Abstract
Newborns are at increased risk of infection due to genetic, epigenetic, and environmental factors. Herein we examine the roles of the neonatal innate immune system in host defense against bacterial and viral infections. Full-term newborns express a distinct innate immune system biased towards TH2/TH17-polarizing and anti-inflammatory cytokine production with relative impairment in TH1-polarizing cytokine production that leaves them particularly vulnerable to infection with intracellular pathogens. In addition to these distinct features, preterm newborns also have fragile skin, impaired TH17-polarizing cytokine production and deficient expression of complement and of antimicrobial proteins and peptides (APPs) that likely contribute to susceptibility to pyogenic bacteria. Ongoing research is identifying APPs, including bacterial/permeability-increasing protein and lactoferrin, as well as pattern recognition receptor (PRR) agonists that may serve to enhance protective newborn and infant immune responses as stand alone immune response modifiers or vaccine adjuvants.
Keywords: adjuvants, neonatal sepsis, pathogen recognition receptors, innate immunity
Introduction
Infection of newborns and infants, including bacterial sepsis, is a major health care issue with an annual global mortality in excess of one million lives. Indeed, infection is the leading cause of mortality among infants in the first days of life1, 2. The incidence of infection can vary widely depending on gestational age and time of onset, with severe infection having higher incidence and mortality in very low birth weight (VLBW) premature neonates, within the first three to seven days of life3, 4. The economic burden of caring for and hospitalizing these infected infants is considerable, and is estimated at approximately $700 million in the US alone5. Infection during the neonatal and infant period has also been recognized as an international issue. In an attempt to counter and improve health conditions for infants globally, the United Nations has outlined a series of eight Millenium Development Goals to decrease by 2/3 the mortality of children under the age of five by 20155.
The purpose of this review is to examine current evidence regarding the role of the innate immune response during neonatal infection and highlight it’s relevance to the practicing clinician. We will discuss distinct aspects of neonatal innate immune signaling pathways in response to infection. Next we explore current insights into efforts to prevent neonatal infection using immune adjuvants and vaccines. Lastly, we will examine future directions and goals in the field and elaborate on exciting new therapeutic possibilities. Prior to our discussion of the role of neonatal-specific innate immunity in neonatal infection, a very focused overview of pertinent immunology will likely be helpful to effectively communicate our objectives. For a more complete review of these topics, the reader is also referred to recent reviews6–9.
The first concept we will discuss relates to innate immune function and pathogen detection. Innate immune cells recognize pathogens via pattern recognition receptors (PRRs), of which the most well-studied are the Toll-like receptors (TLRs). TLRs are expressed on the cell surface and within intracellular vesicles (endosomes). TLRs are stimulated by the presence of pathogen-associated molecular patterns (PAMPs) such as cell wall/membrane components (eg lipopolysaccharide [LPS], peptidoglycan, flagelin) or intracellular components (eg single or double stranded RNA or DNA)6. In general, each TLR has a specific “toll” required for stimulation, and more than one TLR can be stimulated simultaneously allowing for concerted responses to be produced10. Following TLR stimulation, second messenger-specific intracellular signaling cascades are activated that result in gene expression, cytokine/chemokine production and cellular activation6.
A second important piece of immunology background relates to the effects of the cytokines produced following innate immune stimulation. Patterns of cytokine production are important because the cytokine milieu can promote the differentiation of naïve CD4+ T cells into distinct subtypes of TH cells that serve important roles in the clearance of pathogens9. For example, TH1 cells are produced from naïve CD4+ T cells following exposure to interferon (IFN)-γ and IL-12, and support cell-mediated immunity against intracellular pathogens through production of IFN-γ, tumor necrosis factor (TNF), and lymphotoxin. TH2 cells arise in the presence of IL-2 and IL-4, produce IL-4, IL-5, IL-13, down-regulate TH1 responses, and support humoral immunity as well as defense against extracellular parasites. A third subset of TH cells, TH17 cells, are produced in the presence of transforming growth factor (TGF)-β, IL-6, IL-21, IL-23, produce IL-17, IL-22, and are important for defense against extracellular bacteria and fungi. In subsequent sections, we will frequently refer to the cytokine milieu as TH1, TH2, or TH17-polarizing or promoting based on these descriptions.
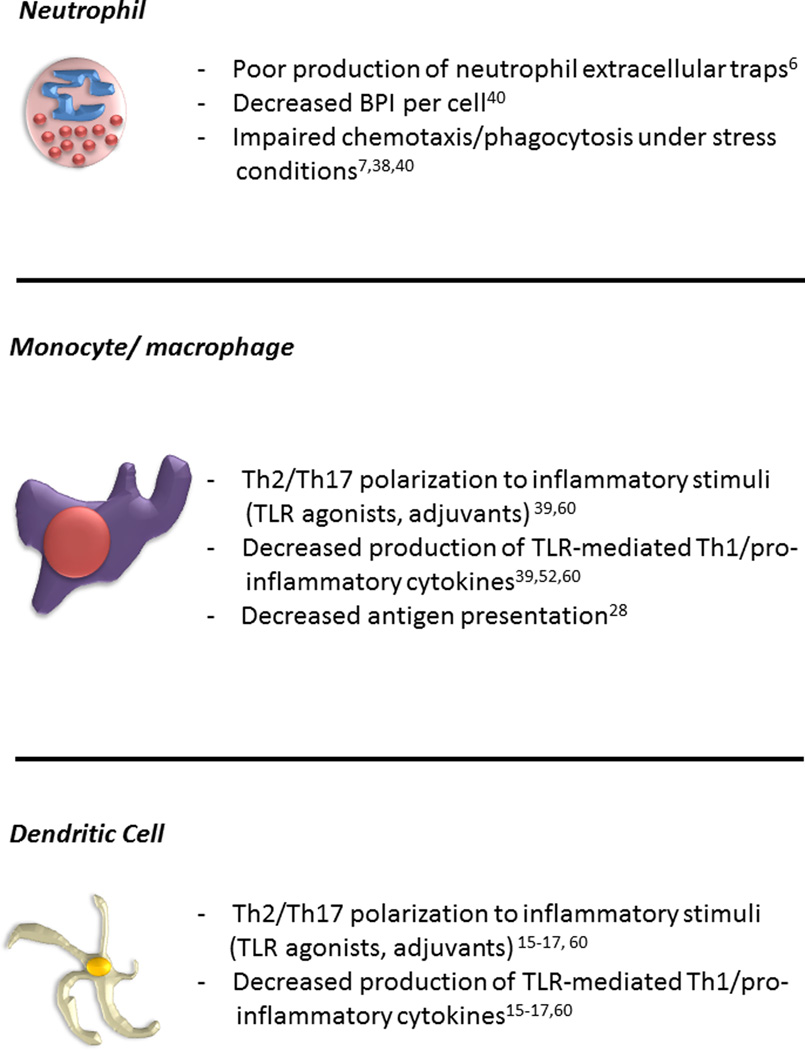
Though there are multiple cells that comprise the innate immune response, the neutrophil (also known as polymorphonuclear leukocyte) and antigen presenting cells (APCs; monocyte, macrophage, and dendritic cells) are each important to the neonatal response to infection (Figure 1)2, 4, 11, 12. Neutrophils have been examined in adult and neonatal infection as one of the primary responders to pathogen-induced inflammation11, 13, 14. The neutrophil is not only able to phagocytose and clear bacteria but it is also is able to release anti-microbial proteins and peptides (APPs), such as lactoferrin (Lf) and bacterial/permeability-increasing protein (BPI), upon activation at infected sites15. The role of the macrophage is similar to the neutrophil in that it functions to clear dead or dying neutrophils and bacteria through phagocytosis but also plays an important role in shaping the adaptive immune response to pathogens, indirectly through cytokine secretion or directly through antigen presentation in secondary lymphoid organs such as lymph nodes and spleen1. The dendritic cell (DC) also plays an important role in the innate immune response to pathogens and is critical in formation of antigen-specific immune responses (e.g. antibody production) and memory responses in both T and B cells16, 17.
