time of 8.7 years, 556 participants died (253 with ChD), and only 89 were lost to follow‐up. ECG variables of independent prognostic value for death in ChD included absence of sinus rhythm, frequent ventricular and supraventricular premature beats, atrial fibrillation, RBBB, old and possible old myocardial infarction, and left ventricular hypertrophy. The presence of any major ECG abnormalities doubled the risk of death in ChD patients (HR: 2.18 [1.35 to 3.53]), but it also increased the risk in non‐ChD subjects (HR: 1.50 [1.07 to 2.10]); the risk of death increased with the number of major abnormalities in the same patient.
Conclusion
ECG abnormalities are more common among elderly Chagas disease patients and strongly predict adverse outcomes.
Keywords: Chagas disease, elderly, electrocardiography
Introduction
Chagas disease (ChD), caused by the protozoan Trypanosoma cruzi, is a systemic chronic infection that may result in cardiac and/or digestive disease in 20% to 40% of cases.1–2 It is endemic in Latin American countries, with about 8 to 10 million infected subjects, and is a leading cause of cardiomyopathy.1,3 As a consequence of immigration from endemic countries and international travel, ChD is now an emerging world health problem.4
In the last 2 decades, ChD control programs have been remarkably successful and interruption of disease transmission has been achieved in many Latin American countries.3 Despite the effectiveness of the control measures, the disease is likely to remain a public health problem for some decades among the elderly, through the operation of a cohort effect.5 Indeed, T. cruzi infection has been shown to be a strong independent predictor of mortality of all causes and stroke mortality among the elderly in areas where the transmission has been interrupted.6–7
Electrocardiography has been considered an important tool in the management of ChD patients,8 and the presence of typical electrocardiographic abnormalities is needed for the recognition of the cardiac chronic form of the disease,9 a condition that indicates the need for more complete diagnostic evaluation and closer follow‐up.10 Specific electrocardiographic abnormalities may eventually lead to specific therapeutic measures, as anticoagulation for atrial fibrillation patients and pacemaker implantation in advanced AV blocks.11 Information on ECG findings in the elderly is scanty and consists mainly of hospital‐ or ambulatory‐based cases series, with no evaluation of the prognostic value of the abnormalities.12–15 Most previous ECG longitudinal studies were performed a long time ago, with young or middle‐age samples of ChD subjects.16–18 Additionally, only one recent cross‐sectional study in middle‐aged adults uses core‐lab reading and classification by the internationally accepted Minnesota Code.8
In the present study, we used baseline data and 10 years of follow‐up of the Bambuí Cohort Study of Aging in Brazil19 to investigate prevalence and prognostic value of electrocardiographic abnormalities, evaluated at a core‐lab using the Minnesota Code, in both T. cruzi chronically infected and non‐infected elderly.
Methods
Study Population
The Bambuí cohort study of aging is an ongoing study conducted in Bambuí city (15 000 inhabitants), which is situated in the Southeast of Brazil. Bambuí is one of the oldest known endemic areas for ChD. Despite the successful interruption of the transmission of T. cruzi infection by 1970, the infection has remained highly prevalent in the elderly due to a cohort effect.5 Procedures used in the cohort study were described in detail elsewhere.5 Briefly, the baseline cohort population comprised all residents aged ≥60 years on January 1, 1997, who were identified by means of a complete census conducted in the city.5 Baseline data collection was performed from February to May, 1997, comprising standardized interviews, blood and clinical tests, and an electrocardiogram (ECG). Cohort members underwent annual follow‐up examinations in arranged clinic visits and death certificates were verified. Participants signed an informed consent and authorized death certificate verification. The Bambuí cohort study was approved by the Ethics Board of the Fundação Oswaldo Cruz, Brazil.
Mortality Data Source
Deaths from any cause occurring from the beginning of the study enrollment in 1997 until December 31, 2007 were included in this analysis. They were reported by next of kin during the annual follow‐up interview and ascertained through the Brazilian System of Information on Mortality, with the permission of the Ministry of Health. Death certificates were obtained for 98.9% of participants who died.
Electrocardiogram
At the baseline examination, a digitally recorded 12‐lead ECG (Hewlett Packard MI700A) was obtained at rest using standardized procedures. ECGs were analyzed by experienced cardiologists at the ECG Reading Center (EPICARE, Wake Forest University) and visually classified by the Minnesota Code (MC) criteria.20 QT interval was corrected to heart rate using the QT index (QTi), calculated as QTi=(QT/656)×(HR+100).21 In this study, major and minor ECG abnormalities were defined as set out in Prineas et al,22 modified to include ECG abnormalities typical of Chagas cardiomyopathy with prognostic significance, as frequent supraventricular or ventricular premature beats.23
Major ECG abnormalities included old myocardial infarction (MI) (major Q‐wave abnormalities [MC 1.1.x or 1.2.x]) or possible MI (minor Q‐waves abnormalities with ST segment or T‐wave abnormalities [1.3.x and 4.1.x, 4.2, 5.1, or 5.2]), complete intraventricular blocks (7.1, 7.2, 7.4, or 7.8), frequent supraventricular or ventricular premature beats (MC 8.1.x, except 8.1.4), major isolated ST segment or T‐wave abnormalities (MC 4.1.x, 4.2, 5.1 or 5.2), atrial fibrillation or flutter or supraventricular tachycardia (MC 8.3.x. or 8.4.2), other major arrhythmias (MC 8.2.x, except 8.2.1), major atrioventricular conduction abnormalities or pacemaker use (MC 6.1, 6.2.x, 6.4, 6.8, 8.6.1 or 8.6.2), major QT prolongation (>115%) and left ventricular hypertrophy (LVH) (MC 3.1 together with [4.1.x, 4.2, 5.1, or 5.2]).
Minor ECG abnormalities included minor isolated Q and QS waves (MC 1.3.x), minor isolated ST segment or T‐wave abnormalities (MC 4.3, 4.4, 5.3 or 5.4), high R waves (MC 3.x), ST segment elevation (MC 9.2.1), incomplete bundle branch blocks or left anterior hemiblock (MC 7.3, 7.6 or 7.7), minor QT prolongation (>111% and <116%), short PR interval (MC 6.5), left (MC 2.1) or right axis deviation (MC 2.2), first degree atrioventricular block (MC 6.3), wandering atrial pacemaker (MC 8.1.4), persistent supraventricular rhythm (MC 8.4.1), and high P‐wave amplitude (MC 9.3).
Trypanosoma cruzi Infection Status and B‐Type Natriuretic Peptide Assay
Diagnosis of chronic ChD relies on serologic methods. Infection with T. cruzi was defined by seropositivity in 3 examinations, a hemaglutination assay (Bio‐Mérieux, France), and 2 enzyme‐linked immunoabsorbent assays (Abbott and Viener). B‐type natriuretic peptide (BNP) was measured using a microparticle‐based immunoassay (MEIA/AxSYM; Abbott).24
Other Baseline Information
Other variables in this study included age, gender, traditional cardiovascular risk factors, body mass index, serum creatinine, anti‐hypertensive medication, and digoxin medication. The cardiovascular risk factors evaluated were (yes=1, no=0): current smoking, systolic blood pressure ≤145 mmHg (upper tertile in the study population), total cholesterol/high‐density lipoprotein (HDL) ratio ≥5.52 mmol/L (upper tertile), and diabetes mellitus. Current smokers were those who had smoked at least 100 cigarettes during their lifetime and were still smokers. Systolic blood pressure was defined as the mean of 2 out of 3 measures using standard protocols.25 Diabetes mellitus was defined as a fasting blood glucose level ≥126 mg/dL and/or current use of insulin or oral antidiabetic drug treatment. Body mass index (BMI) was defined as the weight divided by the squared height. Fasting total cholesterol, HDL cholesterol, blood glucose, and serum creatinine were determined with standardized enzymatic methods. Current medication use was ascertained during the interview by reviewing prescriptions and/or the medication packaging, and they were classified as described elsewhere.26 No participants reported previous use of antitrypanosomal drugs, so this variable was not considered in the present analysis.
