Abstract
Background
Limited information is available on the contemporary and potentially changing trends in the incidence, management, and outcomes of cardiogenic shock complicating ST‐elevation myocardial infarction (STEMI).
Methods and Results
We queried the 2003–2010 Nationwide Inpatient Sample databases to identify all patients ≥40 years of age with STEMI and cardiogenic shock. Overall and age‐, sex‐, and race/ethnicity‐specific trends in incidence of cardiogenic shock, early mechanical revascularization, and intra‐aortic balloon pump use, and inhospital mortality were analyzed. From 2003 to 2010, among 1 990 486 patients aged ≥40 years with STEMI, 157 892 (7.9%) had cardiogenic shock. The overall incidence rate of cardiogenic shock in patients with STEMI increased from 6.5% in 2003 to 10.1% in 2010 (Ptrend<0.001). There was an increase in early mechanical revascularization (30.4% to 50.7%, Ptrend<0.001) and intra‐aortic balloon pump use (44.8% to 53.7%, Ptrend<0.001) in these patients over the 8‐year period. Inhospital mortality decreased significantly, from 44.6% to 33.8% (Ptrend<0.001; adjusted OR, 0.71; 95% CI, 0.68 to 0.75), whereas the average total hospital cost increased from $35 892 to $45 625 (Ptrend<0.001) during the study period. There was no change in the average length of stay (Ptrend=0.394). These temporal trends were similar in patients <75 and ≥75 years of age, men and women, and across each racial/ethnic group.
Conclusions
The incidence of cardiogenic shock complicating STEMI has increased during the past 8 years together with increased use of early mechanical revascularization and intra‐aortic balloon pumps. There has been a concomitant decrease in risk‐adjusted inhospital mortality, but an increase in total hospital costs during this period.
Keywords: cardiogenic shock, early revascularization, inhospital mortality, ST‐elevation myocardial infarction, trends
Introduction
Cardiogenic shock complicates 5% to 10% of cases with acute myocardial infarction (AMI) and remains the leading cause of death in patients hospitalized with AMI.1–2 This usually results from extensive damage to left ventricular myocardium or mechanical complications such as papillary muscle rupture, ventricular septal rupture, free‐wall rupture, or right ventricular infarction. Cardiogenic shock is defined by marked and persistent (>30 minutes) hypotension (systolic arterial pressure <80 mm Hg), marked reduction of cardiac index (<1.8 L/min per square meter) in the face of elevated left ventricular filling pressures (pulmonary capillary wedge pressure >18 mm Hg). Clinically, cardiogenic shock is characterized by low cardiac output, systemic hypotension, and evidence of vital organ hypoperfusion (eg, altered mental status, oliguria, and acidosis).
The Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock (SHOCK) trial demonstrated improved short‐ and long‐term survival with early mechanical revascularization in patients with AMI and cardiogenic shock.3–5 Emergency revascularization with either percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) irrespective of the time delay from onset of AMI is a class I (level of evidence B) recommendation in the current American College of Cardiology Foundation/American Heart Association guidelines for the treatment of patients with cardiogenic shock after ST‐elevation myocardial infarction (STEMI).6 Intra‐aortic balloon pump (IABP) is the most widely used form of mechanical hemodynamic support in patients with cardiogenic shock. But data on the usefulness of IABP in this setting are conflicting. Mortality in cardiogenic shock treated with conservative measures is between 70% and 80%.2 However, since the implementation of guideline‐recommended early revascularization, mortality rates have steadily decreased to <50%.7 Studies on temporal trends in the management and outcomes of cardiogenic shock have traditionally included all patients with AMI (ie, non‐ST‐ and ST‐elevation myocardial infarction). Further, prior studies have involved registries from voluntary participating hospitals or data from single communities, which may not be nationally representative.2,8–10 More recent data on the incidence of cardiogenic shock, early revascularization, IABP use, and outcomes in patients with STEMI in the United States are limited.
The primary objective of this study was to examine the temporal trends in the incidence of cardiogenic shock, early mechanical revascularization, IABP use, and inhospital outcomes in a large real‐world cohort of patients with STEMI included in the 2003–2010 Nationwide Inpatient Sample (NIS) databases. In addition, we aimed to explore differences in the incidence, management, and outcomes of cardiogenic shock according to age, sex, and race/ethnicity, as well as to determine if temporal trends were similar or different in patients <75 and ≥75 years of age, men and women, and each racial/ethnic group.
Methods
Data Source
Data were obtained from the 2003–2010 NIS databases. The NIS, sponsored by the Agency for Healthcare Research and Quality as a part of the Healthcare Cost and Utilization Project, is the largest publicly available all‐payer inpatient care database in the United States. It contains discharge‐level data provided by states (n=45 in 2010) that participate in the Healthcare Cost and Utilization Project. The NIS includes data from ≈8 million hospital stays from about 1000 hospitals designed to approximate a 20% stratified sample of all community hospitals (defined as “all non‐Federal, short‐term, general, and other specialty hospitals, excluding hospital units of institutions”) in the United States. Criteria used for stratified sampling of hospitals include hospital ownership, patient volume, teaching status, urban or rural location, and geographic region. Inpatient stay records in the NIS include clinical and resource use information available from discharge abstracts derived from state‐mandated hospital discharge reports. Discharge weights, provided for each patient discharge record, were used to obtain national estimates.
Study Population
We used the International Classification of Diseases, Ninth Edition, Clinical Modification (ICD‐9‐CM) codes 410.0x, 410.1x, 410.2x, 410.3x, 410.4x, 410.5x, 410.6x, and 410.8x to identify all patients ≥40 years of age with the principal diagnosis of STEMI (N=1 990 486). We chose the principal diagnosis because it is considered the primary reason for hospital admission. Patients with a concomitant diagnosis of cardiogenic shock (present on admission or developing during hospitalization) were identified using the ICD‐9‐CM code 785.51 (n=157 892). Early mechanical revascularization was defined as PCI (ICD‐9‐CM procedure codes 00.66, 36.01, 36.02, 36.05, 36.06, and 36.07) or CABG (ICD‐9‐CM procedure code 36.1x) within 24 hours of admission. Patients undergoing IABP placement were identified using ICD‐9‐CM procedure code 37.61.
Outcomes Measured
We initially studied the overall and age‐, sex‐, and race/ethnicity‐specific trends in the incidence of cardiogenic shock in patients with STEMI. We also examined the trends in early mechanical revascularization and IABP use in patients with cardiogenic shock complicating STEMI. Our primary outcome of interest was all‐cause inhospital mortality, defined as “died” during the hospitalization encounter in the NIS database. We used the average length of stay and total hospital cost as secondary outcomes. We analyzed the overall and age‐, sex‐, and race/ethnicity‐specific trends in inhospital mortality, average length of stay and total hospital cost in patients with cardiogenic shock complicating STEMI.
Patient and Hospital Characteristics
Baseline patient characteristics used included demographics (age, sex, race, primary expected payer, weekday versus weekend admission, median household income for patient zip code), 29 Elixhauser comorbidities as defined by the Agency for Healthcare Research and Quality, other clinically relevant comorbidities (smoking, dyslipidemia, known coronary artery disease, family history of coronary artery disease, prior myocardial infarction, carotid artery disease, and dementia), presentation (anterior wall, inferior wall, or other STEMI), and inhospital procedures (Swan‐Ganz catheterization, PCI, CABG, IABP, and blood transfusion).11–12 A list of ICD‐9‐CM and Clinical Classifications Software codes used to identify comorbidities and inhospital procedures is provided in Table 1. Hospital characteristics such as hospital region (Northeast, Midwest, South, and West), bed size (small, medium, and large), location (rural, urban), and teaching status were also included.
Table 1.
ICD‐9‐CM and CCS Codes Used to Identify Comorbidities and Inhospital Procedures
| Variable | Source | Code(s) |
|---|---|---|
| Comorbidities | ||
| Smoking | ICD‐9‐CM | V15.82, 305.1 |
| Dyslipidemia | CCS | 53 |
| Coronary artery disease | ICD‐9‐CM | 414.00 to 414.07 |
| Family history of coronary artery disease | ICD‐9‐CM | V17.3 |
| Prior myocardial infarction | ICD‐9‐CM | 412 |
| Carotid artery disease | ICD‐9‐CM | 433.10 |
| Dementia | ICD‐9‐CM | 290.xx, 294.1x, 294.2x, 294.8, 331.0 to 331.12, 331.82, 797 |
| STEMI location | ||
| Anterior wall | ICD‐9‐CM | 410.0x, 410.1x |
| Inferior wall | ICD‐9‐CM | 410.2x, 410.3x, 410.4x |
| Other | ICD‐9‐CM | 410.5x, 410.6x, 410.8x, 410.9x |
| Procedures | ||
| Left heart catheterization | ICD‐9‐CM | 88.55, 88.66, 37.22, 37.23 |
| Swan‐Ganz catheterization | CCS | 204 |
| Thrombolysis | ICD‐9‐CM | 99.10, V45.88 |
| Blood transfusion | CCS | 222 |
CCD indicates Clinical Classifications Software; ICD‐9‐CM, International Classification of Diseases, Ninth Edition, Clinical Modification; STEMI, ST‐elevation myocardial infarction.
Statistical Analysis
For trend analysis, we used the Mantel‐Haenszel χ2 test of linear association for categorical variables and linear regression for continuous variables. To determine if there was temporal variability from year to year in the incidence of cardiogenic shock, early mechanical revascularization and IABP placement, or inhospital mortality, we used unadjusted and multivariable adjusted logistic regression models to determine the odds of developing cardiogenic shock, undergoing early mechanical revascularization or IABP placement, or death during hospitalization each year relative to 2003. The regression models adjusted for all demographics (except sex and race/ethnicity for sex‐ and race/ethnicity‐specific trends), hospital characteristics, all Elixhauser and other clinically relevant comorbidities, and presentation. We graphically displayed the unadjusted and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for cardiogenic shock, early mechanical revascularization, IABP placement, and inhospital death over time. Temporal trends (overall and age‐, sex‐, and race/ethnicity specific) in length of stay and total hospital cost were examined using a general linear model with log‐transformed length of stay and total hospital cost, respectively.
Multivariable logistic regression was also used to determine differences in incidence rates, management, and outcomes of cardiogenic shock complicating STEMI between patients <75 and ≥75 years of age, men and women, and each racial/ethnic group (white, African American, Hispanic, and Asian/Pacific Islander).
Statistical analysis was performed using IBM SPSS Statistics 20.0 (IBM Corp, Armonk, NY). All P values were 2 sided with a significance threshold of P<0.001 (a lower than usual P value threshold was selected to correct for the effects of a large sample size as well as inflation of type I error because of repeated testing using a large number of variables). Categorical variables are expressed as percentages and continuous variables as mean±standard deviation. OR and 95% CI are used to report the results of logistic regression.
