Abstract
Both low eGFR and albuminuria are known risk factors for ESRD. This paper focuses on their joint contribution to ESRD and other kidney outcomes.
We performed a collaborative meta-analysis of 9 general population cohorts with 845,125 participants and 8 cohorts with 173,892 participants selected because of high risk for chronic kidney disease. Both eGFR and albuminuria were tested as risk factors for ESRD, acute kidney injury and progressive chronic kidney disease.
In general population cohorts, the risk for ESRD was unrelated to eGFR at values 75–105 ml/min/1.73m2 and increased exponentially at lower eGFR. Hazard ratios (95% confidence interval) at eGFR 60, 45, and 15 (versus 95) ml/min/1.73m2 were 3.69 (2.36–5.76), 29.3 (19.5–44.1) and 454.9 (112.4–1840.2), respectively, after adjustment for albumin-to-creatinine ratio and cardiovascular risk factors. Albuminuria was associated with ESRD risk linearly without thresholds. Adjusted hazard ratios at albumin-to-creatinine ratios 30, 300 and 1000 (versus 5) mg/g were 4.87 (2.30–10.3), 13.4 (5.49–32.7) and 28.4 (14.9–54.2), respectively. eGFR and albuminuria were multiplicatively associated with ESRD, without evidence for interaction. Similar, but numerically less pronounced associations were observed for acute kidney injury and progressive chronic kidney disease. The findings in high risk cohorts were generally comparable to those in general population cohorts.
In conclusion, lower eGFR and higher albuminuria are risk factors for ESRD, acute kidney injury and progressive chronic kidney disease independent of each other and of cardiovascular risk factors, both in the general population and high risk cohorts.
Keywords: Meta-analysis, eGFR (kidney function), albumin-to-creatinine ratio (albuminuria), dipstick (proteinuria), ESRD (end-stage renal disease), acute kidney injury, progressive chronic kidney disease
Introduction
This is the third in a series of four manuscripts to report the results of collaborative meta-analyses of estimated GFR (eGFR) and albuminuria on outcomes of chronic kidney disease (CKD) undertaken by the CKD Prognosis Consortium. These analyses were undertaken in conjunction with the 2009 Controversies Conference sponsored by Kidney Disease Improving Global Outcomes (KDIGO) to evaluate the current definition and classification of chronic kidney disease and proposed alternatives (1). The report of the Consensus Conference is included in this issue of Kidney International (2).
Widespread implementation of the definition and classification of chronic kidney disease, as proposed by Kidney Disease Outcomes Quality Initiative (KDOQI) in 2002 and subsequently endorsed by KDIGO in 2004, has promoted increased attention to chronic kidney disease in clinical practice, research and public health (3–6). It has also generated substantial debate about the appropriateness of recommending the same GFR thresholds for people of all ages, the optimal level of albuminuria for diagnosing kidney damage, and about the value of the 5-stage classification system based on eGFR without consideration of albuminuria (7–11). It was the position of KDOQI and KDIGO that a comprehensive analysis of mortality and kidney outcomes according to eGFR and albuminuria was needed to answer key questions underlying the debate (1,2).
Until recently, most of the data on kidney outcomes were from studies of patients with later stages of chronic kidney disease, rather than from general population cohorts or cohorts at increased risk for chronic kidney disease (12–14). Reports from the general population and high-risk cohorts focused mainly on all-cause and cardiovascular mortality (15–20), with fewer data available on kidney outcomes (19–22). In this manuscript, we describe a collaborative meta-analysis of 9 general population and 8 high-risk cohorts. The outcomes reported in this manuscript include kidney failure treated by dialysis or transplantation (end-stage renal disease) or coded on the death certificate. In addition, we also included acute kidney injury, because it is increasingly recognized as a major cause for (23) and consequence of chronic kidney disease (24), and kidney disease progression, based on fast eGFR decline (progressive chronic kidney disease), because of its clinical importance and potential to lead to ESRD or other complications.
Other papers in this series deal with all-cause and cardiovascular mortality in general population cohorts and high-risk cohorts (25,26). This report describes the kidney outcomes from these cohorts. A fourth manuscript reports mortality and kidney outcomes in chronic kidney disease cohorts (27). A priori we hypothesized that both eGFR and albuminuria would be associated with these outcomes, independent of traditional cardiovascular risk factors and independent of each other, and despite inclusion of diverse study populations.
Methods
Search strategy and study selection
In August 2009, we performed a systematic review of the available literature to retrieve all general population cohorts that might have information on the relation between eGFR and/or albuminuria versus kidney outcomes. Details of the search strategy can be found elsewhere (25). To be eligible for inclusion, studies had to meet the following criteria: 1. prospective, general population based cohort study, 2. information at baseline on eGFR as well as albuminuria levels, 3. at least 1000 subjects included, 4. information on at least one of the three kidney outcome measures, 5. a minimum of 50 events for that outcome measure. The reason to require a minimum sample size is to ensure sufficient outcomes in the reference cell. Ultimately, 21 general population cohorts met these eligibility criteria and were willing to cooperate, of which 9 had data on kidney outcomes (20,28–35).
We also included cohorts of individuals selected because of high risk of chronic kidney disease, including patients with cardiovascular disease risk factors (such as hypertension and diabetes) or a history of cardiovascular disease, because screening for chronic kidney disease is recommended in these groups. However, the associations between eGFR and/or albuminuria and kidney outcomes may differ between high risk populations and the general population. We analyzed 8 high-risk cohorts that met the same eligibility criteria as the general population cohorts (20,36–42).
Study variables
In each cohort, subjects were subdivided according to eGFR and albuminuria. GFR was estimated using the abbreviated MDRD Study equation (43). Each participating study was asked to standardize their serum creatinine to IDMS traceable methods, but calibration methods were not uniform. As recommended in clinical practice guidelines (3,44) albuminuria was assessed as the urine albumin-to-creatinine ratio. If first morning voids were not available, spot urine samples or samples from 24hr urine collections were used. In studies in which no quantitative albuminuria measurements were available, data on urine protein-to-creatinine ratio (41) or dipstick testing for proteinuria (20) were collected. eGFR and albuminuria were measured at the onset of cohort studies.
Besides eGFR and albuminuria, information on demographic factors and cardiovascular risk factors were obtained to compare baseline characteristics of the different cohort studies and to adjust for confounding in multivariable models. Cardiovascular disease history was defined as a history of myocardial infarction, bypass grafting, percutaneous coronary intervention, heart failure or stroke. Hypertension was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or use of antihypertensive medication. Hypercholesterolemia was defined as total cholesterol >5.0 mmol/L in the case of a positive history of cardiovascular disease and as >6.0 mmol/L in the case of a negative history of cardiovascular disease. Diabetes mellitus was defined as fasting glucose ≥ 7.0 mmol/L or non-fasting glucose ≥ 11.1 mmol/L or use of glucose lowering drugs. Smoking habit was dichotomised as current versus not current smoking.
Definition of kidney outcome measures
End-stage renal disease (ESRD) was defined as start of renal replacement therapy or death coded as due to kidney disease other than acute kidney injury. Acute kidney injury was defined as ICD-9 code 584 as primary or additional discharge code. Progressive chronic kidney disease was defined as an average annual decline in eGFR during follow-up of at least 2.5 ml/min/1.73m2 per year and a last eGFR value being less than 45 ml/min/1.73m2, independent of the level of baseline eGFR. The average annual decline in eGFR was calculated as last available eGFR minus baseline eGFR divided by follow-up time (in years, minimum 2) between the two observations.
Statistical analysis
Our primary objective was to evaluate the associations of eGFR and albuminuria, independently and jointly, on kidney outcome measures. To maximize uniformity and minimize bias, investigators from the cohort studies were invited to collaborate in a pooled analysis following an a priori analytic plan using standard statistical code that was provided by the analytic team of the CKD Prognosis Consortium. All analyses were conducted using Stata version 10 or 11 (Stata Corp, College Station, Texas, USA), SAS version 9 (SAS Institute, Inc., Cary, North Carolina, USA), or R version 2.9.2 (R Foundation for Statistical Computing, Vienna, Austria). All data classification was performed separately by analytic teams at the John Hopkins Institute for Public Health, Baltimore, USA (KM, JC, BCA) and the University Medical Center Groningen, Groningen, The Netherlands (MvdV, PEdJ, RTG) and differences were resolved by consensus.