Statistical Analysis
Statistical analyses were conducted using SPSS 18.0 statistical software. A P value of 0.05 was considered statistically significant and all P values are 2‐tailed. To assess the relation between specific ECG findings and chronic T. cruzi infection, unadjusted and adjusted odds ratios were estimated by logistic regression. The analyses were based on 3 models. First, we estimated the crude association between chronic T. cruzi infection and the ECG findings (model 1), and then adjusted for age (continuous) and gender (model 2) and for classical cardiovascular risk factors, including hypertension, diabetes mellitus, current smoking, systolic blood pressure, total cholesterol/HDL ratio, and serum creatinine (model 3). Simple linear regression was used to evaluate changes in major ECG abnormality prevalence by variation of age (in years) in both T. cruzi infected and non‐infected groups. Comparison of linear regression slopes between groups was made with the appropriate test.27
To examine the unadjusted association between ECG findings and all‐cause mortality, cumulative survival curves were computed using Kaplan‐Meier estimates. Hazard ratios (HR) and 95% confidence intervals for mortality were estimated using the Cox proportional hazards model after confirming that the assumption of proportionality was met. The analyses were also based on 4 models, for each ECG abnormality but also for the presence of major and minor ECG abnormalities and the number of major ECG abnormalities in each subject. First we estimated the crude association between ECG findings and mortality (model 1), and then adjusted incrementally for age (continuous) and gender (model 2); for age, gender, and classical cardiovascular risk factors (see above, model 3); and for model 3 plus BNP levels (model 4). We retained all variables in the fully adjusted model because no pairs exhibited significant collinearity.
Results
Study Participants
From 1606 baseline participants, 1496 performed both serological tests for T. cruzi and ECG. From these, 34 had inconclusive results for T. cruzi and/or the quality of the ECG tracing was insufficient for interpretation and were excluded from the present analysis.
Among the 1462 participants in this study, the mean age was 69 years, women predominating (60.9%). The prevalence of T. cruzi infection was 38.1% (n=557). Baseline characteristics of the study participants according to the serological status group are shown in Table 1. In comparison with non‐infected subjects, ChD subjects were slightly older, with a lower proportion of males and diabetics, used digoxin more frequently, had lower values of body mass index, systolic blood pressure, and cholesterol/HDL ratio, but higher HDL cholesterol and BNP values.
Table 1.
Baseline Characteristics of the Study Participants According to the Serological Status Group, The Bambuí Cohort Study of Aging, 1997–2007
| Characteristics | Chagas Disease (n=557) | Non‐Chagas Disease (n=905) | P Value |
|---|---|---|---|
| Age, y | 68 (64 to 74) | 67 (63 to 73) | 0.041 |
| Male gender | 181 (32.5) | 391 (43.2) | <0.001 |
| Systolic blood pressure, mmHg | 133 (119 to 149) | 136 (124 to 161) | 0.003 |
| Treatment for hypertension | 285 (51.2) | 457 (50.5) | 0.829 |
| Diabetes | 57 (10.3) | 152 (16.8) | 0.001 |
| Smoking | 98 (17.6) | 166 (18.3) | 0.727 |
| Digoxin usage | 124 (22.3) | 100 (11) | <0.001 |
| Body mass index, kg2/m | 23.9 (20.8 to 27.3) | 25.4 (22.3 to 28.3) | <0.001 |
| Creatinine, mg/dL | 0.85 (0.75 to 0.97) | 0.85 (0.745 to 0.99) | 0.787 |
| Cholesterol (total), mg/dL | 229 (200 to 266) | 227 (200 to 262) | 0.617 |
| HDL cholesterol, mg/dL | 48.5 (40 to 58) | 46 (38 to 56) | 0.001 |
| Cholesterol/HDL ratio | 4.8 (3.68) | 4.98 (5.96) | 0.017 |
| BNP, pg/mL | 119.5 (63 to 206.5) | 64 (35 to 112) | <0.001 |
Data is presented as median (interquartile range) or numbers (%). Figures in bold represent significant P values. BNP indicates B‐type natriuretic peptide; HDL, high‐density lipoprotein.
Prevalence of ECG Abnormalities
ECG abnormalities were more frequently found in ChD patients (87.6% versus 77.7%, P<0.001). ECG abnormalities significantly associated with T. cruzi infection in the whole sample were sinus bradycardia, frequent supraventricular or ventricular premature beats, atrial fibrillation, right bundle branch block (RBBB) (complete and incomplete), left anterior hemiblock, first‐degree AV block, and prolonged QT interval (Table 2). RBBB, particularly when associated with left anterior hemiblock (LAH) (Figure 1), was strongly associated with the presence of ChD (OR: 11.99 [5.60 to 25.69]). RBBB and/or LAH occurred in 40% of the ChD participants and in only 8% of the non‐ChD elderly.
Table 2.
Association Between ECG Findings and ChD in the Baseline, The Bambuí Cohort Study, 1997–2007
| ECG Variables | N (%) | OR (95% CI) | ||||
|---|---|---|---|---|---|---|
| All Subjects (n=1462) | ChD (n=557) | Non ChD (n=905) | Model 1: Unadjusted | Model 2: Adjusted for Age and Gender | Model 3: Adjusted for Clinical Variables* | |
| Rhythm | ||||||
| Sinus rhythm | 1184 (81.0) | 395 (70.9) | 789 (87.2) | 0.36 (0.27 to 0.47) | 0.35 (0.27 to 0.47) | 0.34 (0.26 to 0.45) |
| Sinus tachycardia | 31 (2.1) | 10 (1.8) | 21 (2.3) | 0.77 (0.36 to 1.65) | 0.74 (0.34 to 1.58) | 0.86 (0.39 to 1.86) |