Results
Trends in Incidence Rates of Cardiogenic Shock
From 2003 to 2010, we identified 1 990 486 patients ≥40 years of age with STEMI. The overall incidence of cardiogenic shock in the study cohort was 7.9% (n=157 892). The proportion of STEMI patients developing cardiogenic shock increased from 6.5% in 2003 to 10.1% in 2010 (unadjusted OR, 1.62; 95% CI, 1.59 to 1.65; P<0.001; Figure 1A). When adjusted for demographics, hospital characteristics, comorbidities, and presentation, we observed a greater than 2‐fold increase in the incidence of cardiogenic shock in patients with STEMI in 2010 as compared with 2003 (adjusted OR, 2.13; 95% CI, 2.07 to 2.18; P<0.001; Figure 1B).
Figure 1.
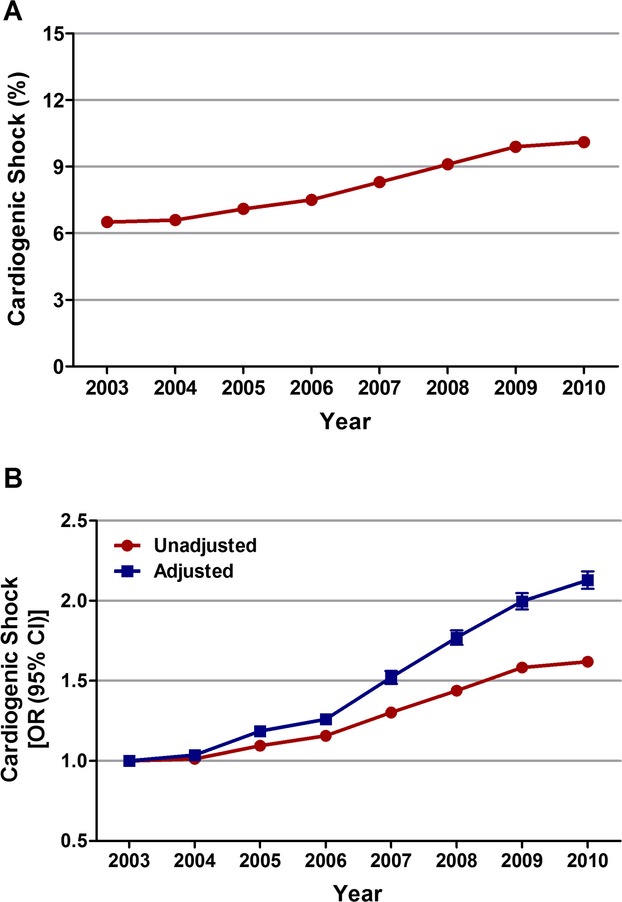
Trends in incidence rates of cardiogenic shock in patients with STEMI. A, Cardiogenic shock (%) was calculated as the number of patients with cardiogenic shock divided by the number of patients with STEMI per year×100; Ptrend<0.001. B, Trends in cardiogenic shock presented as unadjusted and adjusted odds ratios and 95% confidence intervals (CIs) for each year relative to 2003 (reference year). Regression model adjusted for demographics, hospital characteristics, 29 Elixhauser and other clinically relevant comorbidities, and presentation. OR indicates odds ratio; STEMI, ST‐elevation myocardial infarction.
Overall, the incidence of cardiogenic shock was higher in patients aged ≥75 versus <75 years (9.4% versus 7.3%; P<0.001), in women versus men (8.5% versus 7.6%; P<0.001), and in Asian/Pacific Islanders versus other racial/ethnic groups (11.4% versus 8% in whites, 6.9% in African Americans, 8.6% in Hispanics; P<0.001). From 2003 to 2010, the incidence of cardiogenic shock complicating STEMI increased from 5.9% to 9.4% in patients aged <75 years (Ptrend<0.001) and from 7.7% to 12.2% in patients aged ≥75 years (Ptrend<0.001); see Figure 2A. Sex‐specific trend analysis demonstrated an increase in the rates of cardiogenic shock from 6.1% to 9.7% in men (Ptrend<0.001) and from 7.2% to 11% in women (Ptrend<0.001) (Figure 2B) over the 8 years. The incidence of cardiogenic shock remained higher in women than in men throughout the study period. Similarly, from 2003 to 2010, the incidence of cardiogenic shock increased from 6.6% to 10.2% in whites (Ptrend<0.001), from 5.5% to 9% in African Americans (Ptrend<0.001), from 6.5% to 10.1% in Hispanics (Ptrend<0.001), and from 8.6% to 13.1% in Asian/Pacific Islanders (Ptrend<0.001) with STEMI (Figure 2C). The upward trend in the incidence of cardiogenic shock persisted even after adjusting for confounding variables (Table 2).
Figure 2.
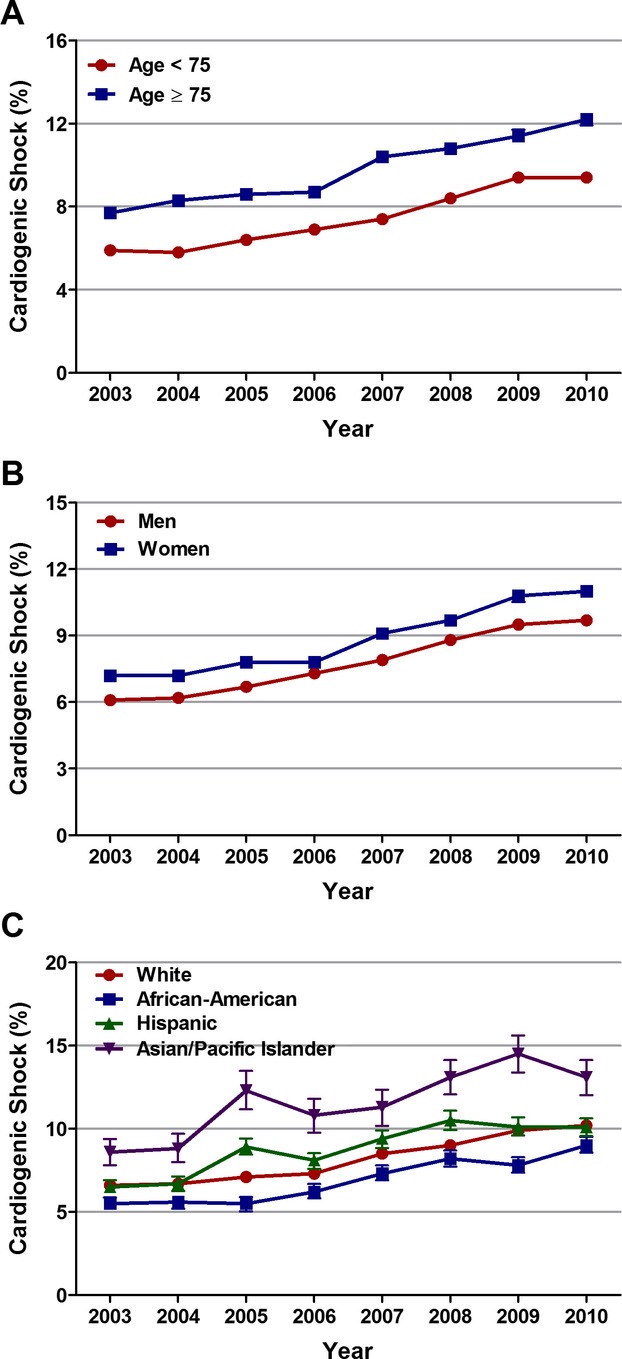
Age‐, sex‐, and race‐specific trends in incidence rates of cardiogenic in patients with ST‐elevation myocardial infarction (STEMI). A, Trends in incidence rates of cardiogenic shock in patients <75 and ≥75 years of age with STEMI; Ptrend<0.001. B, Trends in incidence rates of cardiogenic shock in men and women with STEMI; Ptrend<0.001. C, Trends in incidence rates of cardiogenic shock in whites, African Americans, Hispanics, and Asian/Pacific Islanders with STEMI; Ptrend<0.001.
Table 2.
Overall and Age‐, Sex‐, and Race/Ethnicity‐Specific Trends in Incidence of Cardiogenic Shock in Patients With STEMI
| Year | Overall | Age | Sex | Race/Ethnicity | |||||
|---|---|---|---|---|---|---|---|---|---|
| <75 Years | ≥75 Years | Male | Female | White | African American | Hispanic | Asian/Pacific Islander | ||
| 2003 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2004 | 1.04 (1.01 to 1.06) | 1.00 (0.97 to 1.04) | 1.08 (1.04 to 1.12) | 1.03 (0.99 to 1.07) | 1.05 (1.01 to 1.09) | 1.04 (1.01 to 1.07) | 1.05 (0.95 to 1.17) | 1.11 (1.00 to 1.22) | 1.03 (0.88 to 1.21) |
| 2005 | 1.19 (1.15 to 1.22) | 1.21 (1.17 to 1.25) | 1.17 (1.13 to 1.22) | 1.17 (1.13 to 1.22) | 1.20 (1.16 to 1.25) | 1.15 (1.12 to 1.18) | 1.14 (1.02 to 1.28) | 1.53 (1.39 to 1.67) | 1.66 (1.42 to 1.95) |
| 2006 | 1.26 (1.23 to 1.29) | 1.30 (1.26 to 1.35) | 1.21 (1.16 to 1.26) | 1.32 (1.28 to 1.37) | 1.18 (1.13 to 1.23) | 1.24 (1.20 to 1.27) | 1.33 (1.20 to 1.48) | 1.43 (1.30 to 1.57) | 1.40 (1.19 to 1.64) |
| 2007 | 1.52 (1.48 to 1.56) | 1.53 (1.47 to 1.58) | 1.53 (1.47 to 1.60) | 1.53 (1.48 to 1.59) | 1.51 (1.45 to 1.57) | 1.51 (1.47 to 1.56) | 1.59 (1.43 to 1.76) | 1.74 (1.58 to 1.91) | 1.50 (1.28 to 1.76) |
| 2008 | 1.77 (1.73 to 1.82) | 1.84 (1.78 to 1.90) | 1.67 (1.60 to 1.74) | 1.84 (1.78 to 1.90) | 1.69 (1.62 to 1.75) | 1.74 (1.69 to 1.79) | 1.90 (1.72 to 2.10) | 2.17 (1.98 to 2.39) | 1.78 (1.53 to 2.06) |
| 2009 | 2.00 (1.95 to 2.05) | 2.09 (2.02 to 2.16) | 1.83 (1.76 to 1.91) | 2.04 (1.98 to 2.11) | 1.94 (1.86 to 2.02) | 1.99 (1.94 to 2.05) | 2.02 (1.82 to 2.24) | 2.13 (1.94 to 2.34) | 2.03 (1.74 to 2.36) |
| 2010 | 2.13 (2.07 to 2.18) | 2.18 (2.11 to 2.26) | 2.02 (1.94 to 2.11) | 2.21 (2.13 to 2.18) | 2.02 (1.94 to 2.10) | 2.13 (2.07 to 2.19) | 2.20 (1.99 to 2.43) | 2.29 (2.08 to 2.51) | 1.82 (1.56 to 2.13) |
Trends are expressed as adjusted odds ratios (95% confidence intervals) for each year relative to 2003. Regression model adjusted for demographics (except sex and race/ethnicity for sex‐ and race/ethnicity‐specific trends, respectively), hospital characteristics, all comorbidities, and presentation. STEMI indicates ST‐elevation myocardial infarction.