Figure 1.
Distinct Features of Innate Immune Function of Neonatal Neutrophils and Antigen-presenting Cells
Distinctions between neonatal and adult innate and adaptive immune function likely contribute to the susceptibility of newborns to infection. The neonatal innate immune response has been considered “immature”, as functional impairments in phagocytosis and other bactericidal activity as compared to adults have been noted in neonatal innate immune effector cells, such as neutrophils and macrophages18–20. Neonatal leukocytes also demonstrate decreased responsiveness to agonists of classic PRRs, such as LPS that signals through TLR421, 22. As discussed above, cytokine responses to innate and adaptive stimulation are important for determining TH fates. Neonatal cytokine responses are often TH2 or TH17-polarized8, 13, 23–27, skewed towards anti-inflammatory/innate immune responses, with impaired development of TH1 polarizing responses17. Overall, the immunologic profile of the infant is functionally distinct, possibly as a reflection of the demands of the fetal environment and the need to avoid immune responses to maternal antigens. Prevention or treatment of infection against which TH1-polarized immunity is needed (e.g., against intracellular pathogens), may require at least partial and reversible reorientation of neonatal TH2 polarization.
Why is neonatal innate immunity important to the practicing clinician?
The innate immune response is critical at every stage of human development because it regulates tolerance to self, generates vaccine or memory responses through the interaction with T and B cells, and provides early non-antigen specific pathogen protection to prevent infection. In most cases, defects in post-TLR stimulation second-messenger signaling intermediates critical to effective innate immune function, such as IL-1 receptor activating kinase-4 (IRAK-4) or myeloid differentiation factor 88 (MyD88)-deficiency28, will present early in life, either immediately following birth or within the first few months of life. This early susceptibility to infection is also readily observed in infants with diseases of innate immune system function such as chronic granulomatous disease (CGD) or leukocyte adhesion deficiency (LAD) that typically present early after birth with systemic infection or with persistent mucosal and respiratory infections with encapsulated bacteria throughout infancy. However, even in lieu of congenital innate immune defects, the developing neonatal and infant immune system is distinct at baseline with impaired generation of inflammatory responses to prevent infection.
Innate immune effector cells determine how the host will respond to infection. In particular, effective innate immune responses are critical for the development of adaptive immune responses in the form of protective antibodies. Vaccine-induced antibody responses in neonates demonstrate impairment in both quantity and quality (affinity) due to weak to absent humoral and/or memory T cell responses2, 5. For example, vaccines to Pertussis effectively induce productive humoral responses when given at three weeks of life, but result in inadequate memory responses when given to newborns29, 30. Although vaccines are available for the most common causes of pneumonia, including Streptococcus pneumoniae and Haemophilus influenzae, protective immunity to current conjugate vaccine formulations requires multiple boosters to promote long-lived memory responses2, 5. Importantly, these vaccines are used in infants that are 2 to 12 months of age, with few vaccines given at birth2, the most reliable point of healthcare contact globally. Only three vaccines are given significant immunity during the neonatal period: bacille Calmette-Guérin (BCG, live attenuated Mycobacterium bovis), hepatitis B vaccine (HBV) and oral poliovirus (OPV). Moreover, there are still no vaccines to certain bacterial and viral pathogens, such as respiratory syncytial virus (RSV) which can cause devastating infections in infants2. On-going characterization of the neonatal immune system may inform development of vaccines that better target age-specific aspects of immune function.
Innate immunity and neonatal sepsis
The relative scarcity of early life vaccines is compounded by the fact that despite advances in critical care and therapeutics, few treatments for sepsis, whether in adult, infant, or newborn patients, have yielded any tangible benefit in terms of improved clinical outcome4. A plethora of adjunct immunologic agents have been attempted in infants, such as granulocyte-colony stimulating factor, activated protein C, intravenous immunoglobulin (IVIg) or glutamine31–34. However, due to small patient numbers, discouraging results, and/or poor study design, no adjunctive therapies beyond classic supportive care and broad-spectrum empiric antibiotics, have been approved or instituted for the treatment of neonatal sepsis.
Though neonatal sepsis comprises only a subset of infected infants, mortality related to neonatal sepsis exceeds 1 million newborns a year1, 4. Neonatal sepsis is typically divided into two categories based on timing of onset after birth. Early onset sepsis typically occurs within the first 24 hours and is usually associated with E. coli or group B streptococcal infections35. In contrast, late onset sepsis typically occurs after the first 72-hour period, is particularly prevalent among very low birth weight (VLBW) babies, and is most commonly associated with the nosocomial pathogen, coagulase-negative Staphylococcus epidermidis36, 37. Despite our best efforts and the advancements in critical care and antimicrobials, both early and late onset neonatal sepsis continue to cause significant morbidity and mortality.