| Sinus bradycardia | 51 (3.5) | 28 (5.0) | 23 (2.5) | 2.03 (1.16 to 3.56) | 2.35 (1.33 to 4.16) | 2.09 (1.17 to 3.74) |
| Frequent VPB | 93 (6.4) | 56 (10.1) | 37 (4.1) | 2.62 (1.71 to 4.03) | 2.70 (1.75 to 4.16) | 2.89 (1.86 to 4.49) |
| Frequent SPB | 110 (7.5) | 60 (10.8) | 50 (5.5) | 2.06 (1.40 to 3.05) | 1.98 (1.33 to 2.95) | 1.97 (1.32 to 2.96) |
| Atrial fibrillation or flutter | 51 (3.5) | 34 (6.1) | 17 (1.9) | 3.40 (1.89 to 6.14) | 3.42 (1.88 to 6.22) | 3.43 (1.87 to 6.32) |
| Pacemaker | 6 (0.4) | 6 (1.1) | 0 | — | — | — |
| Intraventricular block | ||||||
| LBBB | 36 (2.5) | 18 (3.2) | 18 (2.0) | 1.65 (0.85 to 3.19) | 1.60 (0.82 to 2.13) | 1.58 (0.80 to 3.11) |
| RBBB | 159 (10.9)* | 129 (23.2)* | 30 (3.3)* | 8.79 (5.81 to 13.30) | 9.42 (6.19 to 14.33) | 9.69 (6.34 to 14.82) |
| LAH+RBBB | 59 (4.0) | 51 (9.2) | 8 (0.9) | 11.30 (5.32 to 24.0) | 11.82 (5.54 to 25.21) | 11.99 (5.60 to 25.69) |
| LAH | 77 (5.3) | 43 (7.7) | 34 (3.8) | 2.14 (1.35 to 3.40) | 2.39 (1.49 to 3.84) | 2.36 (1.47 to 3.80) |
| Incomplete LBBB | 76 (5.2) | 24 (4.3) | 52 (5.7) | 0.74 (0.45 to 1.21) | 0.77 (0.47 to 1.27) | 0.79 (0.48 to 1.32) |
| Incomplete RBBB | 53 (3.6) | 31 (5.6) | 22 (2.4) | 2.37 (1.36 to 4.13) | 2.33 (1.33 to 4.07) | 2.18 (1.24 to 3.83) |
| Atrioventricular block | ||||||
| First degree | 55 (3.8) | 38 (6.8) | 17 (1.9) | 3.83 (2.14 to 6.85) | 3.85 (2.14 to 6.92) | 3.77 (2.09 to 6.81) |
| Second degree | 0 | 0 | 0 | — | — | — |
| Third degree | 4 (0.3) | 3 (0.5) | 1 (0.1) | 4.90 (0.51 to 47.18) | 5.39 (0.55 to 52.74) | 6.04 (0.61 to 60.20) |
| Ischemic | ||||||
| Old MI and possible old MI | 62 (4.2) | 33 (5.9) | 29 (3.2) | 1.90 (1.14 to 3.17) | 1.96 (1.17 to 3.27) | 2.05 (1.21 to 3.46) |
| Major isolated ST‐T abnormalities | 181 (12.4) | 63 (11.3) | 118 (13.0) | 0.85 (0.61 to 1.18) | 0.78 (0.56 to 1.08) | 0.81 (0.58 to 1.14) |
| Minor isolated ST‐T abnormalities | 784 (53.6) | 266 (47.8) | 518 (57.2) | 0.68 (0.55 to 0.84) | 0.59 (0.47 to 0.74) | 0.61 (0.48 to 0.76) |
| ST segment elevation | 19 (1.3) | 5 (0.9) | 14 (1.5) | 0.58 (0.21 to 1.61) | 0.74 (0.26 to 2.09) | 0.71 (0.25 to 2.03) |
| Other | ||||||
| LVH | 46 (3.1) | 11 (2.0) | 35 (3.9) | 0.50 (0.25 to 0.99) | 0.50 (0.25 to 1.0) | 0.55 (0.27 to 1.10) |
| Short PR interval | 7 (0.5) | 5 (0.9) | 2 (0.2) | 4.09 (0.79 to 21.15) | 5.03 (0.96 to 26.27) | 4.26 (0.81 to 22.28) |
| Major QT prolongation | 53 (3.6) | 33 (5.9) | 19 (2.1) | 2.65 (1.55 to 4.52) | 2.93 (1.64 to 5.23) | 2.82 (1.57 to 5.05) |
| Minor QT prolongation | 59 (4.0) | 36 (6.5) | 23 (2.5) | 2.94 (1.65 to 5.22) | 2.49 (1.45 to 4.27) | 2.71 (1.57 to 4.68) |
| Low QRS amplitude | 30 (2.1) | 14 (2.5) | 16 (1.8) | 1.43 (0.69 to 2.96) | 1.27 (0.61 to 2.63) | 1.02 (0.48 to 2.17) |
Figures in bold represent significant values. ChD indicates Chagas disease; LAH, left anterior hemiblock; LBBB, left bundle branch block; LVH, left ventricular hypertrophy; major QT prolongation, QTi≥116%; MI, myocardial infarction; minor QT prolongation, QTi≥112%; QTi, QT prolongation index: (QT/656)×(HR+100); RBBB, right bundle branch block; SPB, supraventricular premature beats; VBP, ventricular premature beats.
Adjusted for age, gender, diabetes mellitus, current smoking, systolic blood pressure, treatment for hypertension, ratio of total cholesterol to high‐density lipoprotein cholesterol and serum creatinine.
LAH+RBBB cases are included in RBBB count.
Figure 1.
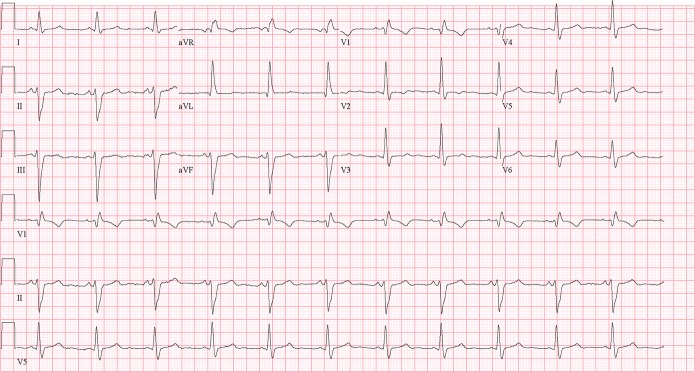
A typical ECG of a Chagas disease subject, showing right bundle branch block and left anterior hemiblock.
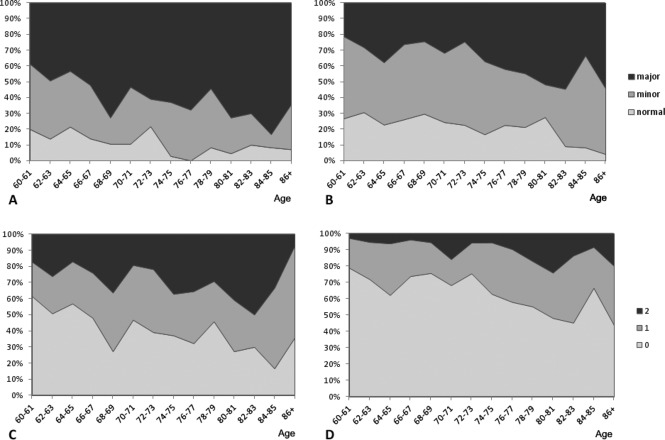
Table 3 shows the prevalence of major and minor abnormalities in both ChD and non‐ChD elderly subjects, as well as the number of participants with major ECG abnormalities. The prevalence and the number of major abnormalities are significantly higher in ChD participants (P<0.001). There is a trend of reduction of the proportion of elderly participants with normal ECG with the advancing of age both in infected and non‐infected subjects, with a concomitant increase of the prevalence of major ECG abnormalities and with participants with ≥2 major abnormalities (Figure 2). The regression slope of the prevalence of major ECG abnormalities by age (in years, from 60 to 88 years or more) was 0.016 (95% CI 0.009 to 0.023) for the T. cruzi‐infected group and 0.014 (95% CI 0.007 to 0.020) for the non‐infected group (P value for comparison of regression slopes=0.59), suggesting a similar rate of development of definite cardiopathy in both groups with albeit higher levels among the ChD group at each age level (Figure 3).
Table 3.
Prevalence of ECG Abnormalities According to the Classification of Severity and Number of Major Abnormalities in Chagas Disease and Non‐Chagas Disease Elderly Patients in the Baseline of The Bambuí Cohort Study, 1997–2007
| ECG Abnormalities | Chagas Disease (n=557) | Non‐Chagas Disease (n=905) | P Value |
|---|---|---|---|
| Classified according to severity | |||
| Normal ECG | 73 (13.1) | 219 (24.2) | <0.001 |
| Minor abnormality | 172 (30.9) | 398 (44.0) | |
| Major abnormality | 312 (56.0) | 288 (31.8) | |
| Classified by the number of major abnormalities | |||
| 0 | 245 (44.0) | 617 (68.2) | <0.001 |
| 1 | 167 (30.0) | 218 (24.1) | |
| 2 or more | 145 (26.0) | 70 (7.7) | |
Data expressed by numbers (%).
Figure 2.