Changing Baseline Characteristics of Patients With STEMI Complicated by Cardiogenic Shock
Table 3 depicts the changes in baseline demographics, hospital characteristics, and comorbidities from 2003 to 2010 in patients with cardiogenic shock complicating STEMI. The mean age decreased from 69.3±12.7 to 67.7±12.9 (Ptrend<0.001). There was an increase in the proportion of men and a decrease in the proportion of women with STEMI and cardiogenic shock over the 8 years (Ptrend<0.001). Whites constituted the highest proportion of patients with cardiogenic shock complicating STEMI. The prevalence of smoking, dyslipidemia, coronary artery disease, prior myocardial infarction, carotid artery disease, diabetes mellitus, hypertension, obesity, peripheral vascular disease, chronic renal failure, alcohol abuse, deficiency anemias, coagulopathy, and fluid/electrolyte disorders increased from 2003 to 2010 (Ptrend<0.001 for all). On the other hand, the prevalence of congestive heart failure and chronic pulmonary disease decreased over the 8 years (Ptrend<0.001). In the overall cohort of patients with STEMI and cardiogenic shock, 42.3% had anterior wall STEMI, 38.6% had inferior wall STEMI, and 19.1% were other location.
Table 3.
Baseline Demographics, Hospital Characteristics, and Comorbidities of Patients With Cardiogenic Shock Complicating ST‐Elevation Myocardial Infarction
| Variable | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | P Value* |
|---|---|---|---|---|---|---|---|---|---|
| No. of cases (weighted) | 21 387 | 19 066 | 18 632 | 19 658 | 18 768 | 20 349 | 20 459 | 19 575 | — |
| Age, y | 69.3±12.7 | 70.0±12.7 | 69.4±13.0 | 68.2±12.7 | 68.7±13.0 | 68.0±13.1 | 67.2±12.8 | 67.7±12.9 | <0.001 |
| Sex | <0.001 | ||||||||
| Male | 57.9% | 58.8% | 58.6% | 62.5% | 60.6% | 62.2% | 63.3% | 63.5% | |
| Female | 42.1% | 41.2% | 41.4% | 37.5% | 39.4% | 37.8% | 36.7% | 36.5% | |
| Race | <0.001 | ||||||||
| White | 79.3% | 80.6% | 79.8% | 79.8% | 78.5% | 77.9% | 78.1% | 77.7% | |
| African American | 6.3% | 6.3% | 4.4% | 5.7% | 6.8% | 6.5% | 6.0% | 7.3% | |
| Hispanic | 7.6% | 6.5% | 8.7% | 7.5% | 7.9% | 6.9% | 6.9% | 7.2% | |
| Asian or Pacific Islander | 2.7% | 2.8% | 2.9% | 2.8% | 2.7% | 3.2% | 3.3% | 3.0% | |
| Other | 4.0% | 3.8% | 4.2% | 4.2% | 4.0% | 5.5% | 5.8% | 4.7% | |
| Primary expected payer | <0.001 | ||||||||
| Medicare | 62.5% | 61.5% | 60.9% | 57.2% | 56.1% | 55.9% | 53.2% | 55.0% | |
| Medicaid | 5.0% | 5.1% | 5.8% | 5.9% | 6.1% | 5.9% | 6.6% | 7.9% | |
| Private insurance | 26.3% | 25.4% | 25.2% | 28.3% | 27.9% | 27.9% | 30.1% | 27.0% | |
| Uninsured | 4.1% | 5.3% | 5.6% | 5.7% | 6.7% | 7.2% | 7.2% | 7.3% | |
| Other | 2.2% | 2.7% | 2.6% | 2.9% | 3.2% | 3.1% | 2.9% | 2.7% | |
| Median household income | 0.002 | ||||||||
| 0 to 25th Percentile | 26.3% | 26.7% | 25.8% | 23.1% | 26.5% | 27.1% | 25.7% | 28.0% | |
| 26th to 50th Percentile | 26.8% | 27.3% | 27.3% | 26.9% | 26.2% | 28.4% | 27.3% | 25.7% | |
| 51st to 75th Percentile | 25.6% | 22.9% | 23.9% | 26.6% | 24.4% | 24.0% | 25.2% | 24.2% | |
| 76th to 100th Percentile | 21.3% | 23.1% | 23.0% | 23.3% | 22.9% | 20.5% | 21.8% | 22.1% | |
| Weekend admission | 27.3% | 26.8% | 26.7% | 27.7% | 27.6% | 27.8% | 28.5% | 29.5% | <0.001 |
| Hospital characteristics | |||||||||
| Region | 0.329 | ||||||||
| Northeast | 17.8% | 16.3% | 17.5% | 15.8% | 18.5% | 17.6% | 17.0% | 19.0% | |
| Midwest | 23.8% | 23.4% | 24.3% | 25.4% | 24.2% | 24.4% | 22.7% | 26.2% | |
| South | 39.9% | 39.2% | 36.9% | 38.3% | 35.3% | 38.5% | 38.6% | 32.0% | |
| West | 18.5% | 21.1% | 21.3% | 20.5% | 22.0% | 19.4% | 21.7% | 22.8% | |
| Bed size | <0.001 | ||||||||
| Small | 6.9% | 7.9% | 4.2% | 9.7% | 7.6% | 7.8% | 6.0% | 8.8% | |
| Medium | 22.7% | 21.9% | 22.9% | 22.5% | 22.4% | 21.5% | 18.4% | 18.2% | |
| Large | 70.4% | 70.3% | 72.8% | 67.8% | 70.1% | 70.7% | 75.6% | 73.0% | |
| Urban location | 89.0% | 90.6% | 92.6% | 94.8% | 91.9% | 92.1% | 93.4% | 91.8% | <0.001 |
| Teaching hospital | 46.7% | 47.6% | 43.9% | 51.7% | 52.4% | 49.1% | 52.2% | 51.0% | <0.001 |
| Comorbidities | |||||||||
| Smoking | 14.1% | 14.6% | 17.4% | 18.2% | 20.5% | 24.4% | 32.3% | 30.6% | <0.001 |
| Dyslipidemia | 16.5% | 17.6% | 20.8% | 23.6% | 27.6% | 33.8% | 40.9% | 41.3% | <0.001 |
| CAD | 53.8% | 54.5% | 56.2% | 59.3% | 63.3% | 69.6% | 73.8% | 75.4% | <0.001 |
| Family history of CAD | 1.6% | 1.7% | 1.7% | 2.5% | 2.6% | 2.9% | 5.2% | 5.3% | <0.001 |
| Prior myocardial infarction | 4.0% | 4.4% | 4.2% | 4.2% | 5.5% | 5.3% | 6.6% | 7.6% | <0.001 |
| Carotid artery disease | 0.4% | 0.3% | 0.5% | 0.4% | 0.5% | 0.7% | 0.7% | 1.2% | <0.001 |
| Dementia | 2.7% | 3.4% | 3.7% | 3.4% | 3.7% | 3.7% | 4.2% | 4.8% | <0.001 |
| AIDS | 0.2% | 0.2% | 0.1% | 0.1% | 0.1% | 0.1% | 0.2% | 0.2% | 0.714 |
| Alcohol abuse | 2.2% | 2.2% | 2.5% | 3.3% | 3.4% | 3.2% | 3.4% | 3.7% | <0.001 |
| Deficiency anemias | 9.0% | 8.6% | 9.3% | 11.1% | 14.3% | 15.8% | 19.3% | 19.2% | <0.001 |
| RA/collagen vascular diseases | 1.4% | 1.4% | 1.3% | 1.5% | 1.8% | 2.0% | 1.4% | 1.7% | <0.001 |
| Chronic blood loss anemia | 1.2% | 1.4% | 1.9% | 1.8% | 2.2% | 1.1% | 1.5% | 1.1% | 0.017 |
| Congestive heart failure | 54.0% | 55.0% | 52.6% | 50.1% | 49.5% | 43.5% | 46.2% | 46.7% | <0.001 |
| Chronic pulmonary disease | 20.4% | 20.6% | 22.2% | 21.9% | 20.7% | 19.3% | 19.9% | 18.8% | <0.001 |
| Coagulopathy | 8.5% | 8.7% | 9.8% | 10.5% | 10.8% | 9.7% | 12.7% | 13.8% | <0.001 |
| Depression | 2.0% | 2.0% | 2.7% | 2.7% | 3.3% | 4.0% | 4.6% | 4.7% | <0.001 |
| Diabetes (uncomplicated) | 19.0% | 19.9% | 20.4% | 21.2% | 22.5% | 24.7% | 25.7% | 27.0% | <0.001 |
| Diabetes (complicated) | 3.7% | 3.6% | 3.6% | 4.0% | 3.9% | 3.9% | 4.2% | 4.3% | <0.001 |
| Drug abuse | 0.6% | 0.8% | 0.9% | 1.2% | 1.4% | 1.3% | 1.7% | 1.3% | <0.001 |
| Hypertension | 36.9% | 36.6% | 40.1% | 41.7% | 43.8% | 51.1% | 54.1% | 55.3% | <0.001 |
| Hypothyroidism | 4.3% | 4.7% | 5.4% | 5.3% | 6.1% | 6.3% | 6.9% | 7.2% | <0.001 |
| Liver disease | 0.9% | 0.9% | 0.6% | 0.8% | 1.3% | 0.9% | 1.4% | 1.3% | <0.001 |
| Lymphoma | 0.4% | 0.5% | 0.4% | 0.3% | 0.5% | 0.4% | 0.3% | 0.7% | 0.153 |
| Fluid and electrolyte disorder | 28.9% | 29.0% | 30.5% | 32.7% | 34.8% | 37.2% | 41.9% | 41.8% | <0.001 |
| Metastatic cancer | 0.7% | 0.7% | 0.8% | 0.8% | 1.0% | 0.6% | 0.9% | 1.1% | <0.001 |
| Other neurological disorders | 5.5% | 5.3% | 5.8% | 5.5% | 6.0% | 6.3% | 6.2% | 6.8% | <0.001 |
| Obesity | 4.3% | 3.8% | 4.5% | 4.8% | 5.8% | 8.7% | 9.8% | 10.1% | <0.001 |
| Paralysis | 1.4% | 1.3% | 1.4% | 1.3% | 1.3% | 2.2% | 1.5% | 2.2% | <0.001 |
| Peripheral vascular disease | 6.1% | 5.9% | 7.0% | 7.2% | 8.6% | 10.3% | 11.1% | 10.5% | <0.001 |
| Psychoses | 1.1% | 1.1% | 0.8% | 1.5% | 1.2% | 1.9% | 2.0% | 2.3% | <0.001 |
| Pulmonary circulation disorders | 0.2% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.3% | 0.3% | <0.001 |
| Renal failure (chronic) | 8.9% | 9.0% | 10.6% | 15.2% | 15.5% | 14.7% | 15.8% | 16.4% | <0.001 |
| Solid tumor without metastasis | 1.3% | 1.5% | 1.4% | 1.3% | 1.5% | 1.6% | 1.3% | 1.7% | 0.008 |
| Peptic ulcer (nonbleeding) | <0.01% | <0.01% | <0.01% | 0.0% | 0.0% | <0.01% | <0.01% | <0.01% | 0.946 |
| Valvular disease | 0.9% | 0.8% | 0.8% | 1.0% | 1.0% | 0.6% | 0.6% | 0.6% | <0.001 |
| Weight loss | 2.0% | 2.5% | 3.0% | 4.0% | 3.3% | 4.8% | 6.3% | 6.1% | <0.001 |
| Presentation | 0.003 | ||||||||
| Anterior wall STEMI | 41.1% | 41.4% | 42.1% | 42.3% | 42.4% | 43.8% | 42.8% | 42.6% | |
| Inferior wall STEMI | 38.6% | 38.4% | 38.3% | 39.3% | 39.3% | 37.7% | 39.0% | 38.2% | |
| Other STEMI | 20.4% | 20.2% | 19.6% | 18.3% | 18.3% | 18.5% | 18.2% | 19.2% |
Data are expressed as mean±standard deviation for continuous variables and number (percentage) for categorical variables. AIDS indicates acquired immunodeficiency syndrome; CAD, coronary artery disease; RA, rheumatoid arthritis; STEMI, ST‐elevation myocardial infarction.
