Abstract
Upon activation, T cells of various subsets are the most important mediators in cell-mediated immune responses. Activated T cells play an important role in immune system related diseases such as chronic inflammatory diseases, viral infections, autoimmune disease, transplant rejection, Crohn disease, diabetes, and many more. Therefore, efforts have been made to both visualize and treat activated T cells specifically. This review summarizes imaging approaches and selective therapeutics for activated T cells and gives an outlook on how tracking and treating can be combined into theragnositc agents for activated T cells.
Keywords: Activated T cells, Imaging, Treatment, Theragnostics, siRNA, Targeting
T cells are lymphocytes that mature in the thymus and can be identified by their expression of the T cell receptor (TCR). Based on their maturation process, T lymphocytes can differentiate into several different subsets, each of which has different effector functions and molecular phenotypes [1]. Examples of subsets are CD4+ T helper cells that can be further differentiated into TH1 cells which mediate cellular immune responses, TH2 cells responsible for humoral immune responses, TH17 cells which play a key role in autoimmune diseases and anti-microbial immunity, TFH cells located in the follicular regions of secondary lymphoid organs, and TH3 cells producing TGFβ [2, 3]. Another subset are the cytotoxic CD8+ cells (CTL cells) that clear tumor cells and cells infected by viruses [4]. Regulatory T cells (TReg cells) suppress the immune response, and natural killer T cells (NKT cells) are cytotoxic for a variety of target cells [2].
T cells are one of the major components in cell-mediated immune responses. The interaction of T cells with antigen-presenting cells (APCs), through the T cell receptor recognition of peptides presented by the major histocompatibility complex and the costimulation by CD28 immunoglobulin superfamily members on T cells binding to B7 family members, initiates a series of signaling cascades resulting in T cell activation [5, 6]. B7 family members on APCs are upregulated after activation and the B7:CD28 costimulatory signal augments the TCR signal, and thereby promotes T cell response [7]. The activation of T cells is triggered either by antigen-presenting cells or other target cells. The activation process itself is complicated and involves the cytoskeleton, as well as integrin-mediated adhesion, receptor sequestration and other intracellular steps [8].
Activated T cells play a key role in immune response and immune system related diseases such as chronic inflammatory diseases, viral infections, autoimmune disease, transplant rejection, Crohn disease, diabetes, and many more [9-12]. Therefore, the selective detection and tracking of activated T cells are very useful for the diagnosis of many inflammatory or infectious diseases as well as for the follow-up of treatment effects. Furthermore, a specific treatment targeting activated T cells bears a therapeutic benefit since the activated rather than naïve T cells play a central role in immune response cascades.
Keeping the importance of activated T cells in mind, in this review, we focus on the selective imaging and treating strategies of only activated T cells, distinguished from naïve T cells or other immune cells.
I. IMAGING ACTIVATED T CELLS
While non-invasiveness, cost-effectiveness and sensitivity are absolutely important, selective detection of activated T cells and differentiation from naïve T cells are the key aims in diagnostic imaging techniques. Therefore, for most studies involving the tracking of activated T cells specific probes were developed to detect markers that are specifically expressed on the cell surface of T cells upon activation.
Once the T cell receptor recognizes a peptide antigen presented by the major histocompatability complex on the antigen-presenting cell, a series of signaling cascades are initiated and result in expression and secretion of several cytokines and up-regulation of their receptors, which can be a good marker for T cell activation [13]. Interleukin-2 (IL-2), a small single-chain glycoprotein expressed and secreted by activated T cells, has been widely used to detect activated T cells [14]. Becker et al. utilized transgenic mice (IL-2/GFPki) that co-express the green fluorescence protein (GFP) gene together with IL-2 after insertion of cDNA coding for GFP into the locus of IL-2 [15]. Activated T cells infiltrating the iris stroma in mice with endotoxin-induced uveitis could thus be visualized by enhanced GFP fluorescence induced by the up-regulation of IL-2 using intravital epifluorescence miscoscopy at multiple time points. Using the specific binding affinity of IL-2 to the IL-2 receptor expressed on activated T cells, radioisotope 123I-labeled IL-2 has also been investigated for in vivo imaging of activated T cells. In a mouse model for the study of the pathogenesis of type 1 insulin-dependent diabetes (IDDM), high accumulation of 123I-IL-2 was observed in the pancreatic region, suggesting the possibility to use nuclear imaging for the early diagnosis of IDDM [16]. Intravenously injected 123I-IL-2 to renal allograft transplanted rats showed the selective and enhanced retention of the radioactivity compared to non-rejecting grafts measured by non-invasive gamma camera imaging [17]. In addition to 123I, 99mTc labeled IL-2 was also studied for the radio-labeling process with a single-step synthesis method to improve cost and time factors [18]. However, in vivo activated T cell imaging with 99mTc-IL-2 has not been reported yet. Positron emission tomography (PET), which is more sensitive and offers higher resolution, was also reported for the visualization of IL-2 receptor positive T cells [12]. Activated T cells which were subcutaneously injected into the shoulder of immune-depressed SCID mice were clearly visualized after intravenous injection of N-(4-18F-fluorobenzoyl) IL-2 by PET imaging. In addition to the IL-2 receptor, interleukin-12 (IL-12) receptor is also a specific target for the detection of activated T cells, since IL-12 receptor is up-regulated upon activation of T cells or NK cells [19]. Radiolabeled 99mTc-IL-12 has shown specific accumulation in inflamed areas of the colon in 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced chronic colitis mice, while accumulation in noninflamed areas or control mice was not significant [20].
