Abstract
Background
Elevated urinary globotriaosylceramide (Gb3) has been considered a hallmark of Fabry disease, an X‐linked lysosomal disorder that is a risk factor for most types of heart disease.
Methods and Results
We screened 1421 consecutive patients with common forms of heart disease for Fabry disease by measuring urinary Gb3 in whole urine using tandem mass spectrometry, α‐galactosidase A activity in dried blood spots, and we looked for GLA mutations by parallel sequencing of the whole gene (exons and introns) in pooled genomic DNA samples followed by Sanger sequencing verification. GLA variants were found in 13 patients. In the 1408 patients without GLA mutations, urinary Gb3 levels were significantly higher in heart disease patients compared to 116 apparently healthy controls (median difference=10.0 ng/mL and P<0.001). Urinary lipid profiling showed that levels of 5 other lipids significantly distinguished between urine of patients with Fabry disease (n=7) and heart disease patients with elevated urinary Gb3 (n=6). Sphingomyelin and Gb3 levels were abnormal in the left ventricular wall of patients with ischemic heart failure. Elevated levels of urinary Gb3 were independently associated with increased risk of death in the average follow‐up of 17 months (hazard ratio=1.59 for increase in Gb3 of 200, 95% CI=1.36 and 1.87, and P<0.0001).
Conclusions
In heart disease patients who do not have Fabry disease or GLA gene mutations, a higher level of urinary Gb3 is positively associated with near‐term mortality. The elevation of urinary Gb3 and that of other lipids suggests that heart disease is associated with multiorgan lipid abnormalities.
Clinical Trial Registration
URL: clinicaltrials.gov. Unique Identifier: NCT01019629.
Keywords: globotriaosylceramide, heart disease, risk factor, sphingolipids
Introduction
Although the number of potential cardiovascular biomarkers continues to grow, most provide only limited added value to established biomarkers for predicting pathology and outcomes. This is probably because the newer markers are not truly mechanistically independent, but rather connected to pathways already known to be associated with cardiovascular disease (eg, inflammation, thrombosis/hemostasis, cholesterol transport).1 Certain known biomarkers are useful. For example, the persistent increases in blood levels of biomarkers such as troponin or natriuretic peptide are known to have prognostic value in post‐myocardial infarction patients.2 To date, no biomarker has emerged as the best screening indicator for cardiovascular disease.3
Fabry disease is an X‐linked genetic disorder (OMIM 301500). The incidence of the disease has been estimated to be at 1 in 117 000 live male births,4 however, recent newborn screening surveys suggest that the incidence may be as high as 1:3100.5 Its major complications are an increased risk of stroke, cardiac disease including cardiomyopathy, atrio‐ventricular conduction defects, arrhythmia, valvular dysfunction, cardiac vascular disease, and progressive renal failure.6 Fabry disease is caused by a deficiency of the lysosomal enzyme α‐galactosidase A and accumulation of the glycosphingolipid globotriaosylceramide (Gb3) in most cells and organs, as well as an increase of Gb3 in urine.7–9 Urinary Gb3 is not primarily in the filtrate, but is mostly in shed renal tubular cells.9–10 Increased urinary Gb3 is considered a hallmark of the disease, is considered to be specific for Fabry, and is often used in screening for this disorder.11–12
In a prospective screening study for Fabry disease among patients with common forms of heart disease, we found that Gb3 is elevated not only in patients with Fabry disease but also in the general population of patients with non‐Fabry–related heart disease. We looked for abnormalities in other urinary lipids in this patient population and investigated their possible relevance to the heart. We then hypothesized that elevated Gb3 may be a risk marker in patients with heart disease and may have prognostic value for assessment of near‐term risk of death.
Materials and Methods
Clinical Study
We screened for Fabry disease in a population of patients with multiple forms of cardiovascular disease (ClinicalTrials.gov Identifier: NCT01019629). These included coronary artery disease (CAD), conduction or rhythm abnormalities, nonischemic cardiomyopathy, or valvular dysfunction. The patients were ambulatory, had to be over 18 years of age and were seen at a number of institutions in Dallas: Baylor Heart and Vascular Hospital in Dallas, the Heart Hospital at Baylor Plano, Soltero Cardiovascular Research Center, and cardiology outpatient clinics in Dallas, Texas. More than 95% of patients who were asked to participate in the study accepted and gave a written informed consent (1421 patients). The Institutional Review Board (IRB) of the Baylor Research Institute provided oversight for the heart disease Gb3 study while the IRB at the University of Colorado had oversight for the heart transplant part of the study. In parallel, healthy control subjects were recruited among friends and relatives of patients with heart disease admitted to the heart hospitals or seen in the clinics. Apparently healthy controls used for comparison of urinary Gb3 levels were subjects recruited to the study who had no history of any cardiac disease and were not taking any cardiac related medications. Screening was performed by measuring urinary Gb3 in randomly collected samples of whole urine using ultra high pressure chromatography‐tandem mass spectrometry (UPLC‐MS/MS), measuring α‐galactosidase A activity in dried blood spots by flow injection analysis‐tandem mass spectrometry (FIA‐MS/MS), and looking for GLA gene mutations by parallel sequencing of the whole gene in pooled genomic DNA samples. Conventional Sanger sequencing was used to further analyze individual samples from selected patient DNA pools. Mortality (all causes) of a patient was determined by accessing the Social Security Death Index.
Urinary Gb3 Analysis by Mass Spectrometry
Urinary Gb3 was measured by UPLC‐MS/MS. We saw no effect on Gb3 levels up to 3 freeze/refreeze cycles. None of the urinary Gb3 measurements were in urine that underwent more than 2 freeze/refreeze cycles. The analytical method was based on a published method13 with some modifications.14 Briefly, 25 μL of C17‐Gb3 at a concentration of 50 μg/mL were added to 1 mL of urine dried on a 5×5 cm filter paper square and were extracted with 4 mL of methanol. Ten microliters were injected into the UPLC‐MS/MS system. Chromatography with a fast methanol/water gradient was performed using a C8 BEH, 1×50 mm, 1.7 μm UPLC column, at 60°C, with a total run time of 3 minutes, including column re‐equilibration. Gb3 was detected with a Quattro Premier tandem mass spectrometer, in positive ion mode. Multiple reaction monitoring (MRM) transitions were: m/z 1060.6→898.6 for C17‐Gb3 and 1046.6→884.6 for C16:0, 1074→912.6 for C18:0, 1102→940.6 for C20:0, 1128→966.6 for C22:1, 1130→968.6 for C22:0, 1156→994.6 for C24:1, 1158→996.6 for C24:0, 1174→1012.6 for C24:0(OH) for a total of 8 Gb3 isoforms. Concentrations of urinary Gb3 were expressed as ng/mL. We had previously determined that it is preferable to express urinary Gb3 concentration per urine volume rather than creatinine concentration.15
α‐Galactosidase A Activity Evaluation by Tandem Mass Spectrometry
The analytical procedure was based on the “Triplex” method.14 A 3‐mm dried blood spot punch was incubated for 18 hours at 37°C in a single assay buffer with substrate and internal standard. Fabry internal standard (α‐galactosidase A [GLA]‐IS) and Fabry substrate were from Drs H. Zhou and V. De Jesus (CDC, Atlanta, GA, USA).
The samples were processed by a simple liquid‐liquid extraction by using ethyl acetate. The extracts were dried and resuspended in 80/20 v/v acetonitrile/water with 0.2% formic acid for injection into the tandem mass spectrometer. Products and internal standard were monitored by MRM.14 Samples were processed in a 96‐well plate and each plate included 6 blank samples and quality controls in duplicate. Quality control DBS samples (low, medium, and high) were obtained from Drs H. Zhou and V. De Jesus at the CDC in Atlanta. Twenty microliters were injected for flow injection analysis—tandem mass spectrometry using a Micromass (Waters) Quattro LC triple quadrupole. The flow rate was 40 μL/min. MRM transitions were m/z 489.3→389.3 for [GLA]‐IS and m/z 483.3→383.3 for GLA product.