P value for trend.
Trends in Inhospital Procedures, Early Mechanical Revascularization, and Utilization of IABP
In patients with cardiogenic shock complicating STEMI, left heart catheterization rates increased from 64.1% in 2003 to 74.4% in 2010 (Ptrend<0.001), whereas Swan‐Ganz catheterization rates decreased from 9.8% in 2003 to 6.2% in 2010 (Ptrend<0.001); see Table 4. Early PCI rates increased significantly, from 26.6% in 2003 to 53.8% in 2010 (Ptrend<0.001). In contrast, early CABG rates increased from 4.5% in 2003 to 6.2% in 2008 (Ptrend<0.001), followed by declines in 2009 and 2010 (Table 2). Overall, among 157 892 patients with STEMI and cardiogenic shock, 67 481 (42.7%) underwent early mechanical revascularization (PCI or CABG within 24 hours of admission). The proportion of patients with STEMI and cardiogenic shock undergoing early mechanical revascularization increased significantly from 30.4% in 2003 to 50.7% in 2010 (unadjusted OR, 3.04; 95% CI, 2.92 to 3.17; P<0.001; Figure 3A). When adjusted for demographics, hospital characteristics, comorbidities, and presentation, we observed a similar 3‐fold increase in early mechanical revascularization rates in patients with STEMI and cardiogenic shock from 2003 to 2010 (adjusted OR, 3.02; 95% CI, 2.87 to 3.19; P<0.001; Figure 3B).
Table 4.
Inhospital Procedures in Patients With Cardiogenic Shock Complicating ST‐Elevation Myocardial Infarction
| Procedure | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | P Value* |
|---|---|---|---|---|---|---|---|---|---|
| No. of cases (weighted) | 21 387 | 19 066 | 18 632 | 19 658 | 18 768 | 20 349 | 20 459 | 19 575 | — |
| Left heart catheterization | 64.1% | 64.5% | 66.4% | 67.2% | 70.0% | 72.5% | 74.9% | 74.4% | <0.001 |
| Swan‐Ganz catheterization | 9.8% | 9.1% | 7.9% | 7.2% | 6.7% | 6.9% | 6.8% | 6.2% | <0.001 |
| Thrombolysis | 2.9% | 2.9% | 2.1% | 2.3% | 1.9% | 2.4% | 3.5% | 3.6% | <0.001 |
| PCI | |||||||||
| Total | 45.2% | 47.7% | 50.4% | 56.4% | 57.1% | 60.9% | 63.3% | 66.0% | <0.001 |
| Early* | 26.6% | 27.7% | 32.5% | 36.6% | 38.4% | 43.2% | 49.3% | 53.8% | <0.001 |
| CABG | |||||||||
| Total | 15.4% | 14.5% | 16.6% | 16.9% | 15.7% | 16.5% | 14.9% | 12.9% | <0.001 |
| Early* | 4.5% | 5.1% | 5.5% | 5.7% | 5.3% | 6.2% | 5.7% | 4.7% | 0.003 |
| IABP placement | |||||||||
| Total | 44.8% | 45.0% | 48.3% | 51.9% | 51.5% | 53.2% | 54.5% | 53.7% | <0.001 |
| Early* | 26.2% | 26.1% | 30.0% | 32.5% | 34.0% | 35.9% | 41.3% | 41.0% | <0.001 |
| Blood transfusion | 10.4% | 12.3% | 12.9% | 11.6% | 14.7% | 14.2% | 15.0% | 12.7% | <0.001 |
CABG indicates coronary artery bypass grafting; IABP, intra‐aortic balloon pump; PCI, percutaneous coronary intervention.
P value for trend.
Within 24 hours of admission.
Figure 3.
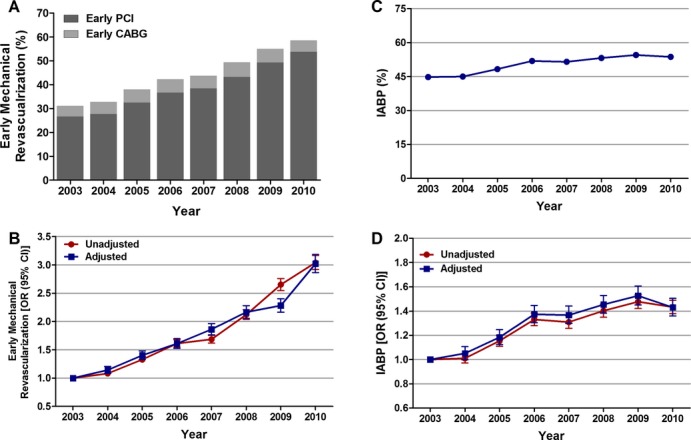
Trends in early mechanical revascularization and IABP use in patients with cardiogenic shock complicating STEMI. A, Early mechanical revascularization was defined as percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) within 24 hours of admission; Ptrend<0.001. B, Trends in early mechanical revascularization presented as unadjusted and adjusted odds ratios and 95% confidence intervals (CIs) for each year relative to 2003 (reference year). Regression model adjusted for demographics, hospital characteristics, 29 Elixhauser and other clinically relevant comorbidities, and presentation. C, IABP (%) was calculated as the number of patients undergoing IABP placement divided by the number of patients with STEMI complicated by cardiogenic shock per year×100; Ptrend<0.001. D, Trends in IABP use presented as unadjusted and adjusted odds ratios and 95% CIs for each year relative to 2003 (reference year). Regression model adjusted for demographics, hospital characteristics, 29 Elixhauser and other clinically relevant comorbidities, and presentation. IABP indicates intra‐aortic balloon pump; OR, odds ratio; STEMI, ST‐elevation myocardial infarction.
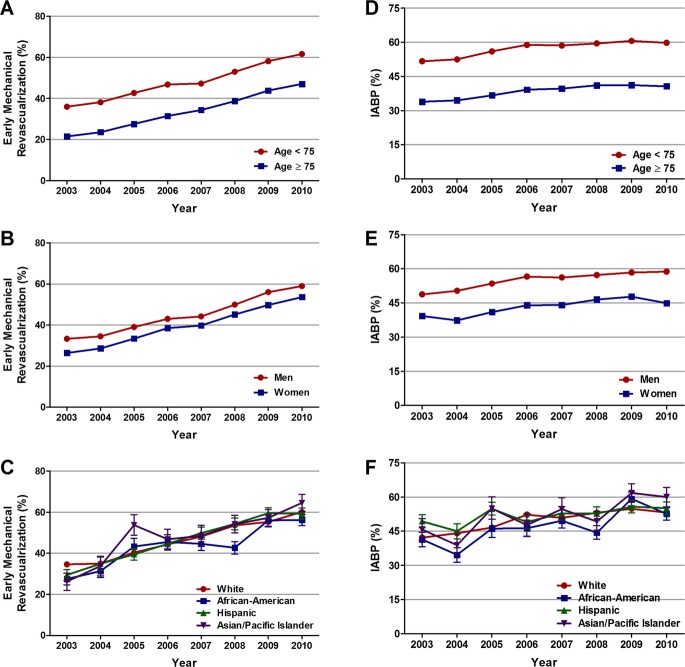
Multivariable logistic regression analysis demonstrated a significantly lower likelihood of receiving early mechanical revascularization in patients aged ≥75 versus <75 years (32.8% versus 48.4%; adjusted OR, 0.68; 95% CI, 0.66 to 0.70; P<0.001), in women versus men (38.9% versus 45.2%; adjusted OR, 0.95; 95% CI, 0.92 to 0.98; P<0.001), and in African Americans versus whites (44.1% versus 47%; adjusted OR, 0.91; 95% CI, 0.87 to 0.96). Despite these differences, trend analysis revealed a similar ≈3‐fold increase in early mechanical revascularization rates over the 8 years in patients <75 and ≥75 years of age, men and women, and in whites, African Americans, and Hispanics (Table 5, Figure 4A through 4C). Interestingly, there was a much steeper increase (6‐fold) in early mechanical revascularization rates in Asian/Pacific Islanders.
Table 5.