Other than cytokines, T cell receptor-dependent nuclear factor of activated T cells (NFAT), which is a major transcription factor downstream of the T cell receptor/CD3 signal cascade, has been used for the tracking of activated T cells with NFAT-inducible reporter systems [21-23]. Na et al. recently reported a dual bioluminescent reporter system consisting of a constitutive reporter and NFAT-activation inducible reporter that can non-invasively monitor in vivo trafficking of activated T cells in a mouse model of graft-versus-host disease (GVHD) [22]. Ponomarev et al. developed the herpes simplex virus type 1 thymidine kinase/GFP protein (TKGFP) dual reporter gene for imaging of NFAT-mediated activated T cells [21]. Using the same reporter system, they demonstrated optical fluorescence imaging as well as PET imaging with 124I-FIAU (2’-fluoro-2’deoxy-1-β-D-arabinofuranosyl-5-iodouracil) to visualize activated subcutaneous Jurkat infitrates transduced with NFAT-TKGFP reporter in nude mice [21].
Furthermore, a therapeutic agent that has a selective toxicity toward T cell lymphoblasts, 9-(β-D-arabinofuranosyl)guanine (AraG) was tested as a PET imaging probe after radiofluorination [24]. Although in vitro or in vivo results of PET imaging with [18F]F-AraG have not been reported, higher uptake of [18F]F-AraG into activated primary T cells suggests a potential to be used as a PET imaging probe in the diagnosis of diseases that involve activated T cells. PET probes like 1-(2’-deoxy-2’[18F]fluroarabinofuranosyl)cytosine ([18F]FAC ) that use the deoxyribonucleotide salvage pathway, which is mostly utilized in lymphoid organs and rapidly proliferating tissues, have shown a higher accumulation in activated T cells ex vivo and an increased lymphoid mass in autoimmune disease model mice compared to wild-type mice [25]. However, the specificity for activated immune cells should be further studied in vivo before these probes can be applied as a specific PET probe for activated T cells.
In addition to the imaging strategies using activated T cell-specific probes described so far, histological analysis, one of the classical imaging techniques, has been employed to confirm the probe-mediated imaging results in some studies [16, 20]. Two-photon laser scanning microscopy (TPLSM) has shown the different migration pattern of activated and naïve CD4+ T cells in autoimmune CNS inflammation models [26, 27]. Magnetic resonance imaging (MRI) is another noninvasive and highly sensitive imaging technique, but in vivo visualizing of activated T cells has not been reported yet to our knowledge. An in vitro study demonstrated the feasibility of imaging activated T cells isolated from rhesus macaques showing successful labeling with monocrystalline iron oxide nanoparticles (MION) [28].
II. TREATMENT OF ACTIVATED T CELLS
Since activated T cells are involved in various inflammatory diseases such as asthma, autoimmune diseases, and acute rejection after organ transplantation, treatment strategies to target activated T cells have been developed using markers on the cell surface of activated T cells. One such approach that is extensively employed and showed successful results is antibody-based. In this approach, the cell surface markers on the activated T cell are blocked by an antibody against a specific marker resulting in inhibition of the signaling pathway or induction of active cell death.