Proportion of ECG abnormalities according to age at baseline in Chagas disease patients (A) and non‐infected participants (B). Proportion of number of major ECG abnormalities (0, 1, 2, or more) according to age at baseline in Chagas disease patients (C) and non‐infected participants (D).
Figure 3.
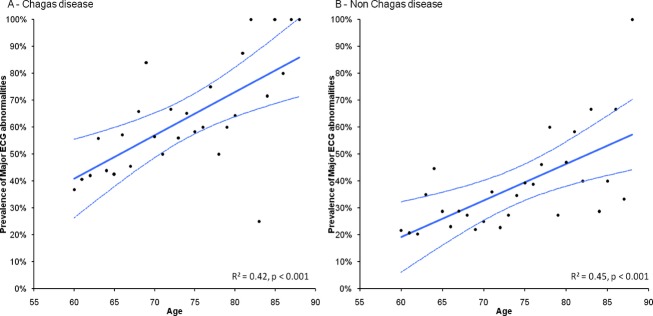
Regression lines of prevalence of major ECG abnormalities by age (in years, varying from 60 to 88 or more) in Chagas disease group (A) and non‐Chagas disease group (B).
Prognostic Significance of ECG Abnormalities
The mean follow‐up time was 8.7 years (median time 11.0 years). During this period, 556 participants died, and only 89 were lost to follow‐up, resulting in a total of 12 795 person‐years of observation. Of the total number of deaths, 253 were in ChD subjects (45%). Several ECG variables were independently associated with increased risk of death in ChD patients (Table 4), including frequent ventricular and supraventricular premature beats, atrial fibrillation, RBBB, possible old MI, major isolated ST‐T abnormalities and LVH; sinus rhythm is related to lower risk of death. Major and minor QT interval prolongation, ST elevation, and low QRS amplitude were related to higher risk of death in univariate analysis, but did not maintain their significance in the fully adjusted model.
Table 4.
Prognostic Value of ECG Findings in Chagas Disease Elderly Patients (n=557) for Mortality, The Bambuí Cohort Study, 1997–2007
| ECG Variables | HR (95% CI) | |||
|---|---|---|---|---|
| Unadjusted | Adjusted for Age and Gender | Adjusted for Clinical Variables* | Fully Adjusted Model* | |
| Rhythm | ||||
| Sinus rhythm | 0.36 (0.28 to 0.47) | 0.42 (0.33 to 0.54) | 0.44 (0.34 to 0.56) | 0.44 (0.34 to 0.56) |
| Sinus tachycardia | 1.56 (0.69 to 3.50) | 1.36 (0.61 to 3.07) | 1.45 (0.64 to 3.27) | 1.52 (0.67 to 3.44) |
| Sinus bradycardia | 1.24 (0.72 to 2.13) | 1.22 (0.71 to 2.10) | 1.10 (0.63 to 1.94) | 1.10 (0.63 to 1.94) |
| Frequent VPB | 1.92 (1.36 to 2.72) | 2.16 (1.53 to 3.06) | 2.15 (1.51 to 3.06) | 2.15 (1.51 to 3.06) |
| Frequent SPB | 2.24 (1.60 to 3.13) | 1.66 (1.18 to 2.38) | 1.64 (1.15 to 2.32) | 1.57 (1.10 to 2.24) |
| Atrial fibrillation or flutter | 2.84 (1.91 to 4.21) | 2.35 (1.57 to 3.50) | 2.38 (1.54 to 3.68) | 2.35 (1.53 to 3.62) |
| Intraventricular block | ||||
| LBBB | 1.60 (0.88 to 2.93) | 1.24 (0.68 to 2.28) | 1.11 (0.60 to 2.06) | 1.08 (0.58 to 2.00) |
| RBBB | 1.55 (1.18 to 2.03) | 1.52 (1.16 to 2.00) | 1.48 (1.12 to 1.96) | 1.48 (1.12 to 1.96) |
| LAH+RBBB | 1.40 (0.95 to 2.06) | 1.34 (0.91 to 1.98) | 1.34 (0.90 to 1.99) | 1.36 (0.91 to 2.02) |
| LAH | 1.32 (0.87 to 2.02) | 1.16 (0.76 to 1.76) | 1.18 (0.77 to 1.81) | 1.38 (0.89 to 2.13) |
| Incomplete LBBB | 1.28 (0.73 to 2.24) | 1.55 (0.89 to 2.72) | 1.44 (0.81 to 2.55) | 1.49 (0.84 to 2.63) |
| Incomplete RBBB | 1.16 (0.70 to 1.93) | 1.20 (0.72 to 1.99) | 1.23 (0.74 to 2.05) | 1.25 (0.75 to 2.09) |
| Atrioventricular block | ||||
| First degree | 1.36 (0.87 to 2.13) | 1.20 (0.77 to 1.88) | 1.10 (0.70 to 1.74) | 1.01 (0.63 to 1.62) |
| Third degree or pacemaker | 1.66 (0.74 to 3.74) | 1.52 (0.67 to 3.42) | 1.07 (0.46 to 2.47) | 0.97 (0.42 to 2.26) |
| Ischemic | ||||
| Old MI and possible old MI | 2.21 (1.44 to 3.40) | 1.83 (1.23 to 2.90) | 1.84 (1.19 to 2.85) | 1.88 (1.22 to 2.91) |
| Major isolated ST‐T abnormalities | 1.27 (0.88 to 1.84) | 1.40 (0.96 to 2.03) | 1.50 (1.03 to 2.19) | 1.38 (0.94 to 2.03) |
| Minor isolated ST‐T abnormalities | 0.86 (0.67 to 1.10) | 0.81 (0.62 to 1.04) | 0.83 (0.64 to 1.08) | 0.80 (0.62 to 1.04) |
| ST segment elevation | 2.80 (1.04 to 7.52) | 3.53 (1.31 to 9.51) | 2.97 (1.09 to 8.12) | 2.63 (0.95 to 7.24) |
| Other | ||||
| LVH | 2.80 (1.44 to 5.46) | 1.89 (0.97 to 3.71) | 1.84 (0.93 to 3.62) | 2.06 (1.04 to 4.08) |
| Short PR interval | 0.34 (0.05 to 2.45) | 0.43 (0.06 to 3.09) | 0.42 (0.06 to 2.99) | 0.39 (0.05 to 2.81) |
| Major QT prolongation | 2.06 (1.34 to 3.16) | 1.62 (1.05 to 2.50) | 1.52 (0.98 to 2.37) | 1.40 (0.89 to 2.20) |
| Minor QT prolongation | 1.60 (1.05 to 2.44) | 1.59 (1.04 to 2.42) | 1.47 (0.96 to 2.26) | 1.46 (0.95 to 2.24) |
| Low QRS amplitude | 2.14 (1.01 to 4.16) | 1.80 (0.92 to 3.51) | 1.77 (0.87 to 3.64) | 1.86 (0.90 to 3.81) |
Figures in bold represent significant values. BNP indicates B‐type natriuretic peptide; LAH, left anterior hemiblock; LBBB, left bundle branch block; LVH, left ventricular hypertrophy; major QT prolongation, QTi>115%; MI, myocardial infarction; minor QT prolongation, QTi>111%; QTi, QT prolongation index: (QT/656)×(HR+100); RBBB, right bundle branch block; SPB, supraventricular premature beats; VBP, ventricular premature beats.
Adjusted for age, gender, diabetes mellitus, current smoking, systolic blood pressure, treatment for hypertension, ratio of total cholesterol to high‐density lipoprotein cholesterol and serum creatinine.
Adjusted for variables in * and BNP levels.
Atrial fibrillation, incomplete left bundle branch block, possible old MI, major isolated ST‐T abnormalities and LVH were independently related to a higher risk of death in the non‐infected population (Table 5).