Together with the findings of poor immune responses at baseline and only a handful of vaccines to prevent infection and/or improve these responses, it is no surprise that this patient population is highly susceptible to sepsis. Part of the problem stems from attempts to apply principles and parameters based on the adult response to neonates, and especially to the premature or low birth weight infant, where these concepts may not be valid4. In addition to poorly defined clinical guidelines for the management of sepsis and shock in preterm neonates, our understanding of the neonatal immune response to sepsis lags far behind what is known in adults. Several facets of the neonatal innate immune response relevant to the development of neonatal sepsis appear to be well defined. There appears to be consensus that neonates have dampened TH1-polarizing/pro-inflammatory responses to purified PRR agonists compared with adults. In fact, many murine and clinical studies have demonstrated decreased TH1-polarizing/pro-inflammatory responses (TNF-α, IFN-g, IL-12p70, IFN-α, IFN-γ), with increased production of TH2 polarizing (e.g., IL-6) and anti-inflammatory cytokine production (IL-10), following in vitro stimulation with bacterial products or septic challenge21, 22, 38. Also, innate phagocyte function is impaired in the neonate as compared to the adult19, 39–41. In addition, neonatal neutrophils produce decreased neutrophil extracellular traps (NETs), which are one of the critical mechanisms by which phagocytes localize and contain pathogens18. Moreover, the neonatal adaptive immune response appears to play only a minor role during neonatal sepsis and is naturally skewed towards TH2 responses13–15, 19. Although these observations might raise concern for the ability to induce protective immune responses early in life, we are now beginning to understand the impact of the innate immune system during neonatal infection, and exploration of stimuli that are able to induce robust responses may hold the key to developing novel interventions.
Innate immune signaling pathways studied in the neonate
The findings described above place innate immunity at the nexus of immune responses in the neonate. Below, we will review key pathways of the neonatal innate immune response. These pathways will also be the target of future therapeutic strategies to enhance pathogen responses and vaccine efficacy. Indeed, further characterization of these pathways will inform development of novel approaches to improve clinical outcomes in this particularly susceptible patient population.
Toll like receptors (TLRs)
As we described briefly above, TLRs are well-characterized PRRs. Currently, there are 13 described TLRs that recognize bacterial (such as lipopolysaccharide (LPS), peptidoglycan), parasitic, or viral (such as Poly I:C) products6, 10 of which are expressed in humans. TLRs are critical for the detection and recognition of pathogens and are expressed on many innate immune effector cells, such as macrophages, neutrophils, and DCs. Downstream second-messenger signaling following ligation of these receptors proceeds through MyD88 and/or TIR domain containing adapter-inducing interferon B (TRIF) adaptor proteins. Signaling through MyD88 typically leads to the production of NF-κB-dependent inflammatory cytokines/chemokines, whereas signaling through TRIF induces production of type I interferons as well as nuclear factor-kappa B (NF-κB) related inflammatory cytokines6. As these receptors are expressed on many immune effector cells, differences in TLR signaling between the infant and adult immune system may contribute to the increased susceptibility to infection.
Due to the severity of Gram-negative septic shock in both critically ill neonates and adults, TLR4, (receptor for LPS or endotoxin signaling) is one of the most intensively studied of the PRRs. TLR4 signaling is not only critical for innate immune activation in leukocytes but is also expressed on many epithelial cells where it is important in the maintenance of intestinal epithelial and gut tolerance42. Neonatal cord blood leukocytes demonstrate reduced MyD88 expression and impaired LPS-induced p38 MAPK phosphorylation21, 22 and impaired LPS-induced pro-inflammatory cytokine production, such as TNF-α, IL-12p70, and IFN-γ43. Conversely newborn cord blood leukocytes demonstrate relatively increased TLR-mediated production of the TH2/TH17-polarizing cytokine IL-6 and of the anti-inflammatory cytokine IL-10 as compared to adults21, 43. This phenomenon of decreased TH1 cytokine responses has been demonstrated in neonatal animals as well44.
Why would the neonatal innate immune system be so functionally different from adults and what ontological mechanisms might regulate this state? Several investigators have turned to epigenetics to describe why the functional consequences of TLR activation in neonates are so markedly different from those in adults, in particular micro RNAs (miRNA). MiRNAs are small inhibitory RNAs that post-transcriptionally inhibit the expression of certain genes. MiRNAs participate in the control of many immunological and biological processes. In particular, micro RNA 146a or miR146a has been found to functionally regulate TLR4 signaling through the inhibition of IRAK4, a kinase that is critical for downstream signaling for TLR4, and appears to have a role in endotoxin tolerance45, 46. Of note, cord blood monocytes have a higher expression of miR146a compared to adults47.
In addition to cell intrinsic factors, neonatal plasma modulates the responses of neonatal mononuclear cells to TLR agonists. Cord blood plasma contains relatively high concentrations of the low molecular weight endogenous purine metabolite adenosine, this is typically released during inflammatory event and hypoxic states. Adenosine acts via leukocyte adenosine receptors resulting in increased intracellular levels of the second messenger cyclic adenosine monophosphate (cAMP) that reduces TLR-mediated TH1-polarizing inflammatory responses48. In addition to the high concentrations of adenosine at birth, neonatal mononuclear cells are also particularly sensitive to adenosine-induced accumulation of cAMP, resulting in impaired TLR-mediated TNF-α and IL-12p70 production in neonates compared to adults49, 50. Recent work by Belderbos et al suggests that additional soluble factors differentially modulate TLR-mediated cytokine production, including the increased secretion of the anti-inflammatory cytokine IL-1051. Overall, soluble plasma factors appear to play an important role in the ontogeny of responses to TLR agonists. Importantly, certain TLR agonists are relatively refractory to the modulating effects of neonatal blood plasma. For example, TLR8 agonists (including small antiviral imidazoquinoline compounds) induce robust, adult-level TNF production from neonatal monocytes52 and are refractory to adenosine50. These observations suggest that TLR8 agonists may be well suited as TH1-polarizing vaccine adjuvants to enhance neonatal immunization53.