One of the chronic inflammatory diseases in which activated T cells play a central role is asthma, which is an inflammatory disorder characterized by chronic airway inflammation caused by infiltration of eosinophils and TH2 cells in the lung [29]. In the airways of asthmatics, many of the inflammatory cascades are orchestrated by CD4+ T cells, which secrete IL-4, IL-5, and IL-13 [30], as shown in Figure 1. It was shown that in asthma-mediated lung inflammation, inflammatory responses caused by activated CD4+ T cells were decreased by using an anti-CD147 antibody that modulated the activated cell surface signaling [31]. Equivalently, a monoclonal anti-E-selectin (agonist) antibody against the cutaneous lymphocyte antigen expressed on activated T cells in atopic skin inflammation helps to reduce inflammatory reactions [32]. In graft versus host type autoimmune disease, upregulation of CD44 expression causes inflammation and rejection. An anti-CD44 monoclonal antibody (mAb) that killed activated T cell was shown to prevent the graft rejection and associated negative responses [33]. And in acute lymphoblastic leukemia, targeting or blocking the mammalian target of rapamycin (mTOR) on activated T cell was reported to inhibit primary leukemia [34, 35]. Bispecific antibodies can also be used in targeting activated T cell and other cell surfaces or two different surface markers on the same activated T cell. A bispecific antibody that was chemically crosslinked and genetically engineered with specificities for tumor and CD3 on activated mouse T cells successfully killed tumor cells in primary breast cancer [36]. Activated T cell targeting was extensively studied and tested clinically in rheumatoid arthritis. Several of these strategies included the cytotoxic T lymphocyte antigen 4 (CTLA4, CD152) on activated cytotoxic T cells, the CD4 surface marker on T helper cells, the T cell receptor on activated T cells in disease state synovial fluid, and co-stimulatory signal molecule blockade of CD28/B7 [37-41].
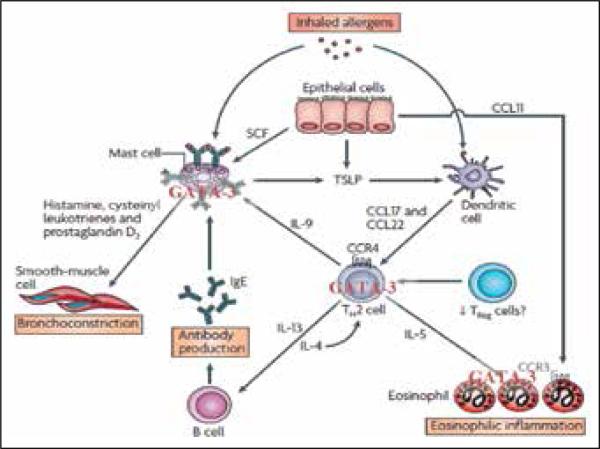
Figure 1.
Pathophysiology of asthma involving activated T cells. Adapted from Barnes, et al [67]. T cell activation by antigen-presenting cells (APCs) induces translocation of GATA-3 into the nucleus and thus upregulates gene expression of TH2 cytokines, which leads to i) increased mucus production in goblet cells, ii) activation of eosinophils and B cells. The latter produce IgE, which is presented on the surface of mast cells to directly interact with the allergen. Both eosinophils and mast cells trigger smooth muscle broncho-obstruction mediated by secretion of second messengers such as leukotriens, cytokines, and histamine. Thus, GATA-3 conducts an orchestra of inflammation.
Activated T cells are also treated by using indirect methods such as modulating cytokines, tumor necrosis factors, peptides, or co-stimulatory molecules [42]. In inflammatory bowel disease, antibodies against TNF caused apoptosis of activated T cells and thus reduced the inflammatory symptoms [43]. Synovial homing peptides that targeted the synovial vasculature have decreased the inflammation in autoimmune rheumatoid arthritis by decreasing T cell trafficking to the synovial region [44]. In cardiac allograft rejection, T cell to T cell mediated co-stimulation in activated T cells was targeted and blocked with a co-stimulatory signal/ligand [45]. Activated CD4+ T cells expressing tumor necrosis factors were also blocked by ligand-mediated interaction (such as OX40 or CD134) and thus minimized autoimmunity [46]. In situations where T cells are activated, combined strategies that employed an antibody conjugated to chemotherapeutic or photodynamic agents that target and kill activated T cells were also shown to be an attractive strategy [47]. Activated T cells showed higher sensitivity to photodynamic treatment with benzoporphyrin derivative monoacid ring A compared to resting T cells [48]. A synthetic peptide analog that targeted T cells and blocked the high voltage-gated potassium channel (Kv1.3) helped in reducing a delayed type of hypersensitive reactions [49-51].
Small compound drugs or natural products have also been tested for their effect on activated T cells. Tacrolimus, an immunosuppressive drug inhibiting activated T cells specifically, reduced the number of activated T cells in cerebrospinal fluid (CSF) and activated cerebellar degeneration-related protein 2 (cdr2)-specific cytotoxic T lymphocytes in the peripheral blood of patients with paraneoplastic cerebellar degeneration [52]. The effects of bromelain, an extract from pineapple stem acting as an anti-inflammatory agent, on activated T cells have been various. Decreased number of activated CD4+ T cells in an allergic airway model and reduced expression of CD25, which is upregulated upon T cell activation, on anti-CD3 stimulated CD4+ T cells were demonstrated, while Hale et al. showed bromelain treatment enhanced CD2-mediated T cell activation [53-55]. These results may be due to the variability in the immunomodulatory properties of natural products [56, 57]. Other natural compounds such as astin C, a plant cyclopeptide isolated from Aster tataricus roots, or fraxinellone, a natural small lactone isolated from Dictamnus dasycarpus root bark, induced apoptosis of activated T cells in vivo in murine colitis or hepatitis models [58, 59].