For each study, a table was generated providing baseline study characteristics. Cox proportional hazard models were used to estimate the hazard ratios for ESRD and acute kidney injury, and logistic regression analysis to estimate odds ratios for progressive chronic kidney disease. These analyses were adjusted for age, sex, race and cardiovascular risk factors. Cardiovascular risk factors taken into account were cardiovascular disease history, smoking status, diabetes mellitus, systolic blood pressure and serum total cholesterol. The independent continuous association of eGFR and of albuminuria with risk for kidney outcomes was evaluated after adjusting for each other and for CVD risk factors. eGFR and albumin-to-creatinine ratio were modelled using linear splines with knots at 45, 60, 75, 90, and 105 ml/min/1.73 m2 and 10, 30, and 300 mg/g, respectively. eGFR splines were also adjusted for albuminuria (adjusted to an albumin-to-creatinine ratio of 5 mg/g and dipstick negative), whereas albuminuria splines were also adjusted for eGFR. For the continuous albuminuria splines only cohorts that had ACR data were taken into account. eGFR 95 ml/min/1.73 m2 and albumin-to-creatinine ratio 5 mg/g were treated as the reference points, respectively. These points were chosen since they reflect the anticipated low risk groups. Interactions between eGFR and both albuminuria and age, were evaluated by likelihood-ratio tests in individual studies, with albuminuria and age treated as continuous variables.
For each outcome variable, information was generated for the joint association of eGFR and albuminuria with kidney outcomes. Eight eGFR categories were defined: <15, 15–29, 30–44, 45–59, 60–74, 75–89, 90–104, and ≥ 105 mL/min/1.73m2). These 15 mL/min/1.73m2 categories were chosen to correspond to current chronic kidney disease stages 1–5 and to evaluate whether these stages require subdivision. For albumin-to-creatinine ratio we defined 4 categories: <10, 10–29, 30–299, and ≥ 300 mg/g. These categories were chosen to correspond to current definitions for microalbuminuria and macroalbuminuria and to evaluate whether the normoalbuminuria category should be subdivided. When information on albumin-to-creatinine ratio was lacking, we used information on dipstick proteinuria. As it has been shown that the majority of subjects with a dipstick trace have high-normal albuminuria, dipstick 1+ microalbuminuria, and dipstick ≥ 2+ macroalbuminuria (45), we defined four dipstick categories as: negative, trace, 1+, and ≥ 2+. We tested whether combining cohorts with data on albumin-to-creatinine ratio and cohorts with data on dipstick proteinuria was valid. Unlike the mortality analyses (24, 25), there were insufficient kidney outcomes in the “optimal” reference cell (eGFR 90–104 mL/min/1.73m2 and albumin-to-creatinine ratio<10 mg/g) for the current analyses. Therefore eGFR ≥ 60 ml/min/1.73m2 and albumin-to-creatinine ratio<30 mg/g or dipstick negative/trace was chosen as the reference cell, since present guidelines classify this group as being free of chronic kidney disease. For all of the 25 eGFR x albumin-to-creatinine ratio categories, information was obtained on the distribution of subjects, and the distribution of incident events. For each study, the unadjusted incidence rate per 1,000 person-years was calculated for each category. Hazard ratios or odds ratios were estimated with adjustment for the aforementioned cardiovascular risk factors. We conducted complementary analyses where eGFR and albumin-to-creatinine ratio were modelled continuously using the same statistical models and adjustments. These models were parameterized with eGFR=95 mL/min/1.73m2 and albumin-to-creatinine ratio=5 mg/g or albumin-to-creatinine ratio=dipstick negative/trace as the reference point (hazard ratio or odds ratio =1.0).
Pooled unadjusted incidence rates were obtained by weighting the individual studies by the number of subjects per category. Pooled estimates of the adjusted hazard ratios and odds ratios, with 95% confidence intervals, were obtained from random effects meta-analyses. Heterogeneity was estimated using the χ2 test for heterogeneity and the I2 statistic (46). Meta-analyses were conducted separately for general population cohorts and high risk cohorts. Since there were few participants (0.1%) with eGFR <15 ml/min/1.73m2, we only report results for participants with eGFR ≥ 15 ml/min/1.73m2. A priori it was considered that age could be an important effect modifier, and hence results were also produced for age <65 and ≥ 65 years. This age subdivision was chosen since guidelines advise to screen for chronic kidney disease in subjects ≥ 65 years.
In all analyses, a p value of <0.05 was considered to indicate statistical significance.
Results
Study and population characteristics
Of the 9 general population cohorts (845,125 subjects), 5 had data on albumin-to-creatinine ratio and 4 on dipstick. Of the 8 high-risk cohorts (173,892 subjects), 5 had data on albumin-to-creatinine ratio and 3 on dipstick (Table 1). Subjects in the high risk cohorts were more often male and these cohorts had a higher prevalence of cardiovascular risk factors than did the general population cohorts. Moreover, the high risk cohorts generally had a lower eGFR and higher albumin-to-creatinine ratio. Not all cohorts had data on all kidney outcomes. There were a total of 2201, 4939 and 11144 participants who developed ESRD, acute kidney injury and progressive chronic kidney disease, respectively. The incidence rates for the kidney outcomes were 2 to 6 fold higher in the high risk cohorts compared to the general population cohorts (1.83 vs 0.31 for ESRD, 4.88 vs 2.21 for acute kidney injury, and 18.44 vs 7.55 events per 1000 person years for progressive chronic kidney disease, respectively) (web appendix Tables 3, 6 and 9, respectively). A total of 13.7% of the subjects of general population cohorts with albumin-to-creatinine ratio data had chronic kidney disease according to the current definition (eGFR<60 ml/min/1.73 m2 or albumin-to-creatinine ratio ≥30 mg/g) (web appendix Table 1). This subgroup accounted for 88.6% of ESRD events (web appendix 2), 61.5% of acute kidney injury events (web appendix Table 5) and 76.7% of subjects with progressive chronic kidney disease (web appendix Table 8).
Table 1.
Characteristics of included studies
| N | Age year | Male % | Black % | CVD % | HT % | HC % | DM % | Smoking % | eGFR ml/min/1.73m2 | ACR mg/g | FU Year | ESRD n | AKI n | pCKD n | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General population cohorts with albumin-to-creatinine ratio data | 147 | 427 | 173 | ||||||||||||
|
| |||||||||||||||
| - ARIC | 11,408 | 62.8 | 44.2 | 22.2 | 8.6 | 47.6 | 34.5 | 16.7 | 14.9 | 82.5 | 3.7 | 8.0 | 92 | 363 | -- |
| - AusDiab | 11,240 | 51.5 | 44.9 | 0 | 8.3 | 32.7 | 70.6 | 8.4 | 15.5 | 78.9 | 4.9 | 5.0 | -- | -- | 72 |
| - CHS | 3,230 | 78.0 | 40.2 | 15.9 | 29.3 | 50.1 | 31.0 | 14.7 | 7.6 | 79.4 | 8.8 | 7.6 | -- | 64 | -- |
| - HUNT2 | 9,525 | 62.0 | 44.8 | 0 | 22.5 | 82.5 | 61.3 | 17.6 | 19.7 | 83.8 | 7.5 | 10.5 | 55 | -- | -- |
| - MESA | 6,728 | 62.2 | 47.2 | 27.5 | 0.0 | 44.8 | 9.0 | 12.6 | 13.0 | 81.2 | 5.3 | 4.7 | -- | 101 | |
|
| |||||||||||||||
| General population cohorts with dipstick data | 713 | 3,438 | 4,624 | ||||||||||||
|
| |||||||||||||||
| - AKDN UDIP | 690,680 | 47.4 | 45.1 | NA | 1.8 | 20.2 | NA | 6.1 | NA | 80.9 | -- | 2.3 | 478 | 3,438 | 4,475 |
| - Beaver Dam | 4,926 | 62.0 | 43.9 | 0 | 14.8 | 50.5 | 53.9 | 10.3 | 19.7 | 76.2 | -- | 11.6 | -- | -- | 149 |
| - Okinawa 83 | 6,659 | 51.9 | 39.5 | NA | NA | NA | NA | 3.8 | NA | 73.9 | -- | 16.8 | 61 | -- | -- |
| - Okinawa 93 | 93,234 | 54.6 | 43.6 | NA | NA | NA | NA | 4.7 | NA | 77.3 | -- | 6.9 | 174 | -- | -- |
|
| |||||||||||||||
| High risk cohorts with albumin-to-creatinine ratio data | 762 | 1,074 | 4,935 | ||||||||||||
|
| |||||||||||||||
| - ADVANCE | 11,140 | 65.8 | 57.5 | NA | 32.2 | 82.2 | 33.0 | 100 | 15.1 | 78.2 | 15.9 | 4.8 | 59 | -- | 822 |
| - AKDN ACR | 67,406 | 55.5 | 56.8 | NA | 5.0 | 46.8 | NA | 49.0 | NA | 76.8 | 11.1 | 2.3 | 191 | 1,013 | 1,572 |
| - ONTARGET | 25,620 | 66.4 | 73.3 | 2.5 | 92 | NA* | NA* | 37.5 | 12.6 | 73.6 | 52.2 | 4.5 | 184 | 61 | 1,914 |
| - Pima | 6,341 | 26.4 | 45.4 | 0 | NA | 12.9 | 4.2 | 20.4 | 27.8 | 144 | 11.9 | 13.5 | 328 | -- | 273 |
| - TRANSCEND | 5,926 | 66.9 | 57 | 1.8 | 92.5 | NA* | NA* | 35.7 | 9.8 | 71.7 | 25.3 | 4.6 | -- | -- | 354 |
|
| |||||||||||||||
| High risk cohorts with dipstick data | 579 | -- | 1,412 | ||||||||||||
|
| |||||||||||||||
| - CARE | 4,098 | 58.6 | 87.2 | 3.2 | 100 | 82.9 | 79.0 | 14.2 | 16.1 | 71.9 | -- | 4.8 | -- | -- | 124 |
| - Hawaii | 40,210 | 59.0 | 50.4 | NA | 17.0 | NA | NA | 48.0 | 13.6 | 71.5 | -- | 2.4 | 331 | -- | 1,288 |
| - MR FIT | 12,851 | 46.2 | 100 | 31.3 | 0.0 | 62.3 | 57.1 | 3.1 | 63.7 | 79.7 | -- | 21.6 | 248 | -- | -- |
Abbreviations are: HT, hypertension; HC, hypercholesterolemia; DM, diabetes mellitus; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; ACR, albumin:creatinine ratio; FU, duration of follow-up; ESRD, end-stage renal disease; pCKD, progressive chronic kidney disease; AKI, acute kidney injury.