Genetic polymorphisms or mutations in key downstream TLR signaling proteins can lead to an increased susceptibility to infection or sepsis. For example, patients with MyD88 deficiency are more susceptible to recurrent bacterial infection28. Similar findings were observed in IRAK-4 deficient patients22, 28, 54. In addition, neutrophils from IRAK-4 deficient patients were shown to be hyporesponsive to LPS via failure to upregulate cell surface activation markers and poor phagocytic function54. Patients with a mutation in NF-κB essential modulator (NEMO), a subunit of IκB kinase that activates NF-κB, were also found to be extremely susceptible to pyogenic infections like the above patients55. As MyD88, IRAK4, and NF-κB are all downstream signaling proteins utilized by many of the TLRs, it is no surprise that these patients manifest similar susceptibilities and clinical phenotypes.
TLR agonists induce inflammatory responses, and accordingly, have been evaluated as stand-alone immune response modifiers and as vaccine adjuvants. For example, administration of monophosphoryl lipid A or MPLA (TLR4 agonist) or CpG (TLR9 ligand) can induce Th1-polarizing cytokines16, 56. Recent studies of neonatal and infant blood stimulated in vitro have begun to shed light on the ontogeny of TLR-mediated cytokine production57. Corbett et al profiled the cytokine responses of different APC subsets to different TLR agonists in neonates, one and two year olds, and adults58. Surprisingly, they found, for example, that in response to TLR2 agonists, cytokine responses did not change in a linear manner: TLR2-mediated IL-10 production was elevated in neonatal APCs, decreased to below adult levels in APCs isolated from a 2 year old, and then increased in adulthood58. Belderbos et al demonstrated that whereas TLR3, 7, and 9-mediated IL-12p70 and IFN-α responses matured to adult levels over the first month of life, responses to endotoxin remained impaired throughout the first month of life43. A study by Burl and colleagues characterized TLR-mediated cytokine production in vitro stimulated whole blood cultures indicates that TLR8 agonists are particularly able to induce TNF-α and IFN-γ at birth (cord blood)59. Overall, these studies suggest that the ontogeny of mononuclear cell cytokine responses is TLR-specific and non-linear.
A growing body of in vitro and in vivo studies suggest that TLR stimulation can modulate responses to subsequent stimuli, in effect re-setting or priming innate immunity in a form of innate memory or “trained immunity”60. For example, we demonstrated that intraperitoneal administration of TLR4 or TLR7/8 agonists to neonatal mice 24 hours prior to infection (polymicrobial peritonitis) resulted in enhanced innate phagocyte and cytokine response and a significant improvement in survival19. Of note, live attenuated vaccines such as BCG activate multiple TLRs and may have beneficial non-specific effects such as reducing all cause neonatal mortality61. Much remains to be learned regarding the mechanisms underlying these TLR-mediated response patterns, to what extent in vitro responses in whole blood correspond with responses to TLR agonists in vivo, and how these may be modulated to treat and/or prevent infection.
C-type Lectin Receptors
Another important component of neonatal innate immunity are the C-type lectin receptors (CLRs). CLRs are conserved PRRs that recognize bacterial, viral, fungal, and parasitic carbohydrate moieties. CLRs are either expressed on the cell surface (e.g., DC-specific intercellular adhesion molecule-3 grabbing non-integrin (DC-SIGN) and Dectin-1) or secreted as soluble proteins (e.g., mannose binding lectin, MBL). Once bound to its carbohydrate ligand, MBL initiates activation of the “lectin complement pathway” to form the membrane attack complex and promote agglutination and phagocytic clearance of pathogens. Plasma MBL concentrations are low at birth (especially in preterm infants), but rise steadily throughout infancy and childhood62. Low levels of MBL are associated with the increased incidence of nosocomial bacterial sepsis in neonates63–65. In addition to decreased concentrations at birth, certain genetic polymorphisms of MBL, namely MBL2, have also been associated with increased infection64. However, this association has not been found in all studies62, 66, 67. Though there are some limited investigations into the role or function of other CLRs, such as the role of surfactant protein A in the phagocytic function of preterm baboon DC precursors68, 69, other CLRs are less well characterized, particularly in relation to human neonatal innate immunity. Further investigation of CLRs and associated pathways may lead to novel therapeutic strategies to enhance neonatal innate immune response and provide protection against infection.
Antimicrobial proteins/peptides
Several APPs are expressed in neonates including Lf, BPI, cathelicidins and defensins52. APPs are often cationic and engage in electrostatic interactions with that negatively charged microbial surfaces resulting in membrane permeabilization. Many APPs are expressed in neutrophil granules where they can be deployed against ingested microbes in the phagolysosome and/or after extracellular release upon degranulation. Lf is a mammalian milk whey protein that exerts antimicrobial activity via both direct, membrane-active and indirect iron-binding mechanisms, thereby depriving microbes of a key nutrient. Lf has been used as a therapeutic intervention in several neonatal sepsis and necrotizing enterocolitis (NEC) trials. Oral supplementation with Lf reduced incidence of late onset neonatal sepsis and of invasive fungal infections in VLBW neonates70. However, the mechanism for this benefit is unknown, and doses were not normalized per surface area such that smaller infants may have received a greater dose than larger study subjects. Oral Lf supplementation was also studied in neonates in an open label design for the prevention of necrotizing enterocolitis (NEC), but have been equivocal perhaps due to lack of well-designed randomized trials71.
BPI’s antimicrobial and anti-infective activities against Gram-negative bacteria are targeted by its high (nanomolar) affinity for the lipid A region of LPS. BPI kills Gram-negative bacteria via membrane permeabilization, opsonizes bacteria for enhanced phagocytic uptake, and neutralizes the endotoxic activity of LPS72. BPI is also expressed in pulmonary mucosal epithelium and in the intestinal epithelial cells73, 74. Of note, neonatal neutrophils have ~3–4 fold less BPI per cell than do adult neutrophils75. Patients with a BPI Taq allele polymorphism had a higher incidence of sepsis than those with the wild-type allele76. Intravenous administration of a recombinant N-terminal fragment of BPI (rBPI) demonstrated some benefit in a pediatric intensive care unit study of meningococcal sepsis77 that included a 2 week-old newborn and multiple infants77 and is in biopharmaceutical development78 for immunocompromised hosts rendered relatively BPI-deficient by immaturity (eg, newborns) or due to radiochemotherapy-induced neutropenia/mucositis with associated endotoxemia due to enhanced gut permeability/translocation79.