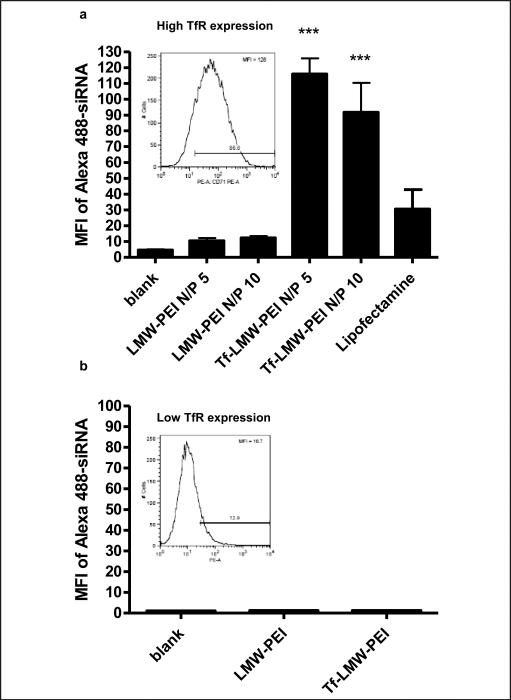
Gene therapy can be another powerful method to treat activated T cells, however, studies about gene-mediated treatment of activated T cells in the literature are very limited because of the inefficient delivery to primary T cells in vitro and in vivo. Various delivery strategies such as electroporation, nucleofection, viral or non-viral vectors, have been studied to transfect T cells [60-63]. Even though some of the studies reported high transfection efficiency in activated primary T cells, specific delivery of DNA or siRNA to activated T cells was not demonstrated. One strategy for targeted delivery to activated T cells is to use transferrin as a targeting ligand for non-viral vectors. Activated T cells highly express the transferrin receptor (TfR), which is internalized into the cell by receptor-mediated endocytosis after binding with transferrin [64]. Using this characteristic, our group has examined the siRNA delivery efficiency with a transferrin-low molecular weight polyethylenimine conjugate (Tf-PEI) into activated primary human T cells [65]. We found the specific uptake of fluorescently-labeled siRNA complexed with Tf-PEI into TfR-overexpressing T cells compared to unconjugated PEI (Figure 2a), while the uptake level was similarly low for both Tf-PEI/siRNA and PEI/siRNA in T cells with low TfR expression level (Figure 2b). Based on this result, we expect that radiolabeled siRNA delivered with Tf-PEI can be used for both therapy and diagnosis of activated T cell related diseases, such as asthma.
Figure 2.
Specific uptake of siRNA in activated T cells with a Tf-PEI conjugate. Uptake of Alexa488-labeled siRNA a) at different N/P ratios into fully activated T cells with high TfR expression (inset in 2a), and b) lack of uptake into T cells with low TfR expression (inset in 2b). The expression of TfR in the T cells was confirmed by anti-CD71 antibody binding assay. The siRNA taken up into the T cells was analyzed by flow cytometry. Lipofectamine was used as a positive control.
While activated T cells have so far only been specifically tracked or treated, a combined theragnostic agent has not been described yet. Most approaches to image activated T cells specifically used probes for IL-2 which were not employed further for therapeutic effects. The most efficient selective therapeutics for activated T cells that are described in the literature are antibody-based. These therapeutics could essentially be radiolabeled and used as theragnostics, for example to image T cell trafficking, recruiting to sites of inflammation or to lymph nodes. To enhance the therapeutic effects, interference with disease-causing transcription factors like GATA-3 in TH2 cells, rather than downregulating single cytokines, has been reported to be a promising approach [66] and will certainly gain importance in the future. Therefore, RNA interference technology will certainly gain importance and can serve as an attractive theragnostic approach in the future.