Gb3 Analysis of Heart Tissue by Mass Spectrometry
Human heart tissue (left ventricular tip, full wall thickness) was obtained at the time of transplantation from patients with end‐stage heart failure due to ischemic heart disease. Control samples from subjects without heart failure were obtained from hearts harvested for transplantation, but unutilized for noncardiac reasons. Both patients and controls were randomly selected. Tissue was flash frozen in liquid nitrogen according to methods we have previously described. The research was approved by the IRBs at the University of Colorado.16
To perform heart tissue Gb3 quantitation, homogenates were prepared by adding 16 μL of ice‐cold deionized water per mg of heart tissue. 50:50 acetone:methanol was added to the homogenate (ratio 20:1), the mixture was vortexed, rehomogenized, and centrifuged at 10 600g for 10 minutes at room temperature. A 50 μL aliquot of each supernatant was transferred to a 13 mL silanized glass tube and prepared for solid phase extraction (SPE); successive additions of 200 μL DMSO, 150 μL of 1:20 water (1:1 acetone:methanol), 50 μL C17‐CTH internal standard (from porcine red blood cell [RBC]; Matreya, LLC) at a final concentration of 1 μg/mL, and 600 μL of water: methanol (13:87) were briefly vortexed and loaded onto a pre‐conditioned Varian Bond Elut 40 μm, 100 mg C‐18 column (Varian Inc). After elution, the column was washed with 67:23:10 methanol:acetone:water. Gb3 was eluted from the column with 1 mL of 9:1 acetone:methanol into silanized glass tubes containing 300 μL of DMSO. Samples were evaporated to the DMSO layer at 40°C for 10 minutes and vortexed. Ten microliter were injected into a LC‐MS/MS system (LC: Shimadzu Corporation; MS/MS: 4000QTRAP LC/MS/MS, Applied Biosystems) at room temperature. The separation was carried out on a C18 analytical column (Phenomenex Aqua 3 μm 100×3.0 mm, 125A; Phenomenex) under gradient elution with acetone/methanol/acetonitrile with sodium acetate binary mobile phase system at a flow rate of 0.5 mL/min. MS/MS analysis was performed in positive ion mode (ESI+): ionspray voltage of +5500 V, a source temperature of 400°C, a curtain gas flow of 20 psi, a Gas1 flow of 60 psi, a Gas2 flow of 40 psi, a de‐clustering potential (DP) in the [+251 to +336] V range, and a collision energy (CE) in the [+83 to + 93] V range. The following 12 transitions were monitored: m/z 1046.70→m/z 884.7 for C16:0; m/z 1074.8→m/z 912.8 for C18:0; m/z 1102.8→m/z 940.8 for C20:0; m/z 1128.8→m/z 966.8 for C22:1; m/z 1130.9→m/z 968.8 for C22:0; m/z 1144.9→m/z 982.8 for C23:0; m/z 1146.9→m/z 984.8 for C22:0(2OH); m/z 1154.9→m/z 992.8 for C24:2; m/z 1156.9→m/z 994.8 for C24:1; m/z 1158.9→m/z 996.9 for C24:0; m/z 1172.9→m/z 1010.8 for C24:1(2OH); m/z 1174.9→m/z 1012.8 for C24:0(2OH); and m/z 1060.7→m/z 898.6 for the C17‐CTH internal standard. The ratio of the total Gb3 area counts (sum of 12 isoforms) to that of the internal standard was used to calculate the concentration of Gb3 in each sample based on a linear equation fitted with the weighting factor 1/x2. Total Gb3 measurements were normalized to wet tissue weight.
Lipid Profiling of Urine and Heart Tissue
Pieces of heart tissue were thawed and homogenized in 1 mL 0.5 mol/L NaCl, 20 mmol/L Tris, pH 7, using a Microson Ultrasonic Cell Disruptor, and total protein was determined by the Lowry method.17 Lipids were extracted from 0.1 mg of protein by the Folch method18 and from 1.5 mL of urine by the Bligh and Dyer19 method with the inclusion of 400 pmol of the following internal standards: bis (monoacylglycero) phosphate (BMP) 14:0/14:0, ceramide 18:1/17:0, dihexosylceramide (DHC) 18:1/16:0 (d3), monohexosylceramide (MHC) 18:1/16:0 (d3), phosphatidylethanolamine (PE) 17:0/17:0, phosphatidylglycerol (PG) 14:0/14:0, phosphatidyinositol (PI) 16:0/16:0, phosphatidylserine (PS) 17:0/17:0, cholesteryl heptadecanoate 17:0 and 100 pmol of lysoPC 13:0 and lyso PE 14:0. Dried lipid extracts were resuspended in 0.2 mL of methanol containing 10 mmol/L NH4COOH and 20 μL were injected onto a 3 μm Alltima C18 column (50×2.1 mm) at a flow rate of 0.2 μL/min in 70% mobile phase A (30% tetrahydrofuran/20%CH3OH/10%H2O in 5 mmol/L NH4COOH). This was then linearly converted to 100% mobile phase B (70% tetrahydrofuran/20%CH3OH/10%H2O in 5 mmol/L NH4COOH) over 7 minutes and maintained for 3 minutes prior to the next injection. A divert valve was used for the first 1.6 minutes. Following chromatography, individual species of ceramide, MHC, DHC, SM, PC, PI, PE, PS and cholesterol were quantified by ESI‐MS/MS as described,20 and BMP and PG as described.21 LysoPC and lysoPE were quantified in positive and negative ion in the MRM mode, respectively. For lysoPC the ion spray voltage was +5500, source temperature 200°C, curtain gas, gas 1 and 2 flow 10 psi, DP 106, CE 37, and 16 isoforms were measured using the m/z product ion of 184 corresponding to the phosphocholine head group. For lysoPE the ion spray voltage was −4500, source temperature 200°C, curtain gas flow 10 psi, gas 1 flow 16 psi and gas 2 flow 10 psi, DP −70, CE −33, and 12 isoforms were measured using the m/z product ion of 196 corresponding to the dilyso‐H2O. Concentrations of individual species were calculated by relating the AUC to that for the corresponding internal standard. The total amount of each lipid was determined by summing each of the isoforms. A similar method was used for lipid profiling in plasma.
Determination of Urine Pellet Size
For each sample, 1.5 mL of thawed frozen urine was aliquoted into an Eppendorf vial in duplicate. The specimens were centrifuged at low speed (1000g) at 4°C for 10 minutes in order to precipitate salts and inorganic matter. The supernatant was transferred into a second preweighed vial. The samples were spun at maximum speed (13 500 rpm) for 30 minutes. The supernatant was removed and discarded. The pellet was dried overnight in a rotary evaporator. The vial containing the dry residue was weighed again and the weight of the dry residue was calculated.
Statistical Methods
Comparison of urinary Gb3 levels between cardiac patients and controls
The difference in urinary Gb3 levels between cardiac patients and apparently healthy controls was assessed. All subjects were recruited to the study between March 31, 2010 and February 3, 2012. Summary statistics are presented by disease status in Tables 1 and 2. Medians with first and third quartiles and frequencies with percentages are given for continuous and categorical variables, respectively. Due to Gb3 being skewed, a Wilcoxon test was used to assess the difference between groups. Furthermore, age, gender, ethnicity, and race were evaluated as confounders. A variance component model on log (base 10) transformed values of Gb3 was used to adjust for the possible confounders and account for the difference in variance between the 2 groups.
Table 1.
Summary Statistics by Disease Status, Age, Gender, Urinary Gb3 Levels (ng/mL) and Ethnic Background
| Variable | Apparently Healthy Controls (N=116) | Cardiac Patients (N=1408) | P Value | ||
|---|---|---|---|---|---|
| N | Median (Q1 to Q3) | N | Median(Q1 to Q3) | ||
| Gb3 | 116 | 90 (72 to 111) | 1406 | 100 (78 to 140) | <0.001 |
| Age, y* | 116 | 44 (33 to 54) | 1406 | 65 (56 to 73) | <0.001 |
| N (%) | N (%) | ||||
| Gender | |||||
| Female | 79 (68.1) | 494 (35.1) | <0.001 | ||
| Male | 37 (31.9) | 914 (64.9) | |||
| Ethnicity | |||||
| Hispanic | 7 (6.0) | 55 (3.9) | 0.322 | ||
| Non‐Hispanic | 109 (94.0) | 1353 (96.1) | |||
| Race | |||||
| Black | 6 (5.2) | 91 (6.5) | 0.021 | ||
| White/Caucasian | 102 (87.9) | 1282 (91.2) | |||
| Other | 8 (6.9) | 33 (2.3) | |||
Gb3 indicates globotriaosylceramide.