The broadly microbicidal ~4kDa defensin peptides function via protein binding to the bacterial cell wall, pore formation, and subsequent lysis of the pathogen72. Preterm and term newborns demonstrated low levels of β-defensin 2 in stool that increased with age80. α-defensin and cryptidin, are important for protection of mice against oral Shigella infections81. Additionally, the role of defensins has been investigated in the transmission of human immunodeficiency virus35. Thus far, published studies suggest that defensins may play dual paradoxical roles with respect to HIV: on one hand protecting the neonate from mother-to-child transmission, while at the same time, facilitating infectivity of the virus82, 83. Further studies are needed to better understand these effects. Overall, future studies of APPs will assess whether there is a significant link between the levels of these proteins/peptides and early life infection as well as the possible therapeutic options of employing congeners of these proteins in neonates and infants to prevent and/or treat infection.
The Inflammasome and NOD like receptors (NLRs)
Nucleotide oligomerization domain (NOD)-like receptors (NLRs) are intracellular PRRs that recognize an array of microbial products. Similar to TLRs, NLRs recognize a conserved portion of viral and bacterial components and participate in the inflammatory response that enables the host to respond to infection. NLRs and the inflammasome are integral in the conversion of pro-IL-1β and pro-IL-18 to mature IL-1β and IL-18 active forms84. The formation of the cytosolic multi-protein complex known as the inflammasome has been ascribed to several different NLRs, such Nalp3/NLRP3, as well as caspase-1 and also involves the ATP receptor, P2X785. The inflammasome may contribute to the response of trophoblasts to preterm infection at maternal-fetal interface and to initiation of preterm labor86. Of note, the NLRP3 inflammasome is activated by the β-hemolysin of the neonatal pathogen Group B Streptococcus (GBS)87. Importantly, some vaccine adjuvants, such as alum, may act in part via inflammasome activation88, 89, 90. Li and colleagues demonstrated that in mice alum-enhanced humoral responses are NLRP3-dependent90. Studies of the ontogeny of stimulus-induced cytokine production in whole blood studied in vitro indicate that Alum-induced IL-1β production is actually high at birth and decreases with age91. A recent study demonstrated that low molecular weight synthetic imidazoquinoline compounds that activate TLR7/8 can also directly activate the inflammasome in human neonatal monocyte-derived dendritic cells with greater potency and efficacy than does alum50, 53. Ongoing or future investigation examining aluminum-containing adjuvants, with or without additional TLR agonists, will determine the significance or utility with respect to improving neonatal vaccine responses.
Conclusions and future directions
Neonatal innate immunity remains incompletely understood. Translational investigation in this field is centered on two areas: 1) modulation of the neonate’s innate immune pathway to overcome immaturity, and 2) development of safe and efficacious adjuvanted vaccines to protect the newborn/infant during the first few months of life. Ongoing studies characterizing neonatal innate immunity and assessing potentially beneficial effects of TLR agonists or live attenuated bacteria (eg, BCG) will inform efforts to extend, enhance, and/or increase available immune response modifiers and adjuvanted vaccines for these vulnerable patients.
Acknowledgements
AGC and LLM supported in part by GM-40586 and GM-81923, awarded by the National Institute of General Medical Sciences (NIGMS). AGC was supported by the Ruth Kirschstein NRSA training grant (T32 GM-08721) in burns and trauma, awarded by NIGMS. AGC was also supported by an individual NRSA training grant (F32 GM-093665), awarded by NIGMS. JLW’s laboratory is supported grants from The Gerber Foundation and the Thrasher Foundation. OL’s laboratory is supported by Bill & Melinda Gates Foundation Global Health Grants OPPGH5284 and OPP1035192, NIH RO1 1R01AI100135-01 and by sponsored research support from VentiRx Pharmaceuticals.
References
- 1.Lawn JE, Cousens S, Zupan J T. Lancet Neonatal Survival Steering. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365(9462):891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez-Schmitz G, Levy O. Development of newborn and infant vaccines. Sci Transl Med. 2011;3(90):90ps27. doi: 10.1126/scitranslmed.3001880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lukacs SL, Schoendorf KC, Schuchat A. Trends in sepsis-related neonatal mortality in the United States, 1985–1998. Pediatr Infect Dis J. 2004;23(7):599–603. doi: 10.1097/01.inf.0000131633.74921.90. [DOI] [PubMed] [Google Scholar]
- 4.Wynn JL, Wong HR. Pathophysiology and treatment of septic shock in neonates. Clin Perinatol. 2010;37(2):439–479. doi: 10.1016/j.clp.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.PrabhuDas M, Adkins B, Gans H, et al. Challenges in infant immunity: implications for responses to infection and vaccines. Nat Immunol. 2011;12(3):189–194. doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
- 6.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7(5):379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 8.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4(7):553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 9.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112(5):1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7(3):179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 11.Wynn JL, Scumpia PO, Delano MJ, et al. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock. 2007;28(6):675–683. doi: 10.1097/SHK.0b013e3180556d09. [DOI] [PubMed] [Google Scholar]