Overall and Age‐, Sex‐, and Race/Ethnicity‐Specific Trends in Early Mechanical Revascularization in Patients With Cardiogenic Shock Complicating STEMI
| Year | Overall | Age | Sex | Race/Ethnicity | |||||
|---|---|---|---|---|---|---|---|---|---|
| <75 Years | ≥75 Years | Male | Female | White | African American | Hispanic | Asian/Pacific Islander | ||
| 2003 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2004 | 1.15 (1.08 to 1.21) | 1.15 (1.07 to 1.22) | 1.17 (1.06 to 1.28) | 1.13 (1.06 to 1.21) | 1.20 (1.10 to 1.31) | 1.12 (1.06 to 1.19) | 1.38 (1.10 to 1.74) | 1.21 (0.98 to 1.49) | 1.61 (1.13 to 2.30) |
| 2005 | 1.40 (1.33 to 1.48) | 1.36 (1.27 to 1.45) | 1.52 (1.39 to 1.67) | 1.42 (1.32 to 1.52) | 1.39 (1.28 to 1.52) | 1.36 (1.28 to 1.45) | 2.15 (1.68 to 2.74) | 1.60 (1.32 to 1.94) | 4.22 (2.98 to 5.97) |
| 2006 | 1.61 (1.53 to 1.70) | 1.59 (1.49 to 1.70) | 1.59 (1.45 to 1.75) | 1.53 (1.43 to 1.63) | 1.77 (1.63 to 1.93) | 1.59 (1.50 to 1.69) | 2.12 (1.68 to 2.68) | 1.91 (1.57 to 2.33) | 3.11 (2.21 to 4.39) |
| 2007 | 1.86 (1.77 to 1.96) | 1.80 (1.68 to 1.92) | 1.98 (1.81 to 2.18) | 1.80 (1.69 to 1.93) | 2.00 (1.84 to 2.18) | 1.82 (1.72 to 1.94) | 1.71 (1.37 to 2.14) | 2.36 (1.94 to 2.86) | 3.11 (2.19 to 4.42) |
| 2008 | 2.17 (2.06 to 2.28) | 2.05 (1.93 to 2.19) | 2.51 (2.29 to 2.74) | 2.11 (1.97 to 2.25) | 2.33 (2.14 to 2.53) | 2.20 (2.08 to 2.33) | 1.80 (1.44 to 2.24) | 2.36 (1.94 to 2.86) | 3.73 (2.69 to 5.18) |
| 2009 | 2.28 (2.17 to 2.40) | 2.22 (2.08 to 2.36) | 2.47 (2.25 to 2.71) | 2.28 (2.13 to 2.43) | 2.34 (2.15 to 2.55) | 2.24 (2.11 to 2.37) | 2.88 (2.30 to 3.62) | 2.96 (2.43 to 3.61) | 3.72 (2.68 to 5.16) |
| 2010 | 3.02 (2.87 to 3.19) | 2.96 (2.78 to 3.16) | 3.29 (3.00 to 3.61) | 2.91 (2.72 to 3.11) | 3.30 (3.02 to 3.59) | 2.98 (2.81 to 3.16) | 3.43 (2.77 to 4.26) | 3.09 (2.54 to 3.77) | 6.62 (4.67 to 9.40) |
Trends are expressed as adjusted odds ratios (95% confidence intervals) for each year relative to 2003. Regression model adjusted for demographics (except sex and race/ethnicity for sex‐ and race/ethnicity‐specific trends, respectively), hospital characteristics, all comorbidities, and presentation. STEMI indicates ST‐elevation myocardial infarction.
Figure 4.
Age‐, sex‐, and race‐specific trends in early mechanical revascularization and IABP use in patients with cardiogenic shock complicating STEMI. Trends in early mechanical revascularization in (A) patients <75 and ≥75 years of age, (B) men and women, and (C) whites, African Americans, Hispanics, and Asian/Pacific Islanders, and IABP use in (D) patients <75 and ≥75 years of age, (E) men and women, and (F) whites, African Americans, Hispanics, and Asian/Pacific Islanders, with cardiogenic shock complicating STEMI; Ptrend<0.001 for all. IABP indicates intra‐aortic balloon pump; STEMI, ST‐elevation myocardial infarction.
Similarly, IABP utilization rates were significantly lower in patients aged ≥75 versus <75 years (38.1% versus 57.3%; adjusted OR, 0.59; 95% CI, 0.57 to 0.60; P<0.001), in women versus men (43% versus 55.1%; adjusted OR, 0.73; 95% CI, 0.72 to 0.75; P<0.001), and in African Americans versus whites (47.3% versus 49.9%; adjusted OR, 0.85; 95% CI, 0.81 to 0.90; P<0.001). Trend analysis demonstrated a significant increase in the overall IABP utilization rates from 44.8% in 2003 to 54.5% in 2009, followed by a small decrease to 53.7% in 2010 (unadjusted OR, 1.43; 95% CI, 1.38 to 1.49; P<0.001; adjusted OR, 1.43; 95% CI, 1.36 to 1.51 P<0.001 for 2010 relative to 2003; Figure 3C and 3D). We observed a similar upward trend in IABP utilization in patients <75 and ≥75 years of age, in men and women, and in whites and African Americans (Table 6, Figure 4D through 4F). However, there was no statistically significant increase in IABP utilization rate among Hispanics and Asian/Pacific Islanders over the past 8 years.
Table 6.
Overall and Age‐, Sex‐, and Race/Ethnicity‐Specific Trends in IABP in Patients With Cardiogenic Shock Complicating STEMI
| Year | Overall | Age | Sex | Race/Ethnicity | |||||
|---|---|---|---|---|---|---|---|---|---|
| <75 Years | ≥75 Years | Male | Female | White | African American | Hispanic | Asian/Pacific Islander | ||
| 2003 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2004 | 1.05 (1.00 to 1.11) | 1.05 (0.98 to 1.12) | 1.08 (0.99 to 1.18) | 1.12 (1.04 to 1.19) | 0.95 (0.87 to 1.03) | 1.11 (1.05 to 1.17) | 0.99 (0.79 to 1.23) | 0.77 (0.63 to 0.95) | 0.63 (0.45 to 0.89) |
| 2005 | 1.18 (1.12 to 1.25) | 1.19 (1.12 to 1.27) | 1.21 (1.11 to 1.33) | 1.23 (1.15 to 1.31) | 1.11 (1.03 to 1.21) | 1.20 (1.14 to 1.28) | 1.30 (1.03 to 1.64) | 1.17 (0.97 to 1.40) | 1.29 (0.93 to 1.80) |
| 2006 | 1.37 (1.30 to 1.45) | 1.40 (1.31 to 1.49) | 1.31 (1.20 to 1.43) | 1.45 (1.36 to 1.55) | 1.25 (1.15 to 1.36) | 1.47 (1.39 to 1.56) | 1.36 (1.09 to 1.69) | 0.79 (0.66 to 0.96) | 1.22 (0.88 to 1.69) |
| 2007 | 1.37 (1.30 to 1.44) | 1.36 (1.27 to 1.45) | 1.41 (1.29 to 1.54) | 1.46 (1.36 to 1.56) | 1.24 (1.14 to 1.34) | 1.44 (1.36 to 1.53) | 1.28 (1.04 to 1.58) | 1.02 (0.84 to 1.23) | 1.50 (1.08 to 2.09) |
| 2008 | 1.45 (1.38 to 1.53) | 1.43 (1.35 to 1.53) | 1.57 1.44 to 1.72) | 1.45 (1.36 to 1.55) | 1.48 (1.36 to 1.60) | 1.55 (1.46 to 1.64) | 1.22 (0.99 to 1.50) | 0.89 (0.74 to 1.06) | 0.87 (0.64 to 1.17) |
| 2009 | 1.53 (1.45 to 1.61) | 1.64 (1.54 to 1.74) | 1.35 (1.24 to 1.48) | 1.59 (1.49 to 1.70) | 1.44 (1.33 to 1.57) | 1.60 (1.51 to 1.70) | 2.31 (1.85 to 2.87) | 0.90 (0.75 to 1.09) | 1.44 (1.06 to 1.95) |
| 2010 | 1.43 (1.36 to 1.50) | 1.42 (1.34 to 1.51) | 1.53 (1.40 to 1.68) | 1.57 (1.47 to 1.67) | 1.22 (1.13 to 1.33) | 1.49 (1.41 to 1.58) | 1.66 (1.35 to 2.03) | 0.92 (0.76 to 1.11) | 1.24 (0.90 to 1.71) |
Trends are expressed as adjusted odds ratios (95% confidence intervals) for each year relative to 2003. Regression model adjusted for demographics (except sex and race/ethnicity for sex‐ and race/ethnicity‐specific trends, respectively), hospital characteristics, all comorbidities, and presentation. IABP indicates intra‐aortic balloon pump; STEMI, ST‐elevation myocardial infarction.
Trends in Inhospital Mortality
Inhospital mortality in the overall cohort of patients with STEMI and cardiogenic shock was 39%. Trend analysis showed a significant decrease in inhospital mortality from 44.6% in 2003 to 33.8% in 2010 (unadjusted OR, 0.63; 95% CI, 0.61 to 0.66; P<0.001; Figure 5A). When adjusted for demographics, hospital characteristics, comorbidities, and presentation, there was a 29% decline in inhospital mortality from 2003 to 2010 (adjusted OR, 0.71; 95% CI, 0.68 to 0.75; P<0.001; Figure 5B).
Figure 5.
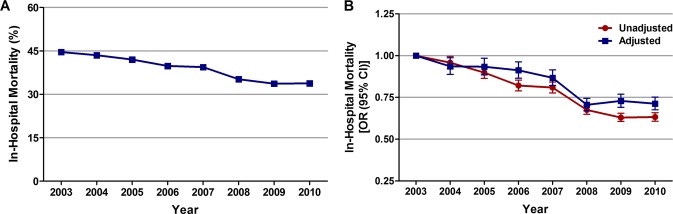
Trends in inhospital mortality in patients with cardiogenic shock complicating STEMI. A, Inhospital mortality (%) was calculated as the number of patients who died during hospitalization divided by the number of patients with STEMI complicated by cardiogenic shock per year×100; Ptrend<0.001. B, Trends in inhospital mortality presented as unadjusted and adjusted odds ratios and 95% confidence intervals (CIs) for each year relative to 2003 (reference year). Regression model adjusted for demographics, hospital characteristics, 29 Elixhauser and other clinically relevant comorbidities, and presentation. OR indicates odds ratio; STEMI, ST‐elevation myocardial infarction.
Inhospital mortality was significantly higher in patients aged ≥75 versus <75 years (55% versus 29.8%; adjusted OR, 2.15; 95% CI, 2.08 to 2.22; P<0.001), in women versus men (44.4% versus 34.5%; adjusted OR, 1.11; 95% CI, 1.08 to 1.14; P<0.001), and in Hispanics versus whites (40.6% versus 38.9%; adjusted OR, 1.11; 95% CI, 1.06 to 1.17; P<0.001) (Table 7). These differences persisted even after adjusting for early mechanical revascularization status. However, similar to the overall inhospital mortality trend, a downward trend in inhospital mortality was observed in patients <75 and ≥75 years of age, in men and women, and in whites, African Americans, and Asian/Pacific Islanders, but not in Hispanics, over the 8‐year period (Figure 6A through 6C, Table 8).