Table 5.
Prognostic Value of ECG Findings in Non‐Infected Elderly Patients (n=905) for Mortality, The Bambuí Cohort Study, 1997–2007
| ECG Variables | HR (95% CI) | |||
|---|---|---|---|---|
| Unadjusted | Adjusted for Age and Gender | Adjusted for Clinical Variables* | Fully Adjusted Model* | |
| Rhythm | ||||
| Sinus rhythm | 0.56 (0.42 to 0.75) | 0.77 (0.57 to 1.03) | 0.83 (0.61 to 1.13) | 0.86 (0.63 to 1.17) |
| Sinus tachycardia | 1.37 (0.71 to 2.66) | 1.57 (0.81 to 3.05) | 1.20 (0.61 to 2.35) | 1.29 (0.65 to 2.53) |
| Sinus bradycardia | 0.58 (0.24 to 1.41) | 0.59 (0.24 to 1.43) | 0.70 (0.29 to 1.71) | 0.71 (0.29 to 1.74) |
| Frequent VPB | 1.23 (0.72 to 2.10) | 1.00 (0.58 to 1.71) | 0.97 (0.56 to 1.66) | 0.98 (0.57 to 1.69) |
| Frequent SPB | 1.70 (1.13 to 2.57) | 1.20 (0.79 to 1.82) | 0.98 (0.63 to 1.53) | 1.01 (0.65 to 1.57) |
| Atrial fibrillation or flutter | 3.73 (2.14 to 6.51) | 2.24 (1.28 to 3.93) | 2.39 (1.35 to 4.21) | 1.92 (1.05 to 3.51) |
| Intraventricular block | ||||
| LBBB | 1.83 (0.94 to 3.54) | 1.69 (0.87 to 3.28) | 1.26 (0.60 to 2.66) | 1.55 (0.78 to 3.08) |
| RBBB | 1.48 (0.85 to 2.58) | 0.86 (0.49 to 1.51) | 1.04 (0.59 to 1.82) | 1.08 (0.61 to 1.90) |
| LAH+RBBB | 2.44 (1.01 to 5.92) | 1.07 (0.44 to 2.61) | 1.32 (0.54 to 3.23) | 1.36 (0.56 to 3.33) |
| LAH | 1.15 (0.66 to 2.00) | 0.70 (0.40 to 1.22) | 0.59 (0.33 to 1.03) | 0.57 (0.32 to 1.01) |
| Incomplete LBBB | 2.22 (1.51 to 3.25) | 2.25 (1.53 to 3.31) | 2.13 (1.44 to 3.14) | 2.07 (1.39 to 3.07) |
| Incomplete RBBB | 1.21 (0.62 to 2.35) | 1.19 (0.72 to 1.91) | 1.32 (0.68 to 2.56) | 1.45 (0.72 to 2.94) |
| Atrioventricular block | ||||
| First degree | 1.63 (0.81 to 3.30) | 1.74 (0.86 to 3.51) | 1.96 (0.96 to 3.99) | 2.00 (0.98 to 4.08) |
| Ischemic | ||||
| Old MI and possible old MI | 2.48 (1.56 to 3.95) | 2.20 (1.38 to 3.51) | 2.03 (1.26 to 3.26) | 1.91 (1.18 to 3.07) |
| Major isolated ST‐T abnormalities | 1.70 (1.27 to 2.28) | 1.64 (1.22 to 2.20) | 1.37 (1.01 to 1.87) | 1.30 (0.94 to 2.03) |
| Minor isolated ST‐T abnormalities | 1.10 (0.88 to 1.39) | 1.20 (0.95 to 1.52) | 1.11 (0.87 to 1.42) | 1.07 (0.84 to 1.37) |
| ST segment elevation | 1.27 (0.52 to 3.07) | 1.50 (0.61 to 3.66) | 1.71 (0.70 to 4.21) | 1.61 (0.65 to 3.97) |
| Other | ||||
| LVH | 2.30 (1.47 to 3.58) | 1.94 (1.25 to 3.02) | 1.66 (1.05 to 2.63) | 1.62 (1.02 to 2.56) |
| Major QT prolongation | 1.39 (0.69 to 2.80) | 1.51 (0.75 to 3.04) | 1.65 (0.81 to 3.34) | 1.71 (0.84 to 3.47) |
| Minor QT prolongation | 2.35 (1.37 to 4.02) | 1.22 (0.70 to 2.13) | 0.97 (0.54 to 1.75) | 0.96 (0.53 to 1.73) |
| Low QRS amplitude | 1.22 (0.54 to 2.73) | 1.01 (0.45 to 2.28) | 0.87 (0.38 to 1.98) | 0.53 (0.20 to 1.42) |
Figures in bold represent significant values. BNP indicates B‐type natriuretic peptide; LAH, left anterior hemiblock; LBBB, left bundle branch block; LVH, left ventricular hypertrophy; major QT prolongation, QTi>115%; MI, myocardial infarction; minor QT prolongation, QTi>111%; QTi, QT prolongation index: (QT/656)×(HR+100); RBBB, right bundle branch block; SPB, supraventricular premature beats; VBP, ventricular premature beats.
Adjusted for age, gender, diabetes mellitus, current smoking, systolic blood pressure, treatment for hypertension, ratio of total cholesterol to high‐density lipoprotein cholesterol and serum creatinine.
Adjusted for variables in * and BNP levels.
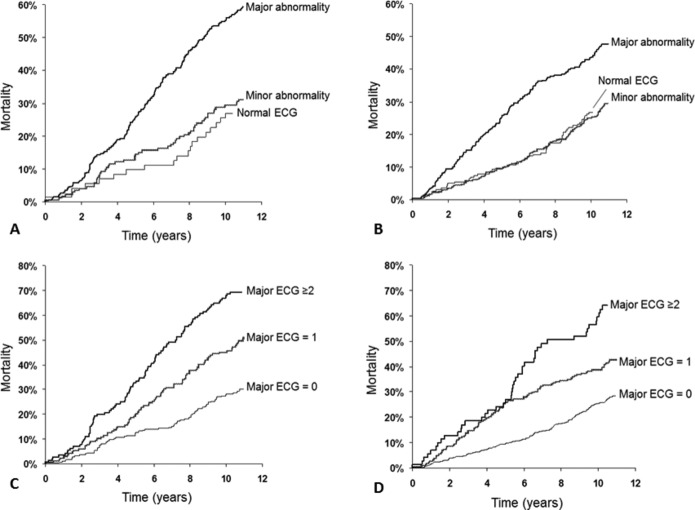
The presence of any major ECG abnormalities doubles the risk of death in ChD patients (HR: 2.18 [1.35 to 3.53]), but it also increases the risk in non‐ChD participants (HR: 1.50 [1.07 to 2.10], see Table 6). Those ChD patients with normal ECG or with minor abnormalities did not show increased risk of death when compared with the non‐infected population. The risk of death increases with the number of major abnormalities in the same patient both in ChD and in non‐ChD participants (Table 6). Figure 4 graphically shows the survival curve of ChD and non‐ChD participants according to the severity of ECG abnormalities and number of major ECG abnormalities in each subject.
Table 6.