, NA* in ONTARGET and TRANSCEND respectively, a history of hypertension was reported by 69% and 76%, and statin use by 62% and 55%.
Independent continuous associations of eGFR and albuminuria with kidney outcomes
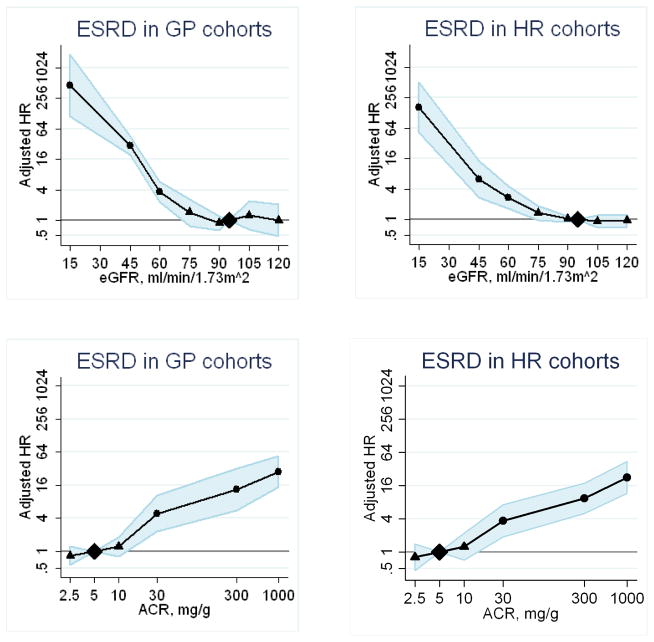
Pooled hazard ratios of ESRD according to eGFR and albuminuria adjusted for each other and covariates in the general population cohorts and the high risk cohorts are shown in Figure 1. ESRD risk was relatively constant between an eGFR 75–120 mL/min/1.73m2 and was exponentially greater at lower eGFR. In the general population cohorts eGFR risk association with ESRD showed hazard ratios at eGFR 60, 45 and 15 mL/min/1.73m2 of 3.69 (2.36–5.76), 29.3 (19.5–44.1) and 454.9 (112.4–1840.2), respectively. The relationship of albumin-to-creatinine ratio to the relative risk of ESRD was monotonic on the log-log scale, without threshold effects. As compared to albumin-to-creatinine ratio 5 mg/g, hazard ratios for ESRD at albumin-to-creatinine ratios of 30, 300 and 1000 mg/g were 4.87 (2.30–10.3), 13.4 (5.49–32.7) and 28.4 (14.9–54.2), respectively. These patterns for ESRD in the high risk cohorts were similar to the general population cohorts (Figure 1). The patterns for acute kidney injury and progressive chronic kidney disease were generally similar to the patterns for ESRD, although less steep (web appendix Figures 1 and 2).
Figure 1.
Pooled hazard ratios (95% confidence intervals) for end-stage renal disease according to spline eGFR (upper panels) and albumin-to-creatinine ratio ( (lower panels), adjusted for each other and for age, sex and cardiovascular risk factors (continuous analyses). Reference categories are eGFR 95 mL/min/1.73 m2 and albumin-to-creatinine ratio 5 mg/g or dipstick negative or trace. Left panels show results for general population cohorts, and right panels for high risk cohorts. Dots represent statistical significance, triangles represent non significance, and shaded areas are 95% confidence intervals. Abbreviations are: HR, hazard ratio; eGFR, estimated glomerular filtration rate; ACR, albumin-to-creatinine ratio; ESRD, end-stage renal disease; AKI, acute kidney injury; pCKD, progressive chronic kidney disease; GP cohorts, general population cohorts and HR cohorts, high risk cohorts.
Interactions
The interaction between eGFR and albuminuria was significant for ESRD in only 1 out of 8 cohorts, for acute kidney injury in 3 out of 5 cohorts, and for progressive chronic kidney disease in 4 out of 11 cohorts (web appendix Table 11). Significant interaction between eGFR and age was found for ESRD in only 1 out of 9 cohorts, for acute kidney injury in 3 out 5 cohorts, and for progressive chronic kidney disease in 4 out of 11 cohorts (web appendix Table 11). Age interactions tended to show lower hazard ratios at older age, but a similar pattern of the associations of eGFR and albumin-to-creatinine ratio with the various kidney outcomes (web appendix Tables 4,7,10). The eGFR * albumin-to-creatinine ratio interaction can be visually assessed in graph 2. At low eGFR the hazard ratio of higher albumin-to-creatinine ratio tended to be less than at high eGFR for ESRD as well as for acute kidney injury, but not for progressive chronic kidney disease.
Joint associations of eGFR and albuminuria with kidney outcomes
As the albumin-to-creatinine ratio and the dipstick cohorts showed similar relationships between eGFR and albuminuria with ESRD, these two type of cohorts were combined to increase power for investigation of the joint associations of eGFR and albuminuria with kidney outcomes, both in general population and in high risk cohorts (web appendix Figure 3). Table 2 shows unadjusted incidence rates of the three kidney outcomes for general population cohorts. Pooled hazard ratios/odds ratios for ESRD, acute kidney injury and progressive chronic kidney disease of the 21 categories of eGFR and albuminuria for the general population cohorts are shown in Tables 3 and 4. Low eGFR showed a similar association with risk across all levels of albuminuria and high albuminuria showed a similar association with risk across all levels of eGFR, indicating multiplicative independent risk for kidney outcomes. At severely reduced eGFR values (15–29 mL/min/1.73m2), the risk associated with higher albuminuria was attenuated. The patterns were much steeper (ie risk increased more rapidly with increasing albuminuria) for ESRD than for acute kidney injury and progressive chronic kidney disease (Tables 3 and 4). Figure 2 shows the continuous analyses (allowing interaction) of the hazard ratios/odds ratios of eGFR and albuminuria for ESRD, acute kidney injury and progressive chronic kidney disease, respectively.
Table 2.
General population cohorts. Unadjusted incidence rates (per 1000 patient years) for end-stage renal disease, acute kidney injury and progressive chronic kidney disease. Shaded areas make up the combined reference groups.
| albumin-to-creatinine ratio (mg/g) or dipstick (classes)
|
||||||
|---|---|---|---|---|---|---|
| <10 Negative | 10–29 trace | 30–299 1+ |
≥300 ≥2+ |
All | ||
|
End-Stage Renal Disease
| ||||||
| eGFR mL/min/1.73m2 | >105 | 0.13 | 0.75 | |||
| 90–104 | 0.04 | 0.05 | 0.57 | 0.06 | ||
| 75–89 | 0.11 | 2.35 | ||||
| 60–74 | 0.27 | 2.66 | ||||
| 45–59 | 0.12 | 0.77 | 1.44 | 5.13 | 0.34 | |
| 30–44 | 1.03 | 1.55 | 9.15 | 27.07 | 4.02 | |
| 15–29 | 9.05 | 19.5 | 37.7 | 128.4 | 43.0 | |
|
| ||||||
| All | 0.09 | 1.61 | 14.9 | 0.31 | ||
|
| ||||||
|
Acute Kidney Injury
| ||||||
| eGFR mL/min/1.73m2 | >105 | 3.55 | 7.57 | |||
| 90–104 | 0.98 | 3.04 | 5.73 | 1.14 | ||
| 75–89 | 3.45 | 5.86 | ||||
| 60–74 | 6.46 | 13.77 | ||||
| 45–59 | 4.73 | 13.10 | 21.40 | 36.08 | 6.48 | |
| 30–44 | 24.49 | 42.53 | 52.09 | 76.62 | 32.65 | |
| 15–29 | 69.66 | 65.82 | 92.93 | 109.6 | 81.37 | |
|
| ||||||
| All | 1.69 | 10.15 | 26.26 | 2.21 | ||
|
| ||||||
|
Progressive Chronic Kidney Disease
| ||||||
| eGFR mL/min/1.73m2 | >105 | 1.56 | 12.6 | |||
| 90–104 | 2.02 | 2.72 | 7.02 | 2.48 | ||
| 75–89 | 5.25 | 25.21 | ||||
| 60–74 | 16.80 | 47.50 | ||||
| 45–59 | 23.91 | 31.91 | 63.61 | 135.1 | 28.78 | |
| 30–44 | 37.53 | 54.60 | 82.27 | 177.5 | 55.37 | |
| 15–29 | 33.12 | 55.36 | 82.08 | 178.9 | 77.14 | |
|
| ||||||
| All | 5.62 | 25.93 | 89.59 | 7.55 | ||
Abbreviations are: eGFR. estimated glomerular filtration rate.