- 12.Canaday DH, Chakravarti S, Srivastava T, et al. Class II MHC antigen presentation defect in neonatal monocytes is not correlated with decreased MHC-II expression. Cell Immunol. 2006;243(2):96–106. doi: 10.1016/j.cellimm.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adkins B, Bu Y, Cepero E, Perez R. Exclusive Th2 primary effector function in spleens but mixed Th1/Th2 function in lymph nodes of murine neonates. J Immunol. 2000;164(5):2347–2353. doi: 10.4049/jimmunol.164.5.2347. [DOI] [PubMed] [Google Scholar]
- 14.Adkins B, Bu Y, Guevara P. Murine neonatal CD4+ lymph node cells are highly deficient in the development of antigen-specific Th1 function in adoptive adult hosts. J Immunol. 2002;169(9):4998–5004. doi: 10.4049/jimmunol.169.9.4998. [DOI] [PubMed] [Google Scholar]
- 15.Rose S, Lichtenheld M, Foote MR, Adkins B. Murine neonatal CD4+ cells are poised for rapid Th2 effector-like function. J Immunol. 2007;178(5):2667–2678. doi: 10.4049/jimmunol.178.5.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duthie MS, Windish HP, Fox CB, Reed SG. Use of defined TLR ligands as adjuvants within human vaccines. Immunol Rev. 2011;239(1):178–196. doi: 10.1111/j.1600-065X.2010.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30(12):585–591. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yost CC, Cody MJ, Harris ES, et al. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood. 2009;113(25):6419–6427. doi: 10.1182/blood-2008-07-171629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wynn JL, Scumpia PO, Winfield RD, et al. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112(5):1750–1758. doi: 10.1182/blood-2008-01-130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gessler P, Nebe T, Birle A, Haas N, Kachel W. Neutrophil respiratory burst in term and preterm neonates without signs of infection and in those with increased levels of C-reactive protein. Pediatr Res. 1996;39(5):843–848. doi: 10.1203/00006450-199605000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Sadeghi K, Berger A, Langgartner M, et al. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J Infect Dis. 2007;195(2):296–302. doi: 10.1086/509892. [DOI] [PubMed] [Google Scholar]
- 22.Yan SR, Qing G, Byers DM, et al. Role of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infect Immun. 2004;72(3):1223–1229. doi: 10.1128/IAI.72.3.1223-1229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forsthuber T, Yip HC, Lehmann PV. Induction of TH1 and TH2 immunity in neonatal mice. Science. 1996;271(5256):1728–1730. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 24.Yip HC, Karulin AY, Tary-Lehmann M, et al. Adjuvant-guided type-1 and type-2 immunity: infectious/noninfectious dichotomy defines the class of response. J Immunol. 1999;162(7):3942–3949. [PubMed] [Google Scholar]
- 25.Aksoy E, Albarani V, Nguyen M, et al. Interferon regulatory factor 3-dependent responses to lipopolysaccharide are selectively blunted in cord blood cells. Blood. 2007;109(7):2887–2893. doi: 10.1182/blood-2006-06-027862. [DOI] [PubMed] [Google Scholar]
- 26.De Wit D, Tonon S, Olislagers V, et al. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J Autoimmun. 2003;21(3):277–281. doi: 10.1016/j.jaut.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Vanden Eijnden S, Goriely S, De Wit D, Goldman M, Willems F. Preferential production of the IL-12(p40)/IL-23(p19) heterodimer by dendritic cells from human newborns. Eur J Immunol. 2006;36(1):21–26. doi: 10.1002/eji.200535467. [DOI] [PubMed] [Google Scholar]
- 28.Picard C, von Bernuth H, Ghandil P, et al. Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine (Baltimore) 2010;89(6):403–425. doi: 10.1097/MD.0b013e3181fd8ec3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Provenzano RW, Wetterlow LH, Sullivan CL. Immunization and antibody response in the newborn infant. I. Pertussis inoculation within twenty-four hours of birth. N Engl J Med. 1965;273(18):959–965. doi: 10.1056/NEJM196510282731804. [DOI] [PubMed] [Google Scholar]
- 30.Wood N, McIntyre P, Marshall H, Roberton D. Acellular pertussis vaccine at birth and one month induces antibody responses by two months of age. Pediatr Infect Dis J. 2009;29(3):209–215. doi: 10.1097/INF.0b013e3181bc98d5. [DOI] [PubMed] [Google Scholar]
- 31.Tarnow-Mordi W, Isaacs D, Dutta S. Adjunctive immunologic interventions in neonatal sepsis. Clin Perinatol. 2010;37(2):481–499. doi: 10.1016/j.clp.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Carr R, Brocklehurst P, Dore CJ, Modi N. Granulocyte-macrophage colony stimulating factor administered as prophylaxis for reduction of sepsis in extremely preterm, small for gestational age neonates (the PROGRAMS trial): a single-blind, multicentre, randomised controlled trial. Lancet. 2009;373(9659):226–233. doi: 10.1016/S0140-6736(09)60071-4. [DOI] [PubMed] [Google Scholar]
- 33.Decembrino L, D'Angelo A, Manzato F, et al. Protein C concentrate as adjuvant treatment in neonates with sepsis-induced coagulopathy: a pilot study. Shock. 2010;34(4):341–345. doi: 10.1097/SHK.0b013e3181e7623e. [DOI] [PubMed] [Google Scholar]
- 34.Wynn JL, Seed PC, Cotten CM. Does IVIg administration yield improved immune function in very premature neonates? J Perinatol. 2010;30(10):635–642. doi: 10.1038/jp.2009.197. [DOI] [PubMed] [Google Scholar]
- 35.Sgro M, Shah PS, Campbell D, et al. Early-onset neonatal sepsis: rate and organism pattern between 2003 and 2008. J Perinatol. 2011 doi: 10.1038/jp.2011.40. [DOI] [PubMed] [Google Scholar]
- 36.van den Hoogen A, Gerards LJ, Verboon-Maciolek MA, Fleer A, Krediet TG. Long-term trends in the epidemiology of neonatal sepsis and antibiotic susceptibility of causative agents. Neonatology. 2009;97(1):22–28. doi: 10.1159/000226604. [DOI] [PubMed] [Google Scholar]
- 37.Hornik CP, Fort P, Clark RH, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev. 2012;88(Suppl 2):S69–S74. doi: 10.1016/S0378-3782(12)70019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vosters O, Lombard C, Andre F, et al. The interferon-alpha and interleukin-10 responses in neonates differ from adults, and their production remains partial throughout the first 18 months of life. Clin Exp Immunol. 2010;162(3):494–499. doi: 10.1111/j.1365-2249.2010.04267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marodi L. Neonatal innate immunity to infectious agents. Infect Immun. 2006;74(4):1999–2006. doi: 10.1128/IAI.74.4.1999-2006.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marodi L. Innate cellular immune responses in newborns. Clin Immunol. 2006;118(2–3):137–144. doi: 10.1016/j.clim.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 41.Carr R. Neutrophil production and function in newborn infants. Br J Haematol. 2000;110(1):18–28. doi: 10.1046/j.1365-2141.2000.01992.x. [DOI] [PubMed] [Google Scholar]