Prognostic Value of ECG Findings in Chagas Disease and Non‐Chagas Disease Elderly Patients According to the Classification of Severity and Number of Major ECG Abnormalities for Mortality, The Bambuí Cohort Study, 1997–2007
| ECG | HR (95% CI) | |||
|---|---|---|---|---|
| Unadjusted | Adjusted for Age and Gender | Adjusted for Clinical Variables* | Fully Adjusted Model* | |
| Classified according to severity | ||||
| Chagas disease (n=557) | ||||
| Normal ECG | 1 | 1 | 1 | 1 |
| Minor abnormality | 1.22 (0.72 to 2.05) | 1.19 (0.70 to 2.02) | 1.15 (0.67 to 1.95) | 1.10 (0.65 to 1.87) |
| Major abnormality | 2.93 (1.82 to 4.70) | 2.50 (1.55 to 4.03) | 2.31 (1.43 to 3.74) | 2.18 (1.35 to 3.53) |
| Non‐Chagas disease (n=905) | ||||
| Normal ECG | 1 | 1 | 1 | 1 |
| Minor abnormality | 1.09 (0.79 to 1.51) | 1.08 (0.78 to 1.49) | 1.04 (0.74 to 1.44) | 1.01 (0.72 to 1.41) |
| Major abnormality | 2.17 (1.59 to 2.97) | 1.77 (1.29 to 2.44) | 1.56 (1.12 to 2.18) | 1.50 (1.07 to 2.10) |
| Classified by the number of major abnormalities | ||||
| T. cruzi infected (n=557) | ||||
| 0 | 1 | 1 | 1 | 1 |
| 1 | 1.98 (1.44 to 2.71) | 1.67 (1.22 to 2.30) | 1.56 (1.13 to 2.16) | 1.53 (1.11 to 2.12) |
| 2 or more | 3.39 (2.49 to 4.60) | 3.01 (2.21 to 4.09) | 2.88 (2.11 to 3.93) | 2.78 (2.03 to 3.79) |
| Non Trypanosoma cruzi infected (n=905) | ||||
| 0 | 1 | 1 | 1 | 1 |
| 1 | 1.77 (1.37 to 2.28) | 1.56 (1.21 to 2.02) | 1.43 (1.09 to 1.86) | 1.37 (1.04 to 1.80) |
| 2 or more | 3.08 (2.21 to 4.30) | 2.05 (1.45 to 2.89) | 1.83 (1.28 to 2.62) | 1.83 (1.28 to 2.63) |
Figures in bold represent significant values. BNP indicates B‐type natriuretic peptide.
Adjusted for age, gender, diabetes mellitus, current smoking, systolic blood pressure, treatment for hypertension, ratio of total cholesterol to high‐density lipoprotein cholesterol and serum creatinine.
Adjusted for variables in * and BNP levels.
Figure 4.
Cumulative incidence rates of mortality in Chagas disease patients and non‐infected participants according to ECG abnormalities (respectively, A and B) according to the number of major ECG abnormalities (respectively, C and D).
Discussion
This large, population‐based, cohort study, with long‐term follow‐up and adjustment for co‐variables, provides unique information about the prevalence and prognostic value of ECG abnormalities in ChD community‐dwelling elderly. One of the most important findings of our study is the high frequency of ECG abnormalities in T. cruzi‐infected elderly: 86.9% of ChD patients had any ECG abnormality, 56.0% with at least 1 major and 26.0% with 2 or more major ECG abnormalities (versus 75.8%, 31.8%, and 7.7%, respectively, of non‐infected subjects). These results suggest that ECG abnormalities and cardiac lesion in aged ChD subjects may be caused by both Chagas cardiomyopathy and common conditions of the elderly, as ischemic and hypertensive heart disease. They indicate also that only a minority of ChD elderly participants is free of ECG abnormalities and, as consequence, in the so‐called indeterminate form of ChD. Infection by T. cruzi occurs early in life and almost all cases evolve into this indeterminate chronic form, defined by the presence of infection, confirmed by either serologic or parasitological tests, and the absence of symptoms and of electrocardiographic and radiologic abnormalities (comprising heart, esophagus and colon evaluation).28 Classical cohort studies in endemic areas (with young and middle‐aged people) showed that indeterminate form constitutes the most common chronic form (50% to 60% of cases), and that 30% to 40% of these ChD patients may persist forever in this clinical situation.29 Our findings show that this statement is not applicable to the elderly infected population, because only 13.1% of infected patients (versus 24.2% in non‐infected) presented with a normal ECG.
In this cohort, the presence and the number of major ECG abnormalities have prognostic significance in both infected and non‐infected elderly, although the risk is always higher in ChD subjects. These findings are in concordance with our previous results in this cohort, showing that T. cruzi infection in the elderly is associated with increased risk for mortality6 and that both BNP levels and ECG abnormalities are related to increased risk of death from all causes24 or from stroke.7 All these findings oppose a previous concept that elderly patients with ChD have less cardiac functional and organic damage, with lower mortality and better survival,18,30–31 and reinforce that the severity of ChD in old ages is probably similar to that observed in young adults.12,14–15,14–33
Our results are also important to further clarify the natural history of ChD, specifically regarding the progression from the indeterminate to the cardiac form. Longitudinal studies in endemic areas have shown that about 2% of middle‐aged ChD patients progress from the indeterminate to the cardiac form every year.28,34–35 Our data (see Figure 3) shows that rate of appearance of new ECG major abnormalities in those without these findings, a surrogate of development of overt cardiac disease, is 1.6%/year in the T. cruzi‐infected elderly group, a figure very close to what has been reported for the middle‐aged population.36 However, this rate is not different from the rate observed in non‐infected subjects in the same community (1.4%/year), reinforcing the role of other cardiopathies in the development of new ECG abnormalities in aged patients, infected or not. As a consequence, the higher mortality observed in ChD elderly may be due to the continuous process of cardiac damage starting with the infection in childhood and during the whole adulthood of these patients, leading to a higher frequency of ECG abnormalities, an established marker of cardiopathy, in the infected elderly, in comparison to non‐infected ones.
Among specific ECG findings, RBBB is the most frequent major abnormality in the infected elderly population (23.2%). The association of RBBB with LAH, atypical finding of chronic ChD,2,16,37–39 is strongly associated (OR: 11.99) with the presence of T. cruzi infection in this cohort. The combined RBBB and/or LAH were present in 30.9% of ChD participants and in only 7.1% of the non‐ChD elderly. Recently, in a cohort of blood donors (8), the combined RBBB and/or LAH was present in 34% of ChD patients aged <60 years. Most important, RBBB is an independent predictor of the risk of death in the elderly, confirming some previous reports in younger subjects.40–41 This prognostic significance is maintained even after full adjustment for gender, age, cardiovascular risk factor, and BNP levels, this last biomarker itself an excellent surrogate of LV dysfunction.42 Some other ECG abnormalities that are also typical of Chagas cardiomyopathy, as frequent ventricular premature beats, atrial fibrillation, and pathological Q‐waves,16,37,39 are more frequent in ChD elderly subjects and have also independent prognostic value in infected subjects, as reported in some previous cohort studies of middle‐age ChD patients.40,43–44 Nonetheless, some features seem to be characteristic of this elderly population. The prevalence of frequent SPB is higher in infected elderly subjects, with independent prognostic meaning. The prognostic significance of SPB in ChD elderly subjects has never been reported before. On the other hand, an ECG abnormality that has been recognized as typical16,37 and of prognostic importance23 in previous studies with ChD populations, low voltage QRS amplitude, was not more prevalent nor was it of prognostic value (after adjustment for at least age and gender) in this cohort of infected elderly subjects. Because this abnormality has been included as a risk factor in the most cited score for risk prediction in Chagas cardiopathy,23 this finding suggests that this score should be reevaluated specifically for the elderly with Chagas cardiopathy, to confirm whether it retains its predictive accuracy in this population.