Table 3.
General population cohorts. Pooled adjusted hazard ratios (95% confidence intervals) for end-stage renal disease and acute kidney injury and pooled adjusted odds ratios (95% confidence intervals) for progressive chronic kidney disease. Shaded areas make up the combined reference groups.
| albumin-to-creatinine ratio (mg/g) or dipstick (classes)
|
||||||
|---|---|---|---|---|---|---|
| <10 Negative | 10–29 trace | 30–299 1+ |
≥300 ≥2+ |
All | ||
|
End-Stage Renal Disease
| ||||||
| eGFR mL/min/1.73m2 | >105 | 7.8 (1.7–35.9) | 18.1 (4.30–75.9) | |||
| 90–104 | Ref | 11.3 (2.7–47.7) | 19.7 (5.84–66.5) | Ref | ||
| 75–89 | 3.83 (1.2–12.3) | 48.1 (28.1–82.3) | ||||
| 60–74 | 7.42 (3.6–15.2) | 67.2 (40.1–113) | ||||
| 45–59 | 5.2 (3.3–8.0) | 21.8 (12.0–39.6) | 40.3 (23.5–69.2) | 147 (98.7–219) | 9.67 (7.07–13.2) | |
| 30–44 | 55.5 (36.0–85.6) | 74.1 (29.3–187) | 293 (199–433) | 763 (563–1035) | 98.1 (61.8–156) | |
| 15–29 | 433 (239–787) | 1044 (524–2077) | 1056 (572–1948) | 2286 (1114–4695) | 573 (241–1362) | |
|
| ||||||
| All | Ref | 12.0 (7.9–18.1) | 72.1 (43.0–121) | |||
|
| ||||||
|
Acute Kidney Injury
| ||||||
| eGFR mL/min/1.73m2 | >105 | 2.7 (0.9–8.5) | 8.4 (5.1–13.8) | |||
| 90–104 | Ref | 2.4 (1.1–5.2) | 5.8 (3.7–9.2) | Ref | ||
| 75–89 | 2.5 (1.9–3.4) | 4.1 (2.8–5.9) | ||||
| 60–74 | 3.3 (2.6–4.1) | 6.4 (5.0–8.2) | ||||
| 45–59 | 2.2 (2.0–2.5) | 4.9 (3.3–7.3) | 6.3 (4.8–8.4) | 5.9 (2.4–14.5) | 2.6 (2.2–3.1) | |
| 30–44 | 7.3 (6.5–8.2) | 10.2 (5.9–17.5) | 12.4 (10.2–15.2) | 19.6 (16.5–23.2) | 7.9 (7.1–8.7) | |
| 15–29 | 16.8 (14.0–20.2) | 16.8 (11.3–25.1) | 21.4 (16.5–27.8) | 28.8 (23.7–35.1) | 16.7 (14.7–18.9) | |
|
| ||||||
| All | Ref | 2.5 (1.7–3.7) | 6.0 (4.5–8.0) | |||
|
| ||||||
|
Progressive Chronic Kidney Disease
| ||||||
| eGFR mL/min/1.73m2 | >105 | 0.7 (0.7–0.8) | 3.0 (0.4–23.7) | |||
| 90–104 | Ref | 0.9 (0.4–2.1) | 3.3 (0.5–23.3) | Ref | ||
| 75–89 | 1.9 (0.6–5.6) | 5.0 (0.9–27.1) | ||||
| 60–74 | 3.2 (1.4–7.5) | 8.1 (5.2–12.8) | ||||
| 45–59 | 3.1 (1.6–6.0) | 4.0 (1.9–8.8) | 9.4 (3.7–23.7) | 56.6 (4.2–767.6) | 3.9 (1.9–7.8) | |
| 30–44 | 3.0 (1.2–7.5) | 19.1 (19.0–19.2) | 14.9 (2.8–78.5) | 22.2 (4.8–103.6) | 3.7 (1.1–12.3) | |
| 15–29 | 4.0 (3.9–4.0) | 11.7 (11.6–11.9) | 21.0 (4.5–99.5) | 7.7 (2.9–20.6) | 7.9 (3.0–21.2) | |
|
| ||||||
| All | Ref | 3.1 (2.5–3.8) | 11.2 (5.8–21.5) | |||
Abbreviations are: eGFR. estimated glomerular filtration rate.
Table 4.
General population cohorts. Pooled adjusted hazard ratios (95% confidence intervals) for end-stage renal disease subdivided for age groups <65 and >65 years. Shaded areas make up the combined reference groups.
| albumin-to-creatinine ratio (mg/g) or dipstick (classes)
|
||||||
|---|---|---|---|---|---|---|
| <10 Negative | 10–29 trace | 30–299 1+ |
≥300 ≥2+ |
All | ||
|
ESRD, younger than 65 years
| ||||||
| eGFR mL/min/1.73m2 | >105 | 12.4 (2.30–66.8) | 28.6 (6.45–127) | |||
| 90–104 | Ref | 14.2 (3.31–61.0) | 13.8 (1.87–101.2) | Ref | ||
| 75–89 | 5.81 (1.40–24.2) | 65.2 (37.3–114) | ||||
| 60–74 | 5.58 (2.00–15.7) | 87.3 (32.3–236) | ||||
| 45–59 | 3.1 (1.1–8.3) | 31.8 (14.3–70.5) | 55.4 (29.6–103) | 261 (112–610) | 9.5 (5.6–15.9) | |
| 30–44 | 101 (54.8–187) | 293 (69.3–1236) | 272 (107–693) | 828 (443–1545) | 110 (49.6–245) | |
| 15–29 | 999 (493–2023) | 3897 (1717–8845) | 2398 (1247–4609) | 5081 (2736–9435) | 1281 (556–2952) | |
|
| ||||||
| All | Ref | 13.7 (8.8–21.3) | 124 (60.2–257) | |||
|
| ||||||
|
ESRD, older than 65 years
| ||||||
| eGFR mL/min/1.73m2 | >105 | 0.0 (0.0–.) | 0.0 (0.0–.) | |||
| 90–104 | Ref | 0.0 (0.0–.) | 0.0 (0.0–.) | Ref | ||
| 75–89 | 0.0 (0.0–.) | 0.0 (0.0–.) | ||||
| 60–74 | 6.61 (1.61–27.2) | 18.8 (5.29–67.1) | ||||
| 45–59 | 3.4 (1.6–7.2) | 9.6 (3.8–24.4) | 16.4 (5.85–45.9) | 41.4 (7.95–215) | 4.5 (3.0–6.75) | |
| 30–44 | 11.5 (5.97–22.1) | 18.1 (3.83–85.9) | 90.8 (48.3–171) | 268 (157–458) | 42.1 (28.7–61.7) | |
| 15–29 | 131 (62.7–274) | 115 (33.8–389) | 413 (222–768) | 1071 (645–1779) | 186 (92.9–372) | |
|
| ||||||
| All | Ref | 10.3 (5.96–17.8) | 47.5 (27.2–82.9) | |||
Abbreviations are: eGFR. estimated glomerular filtration rate.
Figure 2.
Pooled adjusted hazard ratios or Odds Ratios (95% confidence intervals) for end-stage renal disease (upper panel), acute kidney injury (middle panel) and progressive chronic kidney disease (lower panel) according to eGFR and albuminuria based on continuous models with eGFR (splines), albuminuria (log-linear albumin-to-creatinine ratio or categorical dipstick) and their interaction terms. Hazard ratios are adjusted for age, sex and cardiovascular risk factors. Reference category is eGFR 95 mL/min/1.73 m2 plus albumin-to-creatinine ratio 5 mg/g or dipstick negative or trace. Left panels shows results for general population cohorts, and right panels for high risk cohorts. Dots represent statistical significance, triangles represent non significance, and shaded areas are 95% confidence intervals. In this figure albuminuria is treated categorically. Black lines and blue shading represent an albumin-to-creatinine ratio < 30 mg/g or dipstick negative or trace, green lines and green shading an albumin-to-creatinine ratio 30–299 mg/g or dipstick 1+, and red lines and red shading an albumin-to-creatinine ratio ≥ 300 mg/g or dipstick ≥ 2+. Abbreviations are: HR, hazard ratio; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; AKI, acute kidney injury; pCKD, progressive chronic kidney disease; GP cohorts, general population cohorts and HR cohorts, high risk cohorts.