Urinary Gb3 was independent of age.
Table 2.
Summary Statistics for 1408 Heart Disease Patients by Death Status
| Alive (N=1333) | Deceased (N=75) | P Value | |||
|---|---|---|---|---|---|
| Demographic | |||||
| Categorical Variable | N (%) | N (%) | |||
| Gender | |||||
| Female | 473 (35.5) | 20 (26.7) | 0.152 | ||
| Male | 860 (64.5) | 55 (73.3) | |||
| Ethnicity | |||||
| Hispanic | 55 (4.1) | 0 (0.0) | 0.115 | ||
| Non‐Hispanic | 1271 (95.9) | 75 (100.0) | |||
| Race | |||||
| Black | 85 (6.4) | 6 (8.0) | 0.474 | ||
| White/Caucasian | 1213 (91.3) | 69 (92.0) | |||
| Other | 31 (2.3) | 0 (0.0) | |||
| Continuous Variable | N | Mean (SD) | N | Mean (SD) | |
| Age, y | 1331 | 63.2 (12.7) | 75 | 69.5 (9.1) | <0.001 |
| Follow‐up, months | 1331 | 17.1 (10.7) | 75 | 9.7 (8.5) | <0.001 |
| Gb3, ng/mL | 1331 | 122.3 (87) | 75 | 187.2 (308.8) | <0.001 |
| AlphaGAL, mmol/L per hour | 1302 | 6 (3.9) | 74 | 6.2 (5) | 0.673 |
| Main Diagnosis | N (%) | N (%) | |||
| CAD | 859 (64.5) | 59 (78.7) | 0.017 | ||
| Cardiomyopathy | 117 (8.8) | 11 (14.7) | <0.001 | ||
| Valvular disease | 186 (14.0) | 12 (16.0) | 0.753 | ||
| Arrhythmia/conduction abn | 604 (45.4) | 39 (52.0) | 0.320 | ||
| Risk Factors | |||||
| Continuous Factors | N | Mean (SD) | N | Mean (SD) | |
| BMI | 1331 | 29.9 (6.8) | 75 | 28 (5.8) | 0.018 |
| Ejection fraction, % | 917 | 49.4 (15.1) | 59 | 39.5 (17.7) | <0.001 |
| eGFR*, mL/min per 1.73 m2 | 1288 | 69.3 (25.5) | 74 | 53.2 (25.3) | <0.001 |
| HDL, mg/dL | 1079 | 45.4 (17.9) | 57 | 38.6 (12.5) | 0.005 |
| LDL, mg/dL | 1084 | 96.5 (35.5) | 58 | 92 (44.4) | 0.354 |
| Categorical Factors | N (%) | N (%) | |||
| Hypertension* | 1046 (78.6) | 66 (88.0) | 0.071 | ||
| Proteinuria | 130 (9.8) | 4 (5.3) | 0.281 | ||
| Diabetes | 191 (14.4) | 17 (22.7) | 0.073 | ||
| Onset < age 40 | 67 (5.0) | 1 (1.3) | 0.259 | ||
| Medications | N (%) | N (%) | |||
| ACE | 536 (40.3) | 31 (41.3) | 0.955 | ||
| ARB | 261 (19.6) | 20 (26.7) | 0.178 | ||
| Analgesic | 842 (63.4) | 50 (66.7) | 0.180 | ||
| Antihyperlidemic | 947 (71.3) | 55 (73.3) | 0.806 | ||
| Antiplatelet agent | 412 (31.0) | 28 (37.3) | 0.307 | ||
| β‐Blocker | 444 (33.4) | 23 (30.7) | 0.716 | ||
| Anticoagulant | 330 (24.8) | 28 (37.3) | 0.022 | ||
| Calcium channel block | 222 (16.7) | 13 (17.3) | 0.874 | ||
| Cardiac glycoside | 96 (7.2) | 14 (18.7) | <0.001 | ||
| Antiarrhythmics | 215 (16.2) | 22 (29.3) | 0.005 | ||
| Vasodilator | 196 (14.7) | 11 (14.7) | >0.999 | ||
| Diuretics | 280 (21.1) | 35 (46.7) | <0.001 | ||
| Potassium replacement | 197 (14.8) | 24 (32.0) | <0.001 | ||
ACE indicates angiotensin converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CAD, coronary artery disease; CKD‐EPI, chronic kidney disease epidemiology collaboration; eGFR, estimated glomerular filtration rate; GAL, galactosidase; Gb3, globotriaosylceramide; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Using CKD‐EPI formula.40
Whether history of hypertension or current diagnosis.
Urinary Gb3 levels in patients with heart disease
Summary statistics of patients with heart disease are presented by death status in Table 2. Means with standard deviations and frequencies with percentages are given for continuous and categorical variables, respectively. A Cox proportional‐hazards model was implemented to assess the relationship between Gb3 and death. The number of deaths (75) limited the number of variables to include in the model. Therefore, inverse probability weights (IPWs) were used to standardize the populations with differing Gb3 values. The demographic, diagnosis, risk factor, and medication variables listed in Table 2 were included in the standardization. Deciles of Gb3 were calculated to determine the standardization categories. The deciles remained separate if the proportion of deceased varied and collapsed otherwise. The final categories were Gb3 values ≤63 ng/mL (calculated first decile), 63 to 211 ng/mL, and > 211 (calculated ninth decile). Gb3 was assessed as a continuous variable on its original scale in the model with the hazard ratio (HR) being reported in clinically relevant incremental units of 200. Values of 100, 500, and 1000 ng/mL were chosen to display the survival function. The proportional hazards assumption of the Cox model was assessed using the stratified analysis method.22 Missing data were imputed using the expectation‐maximization algorithm.
Urinary lipid profiling comparison in patients with heart disease, Fabry disease, and controls
In order to ascertain that patients with heart disease and high urinary Gb3 have a different lipid pattern from Fabry disease patients, we applied principal component analysis (PCA). PCA23 is a multivariate dimension reduction procedure that linearly converts multiple correlated variables (here lipids and isoforms) into a set of linearly uncorrelated variables called principal components, among which the first principal component accounts for the largest variability, and each succeeding component accounts for the highest variance possible under the constraint that it be orthogonal to (ie, uncorrelated with) the preceding components. PCA was used to supply a lower‐dimensional picture of the data and display 3‐dimensional (3D) scatterplots with axes being the top 3 principal components.
Given that the lipid isoform variables were highly skewed, a log (base 10) transformation was applied prior to principal components. PCA was implemented on the correlation matrix, which is more robust to skewed distributions. Eigenvectors and eigenvalues of the correlation matrix were calculated, for which the eigenvector associated with the largest eigenvalue is the first principal component, and the second largest eigenvalue's corresponding eigenvector is the second principal component, etc.
Normal elliptical contour was employed to estimate the center and radius of the population that a group of samples comes from. If two 90% coverage contours are separated from each other in a 3D scatterplot, there is likely to be a distinction between the corresponding populations. This type of exploratory analysis result positively indicates good power for further statistical inference to discriminate these 2 populations.
Data that were log transformed prior to the first PCA were applied on the correlation matrixes of all lipid isoforms. The lipid groups that showed a separation pattern between Fabry and high Gb3 groups using 90% coverage normal contours ellipsoids were pooled for the second PCA. In order to evaluate individual isoforms and the top 3 principal components for their ability to separate the urinary lipid profile of Fabry disease patients from that of heart disease patients with high Gb3, receiver‐operating characteristic (ROC) curves were plotted. To quantify the differential expression of each isoform between the Fabry group and the group of heart disease patients with elevated urine Gb3, 2 independent sample t‐tests and Wilcoxon tests were applied on all the isoforms simultaneously, and false discovery rate (FDR) multiple comparison corrections were imposed on P values returned by these 2 tests.24
Results
Patient Population
A total of 1421 consecutive patients were recruited. Thirteen patients were excluded because of detected variations in the GLA gene (Table S1). The patients' characteristics are further described in Table 1.