- 42.Chassin C, Kocur M, Pott J, et al. miR-146a mediates protective innate immune tolerance in the neonate intestine. Cell Host Microbe. 2010;8(4):358–368. doi: 10.1016/j.chom.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Belderbos ME, van Bleek GM, Levy O, et al. Skewed pattern of Toll-like receptor 4-mediated cytokine production in human neonatal blood: low LPS-induced IL-12p70 and high IL-10 persist throughout the first month of life. Clin Immunol. 2009;133(2):228–237. doi: 10.1016/j.clim.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chelvarajan L, Popa D, Liu Y, et al. Molecular mechanisms underlying anti-inflammatory phenotype of neonatal splenic macrophages. J Leukoc Biol. 2007;82(2):403–416. doi: 10.1189/jlb.0107071. [DOI] [PubMed] [Google Scholar]
- 45.Nahid MA, Satoh M, Chan EK. Mechanistic role of microRNA-146a in endotoxin-induced differential cross-regulation of TLR signaling. J Immunol. 2010;186(3):1723–1734. doi: 10.4049/jimmunol.1002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nahid MA, Pauley KM, Satoh M, Chan EK. miR-146a is critical for endotoxin-induced tolerance: IMPLICATION IN INNATE IMMUNITY. J Biol Chem. 2009;284(50):34590–34599. doi: 10.1074/jbc.M109.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lederhuber H, Baer K, Altiok I, et al. MicroRNA-146: tiny player in neonatal innate immunity? Neonatology. 2010;99(1):51–56. doi: 10.1159/000301938. [DOI] [PubMed] [Google Scholar]
- 48.Power Coombs MR, Belderbos ME, Gallington LC, Bont L, Levy O. Adenosine modulates Toll-like receptor function: basic mechanisms and translational opportunities. Expert Rev Anti Infect Ther. 2011;9(2):261–269. doi: 10.1586/eri.10.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levy O, Coughlin M, Cronstein BN, et al. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol. 2006;177(3):1956–1966. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Philbin VJ, Dowling DJ, Gallington LC, et al. Imidazoquinoline Toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J Allergy Clin Immunol. 2012;130(1):195–204. e9. doi: 10.1016/j.jaci.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belderbos ME, Levy O, Stalpers F, et al. Neonatal plasma polarizes TLR4-mediated cytokine responses towards low IL-12p70 and high IL-10 production via distinct factors. PLoS One. 2012;7(3):e33419. doi: 10.1371/journal.pone.0033419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levy O. A Pediatric perspective on antimicrobial proteins and peptides: Expression, function, and clinical relevance. London, UK: Horizon Scientific Press; 2004. pp. 305–329. [Google Scholar]
- 53.Philbin VJ, Levy O. Immunostimulatory activity of Toll-like receptor 8 agonists towards human leucocytes: basic mechanisms and translational opportunities. Biochem Soc Trans. 2007;35(Pt 6):1485–1491. doi: 10.1042/BST0351485. [DOI] [PubMed] [Google Scholar]
- 54.Bouma G, Doffinger R, Patel SY, et al. Impaired neutrophil migration and phagocytosis in IRAK-4 deficiency. Br J Haematol. 2009;147(1):153–156. doi: 10.1111/j.1365-2141.2009.07838.x. [DOI] [PubMed] [Google Scholar]
- 55.Orange JS, Jain A, Ballas ZK, et al. The presentation and natural history of immunodeficiency caused by nuclear factor kappaB essential modulator mutation. J Allergy Clin Immunol. 2004;113(4):725–733. doi: 10.1016/j.jaci.2004.01.762. [DOI] [PubMed] [Google Scholar]
- 56.Hoebe K, Janssen EM, Kim SO, et al. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nat Immunol. 2003;4(12):1223–1229. doi: 10.1038/ni1010. [DOI] [PubMed] [Google Scholar]
- 57.Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate Immune Function by Toll-like Receptors: Distinct Responses in Newborns and the Elderly. Immunity. 2012;37(5):771–783. doi: 10.1016/j.immuni.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Corbett NP, Blimkie D, Ho KC, et al. Ontogeny of Toll-like receptor mediated cytokine responses of human blood mononuclear cells. PLoS One. 2010;5(11):e15041. doi: 10.1371/journal.pone.0015041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burl S, Townend J, Njie-Jobe J, et al. Age-dependent maturation of Toll-like receptor-mediated cytokine responses in Gambian infants. PLoS One. 2011;6(4):e18185. doi: 10.1371/journal.pone.0018185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9(5):355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 61.Aaby P, Roth A, Ravn H, et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis. 2011;204(2):245–252. doi: 10.1093/infdis/jir240. [DOI] [PubMed] [Google Scholar]
- 62.Auriti C, Prencipe G, Inglese R, et al. Role of mannose-binding lectin in nosocomial sepsis in critically ill neonates. Hum Immunol. 2010;71(11):1084–1088. doi: 10.1016/j.humimm.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 63.Frakking FN, Brouwer N, Zweers D, et al. High prevalence of mannose-binding lectin (MBL) deficiency in premature neonates. Clin Exp Immunol. 2006;145(1):5–12. doi: 10.1111/j.1365-2249.2006.03093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frakking FN, Brouwer N, van Eijkelenburg NK, et al. Low mannose-binding lectin (MBL) levels in neonates with pneumonia and sepsis. Clin Exp Immunol. 2007;150(2):255–262. doi: 10.1111/j.1365-2249.2007.03479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dzwonek AB, Neth OW, Thiebaut R, et al. The role of mannose-binding lectin in susceptibility to infection in preterm neonates. Pediatr Res. 2008;63(6):680–685. doi: 10.1203/PDR.0b013e31816fdbff. [DOI] [PubMed] [Google Scholar]