Similar data are given for cohorts at high risk for chronic kidney disease (Tables 5, 6 and 7). The patterns for ESRD were less steep in the high risk cohorts (Table 6) compared to the general population cohorts (Table 3), whereas the patterns for acute kidney injury and progressive chronic kidney disease were similar in the general population cohorts and high risk cohorts.
Table 5.
High risk cohorts. Unadjusted incidence rates (per 1000 patient years) for end-stage renal disease, acute kidney injury and progressive chronic kidney disease. Shaded areas make up the combined reference groups.
| albumin-to-creatinine ratio (mg/g) or dipstick (classes)
|
||||||
|---|---|---|---|---|---|---|
| <10 Negative | 10–29 trace | 30–299 1+ |
≥300 ≥2+ |
All | ||
|
End-Stage Renal Disease
| ||||||
| eGFR mL/min/1.73m2 | >105 | 1.22 | 6.52 | |||
| 90–104 | 0.22 | 0.39 | 5.00 | 0.45 | ||
| 75–89 | 0.30 | 4.56 | ||||
| 60–74 | 0.36 | 7.77 | ||||
| 45–59 | 0.25 | 0.36 | 1.65 | 13.38 | 1.44 | |
| 30–44 | 1.56 | 2.42 | 4.33 | 29.80 | 7.35 | |
| 15–29 | 1.57 | 12.78 | 20.93 | 133.0 | 60.98 | |
|
| ||||||
| All | 0.31 | 1.41 | 25.72 | 1.83 | ||
|
| ||||||
|
Acute Kidney Injury
| ||||||
| eGFR mL/min/1.73m2 | >105 | 2.99 | 5.54 | |||
| 90–104 | 1.41 | 3.35 | 5.43 | 2.25 | ||
| 75–89 | 3.09 | 9.92 | ||||
| 60–74 | 6.06 | 13.73 | ||||
| 45–59 | 2.28 | 8.00 | 13.42 | 29.03 | 8.07 | |
| 30–44 | 11.2 | 17.76 | 36.70 | 52.09 | 27.63 | |
| 15–29 | 25.74 | 48.66 | 69.90 | 104.7 | 73.94 | |
|
| ||||||
| All | 2.33 | 9.08 | 26.59 | 4.88 | ||
|
| ||||||
|
Progressive Chronic Kidney Disease
| ||||||
| eGFR mL/min/1.73m2 | >105 | 4.43 | 27.52 | |||
| 90–104 | 5.51 | 5.75 | 14.44 | 7.97 | ||
| 75–89 | 8.59 | 30.90 | ||||
| 60–74 | 19.01 | 68.77 | ||||
| 45–59 | 23.75 | 37.88 | 57.67 | 147.1 | 43.84 | |
| 30–44 | 33.55 | 35.35 | 64.99 | 160.3 | 65.65 | |
| 15–29 | 12.44 | 43.16 | 58.43 | 209.3 | 103.3 | |
|
| ||||||
| All | 10.40 | 25.96 | 105.0 | 18.44 | ||
Abbreviations are: eGFR. estimated glomerular filtration rate.
Table 6.
High risk cohorts. Pooled adjusted hazard ratios (95% confidence intervals) for end-stage renal disease and acute kidney injury and pooled adjusted odds ratios (95% confidence intervals) for progressive chronic kidney disease. Shaded areas make up the combined reference groups.
| albumin-to-creatinine ratio (mg/g) or dipstick (classes)
|
||||||
|---|---|---|---|---|---|---|
| <10 Negative | 10–29 trace | 30–299 1+ |
≥300 ≥2+ |
All | ||
|
End-Stage Renal Disease
| ||||||
| eGFR mL/min/1.73m2 | >105 | 1.1 (0.8–1.6) | 2.0 (0.9–4.5) | |||
| 90–104 | Ref | 2.3 (1.0–5.4) | 10.0 (2.1–47.2) | Ref | ||
| 75–89 | 1.7 (0.9–3.3) | 17.3 (4.0–74.9) | ||||
| 60–74 | 3.1 (1.8–5.3) | 32.2 (11.8–87.8) | ||||
| 45–59 | 2.7 (1.7–4.3) | 3.8 (1.9–7.5) | 14.5 (6.3–33.1) | 55.5 (17.9–172.6) | 5.7 (1.7–4.3) | |
| 30–44 | 23.4 (11.0–49.5) | 33.4 (12.9–86.4) | 56.0 (20.0–156.9) | 139.8 (35.6–549.2) | 27.4 (11.0–49.5) | |
| 15–29 | 32.6 (4.3–248.5) | 308.2 (97.0–978.8) | 387.2 (86.9–1724.9) | 462.7 (31.6–6779.9) | 165.8 (52.4–524.2) | |
|
| ||||||
| All | Ref | 4.3 (2.6–7.1) | 38.1 (15.6–93.5) | |||
|
| ||||||
|
Acute Kidney Injury
| ||||||
| eGFR mL/min/1.73m2 | >105 | 2.2 (1.2–4.2) | 3.8 (1.2–12.0) | |||
| 90–104 | Ref | 2.1 (1.3–3.4) | 3.4 (1.4–8.3) | Ref | ||
| 75–89 | 1.8 (1.3–2.5) | 5.2 (3.2–8.6) | ||||
| 60–74 | 2.8 (1.4–5.6) | 6.3 (4.3–9.2) | ||||
| 45–59 | 1.7 (1.2–2.5) | 3.5 (2.6–4.7) | 6.6 (5.2–8.5) | 13.0 (9.7–17.3) | 3.0 (2.5–3.5) | |
| 30–44 | 8.0 (5.4–11.8) | 7.5 (5.3–10.6) | 14.3 (11.2–18.3) | 26.9 (12.3–58.8) | 10.6 (5.2–21.9) | |
| 15–29 | 12.3 (5.4–27.8) | 1.6 (0.0–.) | 25.3 (18.2–35.3) | 13.7 (0.0–.) | 16.8 (13.5–20.9) | |
|
| ||||||
| All | Ref | 2.7 (2.2–3.4) | 7.4 (5.5–9.8) | |||
|
| ||||||
|
Progressive Chronic Kidney Disease
| ||||||
| eGFR mL/min/1.73m2 | >105 | 0.6 (0.5–0.8) | 4.7 (0.3–69.4) | |||
| 90–104 | Ref | 0.9 (0.7–1.2) | 3.5 (0.5–26.0) | Ref | ||
| 75–89 | 1.0 (0.8–1.1) | 3.5 (2.5–5.0) | ||||
| 60–74 | 2.8 (1.3–6.1) | 9.3 (6.0–14.4) | ||||
| 45–59 | 3.0 (2.1–4.4) | 4.8 (3.7–6.2) | 10.1 (4.9–20.8) | 31.4 (16.1–61.5) | 4.7 (3.3–6.8) | |
| 30–44 | 3.3 (2.7–4.1) | 3.4 (2.5–4.7) | 9.8 (6.3–15.3) | 68.7 (57.6–81.9) | 6.4 (4.3–9.7) | |
| 15–29 | 0.5 (0.4–0.7) | 3.1 (1.2–7.7) | 9.4 (5.3–16.6) | 38.6 (15.7–94.8) | 8.9 (4.8–16.7) | |
|
| ||||||
| All | Ref | 2.2 (1.9–2.7) | 9.9 (6.7–14.5) | |||
Abbreviations are: eGFR. estimated glomerular filtration rate.
Table 7.
High risk cohorts. Pooled adjusted hazard ratios (95% confidence intervals) for end-stage renal disease subdivided for age groups <65 and >65 years. Shaded areas make up the combined reference groups.