Table 7.
Overall and Age‐, Sex‐, and Race/Ethnicity‐Specific Outcomes in Patients With Cardiogenic Shock Complicating STEMI
| Inhospital Mortality (%) | P Value | Length of Stay (Days) | P Value | Total Hospital Cost ($) | P Value | |
|---|---|---|---|---|---|---|
| Overall | 61 448 (39) | — | 8.9±11.8 | — | 41 774±45 252 | — |
| Age | <0.001 | <0.001 | <0.001 | |||
| <75 Years | 29 965 (29.8) | 9.7±12.8 | 46 709±47 435 | |||
| ≥75 Years | 31 483 (55) | 7.4±9.4 | 33 143±39 710 | |||
| Sex | <0.001 | <0.001 | <0.001 | |||
| Male | 34 127 (35.5) | 9.2±12.1 | 44 744±47 676 | |||
| Female | 27 317 (44.4) | 8.3±11.1 | 37 140±40 755 | |||
| Race/ethnicity | <0.001 | <0.001 | <0.001 | |||
| White | 36 817 (38.9) | 8.7±11.6 | 40 784±44 468 | |||
| African American | 2966 (39.9) | 9.7±13.3 | 43 223±48 872 | |||
| Hispanic | 3587 (40.6) | 9.4±13.6 | 48 296±48 961 | |||
| Asian/Pacific Islander | 1332 (37.6) | 10.0±14.7 | 56 077±58 655 |
STEMI indicates ST‐elevation myocardial infarction.
Figure 6.
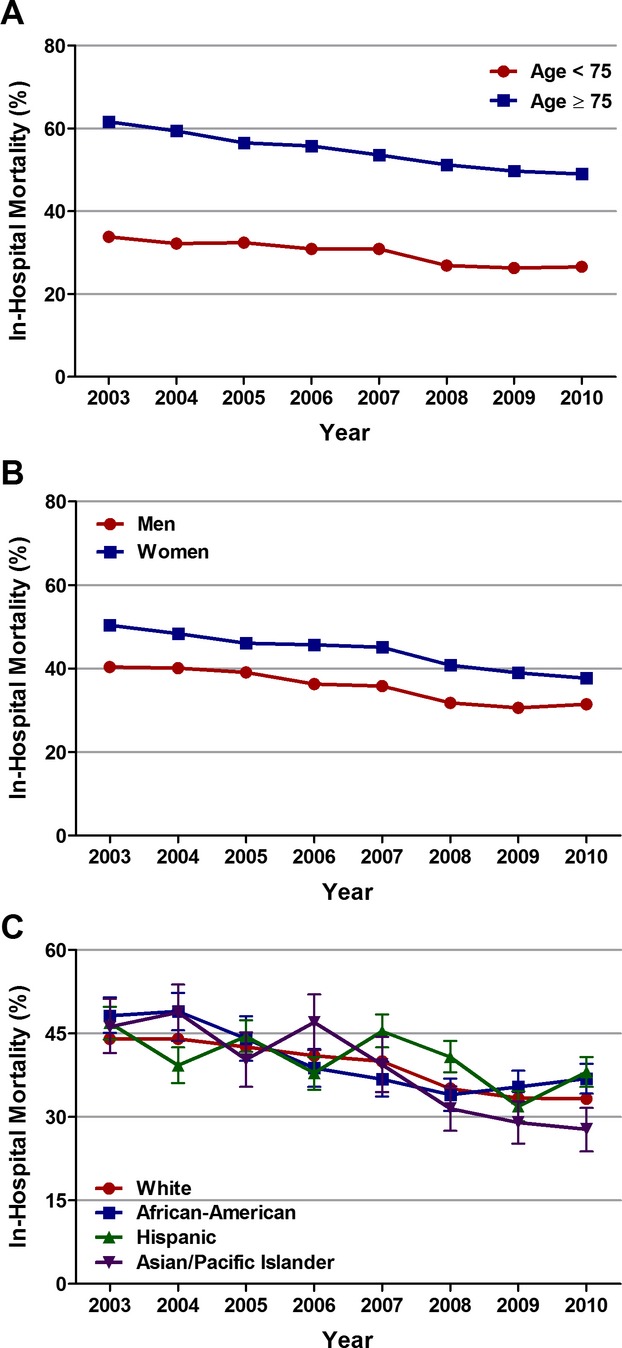
Age‐, sex‐, and race‐specific trends in inhospital mortality in patients with cardiogenic shock complicating ST‐elevation myocardial infarction (STEMI). Trends in inhospital mortality in (A) patients <75 and ≥75 years of age, (B) men and women, and (C) whites, African Americans, Hispanics, and Asian/Pacific Islanders with cardiogenic shock complicating STEMI; Ptrend<0.001 for all.
Table 8.
Overall and Age‐, Sex‐, and Race/Ethnicity‐Specific Trends in Inhospital Mortality in Patients With Cardiogenic Shock Complicating STEMI
| Year | Overall | Age | Sex | Race/Ethnicity | |||||
|---|---|---|---|---|---|---|---|---|---|
| <75 Years | ≥75 Years | Male | Female | White | African American | Hispanic | Asian/Pacific Islander | ||
| Regression Model 1* | |||||||||
| 2003 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2004 | 0.94 (0.89 to 0.99) | 0.94 (0.88 to 1.01) | 0.93 (0.86 to 1.01) | 0.91 (0.85 to 0.97) | 0.97 (0.89 to 1.05) | 0.94 (0.88 to 0.99) | 1.04 (0.84 to 1.28) | 0.80 (0.65 to 0.98) | 1.02 (0.72 to 1.43) |
| 2005 | 0.93 (0.89 to 0.98) | 1.02 (0.95 to 1.10) | 0.82 (0.75 to 0.89) | 0.93 (0.87 to 1.00) | 0.93 (0.86 to 1.01) | 0.94 (0.88 to 0.99) | 0.96 (0.75 to 1.21) | 0.94 (0.78 to 1.13) | 0.73 (0,52 to 1.02) |
| 2006 | 0.91 (0.87 to 0.96) | 0.93 (0.87 to 1.00) | 0.89 (0.82 to 0.97) | 0.90 (0.84 to 0.97) | 0.94 (0.86 to 1.02) | 0.95 (0.90 to 1.01) | 0.74 (0.59 to 0.92) | 0.79 (0.65 to 0.97) | 0.86 (0.62 to 1.20) |
| 2007 | 0.87 (0.82 to 0.92) | 0.94 (0.88 to 1.01) | 0.76 (0.70 to 0.82) | 0.86 (0.80 to 0.92) | 0.88 (0.81 to 0.96) | 0.89 (0.84 to 0.95) | 0.74 (0.60 to 0.92) | 1.12 (0.92 to 1.35) | 0.72 (0.51 to 1.01) |
| 2008 | 0.71 (0.67 to 0.74) | 0.75 (0.70 to 0.80) | 0.64 (0.59 to 0.70) | 0.72 (0.67 to 0.77) | 0.68 (0.63 to 0.74) | 0.72 (0.68 to 0.77) | 0.55 (0.44 to 0.68) | 0.86 (0.71 to 1.04) | 0.68 (0.49 to 0.95) |
| 2009 | 0.73 (0.69 to 0.77) | 0.80 (0.75 to 0.86) | 0.60 (0.56 to 0.66) | 0.73 (0.68 to 0.78) | 0.74 (0.68 to 0.80) | 0.76 (0.71 to 0.80) | 0.72 (0.57 to 0.89) | 0.71 (0.58 to 0.87) | 0.61 (0.44 to 0.85) |
| 2010 | 0.71 (0.68 to 0.75) | 0.77 (0.72 to 0.83) | 0.61 (0.56 to 0.66) | 0.77 (0.72 to 0.82) | 0.63 (0.58 to 0.69) | 0.72 (0.68 to 0.76) | 0.67 (0.54 to 0.83) | 0.94 (0.77 to 1.14) | 0.36 (0.26 to 0.51) |
| Regression Model 2* | |||||||||
| 2003 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2004 | 0.95 (0.90 to 1.00) | 0.96 (0.89 to 1.03) | 0.94 (0.87 to 1.02) | 0.92 (0.86 to 0.99) | 0.99 (0.91 to 1.07) | 0.95 (0.89 to 1.01) | 1.06 (0.86 to 1.32) | 0.83 (0.67 to 1.02) | 1.06 (0.75 to 1.50) |
| 2005 | 0.97 (0.92 to 1.02) | 1.05 (0.98 to 1.13) | 0.85 (0.79 to 0.93) | 0.97 (0.91 to 1.04) | 0.96 (0.89 to 1.04) | 0.97 (0.91 to 1.03) | 1.01 (0.80 to 1.28) | 1.00 (0.83 to 1.21) | 0.88 (0.63 to 1.25) |
| 2006 | 0.96 (0.91 to 1.02) | 0.98 (0.92 to 1.06) | 0.94 (0.86 to 1.02) | 0.95 (0.88 to 1.02) | 0.99 (0.91 to 1.08) | 1.01 (0.95 to 1.07) | 0.78 (0.62 to 0.97) | 0.86 (0.71 to 1.05) | 1.00 (0.71 to 1.40) |
| 2007 | 0.93 (0.88 to 0.98) | 1.00 (0.93 to 1.08) | 0.82 (0.75 to 0.89) | 0.92 (0.86 to 0.99) | 0.95 (0.88 to 1.04) | 0.96 (0.90 to 1.02) | 0.78 (0.63 to 0.96) | 1.27 (1.04 to 1.54) | 0.83 (0.58 to 1.17) |
| 2008 | 0.77 (0.73 to 0.81) | 0.80 (0.75 to 0.86) | 0.71 (0.66 to 0.78) | 0.79 (0.73 to 0.84) | 0.75 (0.69 to 0.81) | 0.79 (0.74 to 0.84) | 0.57 (0.46 to 0.70) | 0.97 (0.79 to 1.18) | 0.81 (0.58 to 1.13) |
| 2009 | 0.80 (0.76 to 0.85) | 0.88 (0.82 to 0.94) | 0.67 (0.62 to 0.73) | 0.80 (0.75 to 0.86) | 0.80 (0.74 to 0.87) | 0.83 (0.78 to 0.88) | 0.78 (0.62 to 0.97) | 0.83 (0.68 to 1.02) | 0.72 (0.52 to 1.00) |
| 2010 | 0.81 (0.77 to 0.85) | 0.86 (0.81 to 0.93) | 0.70 (0.64 to 0.77) | 0.87 (0.81 to 0.94) | 0.72 (0.66 to 0.78) | 0.81 (0.77 to 0.87) | 0.74 (0.60 to 0.91) | 1.11 (0.91 to 1.36) | 0.45 (0.32 to 0.64) |
Trends are expressed as adjusted odds ratios (95% confidence intervals) for each year relative to 2003. STEMI indicates ST‐elevation myocardial infarction.