REFERENCES
- 1.Palmer E. Negative selection - clearing out the bad apples from the T-cell repertoire. Nat. Rev. Immunol. 2003;3:383–391. doi: 10.1038/nri1085. [DOI] [PubMed] [Google Scholar]
- 2.Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat. Rev. Immunol. 2007;7:532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 3.Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Research. 2010;20:4–12. doi: 10.1038/cr.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finlay D, Cantrell DA. Metabolism, migration and memory in cytotoxic T cells. Nat. Rev. Immunol. 2011;11:109–117. doi: 10.1038/nri2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hennecke J, Wiley DC. T cell receptor-MHC interactions up close. Cell. 2001;104:1–4. doi: 10.1016/s0092-8674(01)00185-4. [DOI] [PubMed] [Google Scholar]
- 6.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu. Rev. Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 7.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat. Rev. Immunol. 2002;2:116–26. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 8.Billadeau DD, Nolz JC, Gomez TS. Regulation of T-cell activation by the cytoskeleton. Nat. Rev. Immunol. 2007;7:131–143. doi: 10.1038/nri2021. [DOI] [PubMed] [Google Scholar]
- 9.Krensky AM, Weiss A, Crabtree G, Davis MM, Parham P. T-lymphocyte-antigen interactions in transplant rejection. N. Engl. J. Med. 1990;322:510–7. doi: 10.1056/NEJM199002223220805. [DOI] [PubMed] [Google Scholar]
- 10.Gaston JS, Bacon PA. T-cell induction of autoimmunity. Autoimmunity. 1988;1:151–5. doi: 10.3109/08916938809001928. [DOI] [PubMed] [Google Scholar]
- 11.Modigliani R, Mary JY, Simon JF, Cortot A, Soule JC, Gendre JP, René E. Clinical, biological, and endoscopic picture of attacks of Crohn's disease. Evolution on prednisolone. Groupe d'étude therapeutique des affections inflammatoires digestives. Gastroenterology. 1990;98:811–8. doi: 10.1016/0016-5085(90)90002-i. [DOI] [PubMed] [Google Scholar]
- 12.Di Gialleonardo V, Signore A, Glaudemans AW, Dierckx RA, De Vries EF. N-(4-18F-fluorobenzoyl)interleukin-2 for PET of human-activated T lymphocytes. J. Nucl. Med. 53:679–86. doi: 10.2967/jnumed.111.091306. [DOI] [PubMed] [Google Scholar]
- 13.Swain SL, Croft M, Dubey C, Haynes L, Rogers P, Zhang X, Bradley LM. From naive to memory T cells. Immunol. Rev. 1996;150:143–67. doi: 10.1111/j.1600-065x.1996.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 14.Cohen FE, Kosen PA, Kuntz ID, Epstein LB, Ciardelli TL, Smith KA. Structure-activity studies of interleukin-2. Science. 1986;234:349–52. doi: 10.1126/science.3489989. [DOI] [PubMed] [Google Scholar]
- 15.Becker MD, Crespo S, Martin TM, Planck SR, Naramura M, Rosenbaum JT. Intraocular in vivo imaging of activated T-lymphocytes expressing green-fluorescent protein after stimulation with endotoxin. Graefes Arch. Clin. Exp. Ophthalmol. 2001;239:609–12. doi: 10.1007/s004170100320. [DOI] [PubMed] [Google Scholar]
- 16.Signore A, Chianelli M, Ronga G, Pozzilli P, Beverley PC. In vivo labelling of activated T lymphocytes by i.v. injection of 123I-IL2 for detection of insulitis in type 1 diabetes. Prog. Clin. Biol. Res. 1990;355:229–38. [PubMed] [Google Scholar]
- 17.Abbs IC, Pratt JR, Dallman MJ, Sacks SH. Analysis of activated T cell infiltrates in rat renal allografts by gamma camera imaging after injection of 123iodine-interleukin 2. Transpl. Immunol. 1993;1:45–51. doi: 10.1016/0966-3274(93)90058-g. [DOI] [PubMed] [Google Scholar]
- 18.D'Alessandria C, di Gialleonardo V, Chianelli M, Mather SJ, de Vries EF, Scopinaro F, Dierck RA, Signore A. Synthesis and optimization of the labeling procedure of 99mTc-HYNIC-interleukin-2 for in vivo imaging of activated T lymphocytes. Mol. Imaging Biol. 12:539–46. doi: 10.1007/s11307-009-0285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desai BB, Quinn PM, Wolitzky AG, Mongini PK, Chizzonite R, Gately MK. IL-12 receptor. II. Distribution and regulation of receptor expression. J. Immunol. 1992;148:3125–32. [PubMed] [Google Scholar]
- 20.Annovazzi A, D'Alessandria C, Bonanno E, Mather SJ, Cornelissen B, van de Wiele C, Dierckx RA, Mattei M, Palmieri G, Scopinaro F, Signore A. Synthesis of 99mTc-HYNIC-interleukin-12, a new specific radiopharmaceutical for imaging T lymphocytes. Eur. J. Nucl. Med. Mol. Imaging. 2006;33:474–82. doi: 10.1007/s00259-005-0001-6. [DOI] [PubMed] [Google Scholar]