Urinary Gb3 is Elevated in Patients With Heart Disease
There was a statistically significant difference in median Gb3 between heart disease patients (Gb3=100) and apparently healthy controls (Gb3=90, difference=10, and P<0.001). This difference remained with log‐transformed (base 10) (data difference=0.09, 95% confidence intervals=0.05 and 0.12, and P<0.0001). The distribution of urinary Gb3 before and after log transformation in the overall population, cases, and controls is shown in Figures 1 and 2, respectively. The model included age, gender, ethnicity, and race as possible confounders and they were not statistically significant when included in the model (Table 3). Moreover, the estimates for the difference between cases and controls did not show a meaningful change with and without these variables being included in the model, indicating they are not confounders in this analysis (minimum and maximum difference=0.05 and 0.11 on log [base 10] scale, respectively). Urinary Gb3 levels in apparently healthy controls were independent of age.
Figure 1.
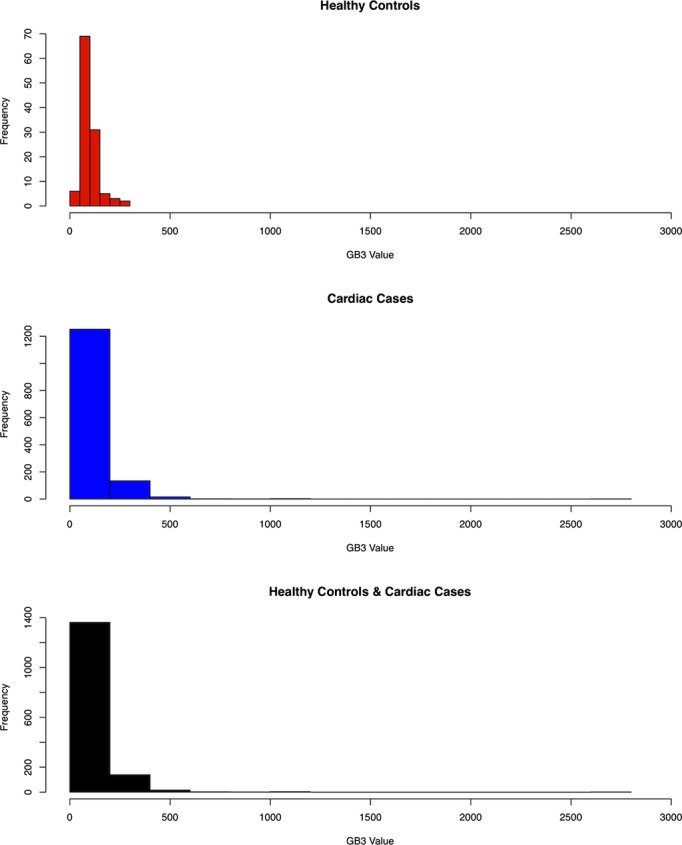
Globotriaosylceramide (Gb3) value of apparently healthy controls, cardiac cases, and all subjects.
Figure 2.
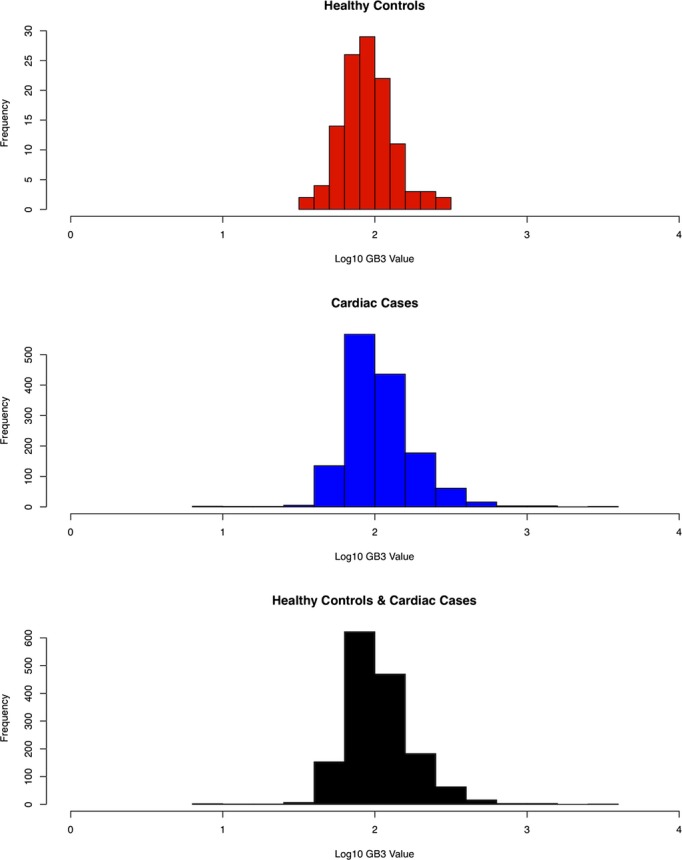
Log 10 globotriaosylceramide (Gb3) value of apparently healthy controls, cardiac cases, and all subjects.
Table 3.
Multiple Regression of Log (10) Gb3 on Group, Gender, Race, and Age
| Effect | Level | Estimate | Standard Error | Num df | Den df | F Value | Pr>F |
|---|---|---|---|---|---|---|---|
| Group | 1 | 1517 | 21.65 | <0.0001 | |||
| Control | 1.9948 | 0.0954 | |||||
| Cardiac | 2.0823 | 0.0966 | |||||
| Gender | Female | −0.0061 | 0.0115 | 1 | 1517 | 0.28 | 0.5966 |
| Race (reference group=other) | 3 | 1517 | 0.44 | 0.7275 | |||
| African American | 0.0037 | 0.0944 | |||||
| Asian/Pacific | 0.0054 | 0.0980 | |||||
| Caucasian | −0.0178 | 0.0919 | |||||
| Age | −0.0005 | 0.00043 | 1 | 1517 | 1.37 | 0.2428 |
Gb3 indicates globotriaosylceramide.
Levels of Other Lipids Are Abnormal in the Urine of Patients With Heart Disease
In order to investigate whether other lipids besides Gb3 are elevated in the urine of patients with heart disease, we performed lipid profiling on randomly collected whole urine and plasma samples on a total of 23 representative samples of urine belonging to patients with heart disease and elevated (478 to 886 ng/mL; n=6) or low (55 to 72 ng/mL; n=5) Gb3, patients with Fabry disease (351 to 9344 ng/mL; n=7) and apparently healthy controls (57 to 105 ng/mL; n=5). The upper limit of normal (99th percentile) urinary Gb3 was 200 ng/mL (Table S2).
PCA showed that the isoforms of MHC PC, SM, PS and PE produced the separation using 90% contours between Fabry disease and heart disease with high urinary Gb3 (Figure 3). These isoforms were pooled together for PCA analysis and the 3D contour plots are shown in Figure 3. The first Eigen vector has all its coefficients nonnegative and accounts for 90.6% of the variation. The coefficients of the top 3 principal components are listed in Table 4. ROC was assessed by AUC on the top 3 principal components and each lipid isoform is shown (Table 5). The first principal component has an AUC of 1—a perfect separation between the Fabry and high Gb3 groups. Some isoforms also had an AUC of 1 (Table 5). However, due to the limited sample size, we cannot assess whether the first principal component is better than other individual isoforms in terms of discriminating between urine from Fabry patients and that of patients with heart disease and elevated Gb3.
Figure 3.