Although the main goal of the study was to evaluate the prevalence and the prognostic significance of ECG abnormalities in the T. cruzi‐infected elderly population, it also shed light on the prevalence and prognostic value of the ECG in the non‐infected elderly in Brazil. Indeed, no previous study addressed the value of ECG in aged people in Brazil and information has to be inferred from foreign studies. We find that atrial fibrillation, major Q‐waves, or minor Q‐waves combined with major ST/T abnormalities and left ventricular hypertrophy have prognostic value in non‐infected elderly, confirming studies from other countries.45–47 Incomplete left bundle branch block is also a prognostic marker in this sample, a finding that has to be further investigated, as this abnormality has been considered a surrogate for left ventricular hypertrophy.48 However, it should be stressed that, in Minnesota Code, incomplete left bundle branch block is defined exclusively by QRS duration ≥0.10 second and <0.12 second in leads I, aVL, and V5 or V6 (code 7 to 6) and may be a nonspecific marker of delayed ventricular depolarization.
The present study has limitations. Left ventricular systolic function, the main prognostic risk factor in most cardiopathies, has not been directly evaluated in this work. However, previous studies42,49–50 showed that BNP levels and LV ejection fraction are highly and inversely correlated, the BNP concentration—used for risk adjustment in this cohort—being a reliable surrogate of the LV systolic function. Other established risk markers in ChD, as functional class, increased cardiothoracic ratio in the chest x‐ray and the presence of non‐sustained ventricular tachycardia in Holter monitoring,23 were not evaluated in this cohort. Moreover, although the linear association between the prevalence of ECG abnormalities and age was used to infer the rate of development of cardiopathy in infected and non‐infected elderly participants, we did not confirm these results by repeating ECGs in individuals.
In conclusion, ECG abnormalities are more frequent in T. cruzi‐infected than in non‐infected, community‐dwelling elderly; a normal ECG is an uncommon finding in infected aged persons. Among ECG abnormalities associated with T. cruzi infection, RBBB, especially in association with left anterior hemiblock, is the most typical finding. ECG variables independently associated with prognosis in ChD included non‐sinus rhythm, frequent ventricular and supraventricular premature beats, atrial fibrillation, RBBB, old MI, possible old MI, and LVH. The presence of any major ECG abnormalities doubles the risk of death in ChD patients, but it also increases the risk in non‐ChD elderly; the risk of death increases with the number of major abnormalities in the same patient. ECG abnormalities are more common among elderly Chagas disease patients and strongly predict adverse outcomes.
Sources of Funding
This work was supported by grants from the Brazilian Research agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), and Financiadora de Estudos e Projetos (FINEP), Brazil. Ribeiro and Lima‐Costa are fellows of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Disclosures
None.
Acknowledgments
The authors would like to thank Alline Beleigoli for her careful review of the final version of this manuscript.
References
- 1.Ribeiro AL, Nunes MP, Teixeira MM, Rocha MO. Diagnosis and management of Chagas disease and cardiomyopathy. Nat Rev Cardiol. 2012; 9:576-589 [DOI] [PubMed] [Google Scholar]
- 2.Biolo A, Ribeiro AL, Clausell N. Chagas cardiomyopathy—where do we stand after a hundred years? Prog Cardiovasc Dis. 2010; 52:300-316 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization First WHO Report on Neglected Tropical Diseases: Working to Overcome the Global Impact of Neglected Tropical Diseases. 2010Geneva: World Health Organization; 1-172 [Google Scholar]
- 4.Schmunis GA, Yadon ZE. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 2010; 115:14-21 [DOI] [PubMed] [Google Scholar]
- 5.Lima‐Costa MF, Barreto SM, Guerra HL, Firmo JO, Uchoa E, Vidigal PG. Ageing with Trypanosoma cruzi infection in a community where the transmission has been interrupted: the Bambui Health and Ageing Study (BHAS). Int J Epidemiol. 2001; 30:887-893 [DOI] [PubMed] [Google Scholar]
- 6.Lima‐Costa MF, Peixoto SV, Ribeiro AL. Chagas disease and mortality in old age as an emerging issue: 10 year follow‐up of the Bambui population‐based cohort study (Brazil). Int J Cardiol. 2010; 145:362-363 [DOI] [PubMed] [Google Scholar]
- 7.Lima‐Costa MF, Matos DL, Ribeiro AL. Chagas disease predicts 10‐year stroke mortality in community‐dwelling elderly: the Bambui Cohort Study of Aging. Stroke. 2010; 41:2477-2482 [DOI] [PubMed] [Google Scholar]
- 8.Ribeiro AL, Sabino EC, Marcolino MS, Salemi VM, Ianni BM, Fernandes F, Nastari L, Antunes A, Menezes M, Oliveira CD, Sachdev V, Carrick DM, Busch MP, Murphy EL. Electrocardiographic abnormalities in Trypanosoma cruzi seropositive and seronegative former blood donors. PLoS Negl Trop Dis. 2013; 7:e2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brasil, Ministério da Saúde Brazilian consensus on Chagas disease. Rev Soc Bras Med Trop. 2005; 38suppl 3:7-29 [PubMed] [Google Scholar]
- 10.Botoni FA, Ribeiro AL, Marinho CC, Lima MM, Nunes MD, Rocha MO. Treatment of Chagas cardiomyopathy. Biomed Res Int. 2013; 2013:849504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nunes MC, Dones W, Morillo CA, Encina JJ, Ribeiro AL. Chagas disease: an overview of clinical and epidemiological aspects. J Am Coll Cardiol. 2013; 62:767-776 [DOI] [PubMed] [Google Scholar]
- 12.Bestetti RB, Ramos CP, Godoy RA, Oliveira JS. Chronic Chagas' heart disease in the elderly: a clinicopathologic study. Cardiology. 1987; 74:344-351 [DOI] [PubMed] [Google Scholar]
- 13.de Almeida EA, Barbosa Neto RM, Guariento ME, Wanderley JS, de Souza ML. Clinical presentation of chronic Chagas disease in elderly individuals. Rev Soc Bras Med Trop. 2007; 40:311-315 [DOI] [PubMed] [Google Scholar]
- 14.Prata SP, da Cunha DF, da Cunha SF, Prata SC, Nogueira N. Prevalence of electrocardiographic abnormalities in 2000 aged and non‐aged chagasic patients. Arq Bras Cardiol. 1993; 60:369-372 [PubMed] [Google Scholar]
- 15.Rocha MO, Correia PC, Barros MV, Torres RM, Ribeiro AL, Teixeira MM. Cardiovascular function in elderly patients with chronic chagasic cardiopathy. Rev Soc Bras Med Trop. 2003; 36:545-550 [DOI] [PubMed] [Google Scholar]
- 16.Porto CC. O eletrocardiograma no prognóstico e evolução da Doença de Chagas. Arq Bras Cardiol. 1964; 17:313-346 [PubMed] [Google Scholar]
- 17.Dias JC, Kloetzel K. The prognostic value of the electrocardiographic features of chronic Chagas' disease. Rev Inst Med Trop Sao Paulo. 1968; 10:158-162 [PubMed] [Google Scholar]
- 18.Maguire JH, Hoff R, Sherlock I, Guimaraes AC, Sleigh AC, Ramos NB, Mott KE, Weller TH. Cardiac morbidity and mortality due to Chagas' disease: prospective electrocardiographic study of a Brazilian community. Circulation. 1987; 75:1140-1145 [DOI] [PubMed] [Google Scholar]
- 19.Lima‐Costa MF, Firmo JO, Uchoa E. Cohort profile: the Bambui (Brazil) Cohort Study of Ageing. Int J Epidemiol. 2011; 40:862-867 [DOI] [PubMed] [Google Scholar]
- 20.Prineas RJ, Crow RS, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings. 1982Littleton, MA: John Wright‐PSG [Google Scholar]
- 21.Rautaharju PM, Warren JW, Calhoun HP. Estimation of QT prolongation. A persistent, avoidable error in computer electrocardiography. J Electrocardiol. 1990; 23suppl:111-117 [DOI] [PubMed] [Google Scholar]
- 22.Prineas RJ, Crow RS, Zhang ZM. The Minnesota Code Manual of Electrocardiographic Findings. 20102nd edLondon: Springer [Google Scholar]
- 23.Rassi A, Jr, Rassi A, Little WC, Xavier SS, Rassi SG, Rassi AG, Rassi GG, Hasslocher‐Moreno A, Sousa AS, Scanavacca MI. Development and validation of a risk score for predicting death in Chagas' heart disease. N Engl J Med. 2006; 355:799-808 [DOI] [PubMed] [Google Scholar]
- 24.Lima‐Costa MF, Cesar CC, Peixoto SV, Ribeiro AL. Plasma B‐type natriuretic peptide as a predictor of mortality in community‐dwelling older adults with Chagas disease: 10‐year follow‐up of the Bambui Cohort Study of Aging. Am J Epidemiol. 2010; 172:190-196 [DOI] [PubMed] [Google Scholar]
- 25. The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V). Arch Intern Med. 1993; 153:154-183 [PubMed] [Google Scholar]
- 26.World Health Organization The Anatomical Therapeutic Chemical Classification System with Defined Daily Doses (ATC/DDD). 2006. Available at: http://www.whocc.no/atcddd/indexdatabase/ Accessed in February 1, 2012.