| albumin-to-creatinine ratio (mg/g) or dipstick (classes)
|
||||||
|---|---|---|---|---|---|---|
| <10 Negative | 10–29 trace | 30–299 1+ |
≥300 ≥2+ |
All | ||
|
Younger than 65 years
| ||||||
| eGFR mL/min/1.73m2 | >105 | 1.1 (0.8–1.7) | 1.4 (0.9–3.6) | |||
| 90–104 | Ref | 2.6 (1.0–6.9) | 10.5 (2.0–55.3) | Ref | ||
| 75–89 | 1.7 (0.8–3.8) | 16.3 (2.3–118.6) | ||||
| 60–74 | 4.0 (2.0–7.7) | 39.0 (10.3–148.1) | ||||
| 45–59 | 2.4 (1.4–4.2) | 5.3 (2.3–12.2) | 16.9 (4.7–60.5) | 66.9 (20.1–222.3) | 7.0 (4.3–11.6) | |
| 30–44 | 15.9 (1.9–133.4) | 73.6 (20.5–263.9) | 90.9 (27.6–299.4) | 161.3 (26.3–988.9) | 33.9 (14.6–78.9) | |
| 15–29 | # | 656.3 (171.8–2507.0) | 791.6 (210.1–2982.2) | 998.3 (105.4–9454.7) | 222.7 (69.9–709.3) | |
|
| ||||||
| All | Ref | 4.5 (2.4–8.5) | 43.8 (16.4–116.7) | |||
|
| ||||||
|
Older than 65 years
| ||||||
| eGFR mL/min/1.73m2 | >105 | 0.0 (0.0–.) | 20.6 (2.4–172.9) | |||
| 90–104 | Ref | 0.0 (0.0–.) | 15.5 (2.0–122.2) | Ref | ||
| 75–89 | 1.9 (0.6–5.9) | 16.2 (3.1–84.6) | ||||
| 60–74 | 1.7 (0.6–4.7) | 20.7 (9.4–45.8) | ||||
| 45–59 | 2.8 (1.1–7.2) | 1.8 (0.5–6.4) | 10.0 (5.5–18.1) | 31.2 (10.9–89.5) | 3.8 (2.5–5.8) | |
| 30–44 | 16.1 (6.7–38.8) | 18.1 (7.5–43.6) | 24.3 (9.3–63.4) | 92.7 (46.3–185.7) | 20.7 (14.0–30.6) | |
| 15–29 | 25.0 (3.2–196.3) | 174.7 (42.5–718.2) | 125.1 (43.0–363.2) | 505.8 (157.9–1619.9) | 146.6 (46.3–464.3) | |
|
| ||||||
| All | Ref | 4.1 (2.5–6.8) | 43.3 (13.0–144.5) | |||
Abbreviations are: albumin:creatinine ratio; eGFR. estimated glomerular filtration rate; #, insufficient number of events for reliable estimates.
Joint associations of eGFR and albuminuria with kidney outcomes per age group
The overall incidence rates for the kidney outcomes were 3 to 9 fold higher in the subgroup of subjects with age ≥65 years compared the subgroup with age <65 years (web appendix Tables 3, 6, 9, respectively). Pooled hazard ratios for ESRD of the 21 categories of eGFR and albuminuria according to age group are shown in Table 4 for the general population cohorts and in Table 5 for the high risk cohorts. The general pattern of higher risk for a lower eGFR independent of albuminuria level and of a higher albuminuria independent of eGFR level was observed in both age groups. However, in general relative hazards were smaller among participants ≥ 65 years than among participants < 65 years. Similar findings were obtained for acute kidney injury (web appendix Table 7) and progressive chronic kidney disease (web appendix Table 10).
Heterogeneity
eGFR x albumin-to-creatinine ratio categories with significant heterogeneity are shown in the web appendix Tables 4, 7 and 10. Quantitative heterogeneity, rather than qualitative heterogeneity, was observed in several categories, reflecting numerical differences in the hazard ratios between cohorts, but the direction of the risk was similar in all cohorts (increased risk with lower eGFR categories and with higher albuminuria categories). However, in all cohorts, the direction of the risk was similar (increased risk with lower eGFR categories and with higher albuminuria categories). Moreover, significant heterogeneity was limited to the lowest eGFR and the highest albuminuria categories. There was no significant heterogeneity in the groups with eGFR of 45–60 mL/min/1.73m2 and in the groups with microalbuminuria (albumin-to-creatinine ratio 30–299 mg/g, or dipstick 1+), either in the general population, or in the high risk population.
Meta-regression analysis was performed that showed that the association between eGFR and albuminuria with kidney outcomes was not dependent on the proportion of diabetic subjects included in the individual cohorts. This held true for both general population and high risk cohorts and for all three kidney outcomes.
Discussion
In this collaborative meta-analysis of 9 general population and 8 high risk cohorts, including a total of more than 1 million subjects, we found that lower eGFR and higher albuminuria were associated with a higher risk for ESRD, independent of each other and independent of traditional CVD risk factors. A similar association of eGFR and albuminuria was found with the risk for acute kidney injury and for progressive chronic kidney disease, although the relative hazards were higher for ESRD.
The risk for ESRD based on eGFR and albuminuria have been reported in a limited number of follow-up studies from general population cohorts (20,22,35,42, 47). The current meta-analysis confirms these studies and extends the generalizability of these data to other populations world wide. Furthermore, our collaborative meta-analysis includes 2.201 ESRD outcomes, substantially more than the number of events in reports of individual studies, thereby allowing evaluation of the independent and joint associations of eGFR and albuminuria with this outcome. In addition, we included data on acute kidney injury and progressive chronic kidney disease, other kidney disease outcomes of clinical and epidemiologic interest.
We found similar patterns in studies that had data on albumin-to-creatinine ratio and in the studies that only had semi-quantitative information available on dipstick proteinuria. These findings suggest that measurement of dipstick proteinuria is useful for risk stratification, despite being a less precise measure of albuminuria. This is of importance considering the lower cost of dipstick compared to albumin-to-creatinine ratio measurement. However, studies directly comparing dipstick testing with more accurate albuminuria measurements are needed to investigate sensitivity, specificity, negative and positive predictive value, to make definite recommendations for screening. Also, it is important to bear in mind, that most studies had measured albuminuria only once, thus raising questions regarding reproducibility and chronicity of albuminuria. However, the finding that a single urine test has significant prognostic implication strengthens the conclusion that albuminuria is an important risk factor. In addition, a single test may underestimate rather than overestimate the risk associated with albumin-to-creatinine ratio due to regression dilution bias (36).
The general pattern of a graded increase in relative risk for the various kidney outcomes with higher albuminuria and lower eGFR was observed in both cohorts at high risk for chronic kidney disease as well as cohorts derived from the general population. Although the absolute incidence of ESRD was higher in the high risk population compared to the general population, the increase in relative hazards for a lower eGFR and a higher albuminuria was more pronounced in the general population than the high risk population. The consistency of our findings in both cohorts with albumin-to-creatinine ratio and dipstick proteinuria data, in both general population and high risk cohorts, and in both continuous and categorical models for eGFR and albumin-to-creatinine ratio, demonstrates the robustness of our findings. The finding of only quantitative, but not qualitative heterogeneity, and that heterogeneity was not observed in the categories of most clinical interest, that is eGFR 45–60 ml/min/1.73m2 and albumin-to-creatinine ratio 30–299 mg/g or dipstick >1+, further underscores the strengths of our observations. Of note, our meta-regression analyses showed that the associations of eGFR and albuminuria with adjusted hazard rates for all three kidney outcomes were not related to the proportion of diabetic subjects included in the various cohorts. This provides no evidence for the assumption of some investigators that diabetic and non-diabetic kidney disease should be regarded as separate entities.
The statistical code that was sent to the participating cohorts rendered output that did not permit computation of a meta-analytic result for interactions. However, tables 3 and 4 show that the pattern of higher relative hazards for ESRD for a lower eGFR and for a higher albuminuria is less steep in subgroups older than ≥65 than in those <65 years. The relationship of higher albuminuria with higher unadjusted incidence rate of ESRD is comparable for both age groups, but less steep with lower eGFR in the elderly when compared to the young (web appendix Table 3). The less steep relationship with lower eGFR needs to be balanced against the higher incidence rates in the older subgroup. Although in elderly the increase in adjusted relative risk with lower eGFR is less than in the young, the increase in unadjusted incidence rates is higher. The age-eGFR interaction will be studied in-depth in later analyses by the CKD Prognosis Consortium.
The observed relative risk increase for ESRD with lower eGFR is more pronounced than the relative risk increase for all-cause and cardiovascular mortality, as described separately (24). The hazard ratios for ESRD at eGFR 60, 45 and 15 ml/min/1.73m2 were 3.69 (2.36–5.76), 29.3 (19.5–44.1) and 454.9 (112.4–1840.2), respectively, compared to 1.16 (1.04–1.30), 1.49 (1.28–1.72) and 3.18 (2.45–4.14), respectively, for all cause mortality (25). Interestingly, the increase in relative risk for higher albuminuria is also substantially higher for ESRD compared to all-cause mortality, with hazard ratios for ESRD at albumin-to-creatinine ratio 30, 300 and 1000 mg/g of 4.87 (2.30–10.3), 13.4 (5.49–32.72), respectively, and 28.4 (14.9–54.2) compared to 1.16 (1.08–1.25), 1.51 (1.34–1.70), and 2.15 (1.80–2.58), respectively, for all-cause mortality (25). For kidney outcomes eGFR and albumin-to-creatinine ratio were the strongest risk factors examined, often stronger than age, which differs from all-cause mortality and cardiovascular mortality where age is the dominant factor. The higher relative risks for kidney outcomes than for mortality, likely reflect a greater specificity of association of eGFR and albumin-to-creatinine ratio with these outcomes. The implications of the more steep relationship of low eGFR and high albuminuria with relative risk for ESRD than for mortality should be considered in view of the relative low incidence rates of the kidney outcomes. Lastly, these data are not consistent with the suggestion by others that microalbuminuria is only a marker for increased CVD risk (11), since it also indicates substantially increased risk for all kidney outcomes examined.