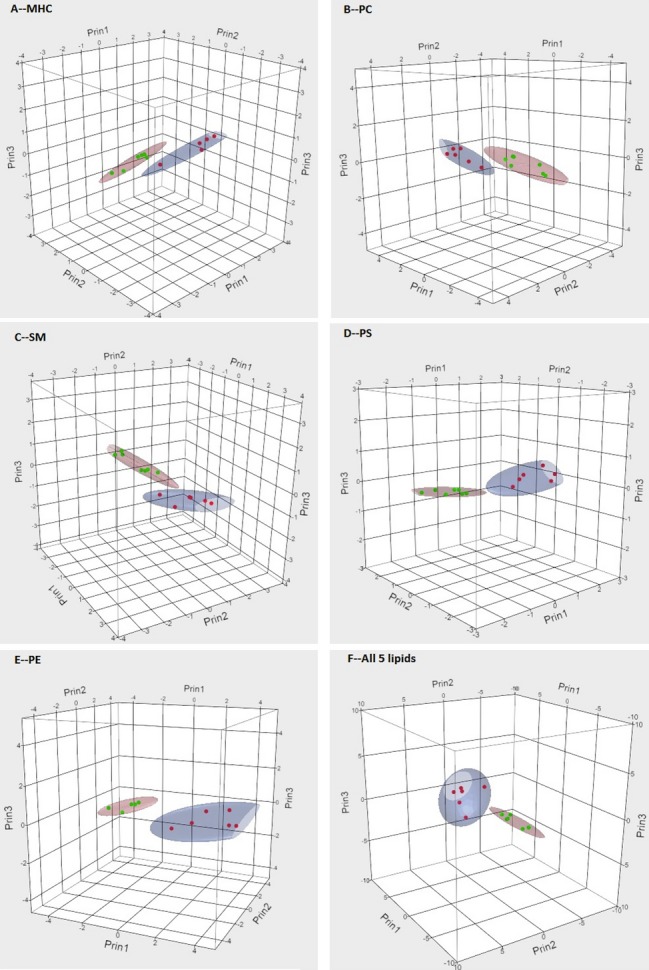
3D scatter plot and 90% coverage contour ellipsoids of Fabry and high globotriaosylceramide (Gb3) heart disease patients in spaces spanned by top 3 principal components of (A) MHC, (B) PC, (C) SM, (D) PS, and (E) PE isoforms. Red dots represent heart disease patients with elevated urinary Gb3 and green dots patients with Fabry disease. MHC indicates monohexosylceramide; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PS, phosphatidylserine; SM, sphingomyelin.
Table 4.
Eigen Value and Eigenvectors on the Top 3 Principal Components
| Prin1 | Prin2 | Prin3 | |
|---|---|---|---|
| Eigen value | 29.00 | 1.35 | 0.61 |
| Percent of variation | 90.6% | 4.2% | 1.9% |
| Eigen vector | |||
| GC.C18.1.16.0 | 0.16878 | −0.22969 | 0.34193 |
| GC.C18.1.20.0 | 0.15490 | −0.37029 | 0.40592 |
| GC.C18.1.22.0 | 0.16710 | −0.29992 | 0.32164 |
| GC.C18.1.24.0 | 0.17468 | −0.16814 | 0.33222 |
| PC.C32.0 | 0.18357 | 0.00830 | 0.04633 |
| PC.C32.1 | 0.18296 | 0.06891 | −0.02844 |
| PC.C34.1 | 0.18366 | 0.04696 | 0.01634 |
| PC.C34.2 | 0.17742 | 0.01040 | −0.07155 |
| PC.C36.2 | 0.17950 | 0.13986 | 0.00732 |
| PC.C36.4 | 0.17582 | 0.01796 | −0.10695 |
| PC.C38.4 | 0.17449 | −0.08411 | −0.18098 |
| SM.C18.0.20.0 | 0.18358 | 0.05705 | 0.01530 |
| SM.C18.1.16.0 | 0.18495 | −0.05186 | −0.03634 |
| SM.C18.1.16.1 | 0.17442 | −0.16272 | −0.26107 |
| SM.C18.1.18.0 | 0.18332 | −0.08620 | −0.05964 |
| SM.C18.1.18.1 | 0.17851 | −0.09876 | −0.22643 |
| SM.C18.1.22.0 | 0.17894 | 0.14567 | 0.01782 |
| SM.C18.1.24.0 | 0.18120 | −0.12693 | −0.02211 |
| SM.C18.1.24.1 | 0.17899 | −0.17291 | −0.17461 |
| PS.C18.0.18.2 | 0.18305 | 0.01671 | 0.02929 |
| PS.C18.0.20.4 | 0.17774 | −0.10889 | −0.17537 |
| PS.C18.1.18.0 | 0.18076 | 0.06609 | 0.11805 |
| PS.C18.1.18.1 | 0.18141 | 0.09424 | 0.04303 |
| PE.C16.0.22.4 | 0.17982 | −0.07114 | −0.20636 |
| PE.C18.0.18.2 | 0.18062 | 0.09980 | −0.06485 |
| PE.C18.0.20.4 | 0.18165 | −0.05554 | −0.17645 |
| PE.C18.1.16.0 | 0.16224 | 0.36519 | 0.23054 |
| PE.C18.1.16.1 | 0.15487 | 0.42350 | 0.06496 |
| PE.C18.1.18.0 | 0.17857 | 0.20499 | 0.11338 |
| PE.C18.1.18.1 | 0.16484 | 0.35987 | 0.17578 |
| PE.C18.1.18.2 | 0.18026 | 0.07402 | −0.11517 |
| PE.C18.1.20.4 | 0.17869 | −0.08820 | −0.23740 |
Table 5.
AUC for Each Isoform and the Top 3 Principal Components in ROC Curve
| ROC Variable | AUC |
|---|---|
| PC1 | 1.000 |
| PC2 | 0.643 |
| PC3 | 0.500 |
| GC.C18.1.16.0 | 0.952 |
| GC.C18.1.20.0 | 0.810 |
| GC.C18.1.22.0 | 0.929 |
| GC.C18.1.24.0 | 1.000 |
| PC.C32.0 | 1.000 |
| PC.C32.1 | 1.000 |
| PC.C34.1 | 1.000 |
| PC.C34.2 | 1.000 |
| PC.C36.2 | 1.000 |
| PC.C36.4 | 1.000 |
| PC.C38.4 | 0.976 |
| SM.C18.0.20.0 | 1.000 |
| SM.C18.1.16.0 | 1.000 |
| SM.C18.1.16.1 | 0.905 |
| SM.C18.1.18.0 | 1.000 |
| SM.C18.1.18.1 | 0.929 |
| SM.C18.1.22.0 | 1.000 |
| SM.C18.1.24.0 | 1.000 |
| SM.C18.1.24.1 | 0.905 |
| PS.C18.0.18.2 | 1.000 |
| PS.C18.0.20.4 | 0.964 |
| PS.C18.1.18.0 | 1.000 |
| PS.C18.1.18.1 | 1.000 |
| PE.C16.0.22.4 | 0.964 |
| PE.C18.0.18.2 | 1.000 |
| PE.C18.0.20.4 | 1.000 |
| PE.C18.1.16.0 | 1.000 |
| PE.C18.1.16.1 | 0.976 |
| PE.C18.1.18.0 | 1.000 |
| PE.C18.1.18.1 | 1.000 |
| PE.C18.1.18.2 | 1.000 |
| PE.C18.1.20.4 | 0.988 |
AUC indicates area under the curve; ROC, receiver‐operating characteristic.
Multiple comparisons for lipid group summation values and individual isoforms confirmed that urinary MHC, SM, and PE were significantly different between patients with Fabry disease and those with heart disease and elevated urinary Gb3 levels (Table 6). No significant differences in lipid profiling were found in plasma from these 2 patient groups (data not shown).
Table 6.