Adjusted for demographics (except sex and race/ethnicity for sex‐ and race/ethnicity‐specific trends, respectively), hospital characteristics, all comorbidities, and presentation.
Adjusted for demographics (except sex and race/ethnicity for sex‐ and race/ethnicity‐specific trends, respectively), hospital characteristics, all comorbidities, presentation, and early mechanical revascularization.
Trends in Length of Stay and Total Hospital Cost
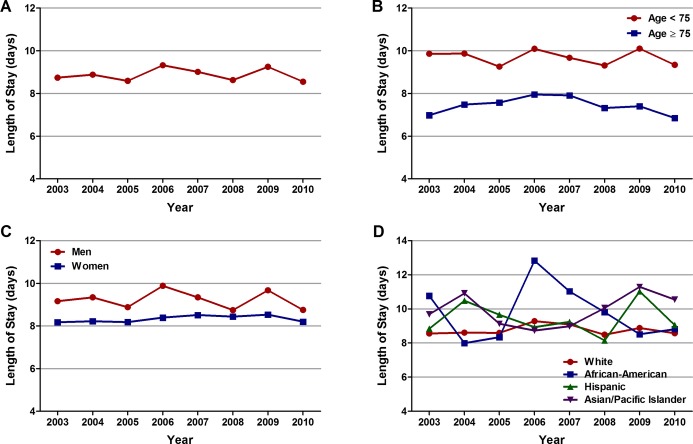
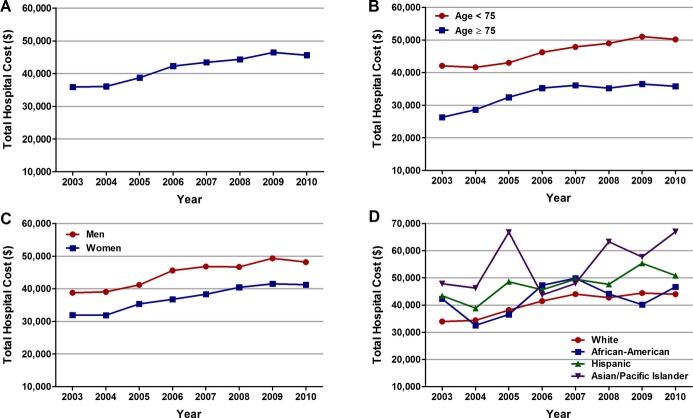
The average length of stay and total hospital cost for the overall cohort of patients with STEMI and cardiogenic shock was 8.9±11.8 days and $41 774±45 252, respectively. The average length of stay was shorter in patients aged ≥75 years and in women, most likely as a consequence of higher inhospital mortality in these groups (Table 7). Trend analysis revealed no significant change in the average length of stay over the 8 years, even across age, sex, and each racial/ethnic group (Ptrend>0.001; Figure 7A through 7D). On the other hand, the average total hospital cost increased from $35 892 in 2003 to $46 454 in 2009 (Ptrend<0.001), followed by a small decline to $45 625 in 2010 (Figure 8A). A similar trend was observed in patients <75 and ≥75 years of age, men and women, and across each racial/ethnic group (Figure 8B through 8D). Asian/Pacific Islanders accrued the highest total hospital cost (Table 7).
Figure 7.
Overall and age‐, sex‐, and race‐specific trends in average length of stay in patients with cardiogenic shock complicating ST‐elevation myocardial infarction (STEMI). Trends in average length of stay (days) in patients with cardiogenic shock complicating STEMI (A) overall, (B) in patients <75 and ≥75 years of age, (C) in men and women, and (D) in whites, African Americans, Hispanics, and Asian/Pacific Islanders; Ptrend>0.001 for all.
Figure 8.
Overall and age‐, sex‐, and race‐specific trends in average total hospital cost in patients with cardiogenic shock complicating ST‐elevation myocardial infarction (STEMI). Trends in average total hospital cost ($) in patients with cardiogenic shock complicating STEMI (A) overall, (B) in patients <75 and ≥75 years of age, (C) in men and women, and (D) in whites, African Americans, Hispanics, and Asian/Pacific Islanders; Ptrend<0.001 for all.
Discussion
In this large United States population‐based observational study, we found increasing rates of cardiogenic shock in patients with STEMI from 2003 to 2008. This was also accompanied by an increase in early mechanical revascularization and IABP use, a decline in inhospital mortality, and an increase in average total hospital cost over the study period. Despite similar trends in patients <75 and ≥75 years of age, men and women, and racial/ethnic groups over the past 8 years, we observed significant age, sex, and racial/ethnic differences in the treatment and outcomes of cardiogenic shock after STEMI.
Incidence Rates of Cardiogenic Shock in STEMI
In our study, the overall rate of cardiogenic shock complicating STEMI was 7.9%, and the proportion of STEMI patients developing cardiogenic shock increased from 6.5% in 2003 to 10.1% in 2010. The incidence of cardiogenic shock in AMI has ranged from 5% to 10% in previously published studies.1–2 Previous studies also provided conflicting information about the temporal trends in cardiogenic shock. Results from the National Hospital Discharge Survey in the United States, the population‐based study of residents in the Worcester metropolitan area (Massachusetts), and the AMIS (Acute Myocardial Infarction in Switzerland) Plus Registry in Switzerland showed decreasing rates of cardiogenic shock in patients with AMI (non‐ST‐ and ST‐elevation myocardial infarction) from 1979 to 2004, 1975 to 2005, and 1997 to 2006, respectively.8–10 On the other hand, data from the NRMI (National Registry of Myocardial Infarction) showed a slight but statistically significant upward trend in rates of cardiogenic shock complicating STEMI in patients <75 but not for those ≥75 years of age from 1995 to 2004.7 In our study we observed an increasing trend in cardiogenic shock in STEMI patients <75 as well as ≥75 years of age. These differences in the overall incidence rates and temporal trends could be a result of variation in the definitions of AMI (inclusion of both non‐ST‐ and ST‐elevation versus ST‐elevation myocardial infarction alone in the current study) and cardiogenic shock (clinical versus ICD‐9‐CM code) used, the use of representative versus more selected patient population, and the periods under study. Nonetheless, the increasing rates of cardiogenic shock complicating STEMI are indeed surprising. One potential explanation could be a result of more frequent diagnosis, either as a result of “diagnosis‐related groups (DRG) creep” (defined as changes in hospital record documentation to increase case mix and reimbursement), early recognition, or increased diagnosis to improve operator/hospital outcomes because in some states (eg, New York) STEMI with cardiogenic shock is excluded from outcomes reporting.13–14 Because prior studies have shown that ≈70% of patients with cardiogenic shock complicating AMI develop shock after hospitalization, one might also speculate that iatrogenic cardiogenic shock caused by increasing utilization of proven therapies such as PCI, β‐blockers, angiotensin‐converting enzyme inhibitors, or diuretics might be contributing to the increasing trend observed in this study.7,15 However, because NIS is an administrative database, it is difficult to evaluate these individual hypotheses and to provide a single valid explanation for the observed increase in incidence of cardiogenic shock complicating STEMI.
The incidence of cardiogenic shock after STEMI has been shown to be higher in women than in men.16–18 Also in our study, incidence rates of cardiogenic shock complicating STEMI were higher in women than in men throughout the 8‐year period. To our knowledge, ours is the first study to demonstrate racial/ethnic variation in the incidence of cardiogenic shock after STEMI. The incidence of cardiogenic shock was highest in Asian/Pacific Islanders (11.4% versus 8% in whites, 6.9% in African Americans, and 8.6% in Hispanics, P<0.001).
Early Mechanical Revascularization and Inhospital Mortality in Cardiogenic Shock
Regardless of the wide variation in the incidence rates and differences in temporal trends of cardiogenic shock complicating AMI, previous studies have consistently demonstrated an increasing trend in early mechanical revascularization and a decreasing trend in inhospital mortality over the past years in these patients.8–10 In our current analysis of the NIS database, there was a significant increase in total and early PCI rates from 2003 to 2010 (Table 4). On the other hand, total CABG rates decreased from 15.4% to 12.9%. Early CABG rates increased from 4.5% in 2003 to 6.2% in 2008, followed by declines in 2009 and 2010. These trends parallel the revascularization trends observed from 1994 to 2003 in patients with STEMI and cardiogenic shock included in the NRMI.7 Although the SHOCK trial demonstrated no difference in 30‐day or 1‐year mortality between patients undergoing emergency PCI versus emergency CABG, the observed trends could be a reflection of the overall increasing utilization of PCI and decreasing CABG rates in patients with AMI (irrespective of the presence of cardiogenic shock) over the past decade.19–23 Nonetheless, we observed a steady and significant decline in inhospital mortality in patients with STEMI and cardiogenic shock (Figure 5), which is likely a result of the overall increasing use of early mechanical revascularization in these patients.
Data on age, sex, and racial/ethnic differences in early revascularization and outcomes among patients with cardiogenic shock after STEMI are conflicting. The SHOCK trial showed no survival benefit with early revascularization in the small subgroup of patients aged ≥75 years.3 However, data from the SHOCK and NRMI registries showed decreased mortality with early revascularization in these patients.7,24 Prior studies have demonstrated that compared with men, women receive less evidence‐based medical care and have higher mortality rates after STEMI, even in the era of reperfusion therapy.25 The SHOCK registry as well as a recent single‐center study in the United Kingdom showed no sex differences in inhospital mortality in patients with cardiogenic shock after AMI.26–27 The SHOCK registry demonstrated lower revascularization rates and higher inhospital mortality in Hispanics (followed by African Americans) compared with whites with cardiogenic shock after AMI.28 Even though there was a similar increase in early mechanical revascularization rates and decrease in inhospital mortality across all age, sex, and racial/ethnic groups over the past 8 years, we observed significantly lower early mechanical revascularization rates in patients aged ≥75 versus <75 years, women versus men, and African Americans versus whites. Inhospital mortality was also significantly higher in patients aged ≥75 versus <75 years, women versus men, and Hispanics versus whites.
