Abstract
We have devised an efficient procedure for the synthesis of 5α-dihydrotestosterone (DHT) (1) starting from 3β-hydroxy-5α-androstan-17-one, providing the product in unprecedented 82% yield. A reported method of using toxic Jones reagent is replaced by milder oxidizing agent (NMO/TPAP) in the synthesis of a key intermediate 17β-[(tert-butyldimethylsilyl)oxy]-5α-androstan-3-one (18). This new procedure is simple, does not require special apparatus/precautions or chromatographic purification in most of the steps.
Keywords: Androgen receptor (AR), 5α-dihydrotestosterone, (DHT), improved synthetic procedure, prostate cancer (PC), steroids
1. Introduction
17β-Hydroxy-5α-androstane-3-one or 5α-dihydrotestosterone (5α-DHT, hereafter referred to as DHT) (1) and 17-β-hydroxy-5β-androstane-3-one (5β-DHT) (2) are the prominent metabolites of testosterone (T) (Chart 1) [1, 2]. DHT is important for in utero differentiation and growth of the prostate gland, male sex organ and pubertal growth of facial and body hair. It also plays an important role in several human diseases, including acne, hirsutism, male pattern baldness, benign prostate hyperplasia (BHP), and prostate cancer (PC) [3]. DHT has 2–5 times higher binding affinity for the androgen receptor (AR) and 10-fold higher potency of inducing AR signaling than T [4]. Because of its potent androgenic property it was misused in sports by athletes to benefit from its anabolic and psychotropic effects before it was banned by the International Olympic Committee and National and International Sports Federations [5]. DHT and its derivatives are also used in analytical chemistry as authentic standards for its determination, its metabolites and other related androgenic compounds in blood and urine samples to control its abuse [5, 6]. The compound is also an important starting material in the synthesis of various pharmaceutical agents of biological interest such as anticancer [7–11], androgenic [12, 13], cardiovascular [14], antifungal [15] and antimicrobial [15] agents. Due to its potent AR-binding property, it is also preferred over T in Luciferase assay (AR competitive binding assay) to determine AR binding affinity of new chemical entities in drug discovery and development research [16–18]. Thus, any ready access of this compound would be of great interest and highly desirable for the wide medicinal chemistry and steroid audience. Because of our continued interest in the discovery and development of novel steroidal and non-steroidal anti-PC agents (AR down regulating agents) [16, 18, 19], we required large amounts of DHT, that provided the impetus to our discovery of a facile, expeditious, and high-yield synthesis of DHT that is the subject of this report.
Chart 1.
Structures of Testosterone, 5α-Dihydrotestosterone and 5β-Dihydrotestosterone
2. Experimental
2.1. General Procedures
Melting points (mp) were determined with a Fischer-Johns melting point apparatus and are uncorrected. IR spectra were recorded neat on a Perkin Elmer spectrum65 FT IR spectrometer. 1H NMR spectra were recorded on a Varian Inova 500 MHz spectrometer using CDCl3 as solvent. Chemical shifts are given in parts per million (ppm), and TMS was used as an internal standard. 13C NMR spectra were recorded in CDCl3 using Bruker 500 MHz spectrometer. High-resolution mass spectra (HRMS) were determined on a Bruker 12Tesla APEX-Qe FTICR-MS by positive ion ESI mode by Ms. Susan A. Hatcher, Facility Director, College of Sci-ences Major Instrumentation Cluster, Old Dominion University, Norfolk, VA. Values of [α]DT were determined with Rudholph Res Analytical: Autopol III automatic polarimeter. Flash Column chromatography (FCC) was performed using silica gel (230–400 mesh, 60 Å), and progress of reactions were monitored by TLC analysis on silica gel plates (Merck F254) (detection by charring reagent of conc. H2SO4 in ethanol 5% w/v). The starting material 3β-hydroxy-5α-androstan-17-one and other reagents were purchased from Sigma Aldrich.