- 21.Ponomarev V, Doubrovin M, Lyddane C, Beresten T, Balatoni J, Bornman W, Finn R, Akhurst T, Larson S, Blasberg R, Sadelain M, Tjuvajev JG. Imaging TCR-dependent NFAT-mediated T-cell activation with positron emission tomography in vivo. Neoplasia. 2001;3:480–8. doi: 10.1038/sj.neo.7900204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Na IK, Markley JC, Tsai JJ, Yim NL, Beattie BJ, Klose AD, Holland AM, Ghosh A, Rao UK, Stephan MT, Serganova I, Santos EB, Brentjens RJ, Blasberg RG, Sadelain M, van den Brink MR. Concurrent visualization of trafficking, expansion, and activation of T lymphocytes and T-cell precursors in vivo. Blood. 2010;116:e18–25. doi: 10.1182/blood-2009-12-259432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 2005;5:472–84. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 24.Namavari M, Chang YF, Kusler B, Yaghoubi S, Mitchell BS, Gambhir SS. Synthesis of 2′-deoxy-2′-[18F]fluoro-9-beta-D-arabinofuranosylguanine: a novel agent for imaging T-cell activation with PET. Mol. Imaging Biol. 13:812–8. doi: 10.1007/s11307-010-0414-x. [DOI] [PubMed] [Google Scholar]
- 25.Radu CG, Shu CJ, Nair-Gill E, Shelly SM, Barrio JR, Satyamurthy N, Phelps ME, Witte ON. Molecular imaging of lymphoid organs and immune activation by positron emission tomography with a new [18F]-labeled 2′-deoxycytidine analog. Nat. Med. 2008;14:783–8. doi: 10.1038/nm1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siffrin V, Brandt AU, Radbruch H, Herz J, Boldakowa N, Leuenberger T, Werr J, Hahner A, Schulze-Topphoff U, Nitsch R, Zipp F. Differential immune cell dynamics in the CNS cause CD4+ T cell compartmentalization. Brain. 2009;132:1247–58. doi: 10.1093/brain/awn354. [DOI] [PubMed] [Google Scholar]
- 27.Herz J, Paterka M, Niesner RA, Brandt AU, Siffrin V, Leuenberger T, Birkenstock J, Mossakowski A, Glumm R, Zipp F, Radbruch H. In vivo imaging of lymphocytes in the CNS reveals different behaviour of naive T cells in health and autoimmunity. J. Neuroinflammation. 2011;8:131. doi: 10.1186/1742-2094-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sundstrom JB, Mao H, Santoianni R, Villinger F, Little DM, Huynh TT, Mayne AE, Hao E, Ansari AA. Magnetic resonance imaging of activated proliferating rhesus macaque T cells labeled with superparamagnetic monocrystalline iron oxide nanoparticles. J. Acquir. Immune Defic. Syndr. 2004;35:9–21. doi: 10.1097/00126334-200401010-00002. [DOI] [PubMed] [Google Scholar]
- 29.Jeffery PK. Pathology of asthma. Br. Med. Bull. 1992;48:23–39. doi: 10.1093/oxfordjournals.bmb.a072537. [DOI] [PubMed] [Google Scholar]
- 30.Ray A, Cohn L. Th2 cells and GATA-3 in asthma: new insights into the regulation of airway inflammation. J. Clin. Invest. 1999;104:985–93. doi: 10.1172/JCI8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gwinn WM, Damsker JM, Falahati R, Okwumabua I, Kelly-Welch A, Keegan AD, Vanpouille C, Lee JJ, Dent LA, Leitenberg D, Bukrinsky MI, Constant SL. Novel approach to inhibit asthma-mediated lung inflammation using anti-CD147 intervention. J. Immunol. 2006;177:4870–9. doi: 10.4049/jimmunol.177.7.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biedermann T, Schwarzler C, Lametschwandtner G, Thoma G, Carballido-Perrig N, Kund J, de Vries JE, Rot A, Carballido JM. Targeting CLA/E-selectin interactions prevents CCR4-mediated recruitment of human Th2 memory cells to human skin in vivo. European Journal of Immunology. 2002;32:3171–80. doi: 10.1002/1521-4141(200211)32:11<3171::AID-IMMU3171>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 33.McKallip RJ, Do Y, Fisher MT, Robertson JL, Nagarkatti PS, Nagarkatti M. Role of CD44 in activation-induced cell death: CD44-deficient mice exhibit enhanced T cell response to conventional and superantigens. International Immunology. 2002;14:1015–26. doi: 10.1093/intimm/dxf068. [DOI] [PubMed] [Google Scholar]