A strength of this pooled analysis is that it includes data on acute kidney injury and progressive chronic kidney disease as well as on ESRD. A disadvantage of limiting study of kidney outcomes to only ESRD is that it will predispose to identification of low eGFR values as the most important risk predictor, as the decision to start renal replacement therapy is for a large part based on eGFR. For clinical practice however, it is also important to identify risk predictors in subjects with relatively preserved renal function, who may benefit from early initiation of therapies to slow progression of chronic kidney disease, thereby delaying or even preventing ESRD and other complications. Therefore incident acute kidney injury and progressive chronic kidney disease were studied as earlier kidney outcomes than ESRD. For acute kidney injury the ICD hospital discharge code 584 was adopted as defining criterion. For progressive chronic kidney disease different definitions have been used in the literature. Our definition required loss of eGFR of more than 2.5 ml/min/1.73m2 per year (approximately 3–5 times faster than the rate of renal function decline in the general population (21,47)), and a final eGFR during follow up of ≤ 45 ml/min/1.73m2 (since it is widely acknowledged that this threshold is of clinical significance). Such a combination of a relative decrease and an absolute threshold has been used before in epidemiological studies (48) to increase specificity with a recognized loss of sensitivity. Of note, the weaker associations of eGFR and albuminuria for progressive chronic kidney disease in comparison to the two other kidney outcomes can be partially explained by misclassification of the outcome and regression to the mean.
Some limitations of this meta-analysis should be mentioned. First, we included only a relatively limited number of cohorts, and measurements of serum creatinine and albuminuria were not centrally standardized across these cohorts. The present analysis however, is to the best of our knowledge the largest and most comprehensive assessment of the relation between eGFR, albuminuria and kidney outcomes yet performed. Second, no data on treatment effects could be taken into account. Thus it cannot be excluded that the observed associations are influenced by the start of specific treatments. However, if such treatment is effective in preventing kidney disease progression, then it would be expected to lead to an underestimation of the true relative risk of low eGFR and high albuminuria for these outcomes. Finally, we used a restrictive definition of progressive chronic kidney disease and alternative definitions should be explored.
What do these findings mean for the current debate on the definition and classification of chronic kidney disease? First, since albuminuria is a risk factor for kidney outcomes independent of eGFR and conventional cardiovascular risk factors, this suggests that albuminuria could be used for risk stratification at each level of eGFR. Furthermore, since the risk for kidney outcomes is higher for subjects with macroalbuminuria (≥300 mg/g ) than for subjects with microalbuminuria (30–299 mg/g) it seems prudent to define not only one, but several thresholds for albuminuria to indicate increased risk for kidney outcomes. Second, our finding that risk for kidney outcomes is substantially higher in subjects with eGFR 30–45 as compared to 45–60 ml/min/1.73m2, suggests that it may be appropriate to subdivide the present stage 3 chronic kidney disease into two stages, as has been proposed by others (44). Our finding of increased relative risk for all three kidney outcomes for eGFR below 60 ml/min/1.73 m2 and albuminuria (albumin-to-creatinine ratio >30 mg/g or dipstick >trace) are consistent with the current thresholds for the definition of chronic kidney disease. Some have suggested age-specific thresholds, arguing that lower eGFR at older age is a reflection of ageing (11) and less associated with risk for adverse outcomes (49,50). Although we found a less steep pattern of risk for kidney outcomes with lower eGFR in older subjects compared to younger subjects, the pattern of incidence rates was similar in older and younger subjects. These data do not provide clear cut evidence for the use of age-specific eGFR thresholds to define chronic kidney disease. In general, decisions about the threshold levels for decreased GFR and albuminuria to define and classify chronic kidney disease should consider the prevalence and absolute risk of decreased eGFR and albuminuria, as well as relative risk.
In conclusion, our data show that both albuminuria and eGFR are associated with all three kidney outcomes, independent of each other and independent of cardiovascular risk factors. These findings provide a quantitative basis for including these two kidney measures for risk stratification, and chronic kidney disease definition and staging.
Supplementary Material
Acknowledgments
The CKD Prognosis Consortium is supported by KDIGO and the US National Kidney Foundation. The meta-analyses work conducted jointly at Johns Hopkins School of Public Health, Baltimore, USA and University Medical Center Groningen, Groningen, The Netherlands, were supported by the US National Kidney Foundation and the Dutch Kidney Foundation, respectively. The Consensus Conference that led to these studies was funded by KDIGO. A variety of institutions have supported the cohorts contributing to the CKD Prognosis Consortium and are described in publications on these cohorts
CKD Prognosis Consortium
Writing Committee: Ron T. Gansevoort, Kunihiro Matsushita, Marije van der Velde, Brad C. Astor, Mark Woodward, Andrew S. Levey, Paul E de Jong, Josef Coresh
KDIGO Controversies Conference Planning Committee: Andrew Levey, Meguid El-Nahas, Paul E. de Jong, Josef Coresh, Kai-Uwe Eckardt, Bertram L. Kasiske.
CKD Prognosis Consortium investigators/collaborators: ADVANCE: Mark Woodward, Toshiharu Ninomiya, John Chalmers, Stephen MacMahon; AKDN: Marcello Tonelli, Brenda Hemmelgarn; ARIC: Josef Coresh, Brad Astor, Kunihiro Matsushita, Yaping Wang; AusDiab: Robert C. Atkins, Kevan R. Polkinghorne; Steven J. Chadban; Beaver Dam: Anoop Shankar, Ronald Klein, Barbara EK Klein; CARE: Marcello Tonelli, Frank Sacks, Gary Curhan; CHS: Michael Shlipak, Mark J. Sarnak, Ronit Katz, Linda P. Fried; HUNT: Stein Hallan, Stian Lydersen, Jostein Holmen; KP-Hawaii Cohort: Brian J. Lee; MESA: Michael Shlipak, Mark J. Sarnak, Ronit Katz, Linda P. Fried; MR-FIT: Areef Ishani, James Neaton, Ken Svendsen; OGHMA-OKID 83 and 93: Kunitoshi Iseki; ONTARGET: Johannes F.E. Mann, Salim Yusuf, Koon K. Teo, Peggy Gao; Pima Indian: Robert G Nelson, William C Knowler; TRANSCEND: Johannes F.E. Mann, Salim Yusuf, Koon K. Teo, Peggy Gao
CKD Prognosis Consortium Analytic Team: Brad C. Astor, Priscilla Auguste, Josef Coresh, Ron T. Gansevoort, Paul E de Jong, Kunihiro Matsushita, Marije van der Velde, Kasper Veldhuis, Yaping Wang, Mark Woodward.
CKD Prognosis Consortium Administration Staff: Laura Camarata, Beverly Thomas.
National Kidney Foundation Staff: Tom Manley.
Footnotes
Contributors
All members of the writing committee contributed to the collection and analysis of the data, and to the preparation of the report. All collaborators are responsible for the collection and analysis of their individual data, and were sent the paper as prepared for submission and given the opportunity to comment on the draft manuscript. The writing committee and all collaborators accept responsibility for the content of this paper.
Conflict of interest statement
The members of the Writing Committee declare that they have no conflict of interests.