P Value of Top 3 Principal Components, Individual Isoform, and Lipid Group Summation Value in MHC, PC, SM, PS, and PE After Correction of Multiple Comparisons (Benjamini and Hochberg)
| ROC Variable | P Value After Correction | Lipids Summation P Value After Correction |
|---|---|---|
| PC1 | 0.003 | — |
| PC2 | 0.471 | — |
| PC3 | 1.000 | — |
| MHC | 0.025 | |
| GC.C18.1.16.0 | 0.018 | |
| GC.C18.1.20.0 | 0.114 | |
| GC.C18.1.22.0 | 0.024 | |
| GC.C18.1.24.0 | 0.011 | |
| PC | 0.003 | |
| PC.C32.0 | 0.011 | |
| PC.C32.1 | 0.010 | |
| PC.C34.1 | 0.011 | |
| PC.C34.2 | 0.010 | |
| PC.C36.2 | 0.011 | |
| PC.C36.4 | 0.010 | |
| PC.C38.4 | 0.011 | |
| SM | 0.003 | |
| SM.C18.0.20.0 | 0.010 | |
| SM.C18.1.16.0 | 0.010 | |
| SM.C18.1.16.1 | 0.027 | |
| SM.C18.1.18.0 | 0.011 | |
| SM.C18.1.18.1 | 0.018 | |
| SM.C18.1.22.0 | 0.011 | |
| SM.C18.1.24.0 | 0.011 | |
| SM.C18.1.24.1 | 0.027 | |
| PS | 0.003 | |
| PS.C18.0.18.2 | 0.011 | |
| PS.C18.0.20.4 | 0.016 | |
| PS.C18.1.18.0 | 0.011 | |
| PS.C18.1.18.1 | 0.010 | |
| PE | 0.003 | |
| PE.C16.0.22.4 | 0.016 | |
| PE.C18.0.18.2 | 0.010 | |
| PE.C18.0.20.4 | 0.011 | |
| PE.C18.1.16.0 | 0.011 | |
| PE.C18.1.16.1 | 0.014 | |
| PE.C18.1.18.0 | 0.011 | |
| PE.C18.1.18.1 | 0.011 | |
| PE.C18.1.18.2 | 0.010 | |
| PE.C18.1.20.4 | 0.014 |
MHC indicates monohexosylceramide; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PS, phosphatidylserine; ROC, receiver‐operating characteristic; SM, sphingomyelin.
Confirmation of Sphingolipid Abnormalities in Heart Disease Patients
In order to verify our initial lipid profiling findings described above we studied urinary MHC, SM, and lactosylceramide (LC) in 8 heart disease patients (Table S2) with elevated urinary Gb3 randomly selected to represent the full spectrum of Gb3 values, and 6 randomly selected patients from each of the following: heart disease with normal urine Gb3 and patients with overt Fabry disease. We confirmed that MHC levels separated Fabry patients from the heart disease high Gb3 group (Figure 4). Importantly, expression of lipid levels per unit of volume or per mg creatinine gave consistent results.
Figure 4.
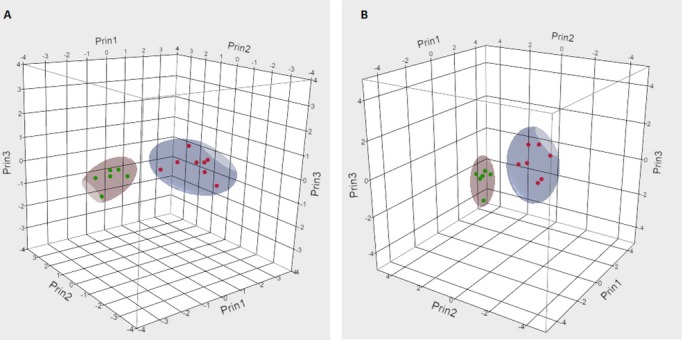
3D 90% contour plots of MHC expressed as per unit of urine volume (A) or per mg of creatinine (B) of Fabry and high globotriaosylceramide (Gb3) heart disease patients. Red dots represent heart disease patients with elevated urinary Gb3 and green dots patients with Fabry disease.
Urinary Gb3 Levels Are Independent of the Size of the Membrane Pellet
In order to determine whether the variation of urinary Gb3 and other lipids in patients with heart disease reflects the amount of sloughed cellular debris rather than membrane lipid composition, we measured the size of the pellet in previously frozen urine samples of 22 patients with heart disease and 6 patients with Fabry disease (Table S2). Urine Gb3 in those samples ranged from undetectable to 478 ng/mL. There was no significant correlation between the size of the pellet and the Gb3 concentration in the urine of patients with heart disease only (P=0.15) and when samples from patients with Fabry disease were included (P=0.25).
Lipid Abnormalities in Failing Ischemic Heart
In order to determine whether lipid abnormalities seen in urine reflect an abnormal lipid profile in the heart itself, we performed lipid profiling on samples of the left ventricle of 20 patients with end‐stage ischemic heart failure (Table S3).16 Gb3 levels were significantly decreased in these hearts compared to 20 controls (Figure 5A) while sphingomyelin levels were significantly higher compared to controls (Figure 5B).
Figure 5.
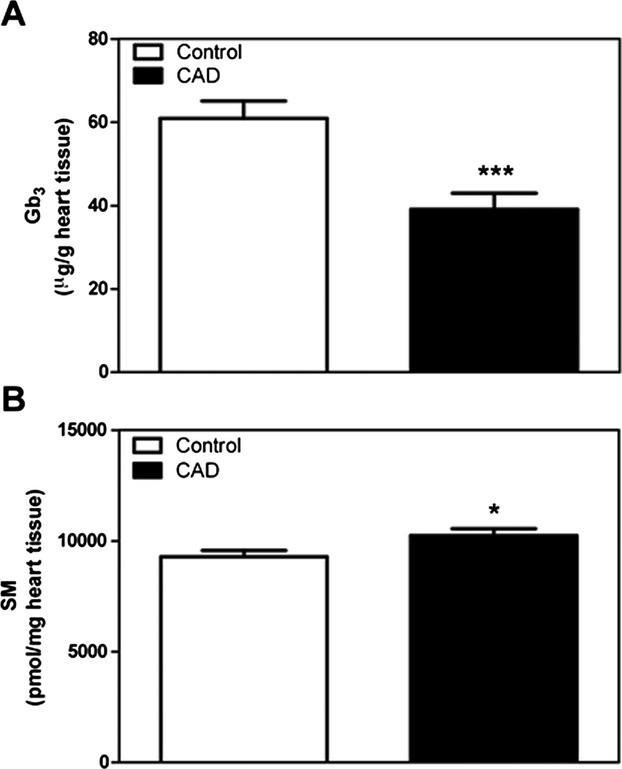
Globotriaosylceramide (Gb3) and sphingomyelin (SM) levels in the left ventricle of failing ischemic hearts. A, Heart Gb3 levels in control vs coronary artery disease (CAD). N=20 per group. B, Heart SM levels in control vs CAD. N=10 per group. In both panels, *P<0.05, ***P<0.001 by Welch's t‐test.
Gb3 Levels Are Significantly Associated With the Risk of Near‐Term Death
Of the 1408 patients with cardiovascular disease, 75 died during the follow‐up period (Table 2). The total follow‐up time, average follow‐up time, and average time to death was 36, 17, and 10 months, respectively. In order to adjust the analysis for demographic, clinical, and medication variables listed in Table 2, a Cox proportional hazard analysis was used. In this analysis there was a significant association between urinary Gb3 levels and risk of death (HR=1.59 for increase in Gb3 of 200, 95% confidence intervals=1.36 and 1.87, and P<0.0001). The rate of death increases by 60% for every increase of 200 units of urinary Gb3. Based on this survival analysis, the risk estimates for death over time are presented in Figure 6 using urine Gb3 values of 100, 500, and 1000 ng/mL. The unadjusted HR (without IPW) produced similar results (HR=1.52 for increase in Gb3 of 200, 95% confidence intervals=1.30 and 1.77, and P<0.0001). Furthermore, the robustness of the results was assessed by analyzing 120 different models in which Gb3 was adjusted for each demographic, diagnosis, risk factor, and medication variable individually, with and without the IPWs, and with and without outlier removed. The mean HR from these analyses was 1.49 and ranged from 1.27 to 1.77.
Figure 6.