- 34.Batista A, Barata JT, Raderschall E, Sallan SE, Carlesso N, Nadler LM, Cardoso AA. Targeting of active mTOR inhibits primary leukemia T cells and synergizes with cytotoxic drugs and signaling inhibitors. Experimental Hematology. 2011;39:457–472. e3. doi: 10.1016/j.exphem.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, Powell JD. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J. Immunol. 2007;178:2163–70. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- 36.Moreno MB, Titus JA, Cole MS, Tso JY, Le N, Paik CH, Bakacs T, Zacharchuk CM, Segal DM, Wunderlich JR. Bispecific antibodies retarget murine T cell cytotoxicity against syngeneic breast cancer in vitro and in vivo. Cancer Immunology, Immunotherapy: CII. 1995;40:182–90. doi: 10.1007/BF01517350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramos-Casals M, Brito-Zeron P. Emerging biological therapies in primary Sjogren's syndrome. Rheumatology (Oxford, England) 2007;46:1389–96. doi: 10.1093/rheumatology/kem078. [DOI] [PubMed] [Google Scholar]
- 38.Smolen JS. What is the place of recently approved T cell-targeted and B cell-targeted therapies in the treatment of rheumatoid arthritis? Lessons from global clinical trials. The Journal of Rheumatology, Supplement. 2007;79:15–20. [PubMed] [Google Scholar]
- 39.Toh ML, Miossec P. The role of T cells in rheumatoid arthritis: new subsets and new targets. Current Opinion in Rheumatology. 2007;19:284–8. doi: 10.1097/BOR.0b013e32805e87e0. [DOI] [PubMed] [Google Scholar]
- 40.VanderBorght A, De Keyser F, Geusens P, De Backer M, Malaise M, Baeten D, Van den Bosch F, Veys EM, Raus J, Stinissen P. Dynamic T cell receptor clonotype changes in synovial tissue of patients with early rheumatoid arthritis: effects of treatment with cyclosporin A (Neoral). The Journal of Rheumatology. 2002;29:416–26. [PubMed] [Google Scholar]
- 41.Weyand CM, Goronzy JJ. T-cell-targeted therapies in rheumatoid arthritis. Nature Clinical Practice. Rheumatology. 2006;2:201–10. doi: 10.1038/ncprheum0142. [DOI] [PubMed] [Google Scholar]
- 42.O'Reilly S, Hugle T, van Laar JM. T cells in systemic sclerosis: a reappraisal. Rheumatology (Oxford, England) 2012 doi: 10.1093/rheumatology/kes090. [DOI] [PubMed] [Google Scholar]
- 43.Atreya R, Zimmer M, Bartsch B, Waldner MJ, Atreya I, Neumann H, Hildner K, Hoffman A, Kiesslich R, Rink AD, Rau TT, Rose-John S, Kessler H, Schmidt J, Neurath MF. Antibodies against tumor necrosis factor (TNF) induce T-cell apoptosis in patients with inflammatory bowel diseases via TNF receptor 2 and intestinal CD14(+) macrophages. Gastroenterology. 2011;141:2026–38. doi: 10.1053/j.gastro.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 44.Yang YH, Rajaiah R, Ruoslahti E, Moudgil KD. Peptides targeting inflamed synovial vasculature attenuate autoimmune arthritis. Proc. Natl. Acad. Sci. USA. 2011;108:12857–62. doi: 10.1073/pnas.1103569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye Q, Fraser CC, Gao W, Wang L, Busfield SJ, Wang C, Qiu Y, Coyle AJ, Gutierrez-Ramos JC, Hancock WW. Modulation of LIGHT-HVEM costimulation prolongs cardiac allograft survival. The Journal of Experimental Medicine. 2002;195:795–800. doi: 10.1084/jem.20012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinberg AD. OX40: targeted immunotherapy--implications for tempering autoimmunity and enhancing vaccines. Trends in Immunology. 2002;23:102–9. doi: 10.1016/s1471-4906(01)02127-5. [DOI] [PubMed] [Google Scholar]
- 47.Boumedine RS, Roy DC. Elimination of alloreactive T cells using photodynamic therapy. Cytotherapy. 2005;7:134–43. doi: 10.1080/14653240510027109. [DOI] [PubMed] [Google Scholar]
- 48.Hunt DW, Jiang H, Granville DJ, Chan AH, Leong S, Levy JG. Consequences of the photodynamic treatment of resting and activated peripheral T lymphocytes. Immunopharmacology. 1999;41:31–44. doi: 10.1016/s0162-3109(98)00051-4. [DOI] [PubMed] [Google Scholar]
- 49.Beeton C, Wulff H, Barbaria J, Clot-Faybesse O, Pennington M, Bernard D, Cahalan MD, Chandy KG, Beraud E. Selective blockade of T lymphocyte K(+) channels ameliorates experimental autoimmune encephalomyelitis, a model for multiple sclerosis. Proc. Natl. Acad. Sci. USA. 2001;98:13942–7. doi: 10.1073/pnas.241497298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beeton C, Pennington MW, Wulff H, Singh S, Nugent D, Crossley G, Khaytin I, Calabresi PA, Chen CY, Gutman GA, Chandy KG. Targeting effector memory T cells with a selective peptide inhibitor of Kv1.3 channels for therapy of autoimmune diseases. Molecular Pharmacology. 2005;67:1369–81. doi: 10.1124/mol.104.008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vianna-Jorge R, Suarez-Kurtz G. Potassium channels in T lymphocytes: therapeutic targets for autoimmune disorders? BioDrugs : Clinical Immunotherapeutics, Biopharmaceuticals and Gene Therapy. 2004;18:329–41. doi: 10.2165/00063030-200418050-00005. [DOI] [PubMed] [Google Scholar]