References
- 1.Eckardt KU, Berns JS, Rocco MV, Kasiske BL. Definition and classification of CKD: the debate should be about patient prognosis--a position statement from KDOQI and KDIGO. Am J Kidney Dis. 2009;53:915–20. doi: 10.1053/j.ajkd.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske B, Eckardt K. Chronic Kidney Disease - Definition, Classification and Prognosis: A KDIGO Controversies Conference Reaches a Consensus. Kidney Int. 2010 doi: 10.1038/ki.2010.483. (submitted) [DOI] [PubMed] [Google Scholar]
- 3.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 4.Levey AS, Coresh J, Balk, et al. NKF Practice Guidelines for CKD: evaluation, classification and stratification. Arch Int Med. 2003;139:137–47. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–59. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 7.Gansevoort RT, de Jong PE. The case for using albuminuria in staging chronic kidney disease. J Am Soc Nephrol. 2009;20:465–8. doi: 10.1681/ASN.2008111212. [DOI] [PubMed] [Google Scholar]
- 8.Glassock RJ, Winearls C. An epidemic of chronic kidney disease: fact or fiction? Nephrol Dial Transpl. 2008;23:1117–1123. doi: 10.1093/ndt/gfn086. [DOI] [PubMed] [Google Scholar]
- 9.Ikizler TA. CKD classification: time to move beyond KDOQI. J Am Soc Nephrol. 2009;20:929–30. doi: 10.1681/ASN.2009030309. [DOI] [PubMed] [Google Scholar]
- 10.Wetzels JF, Willems HL, den Heijer M. Age- and gender-specific reference values of estimated glomerular filtration rate in a Caucasian population: Results of the Nijmegen Biomedical Study. Kidney Int. 2008;73:657–8. doi: 10.1038/sj.ki.5002755. [DOI] [PubMed] [Google Scholar]
- 11.Winearls CG, Glassock RJ. Dissecting and refining the staging of chronic kidney disease. Kidney Int. 2009;75:1009–14. doi: 10.1038/ki.2009.49. [DOI] [PubMed] [Google Scholar]
- 12.Ruggenenti P, Perna A, Mosconi L, Pisoni R, Remuzzi G. Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies. “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN) Kidney Int. 1998;53:1209–16. doi: 10.1046/j.1523-1755.1998.00874.x. [DOI] [PubMed] [Google Scholar]
- 13.Keane WF, Zhang Z, Lyle PA, Cooper ME, de Zeeuw D, Grunfeld JP, Lash JP, McGill JB, Mitch WE, Remuzzi G, Shahinfar S, Snapinn SM, Toto R, Brenner BM RENAAL Study Investigators. Risk scores for predicting outcomes in patients with type 2 diabetes and nephropathy: the RENAAL study. Clin J Am Soc Nephrol. 2006;1:761–7. doi: 10.2215/CJN.01381005. [DOI] [PubMed] [Google Scholar]
- 14.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS AIPRD Study Group. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139:244–52. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 15.Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371:2173–82. doi: 10.1016/S0140-6736(08)60952-6. [DOI] [PubMed] [Google Scholar]
- 16.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 17.Hallan S, Astor B, Romundstad S, Aasarod K, Kvenild K, Coresh J. Association of Kidney Function and Albuminuria With Cardiovascular Mortality in Older vs Younger Individuals: The HUNT II Study. Arch Intern Med. 2007;167:2490–6. doi: 10.1001/archinte.167.22.2490. [DOI] [PubMed] [Google Scholar]
- 18.Astor BC, Hallan SI, Miller ER, 3rd, Yeung E, Coresh J. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol. 2008;167:1226–34. doi: 10.1093/aje/kwn033. [DOI] [PubMed] [Google Scholar]
- 19.Brantsma AH, Bakker SJ, Hillege HL, de Zeeuw D, de Jong PE, Gansevoort RT PREVEND Study Group. Cardiovascular and renal outcome in subjects with K/DOQI stage 1–3 chronic kidney disease: the importance of urinary albumin excretion. Nephrol Dial Transplant. 2008;23:3851–8. doi: 10.1093/ndt/gfn356. [DOI] [PubMed] [Google Scholar]
- 20.Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423–9. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 21.van der Velde M, Halbesma N, de Charro FT, Bakker SJ, de Zeeuw D, de Jong PE, Gansevoort RT. Screening for albuminuria identifies individuals at increased renal risk. J Am Soc Nephrol. 2009;20:852–62. doi: 10.1681/ASN.2008060655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20:1069–77. doi: 10.1681/ASN.2008070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coca SG. Long-term outcomes of acute kidney injury. Curr Opin Nephrol Hypertens. 2010 doi: 10.1097/MNH.0b013e3283375538. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 24.Goldberg R, Dennen P. Long-term outcomes of acute kidney injury. Adv Chronic Kidney Dis. 2008;15:297–307. doi: 10.1053/j.ackd.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 25.The Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality: a collaborative meta-analysis of general population cohorts. Lancet. 2010 May 24; doi: 10.1016/S0140-6736(10)60674-5. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality: a collaborative meta-analysis of high risk cohorts. Kidney Int. doi: 10.1038/ki.2010.536. (submitted) [DOI] [PubMed] [Google Scholar]
- 27.The Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with mortality and end-stage renal disease: a collaborative meta-analysis of kidney disease cohorts. Kidney Int. (submitted) [Google Scholar]
- 28.Matsushita K, Selvin E, Bash LD, Franceschini N, Astor BC, Coresh J. Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol. 2009;20:2617–24. doi: 10.1681/ASN.2009010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White SL, Polkinghorne KR, Atkins RC, Chadban SJ. Comparison of the Prevalence and Mortality Risk of CKD in Australia Using the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) Study GFR Estimating Equations: The AusDiab (Australian Diabetes, Obesity and Lifestyle) Study. Am J Kidney Dis. 2010;55:660–70. doi: 10.1053/j.ajkd.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Shankar A, Klein R, Klein BEK. The association among smoking, heavy drinking and chronic kidney disease. American Journal of Epidemiology. 2006;164:263–71. doi: 10.1093/aje/kwj173. [DOI] [PubMed] [Google Scholar]
- 31.Shlipak MG, Katz R, Kestenbaum B, Fried LF, Newman AB, Siscovick DS, et al. Rate of kidney function decline in older adults: a comparison using creatinine and cystatin C. Am J Nephrol. 2009;30:171–8. doi: 10.1159/000212381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20:1069–77. doi: 10.1681/ASN.2008070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bui AL, Katz R, Kestenbaum B, de Boer IH, Fried LF, Polak JF, et al. Cystatin C and carotid intima-media thickness in asymptomatic adults: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2009;53:389–98. doi: 10.1053/j.ajkd.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iseki K, Ikemiya Y, Iseki C, Takishita S. Proteinuria and the risk of developing end-stage renal disease. Kidney Int. 2003;63:1468–74. doi: 10.1046/j.1523-1755.2003.00868.x. [DOI] [PubMed] [Google Scholar]
- 35.Iseki K, Kinjo K, Iseki C, Takishita S. Relationship between predicted creatinine clearance and proteinuria and the risk of developing ESRD in Okinawa, Japan. Am J Kidney Dis. 2004;44:806–14. [PubMed] [Google Scholar]
- 36.Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Macmahon S, Chalmers J for the ADVANCE Collaborative Group. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813–21. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tonelli M, Jose P, Curhan G, Sacks F, Braunwald E, Pfeffer M Cholesterol and Recurrent Events (CARE) Trial Investigators. Proteinuria, impaired kidney function, and adverse outcomes in people with coronary disease: analysis of a previously conducted randomised trial. BMJ. 2006;332:1426–31. doi: 10.1136/bmj.38814.566019.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, et al. ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–53. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 39.Pavkov ME, Knowler WC, Hanson RL, Bennett PH, Nelson RG. Predictive power of sequential measures of albuminuria for progression to ESRD or death in Pima Indians with type 2 diabetes. Am J Kidney Dis. 2008;51:759–66. doi: 10.1053/j.ajkd.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mann JFE, Schmieder RE, Dyal L, McQueen M, Schumacher H, Pogue J, Wang X, Probstfield JL, Avezum A, Carduna-Munoz E, Dagenais GR, Diaz R, Fodor G, Maillon JM, Ryden LR, Yu CM, Teo KK, Yusuf S. Effects of telmisartan on renal outcomes. Ann Int Med. 2009;151:1–10. doi: 10.7326/0003-4819-151-1-200907070-00122. [DOI] [PubMed] [Google Scholar]
- 41.Lee BJ, Forbes K. The role of specialists in managing the health of populations with chronic illness: the example of chronic kidney disease. BMJ. 2009;339:800–2. doi: 10.1136/bmj.b2395. [DOI] [PubMed] [Google Scholar]
- 42.Ishani A, Grandits GA, Grimm RH, Svendsen KH, Collins AJ, Prineas RJ, Neaton JD. Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol. 2006;17:1444–52. doi: 10.1681/ASN.2005091012. [DOI] [PubMed] [Google Scholar]
- 43.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 44.Crowe E, Halpin D, Stevens P on behalf of the Guideline Development G. Early identification and management of chronic kidney disease: summary of NICE guidance. BMJ. 2008;337:812–5. doi: 10.1136/bmj.a1530. [DOI] [PubMed] [Google Scholar]
- 45.Konta T, Hao Z, Takasaki S, Abiko H, Ishikawa M, Takahashi T, Ikeda A, Ichikawa K, Kato T, Kawata S, Kubota I. Clinical utility of trace proteinuria for microalbuminuria screening in the general population. Clin Exp Nephrol. 2007;11:51–5. doi: 10.1007/s10157-006-0458-z. [DOI] [PubMed] [Google Scholar]
- 46.Woodward M. Epidemiology: study design and data analysis. 2. Boca Raton: Chapman & Hall/CRC; 2005. [Google Scholar]
- 47.Imai E, Horio M, Yamagata K, Iseki K, Hara S, Ura N, Kiyohara Y, Makino H, Hishida A, Matsuo S. Slower decline of glomerular filtration rate in the Japanese general population: a longitudinal 10-year follow-up study. Hypertens Res. 2008;31:433–41. doi: 10.1291/hypres.31.433. [DOI] [PubMed] [Google Scholar]
- 48.Kshirsagar AV, Bang H, Bomback AS, Vupputuri S, Shoham DA, Kern LM, et al. A simple algorithm to predict incident kidney disease. Arch Intern Med. 2008;168:2466–73. doi: 10.1001/archinte.168.22.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, Walter LC, Mehta KM, Steinman MA, Allon M, McClellan WM, Landefeld CS. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18:2758–65. doi: 10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]
- 50.O’Hare AM, Bertenthal D, Covinsky KE, Landefeld CS, Sen S, Mehta K, Steinman MA, Borzecki A, Walter LC. Mortality risk stratification in chronic kidney disease: one size for all ages? J Am Soc Nephrol. 2006;17:846–53. doi: 10.1681/ASN.2005090986. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.