- 27.Kleinbaum DW, Kupper LL, Muller KE, Nizam A. In: Kleinbaum DW, Kupper LL, Muller KE, Nizam A. (eds.). Methods for comparing two straight lines. Applied Regression Analysis and Other Multivariable Methods. 1998Pacific Grove, CA: Duxbury Press; 321-330 [Google Scholar]
- 28.Ribeiro AL, Rocha MO. Indeterminate form of Chagas disease: considerations about diagnosis and prognosis. Rev Soc Bras Med Trop. 1998; 31:301-314 [DOI] [PubMed] [Google Scholar]
- 29.Dias JC. The indeterminate form of human chronic Chagas' disease a clinical epidemiological review. Rev Soc Bras Med Trop. 1989; 22:147-156 [DOI] [PubMed] [Google Scholar]
- 30.Carneiro O, De Rezende JM. Chagas' disease and longevity. Arq Bras Cardiol. 1982; 38:381-384 [PubMed] [Google Scholar]
- 31.Menezes M, Rocha A, da Silva AC, da Silva AM. Basic causes of death in elderly patients with Chagas' disease. Arq Bras Cardiol. 1989; 52:75-78 [PubMed] [Google Scholar]
- 32.Lima‐Costa MF, Barreto SM, Guerra HL. Chagas' disease among older adults: branches or mainstream of the present burden of Trypanosoma cruzi infection? Int J Epidemiol. 2002; 31:688-689 [DOI] [PubMed] [Google Scholar]
- 33.Ribeiro AL, Cassini P, Peixoto SV, Lima‐Costa MF. Vagal impairment in elderly Chagas disease patients: a population‐based study (the Bambui study). Int J Cardiol. 2011; 147:359-365 [DOI] [PubMed] [Google Scholar]
- 34.Macedo V. Indeterminate form of Chagas disease. Mem Inst Oswaldo Cruz. 1999; 94suppl 1:311-316 [DOI] [PubMed] [Google Scholar]
- 35.Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis. 2001; 1:92-100 [DOI] [PubMed] [Google Scholar]
- 36.Sabino EC, Ribeiro AL, Salemi VM, Di Lorenzo OC, Antunes AP, Menezes MM, Ianni BM, Nastari L, Fernandes F, Patavino GM, Sachdev V, Capuani L, de Almeida‐Neto C, Carrick DM, Wright D, Kavounis K, Goncalez TT, Carneiro‐Proietti AB, Custer B, Busch MP, Murphy EL. Ten‐year incidence of Chagas cardiomyopathy among asymptomatic Trypanosoma cruzi‐seropositive former blood donors. Circulation. 2013; 127:1105-1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garzon SA, Lorga AM, Nicolau JC. Electrocardiography in Chagas' heart disease. Sao Paulo Med J. 1995; 113:802-813 [DOI] [PubMed] [Google Scholar]
- 38.Williams‐Blangero S, Magalhaes T, Rainwater E, Blangero J, Correa‐Oliveira R, VandeBerg JL. Electrocardiographic characteristics in a population with high rates of seropositivity for Trypanosoma cruzi infection. Am J Trop Med Hyg. 2007; 77:495-499 [PubMed] [Google Scholar]
- 39.Ribeiro AL. Eletrocardiografia na doença de Chagas. Rev Soc Bras Med Trop. 1994; 27:52-54 [Google Scholar]
- 40.Goncalves JG, Dias Silva VJ, Calzada Borges MC, Prata A, Correia D. Mortality indicators among chronic Chagas patients living in an endemic area. Int J Cardiol. 2010; 143:235-242 [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez‐Salas LA, Klein E, Acquatella H, Catalioti F, Davalos V, Gomez‐Mancebo JR, Gonzalez H, Bosch F, Puigbo JJ. Echocardiographic and clinical predictors of mortality in chronic Chagas' disease. Echocardiography. 1998; 15:271-278 [DOI] [PubMed] [Google Scholar]
- 42.Ribeiro AL, dos Reis AM, Barros MV, de Sousa MR, Rocha AL, Perez AA, Pereira JB, Machado FS, Rocha MO. Brain natriuretic peptide and left ventricular dysfunction in Chagas' disease. Lancet. 2002; 360:461-462 [DOI] [PubMed] [Google Scholar]
- 43.Espinosa RA, Pericchi LR, Carrasco HA, Escalante A, Martinez O, Gonzalez R. Prognostic indicators of chronic chagasic cardiopathy. Int J Cardiol. 1991; 30:195-202 [DOI] [PubMed] [Google Scholar]
- 44.Salles G, Xavier S, Sousa A, Hasslocher‐Moreno A, Cardoso C. Prognostic value of QT interval parameters for mortality risk stratification in Chagas' disease: results of a long‐term follow‐up study. Circulation. 2003; 108:305-312 [DOI] [PubMed] [Google Scholar]
- 45.Kahn S, Frishman WH, Weissman S, Ooi WL, Aronson M. Left ventricular hypertrophy on electrocardiogram: prognostic implications from a 10‐year cohort study of older subjects: a report from the Bronx Longitudinal Aging Study. J Am Geriatr Soc. 1996; 44:524-529 [DOI] [PubMed] [Google Scholar]
- 46.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes. 2012; 5:85-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tervahauta M, Pekkanen J, Punsar S, Nissinen A. Resting electrocardiographic abnormalities as predictors of coronary events and total mortality among elderly men. Am J Med. 1996; 100:641-645 [DOI] [PubMed] [Google Scholar]
- 48.Terasawa F, Kuramochi M, Yazaki Y, Kuramoto K, Ikeda M, Seki M. Clinical and pathological studies on incomplete left bundle branch block in the aged. Isr J Med Sci. 1969; 5:732-735 [PubMed] [Google Scholar]
- 49.Ribeiro AL, Teixeira MM, Reis AM, Talvani A, Perez AA, Barros MV, Rocha MO. Brain natriuretic peptide based strategy to detect left ventricular dysfunction in Chagas disease: a comparison with the conventional approach. Int J Cardiol. 2006; 109:34-40 [DOI] [PubMed] [Google Scholar]
- 50.Talvani A, Rocha MO, Cogan J, Maewal P, de Lemos J, Ribeiro AL, Teixeira MM. Brain natriuretic peptide and left ventricular dysfunction in chagasic cardiomyopathy. Mem Inst Oswaldo Cruz. 2004; 99:645-649 [DOI] [PubMed] [Google Scholar]