- 66.Szala A, Paradowska E, Nowakowska D, et al. Mannan-binding lectin-2 (MBL2) gene polymorphisms in prenatal and perinatal cytomegalovirus infections. Mol Immunol. 2011 doi: 10.1016/j.molimm.2011.06.220. [DOI] [PubMed] [Google Scholar]
- 67.van der Zwet WC, Catsburg A, van Elburg RM, Savelkoul PH, Vandenbroucke-Grauls CM. Mannose-binding lectin (MBL) genotype in relation to risk of nosocomial infection in preterm neonates in the neonatal intensive care unit. Clin Microbiol Infect. 2008;14(2):130–135. doi: 10.1111/j.1469-0691.2007.01886.x. [DOI] [PubMed] [Google Scholar]
- 68.Awasthi S, Brown K, King C, Awasthi V, Bondugula R. A toll-like receptor-4-interacting surfactant protein-A-derived peptide suppresses tumor necrosis factor-alpha release from mouse JAWS II dendritic cells. J Pharmacol Exp Ther. 2011;336(3):672–681. doi: 10.1124/jpet.110.173765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Awasthi S, Madhusoodhanan R, Wolf R. Surfactant protein-A and toll-like receptor-4 modulate immune functions of preterm baboon lung dendritic cell precursor cells. Cell Immunol. 2011;268(2):87–96. doi: 10.1016/j.cellimm.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manzoni P, Rinaldi M, Cattani S, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA. 2009;302(13):1421–1428. doi: 10.1001/jama.2009.1403. [DOI] [PubMed] [Google Scholar]
- 71.Venkatesh MP, Abrams SA. Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2010;(5):CD007137. doi: 10.1002/14651858.CD007137.pub2. [DOI] [PubMed] [Google Scholar]
- 72.Krasity BC, Troll JV, Weiss JP, McFall-Ngai MJ. LBP/BPI proteins and their relatives: conservation over evolution and roles in mutualism. Biochem Soc Trans. 2011;39(4):1039–1044. doi: 10.1042/BST0391039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Canny G, Levy O, Furuta GT, et al. Lipid mediator-induced expression of bactericidal/permeability-increasing protein (BPI) in human mucosal epithelia. Proc Natl Acad Sci U S A. 2002;99(6):3902–3907. doi: 10.1073/pnas.052533799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Canny G, Cario E, Lennartsson A, et al. Functional and biochemical characterization of epithelial bactericidal/permeability-increasing protein. Am J Physiol Gastrointest Liver Physiol. 2006;290(3):G557–G567. doi: 10.1152/ajpgi.00347.2005. [DOI] [PubMed] [Google Scholar]
- 75.Levy O, Martin S, Eichenwald E, et al. Impaired innate immunity in the newborn: newborn neutrophils are deficient in bactericidal/permeability-increasing protein. Pediatrics. 1999;104(6):1327–1333. doi: 10.1542/peds.104.6.1327. [DOI] [PubMed] [Google Scholar]
- 76.Michalek J, Svetlikova P, Fedora M, et al. Bactericidal permeability increasing protein gene variants in children with sepsis. Intensive Care Med. 2007;33(12):2158–2164. doi: 10.1007/s00134-007-0860-3. [DOI] [PubMed] [Google Scholar]
- 77.Levin M, Quint PA, Goldstein B, et al. Recombinant bactericidal/permeability-increasing protein (rBPI21) as adjunctive treatment for children with severe meningococcal sepsis: a randomised trial. rBPI21 Meningococcal Sepsis Study Group. Lancet. 2000;356(9234):961–967. doi: 10.1016/s0140-6736(00)02712-4. [DOI] [PubMed] [Google Scholar]
- 78.Guinan EC, Barbon CM, Kalish LA, et al. Bactericidal/permeability-increasing protein (rBPI21) and fluoroquinolone mitigate radiation-induced bone marrow aplasia and death. Sci Transl Med. 2011;3(110):110ra118. doi: 10.1126/scitranslmed.3003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Palmer CD, Guinan EC, Levy O. Deficient expression of bactericidal/permeability-increasing protein in immunocompromised hosts: translational potential of replacement therapy. Biochem Soc Trans. 2011;39(4):994–999. doi: 10.1042/BST0390994. [DOI] [PubMed] [Google Scholar]
- 80.Richter M, Topf HG, Groschl M, et al. Influence of gestational age, cesarean section, and type of feeding on fecal human beta-defensin 2 and tumor necrosis factor-alpha. J Pediatr Gastroenterol Nutr. 2010;51(1):103–105. doi: 10.1097/MPG.0b013e3181cd26f9. [DOI] [PubMed] [Google Scholar]
- 81.Shim DH, Ryu S, Kweon MN. Defensins play a crucial role in protecting mice against oral Shigella flexneri infection. Biochem Biophys Res Commun. 2010;401(4):554–560. doi: 10.1016/j.bbrc.2010.09.100. [DOI] [PubMed] [Google Scholar]
- 82.Ricci E, Malacrida S, Zanchetta M, et al. Role of beta-defensin-1 polymorphisms in mother-to-child transmission of HIV-1. J Acquir Immune Defic Syndr. 2009;51(1):13–19. doi: 10.1097/QAI.0b013e31819df249. [DOI] [PubMed] [Google Scholar]
- 83.Rapista A, Ding J, Benito B, et al. Human defensins 5 and 6 enhance HIV-1 infectivity through promoting HIV attachment. Retrovirology. 2011;8:45. doi: 10.1186/1742-4690-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Skeldon A, Saleh M. The inflammasomes: molecular effectors of host resistance against bacterial, viral, parasitic, and fungal infections. Front Microbiol. 2011;2:15. doi: 10.3389/fmicb.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kanneganti TD. Central roles of NLRs and inflammasomes in viral infection. Nat Rev Immunol. 2010;10(10):688–698. doi: 10.1038/nri2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gotsch F, Romero R, Chaiworapongsa T, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med. 2008;21(9):605–616. doi: 10.1080/14767050802212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Costa A, Gupta R, Signorino G, et al. Activation of the NLRP3 inflammasome by group B streptococci. J Immunol. 2012;188(4):1953–1960. doi: 10.4049/jimmunol.1102543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453(7198):1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marichal T, Ohata K, Bedoret D, et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med. 2011;17(8):996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- 90.Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol. 2008;181(1):17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lisciandro JG, Prescott SL, Nadal-Sims MG, et al. Ontogeny of Toll-like and NOD-like receptor-mediated innate immune responses in Papua New Guinean infants. PLoS One. 2012;7(5):e36793. doi: 10.1371/journal.pone.0036793. [DOI] [PMC free article] [PubMed] [Google Scholar]