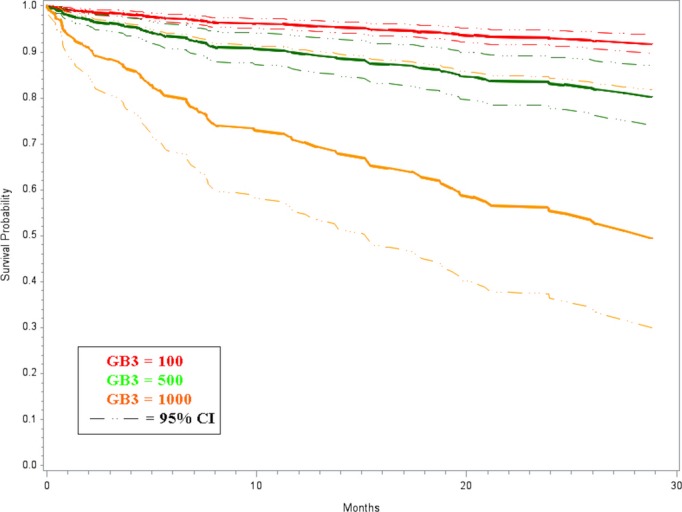
Adjusted estimated survivor functions using Cox proportional hazard model for increasing urinary globotriaosylceramide (Gb3) values. Gb3 was analyzed as a continuous variable. The values of 100, 500, and 1000 along with their 95% confidence intervals (CI) were chosen to graphically represent the survival function. The urinary Gb3 upper limit of normal (99th percentile) is 200 ng/mL in our laboratory.
Discussion
In this study we found that glycosphingolipid Gb3 levels in urine are elevated in some patients with common heart disease who do not have GLA gene mutations (Fabry disease). The highest urinary Gb3 levels in patients with heart disease were in the range seen in the most severe forms of Fabry disease (Table S2). Furthermore, increasing urinary Gb3 concentrations were significantly associated with increased risk of death in the subsequent mean follow‐up of 17 months. This result was seen even after adjusting for the type of primary cardiac diagnosis, medications, and other known risk factors and confounding factors, suggesting that elevated urinary Gb3 is associated with relatively near‐term death in patients with heart disease.
Gb3 in urine is primarily found in sloughed debris of renal tissue, mostly renal tubular cells.9–10,25 Because lipid profiling showed that elevation of urinary Gb3 in patients with heart disease was associated with other lipid abnormalities, it likely reflects abnormal lipid composition of renal cell membranes. We found support for this hypothesis in the abnormal lipid profiles seen in cardiac tissue from patients with end‐stage ischemic cardiomyopathy and in the fact that the urinary Gb3 elevation was independent of the size of the pellet. Although urinary lipid profiling in patients with common heart disease has not been previously published, our results in urine of patients with Fabry disease were similar to previously published data.26 Glycosphingolipids have been suspected of being involved in heart disease. Sphingomyelin and ceramide have been isolated from atherosclerotic plaques in both humans and animals.27–28 Lowering several sphingolipids (including sphingomyelin, ceramide, sphingosine‐1‐phosphate, and glycosphingolipids) inhibits and even induces regression of atherosclerotic plaques in animal studies.29 It is thought that higher levels of sphingolipids are the result of increased amounts of substrates such as triglycerides.27–28,30 Elevation of enzymes that catalyze the synthesis of sphingolipids has been found in samples of the right atrial appendage obtained from patients at the time of coronary bypass surgery.30 However, to our knowledge, a comprehensive measurement of lipid levels in the heart of patients with heart disease has not been published.
Early studies suggested that elevated plasma sphingomyelin is associated with a higher rate of complications from CAD, but recent evidence has not confirmed those findings.31 Current evidence suggests that glycosphingomyelins such as the sphingomyelin in atherosclerotic plaques are synthesized in the atherosclerotic plaques rather than taken up from the plasma. Since many of the lipids in urine are derived from sloughed kidney cells, perhaps it is not surprising that the levels of sphingolipids in urine more closely reflect abnormalities in different organs. Furthermore, sphingolipids such as ceramide, sphingosine, sphingosine‐1‐phosphate, and lactosylceramide are known to have biological cell signaling effects, and are involved in pathophysiological processes in endothelial cells, smooth muscle cells, myocytes, platelets, and leukocytes.32 In our study, the type of primary cardiac diagnosis did not significantly affect the association of urinary Gb3 level with the likelihood of death. This suggests that the lipid abnormalities we identified in patients with heart disease represent a systemic lipid aberration that contributes to the poor prognosis in general by a yet unknown mechanism. We cannot at present fully explain how urinary Gb3 levels could predict outcomes for a group of diseases as heterogeneous as CAD, valvular heart disease, and nonischemic cardiomyopathy. However, these complications frequently coexist in patients and are thought to have common mechanisms. For example, atherosclerotic vascular disease increases the risk of atrial fibrillation and the latter is a major risk factor for vascular disease. CAD and atrial fibrillation share a number of risk factors (eg, increasing age, obesity, diabetes, heart failure, and hypertension), and these complications often coexist33–34 and are associated with most cardiac disorders.35 Remodeling of ion channels in patients with coronary heart disease and other heart ailments causes conduction defects possibly due to sphingolipid abnormalities of lipid rafts.34,36 Unlike existing risk markers in heart disease that are expressed or released by cardiovascular tissue in response to mechanical or pathological stress,37 the Gb3 and other lipid abnormalities we identified here may indicate risk via a different mechanism.
There were several limitations to the study. Although the sample size was adequate, there were a relatively small number of deaths. This limited the number of variables we could simultaneously control for in the Cox proportional hazards model. Therefore, IPWs were used in the analysis. Despite this limitation, the results were robust given the fact that a positive association remained in all 120 models which varied the variables included in the model (ie, the use of IPW and outlier removal). The healthy control population of 116 is relatively small and is younger than the population of patients with heart disease. This is due to the difficulty of finding completely healthy subjects of an older age. These differences probably had no influence on the results since we included Gb3, age, and gender in the statistical models. We used the Social Security Death Index to identify the patients who had died. Although it is possible that this index is not complete and that the number of deaths is actually higher, we supplemented our findings with obituaries and medical records. This database did not allow us to ascertain whether the patient died from heart disease or from another cause. However, the yearly death rate in our study of about 1.7% was similar to the cumulative all causes mortality rate found in the Dallas Heart Study.38 Our findings need to be confirmed by a study in another population with heart disease. In addition, since we sampled urine only once per patient in our study, it is possible that repeated sampling of Gb3 in combination with the measurement of other lipids would improve the predictive value of urinary Gb3 levels. Nevertheless, the data presented here provide proof of concept for a potentially new class of risk markers, and predicts future outcome beyond standard risk factors in cardiovascular disease.39 An understanding of the mechanisms involved in the abnormal lipid levels in the urine and in organs will likely yield novel therapeutic interventions for heart disease.
Sources of Funding
The study was funded in part by The Lysosomal Disease Network (U54NS065768), a part of the National Institutes of Health (NIH) Rare Diseases Clinical Research Network (RDCRN), supported through collaboration between the NIH Office of Rare Diseases Research (ORDR) at the National Center for Advancing Translational Science (NCATS), the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Shire Plc, Amicus Therapeutics and the Baylor Health Care System.
Disclosures
Dr Schiffmann received research funds from Amicus Therapeutics, Inc. Drs Brignol, Wu, Lockhart, and Benjamin are employees of Amicus Therapeutics, Inc. The other authors have no disclosures.
Acknowledgments
We thank the staff at The Heart Hospital at Baylor Plano, Baylor Heart and Vascular Hospital and the Soltero Cardiovascular Research Center for their help with this study. We thank Monica Mendez for technical help, Monica Anand for the creation of an analytical dataset and SAS programming used for the statistical analysis, and Yahya Daoud for the discussions around the statistical analysis plan.