- 52.Albert ML, Austin LM, Darnell RB. Detection and treatment of activated T cells in the cerebrospinal fluid of patients with paraneoplastic cerebellar degeneration. Ann. Neurol. 2000;47:9–17. [PubMed] [Google Scholar]
- 53.Secor ER, Jr., Singh A, Guernsey LA, McNamara JT, Zhan L, Maulik N, Thrall RS. Bromelain treatment reduces CD25 expression on activated CD4+ T cells in vitro. Int. Immunopharmacol. 2009;9:340–6. doi: 10.1016/j.intimp.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Secor ER, Carson WF, Singh A, Pensa M, Guernsey LA, Schramm CM, Thrall RS. Oral bromelain attenuates inflammation in an ovalbumin-induced murine model of asthma. Evid Based Complement Alternat. Med. 2008;5:61–9. doi: 10.1093/ecam/nel110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hale LP, Haynes BF. Bromelain treatment of human T cells removes CD44, CD45RA, E2/MIC2, CD6, CD7, CD8, and Leu 8/ LAM1 surface molecules and markedly enhances CD2-mediated T cell activation. J. Immunol. 1992;149:3809–16. [PubMed] [Google Scholar]
- 56.Barnes J. Quality, efficacy and safety of complementary medicines: fashions, facts and the future. Part II: Efficacy and safety. Br. J. Clin. Pharmacol. 2003;55:331–40. doi: 10.1046/j.1365-2125.2003.01811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khan IA. Issues related to botanicals. Life Sci. 2006;78:2033–8. doi: 10.1016/j.lfs.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 58.Shen Y, Luo Q, Xu H, Gong F, Zhou X, Sun Y, Wu X, Liu W, Zeng G, Tan N, Xu Q. Mitochondria-dependent apoptosis of activated T lymphocytes induced by astin C, a plant cyclo-peptide, for preventing murine experimental colitis. Biochem. Pharmacol. 2011;82:260–8. doi: 10.1016/j.bcp.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 59.Sun Y, Qin Y, Gong FY, Wu XF, Hua ZC, Chen T, Xu Q. Selective triggering of apoptosis of concanavalin A-activated T cells by fraxinellone for the treatment of T-cell-dependent hepatitis in mice. Biochem. Pharmacol. 2009;77:1717–24. doi: 10.1016/j.bcp.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Dardalhon V, Herpers B, Noraz N, Pflumio F, Guetard D, Leveau C, Dubart-Kupperschmitt A, Charneau P, Taylor N. Lentivirus-mediated gene transfer in primary T cells is enhanced by a central DNA flap. Gene Ther. 2001;8:190–8. doi: 10.1038/sj.gt.3301378. [DOI] [PubMed] [Google Scholar]
- 61.Tervo HM, Allespach I, Keppler OT. High-level transfection of primary rabbit T lymphocytes. J. Immunol. Methods. 2008;336:85–9. doi: 10.1016/j.jim.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 62.Gust TC, Neubrandt L, Merz C, Asadullah K, Zugel U, von Bonin A. RNA interference-mediated gene silencing in murine T cells: in vitro and in vivo validation of proinflammatory target genes. Cell Commun. Signal. 2008;6:3. doi: 10.1186/1478-811X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee J, Yun KS, Choi CS, Shin SH, Ban HS, Rhim T, Lee SK, Lee KY. T cell-specific siRNA delivery using antibody-conjugated chitosan nanoparticles. Bioconjug Chem. doi: 10.1021/bc2006219. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 64.Neckers LM, Cossman J. Transferrin receptor induction in mitogen-stimulated human T lymphocytes is required for DNA synthesis and cell division and is regulated by interleukin 2. Proc. Natl. Acad. Sci. USA. 1983;80:3494–8. doi: 10.1073/pnas.80.11.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Debus H, Kilic A, Schiller M, Zheng M, Renz H, Kissel T, Merkel OM. Targeting human and murine T-cells using novel low molecular weight poly(ethylene imine)-transferrinbioconjugates. In preparation. [Google Scholar]
- 66.Sel S, Wegmann M, Dicke T, Sel S, Henke W, Yildirim AÖ, Renz H, Garn H. Effective prevention and therapy of experimental allergic asthma using a GATA-3-specific DNAzyme. Journal of Allergy and Clinical Immunology. 2008;121:910–916. e5. doi: 10.1016/j.jaci.2007.12.1175. [DOI] [PubMed] [Google Scholar]
- 67.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]