IABP Trends
IABP is the most widely used form of mechanical hemodynamic support in patients with cardiogenic shock. But data on the usefulness of IABP in this setting are conflicting. A meta‐analysis of 7 randomized trials comparing IABP use with no IABP use in STEMI patients with cardiogenic shock showed neither a 30‐day survival benefit nor improved left ventricular ejection fraction with IABP use, while being associated with significantly higher stroke and bleeding rates.29 Similarly, in a meta‐analysis of 9 cohort studies, IABP was associated with a decrease in 30‐day mortality in patients treated with thrombolysis but not in those treated with primary PCI.29 More recently, the IABP SHOCK II trial showed that use of IABP in patients with cardiogenic shock complicating AMI who underwent early revascularization did not reduce 30‐day mortality compared with medical therapy alone.30 In the present study, we observed an increasing trend in IABP use from 2003 to 2009, followed by a minor decline in 2010. We observed a similar upward trend in IABP use in patients <75 and ≥75 years of age, men and women, and in whites and African Americans.
Study Limitations
Our study has certain limitations. First, because this is a retrospective observational study, the possibility of selection bias and residual measured and unmeasured confounding cannot be completely eliminated. Second, as NIS is an administrative database, the accuracy and consistency of the data depend heavily on the training and expertise of the coders. Hence, given the abstracted nature of the database, there may have been wide variation in the data because of unrecognized miscoding of diagnostic and procedure codes. However, these potential limitations may be partially compensated for by the large size of the database and the ability to obtain nationwide estimates using the discharge weights provided. There is also the potential that the criteria for identifying and coding cardiogenic shock have changed over time. Third, the NIS database does not allow differentiation of cardiogenic shock present on admission with that developing during hospitalization. Fourth, why early mechanical revascularization was not undertaken in individual patients is difficult to ascertain.
Conclusion
Cardiogenic shock remains the leading cause of death in patients hospitalized with AMI. During the last 8 years, we observed an increase in the incidence of cardiogenic shock in patients hospitalized with STEMI in the United States. There was a significant increase in early mechanical revascularization and IABP use in patients with cardiogenic shock complicating STEMI during this period. This was associated with a declining trend in risk‐adjusted inhospital mortality, but increased total hospital costs over the past 8 years. Despite similar trends in patients <75 and ≥75 years of age, men and women, and racial/ethnic groups over the past 8 years, there remain significant age, sex, and racial/ethnic differences in the treatment and outcomes of cardiogenic shock after STEMI.
Disclosures
None.
References
- 1.Goldberg RJ, Samad NA, Yarzebski J, Gurwitz J, Bigelow C, Gore JM. Temporal trends in cardiogenic shock complicating acute myocardial infarction. N Engl J Med. 1999; 340:1162-1168 [DOI] [PubMed] [Google Scholar]
- 2.Goldberg RJ, Gore JM, Thompson CA, Gurwitz JH. Recent magnitude of and temporal trends (1994–1997) in the incidence and hospital death rates of cardiogenic shock complicating acute myocardial infarction: the second national registry of myocardial infarction. Am Heart J. 2001; 141:65-72 [DOI] [PubMed] [Google Scholar]
- 3.Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, McKinlay SM, LeJemtel TH. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999; 341:625-634 [DOI] [PubMed] [Google Scholar]
- 4.Hochman JS, Sleeper LA, White HD, Dzavik V, Wong SC, Menon V, Webb JG, Steingart R, Picard MH, Menegus MA, Boland J, Sanborn T, Buller CE, Modur S, Forman R, Desvigne‐Nickens P, Jacobs AK, Slater JN, LeJemtel TH. One‐year survival following early revascularization for cardiogenic shock. JAMA. 2001; 285:190-192 [DOI] [PubMed] [Google Scholar]
- 5.Hochman JS, Sleeper LA, Webb JG, Dzavik V, Buller CE, Aylward P, Col J, White HD. Early revascularization and long‐term survival in cardiogenic shock complicating acute myocardial infarction. JAMA. 2006; 295:2511-2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis‐Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction. Circulation. 2013; 127:e362-e425 [DOI] [PubMed] [Google Scholar]
- 7.Babaev A, Frederick PD, Pasta DJ, Every N, Sichrovsky T, Hochman JS. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2005; 294:448-454 [DOI] [PubMed] [Google Scholar]
- 8.Fang J, Mensah GA, Alderman MH, Croft JB. Trends in acute myocardial infarction complicated by cardiogenic shock, 1979–2003, United States. Am Heart J. 2006; 152:1035-1041 [DOI] [PubMed] [Google Scholar]
- 9.Jeger RV, Radovanovic D, Hunziker PR, Pfisterer ME, Stauffer JC, Erne P, Urban P. Ten‐year trends in the incidence and treatment of cardiogenic shock. Ann Intern Med. 2008; 149:618-626 [DOI] [PubMed] [Google Scholar]
- 10.Goldberg RJ, Spencer FA, Gore JM, Lessard D, Yarzebski J. Thirty‐year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population‐based perspective. Circulation. 2009; 119:1211-1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998; 36:8-27 [DOI] [PubMed] [Google Scholar]
- 12.HCUP Comorbidity Software Healthcare Cost and Utilization Project (HCUP). 2013Rockville, MD: Agency for Healthcare Research and Quality; www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp [PubMed] [Google Scholar]
- 13.Steinwald B, Dummit LA. Hospital case‐mix change: sicker patients or DRG creep? Health Aff (Millwood). 1989; 8:35-47 [DOI] [PubMed] [Google Scholar]
- 14.Apolito RA, Greenberg MA, Menegus MA, Lowe MA, Sleeper MA, Goldberger MH, Remick J, Radford MJ, Hochman JS. Impact of the New York State Cardiac Surgery and Percutaneous Coronary Intervention Reporting System on the management of patients with acute myocardial infarction complicated by cardiogenic shock. Am Heart J. 2008; 155:267-273 [DOI] [PubMed] [Google Scholar]
- 15.Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation. 2008; 117:686-697 [DOI] [PubMed] [Google Scholar]
- 16.Goldberg RJ, Gore JM, Alpert JS, Osganian V, de Groot J, Bade J, Chen Z, Frid D, Dalen JE. Cardiogenic shock after acute myocardial infarction. Incidence and mortality from a community‐wide perspective, 1975 to 1988. N Engl J Med. 1991; 325:1117-1122 [DOI] [PubMed] [Google Scholar]
- 17.Akhter N, Milford‐Beland S, Roe MT, Piana RN, Kao J, Shroff A. Gender differences among patients with acute coronary syndromes undergoing percutaneous coronary intervention in the American College of Cardiology‐National Cardiovascular Data Registry (ACC‐NCDR). Am Heart J. 2009; 157:141-148 [DOI] [PubMed] [Google Scholar]
- 18.Abdel‐Qadir HM, Ivanov J, Austin PC, Tu JV, Dzavik V. Sex differences in the management and outcomes of Ontario patients with cardiogenic shock complicating acute myocardial infarction. Can J Cardiol. 2013; 29:691-696 [DOI] [PubMed] [Google Scholar]
- 19.White HD, Assmann SF, Sanborn TA, Jacobs AK, Webb JG, Sleeper LA, Wong CK, Stewart JT, Aylward PE, Wong SC, Hochman JS. Comparison of percutaneous coronary intervention and coronary artery bypass grafting after acute myocardial infarction complicated by cardiogenic shock: results from the Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock (SHOCK) trial. Circulation. 2005; 112:1992-2001 [DOI] [PubMed] [Google Scholar]
- 20.Dunlay SM, Rihal CS, Sundt TM, Gerber Y, Roger VL. Current trends in coronary revascularization. Curr Treat Options Cardiovasc Med. 2009; 11:61-70 [DOI] [PubMed] [Google Scholar]
- 21.Frutkin AD, Lindsey JB, Mehta SK, House JA, Spertus JA, Cohen DJ, Rumsfeld JS, Marso SP. Drug‐eluting stents and the use of percutaneous coronary intervention among patients with class I indications for coronary artery bypass surgery undergoing index revascularization: analysis from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv. 2009; 2:614-621 [DOI] [PubMed] [Google Scholar]
- 22.Epstein AJ, Polsky D, Yang F, Yang L, Groeneveld PW. Coronary revascularization trends in the United States, 2001–2008. JAMA. 2011; 305:1769-1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khera S, Kolte D, Palaniswamy C, Mujib M, Aronow WS, Singh T, Gotsis W, Silverman G, Frishman WH. ST‐elevation myocardial infarction in the elderly—temporal trends in incidence, utilization of percutaneous coronary intervention and outcomes in the United States. Int J Cardiol. 2013; 168:3683-3690 [DOI] [PubMed] [Google Scholar]
- 24.Dzavik V, Sleeper LA, Cocke TP, Moscucci M, Saucedo J, Hosat S, Jiang X, Slater J, LeJemtel T, Hochman JS. Early revascularization is associated with improved survival in elderly patients with acute myocardial infarction complicated by cardiogenic shock: a report from the SHOCK Trial Registry. Eur Heart J. 2003; 24:828-837 [DOI] [PubMed] [Google Scholar]
- 25.Jneid H, Fonarow GC, Cannon CP, Hernandez AF, Palacios IF, Maree AO, Wells Q, Bozkurt B, Labresh KA, Liang L, Hong Y, Newby LK, Fletcher G, Peterson E, Wexler L. Sex differences in medical care and early death after acute myocardial infarction. Circulation. 2008; 118:2803-2810 [DOI] [PubMed] [Google Scholar]
- 26.Wong SC, Sleeper LA, Monrad ES, Menegus MA, Palazzo A, Dzavik V, Jacobs A, Jiang X, Hochman JS. Absence of gender differences in clinical outcomes in patients with cardiogenic shock complicating acute myocardial infarction. A report from the SHOCK Trial Registry. J Am Coll Cardiol. 2001; 38:1395-1401 [DOI] [PubMed] [Google Scholar]
- 27.Kunadian V, Qiu W, Bawamia B, Veerasamy M, Jamieson S, Zaman A. Gender comparisons in cardiogenic shock during ST elevation myocardial infarction treated by primary percutaneous coronary intervention. Am J Cardiol. 2013; 112:636-641 [DOI] [PubMed] [Google Scholar]
- 28.Palmeri ST, Lowe AM, Sleeper LA, Saucedo JF, Desvigne‐Nickens P, Hochman JS. Racial and ethnic differences in the treatment and outcome of cardiogenic shock following acute myocardial infarction. Am J Cardiol. 2005; 96:1042-1049 [DOI] [PubMed] [Google Scholar]
- 29.Sjauw KD, Engstrom AE, Vis MM, van der Schaaf RJ, Baan J, Jr, Koch KT, de Winter RJ, Piek JJ, Tijssen JG, Henriques JP. A systematic review and meta‐analysis of intra‐aortic balloon pump therapy in ST‐elevation myocardial infarction: should we change the guidelines? Eur Heart J. 2009; 30:459-468 [DOI] [PubMed] [Google Scholar]
- 30.Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Böhm M, Ebelt H, Schneider S, Schuler G, Werdan K. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012; 367:1287-1296 [DOI] [PubMed] [Google Scholar]