References
- 1.Rhee EP, Gerszten RE. Metabolomics and cardiovascular biomarker discovery. Clin Chem. 2012; 58:139-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eggers KM, Lagerqvist B, Oldgren J, Venge P, Wallentin L, Lindahl B. Pathophysiologic mechanisms of persistent cardiac troponin I elevation in stabilized patients after an episode of acute coronary syndrome. Am Heart J. 2008; 156:588-594 [DOI] [PubMed] [Google Scholar]
- 3.Gilstrap LG, Wang TJ. Biomarkers and cardiovascular risk assessment for primary prevention: an update. Clin Chem. 2012; 58:72-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999; 281:249-254 [DOI] [PubMed] [Google Scholar]
- 5.Spada M, Pagliardini S, Yasuda M, Tukel T, Thiagarajan G, Sakuraba H, Ponzone A, Desnick RJ. High incidence of later‐onset Fabry disease revealed by newborn screening. Am J Hum Genet. 2006; 79:31-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiffmann R. Fabry disease. Pharmacol Ther. 2009; 122:65-77 [DOI] [PubMed] [Google Scholar]
- 7.Brady R, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L. Enzymatic defect in Fabry disease: ceramide trihexosidase deficiency. N Engl J Med. 1967; 276:1163-1167 [DOI] [PubMed] [Google Scholar]
- 8.Auray‐Blais C, Cyr D, Ntwari A, West ML, Cox‐Brinkman J, Bichet DG, Germain DP, Laframboise R, Melancon SB, Stockley T, Clarke JT, Drouin R. Urinary globotriaosylceramide excretion correlates with the genotype in children and adults with Fabry disease. Mol Genet Metab. 2008; 93:331-340 [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee S, Gupta P, Pyeritz RE, Kwiterovich PO., Jr Immunohistochemical localization of glycosphingolipid in urinary renal tubular cells in Fabry's disease. Am J Clin Pathol. 1984; 82:24-28 [DOI] [PubMed] [Google Scholar]
- 10.Clarke JT, Guttmann RD, Wolfe LS, Beaudoin JG, Morehouse DD. Enzyme replacement therapy by renal allotransplantation in Fabry's disease. N Engl J Med. 1972; 287:1215-1218 [DOI] [PubMed] [Google Scholar]
- 11.Winchester B, Young E. Biochemical and Genetic Diagnosis of Fabry Disease. 2006Oxford, UK: Oxford PharmaGenesis; [PubMed] [Google Scholar]
- 12.Auray‐Blais C, Ntwari A, Clarke JT, Warnock DG, Oliveira JP, Young SP, Millington DS, Bichet DG, Sirrs S, West ML, Casey R, Hwu WL, Keutzer JM, Zhang XK, Gagnon R. How well does urinary lyso‐Gb3 function as a biomarker in Fabry disease? Clin Chim Acta. 2010; 411:1906-1914 [DOI] [PubMed] [Google Scholar]
- 13.Auray‐Blais C, Cyr D, Mills K, Giguere R, Drouin R. Development of a filter paper method potentially applicable to mass and high‐risk urinary screenings for Fabry disease. J Inherit Metab Dis. 2007; 30:106. [DOI] [PubMed] [Google Scholar]
- 14.Duffey TA, Bellamy G, Elliott S, Fox AC, Glass M, Turecek F, Gelb MH, Scott CR. A tandem mass spectrometry triplex assay for the detection of Fabry, Pompe, and mucopolysaccharidosis‐I (Hurler). Clin Chem. 2010; 56:1854-1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forni S, Fu X, Schiffmann R, Sweetman L. Falsely elevated urinary Gb3 (globotriaosylceramide, CTH, Gl3). Mol Genet Metab. 2009; 97:91. [DOI] [PubMed] [Google Scholar]
- 16.Ambardekar AV, Walker JS, Walker LA, Cleveland JC, Jr, Lowes BD, Buttrick PM. Incomplete recovery of myocyte contractile function despite improvement of myocardial architecture with left ventricular assist device support. Circ Heart Fail. 2011; 4:425-432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951; 193:265-275 [PubMed] [Google Scholar]
- 18.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497-509 [PubMed] [Google Scholar]
- 19.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959; 37:911-917 [DOI] [PubMed] [Google Scholar]
- 20.Hein LK, Duplock S, Hopwood JJ, Fuller M. Lipid composition of microdomains is altered in a cell model of gaucher disease. J Lipid Res. 2008; 49:1725-1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meikle PJ, Duplock S, Blacklock D, Whitfield PD, Macintosh G, Hopwood JJ, Fuller M. Effect of lysosomal storage on bis(monoacylglycero)phosphate. Biochem J. 2008; 411:71-78 [DOI] [PubMed] [Google Scholar]
- 22.Lee ET, Wang JW. Statistical Methods for Survival Data Analysis. 2003New York: John Wiley [Google Scholar]
- 23.Jolliffe IT. Principal Component Analysis. 2002New York: Springer‐Verlag [Google Scholar]
- 24.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995; 57:289-300 [Google Scholar]
- 25.Gubler MC, Lenoir G, Grunfeld JP, Ulmann A, Droz D, Habib R. Early renal changes in hemizygous and heterozygous patients with Fabry's disease. Kidney Int. 1978; 13:223-235 [DOI] [PubMed] [Google Scholar]
- 26.Fuller M, Sharp PC, Rozaklis T, Whitfield PD, Blacklock D, Hopwood JJ, Meikle PJ. Urinary lipid profiling for the identification of Fabry hemizygotes and heterozygotes. Clin Chem. 2005; 51:688-694 [DOI] [PubMed] [Google Scholar]
- 27.Cowart LA. Sphingolipids: players in the pathology of metabolic disease. Trends Endocrinol Metab. 2009; 20:34-42 [DOI] [PubMed] [Google Scholar]
- 28.Brice SE, Cowart LA. Sphingolipid metabolism and analysis in metabolic disease. Adv Exp Med Biol. 2011; 721:1-17 [DOI] [PubMed] [Google Scholar]
- 29.Hojjati MR, Li Z, Zhou H, Tang S, Huan C, Ooi E, Lu S, Jiang XC. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE‐deficient mice. J Biol Chem. 2005; 280:10284-10289 [DOI] [PubMed] [Google Scholar]
- 30.Baranowski M, Blachnio‐Zabielska A, Hirnle T, Harasiuk D, Matlak K, Knapp M, Zabielski P, Gorski J. Myocardium of type 2 diabetic and obese patients is characterized by alterations in sphingolipid metabolic enzymes but not by accumulation of ceramide. J Lipid Res. 2010; 51:74-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeboah J, McNamara C, Jiang XC, Tabas I, Herrington DM, Burke GL, Shea S. Association of plasma sphingomyelin levels and incident coronary heart disease events in an adult population: multi‐ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2010; 30:628-633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levade T, Auge N, Veldman RJ, Cuvillier O, Negre‐Salvayre A, Salvayre R. Sphingolipid mediators in cardiovascular cell biology and pathology. Circ Res. 2001; 89:957-968 [DOI] [PubMed] [Google Scholar]
- 33.Depta JP, Bhatt DL. Atherothrombosis and atrial fibrillation: important and often overlapping clinical syndromes. Thromb Haemost. 2010; 104:657-663 [DOI] [PubMed] [Google Scholar]
- 34.Olesen JB, Gislason GH, Torp‐Pedersen C, Lip GY. Atrial fibrillation and vascular disease—a bad combination. Clin Cardiol. 2012; 35Suppl 1:15-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez FL, Agarwal SK, Maclehose RF, Soliman EZ, Sharrett AR, Huxley RR, Konety S, Ballantyne CM, Alonso A. Blood lipid levels, lipid‐lowering medications, and the incidence of atrial fibrillation: the atherosclerosis risk in communities study. Circ Arrhythm Electrophysiol. 2012; 5:155-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maguy A, Hebert TE, Nattel S. Involvement of lipid rafts and caveolae in cardiac ion channel function. Cardiovasc Res. 2006; 69:798-807 [DOI] [PubMed] [Google Scholar]
- 37.Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, Ho JE, Fradley MG, Ghorbani A, Xanthakis V, Kempf T, Benjamin EJ, Levy D, Vasan RS, Januzzi JL. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham heart study. Circulation. 2012; 126:1596-1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathieu RAT, Powell‐Wiley TM, Ayers CR, McGuire DK, Khera A, Das SR, Lakoski SG. Physical activity participation, health perceptions, and cardiovascular disease mortality in a multiethnic population: the Dallas heart study. Am Heart J. 2012; 163:1037-1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, Go AS, Harrell FE, Jr, Hong Y, Howard BV, Howard VJ, Hsue PY, Kramer CM, McConnell JP, Normand SL, O'Donnell CJ, Smith SC, Jr, Wilson PW. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009; 119:2408-2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006; 145:247-254 [DOI] [PubMed] [Google Scholar]