2.2. 5α-Androstan-3β-acetoxy-17-one (14)
To an ice cold solution of commercially available 3β-hydroxy-5α-androstan-17-one (2.5g, 8.6 mmol) in pyridine (15 ml) was added acetic anhydride (2.64g, 25.8 mmol) followed by stirring for 12 h. at rt. The reaction mixture was then poured to a mixture of ice-water (200 mL) and the resulting white precipitate was filtered, washed with water and dried under suction to afford pure compound (2.78g, 97%) of 14: mp 114–115 °C (lit.[20] 111–113 °C); Rf = 0.6 (3.8% EtOH in MDC); IR (neat) 1729, 1240, and 1028; 1H NMR (500 MHz, CDCl3) δ 0.851 (s, 3H, 18-CH3), 0.857 (s, 3H, 19-CH3), 2.02 (s, 3H, 3β-OAc), 4.68 (m, 1H, 3α-H); 13C NMR (500 MHz, CDCl3) δ 221.34 (C-17), 170.81 (COCH3), 73.66 (C-3), 54.49 (C-9), 51.53 (C-14), 47.94 (C-13), 44.82 (C-5), 36.88 (C-1), 36.01 (C-8), 35.81 (C-16), 35.20 (C-10), 34.12 (C-4), 31.70 (C-12), 30.97 (C-7), 28.44 (C-2), 27.58 (C-6), 21.94 (C-15), 21.61 (COCH3), 20.63 (C-11), 13.99 (C-18), 12.38 (C-19); [α]D29 +66.8 [1% in CHCl3]
2.3. 3β-Acetoxy-5α-androstan-17β-ol (15)
To an ice cold solution of 5α-androstan-3β-acetoxy-17-one (14) (2.5g, 7.53 mmol) in methanol (25 mL) was added sodium borohydride (0.23g, 6.07 mmol) over a period of 30 min in three portions. The reaction mixture was stirred for another hour, neutralized with dil. HCl and concentrated under reduced pressure. The residue was stirred with water, filtered, washed with water, dried under suction and recrystallized from methanol to afford white powder (2.48g, 98.7%) of 15: mp 103–104 °C (lit.[20] 106–107 ° C); Rf = 0.51 (4% EtOH in MDC); IR (neat) 3316, 1730, 1237, and 1021; 1H NMR (500 MHz, CDCl3) δ 0.730 (s, 3H, 18-CH3), 0.833 (s, 3H, 19-CH3), 2.01 (s, 3H, 3β-OAc), 3.63 (m, 1H, 17α-H), 4.68 (m, 1H, 3α-H); 13C NMR (500 MHz, CDCl3) δ 170.97 (CO), 82.03 (C-17), 73.99 (C-3), 54.55 (C-9), 51.14 (C-14), 44.90 (C-13), 43.17 (C-5), 36.97 (C-12), 36.90 (C-1), 35.70 (2x, C-8 and C10), 34.18 (C-4), 31.71 (C-7), 30.61 (C-16), 28.63 (C-6), 27.63 (C-2), 23.56 (C-15), 21.64 (COCH3), 20.97 (C-11), 12.42 (C-19), 11.34 (C-18); [α]D29 +2.4 [1% in CHCl3].
2.4. 3β-Acetoxy-5α-androstan-17β-yl Dimethyl-tert-butylsilyl ether (16)
To a dry solution of dry DMF (15 mL) containing 3β-acetoxy-5α-androstan-17β-ol (15) (4.5g, 13.5 mmol) and imidazole (1.37g, 20.25 mmol) was added tert-butyldimethylsilyl chloride (2.03g, 13.5 mmol) and stirred at rt. under argon. Within 20 min, a dense white precipitate observed. The reaction continued for one more hour, followed by dilution with water (150 mL) and then extracted with diethyl ether. The organic phase washed with water, brine and dried with CaCl2. Upon removal of solvent under reduced pressure and air drying resulted in a white solid of 16 (2.9g, 96%): mp 119–121 °C; Rf = 0.77 (2% EtOH in MDC); IR (neat) 1736, 1240, 1195, and 1026; 1H NMR (500 MHz, CDCl3) δ 0.010 and 0.016 (s, 6H, Si(CH3)2), 0.699 (s, 3H, 18-CH3), 0.838 (s, 3H, 19-CH3), 0.886 (s, 9H, tert-C4H9), 2.03 (s, 3H, 3β-OAc), 3.55(m, 1H, 17α-H), 4.68 (m, 1H, 3α-H); 13C NMR (500 MHz, CDCl3) δ 170.89 (CO), 82.01 (C-17), 73.91 (C-3), 54.73 (C-9), 50.82 (C-14), 44.95 (C-13), 43.50 (C-5), 37.35 (C-12), 37.00 (C-1), 35.76 (2x, C-8 and C10), 34.23 (C-4), 31.80 (C-7), 31.12 (C-16), 28.69 (C-6), 27.61 (C-2), 26.06 (3x, CH3)3), 23.72 (C-15), 21.66 (COCH3), 21.04 (C-11), 18.30 (C(CH3)3), 12.44 (C-19), 11.59 (C-18), −4.29 and −4.6 (SiCH3); [α]D29 +4.2 [1% in CHCl3].
2.5. 3β-Hydroxy-5α-androstan-17β-yl Dimethyl-tert-butylsilyl ether (17)
To a solution of 3β-acetoxy-5α-androstan-17β-yl dimethyl-tert-butylsilyl ether (16) (2.5g, 5.58 mmol) in methanol (15 mL) was added 10% methanolic-KOH solution (10 mL) and refluxed for 2 hrs. The reaction mixture was concentrated under reduced pressure and the residue treated with water (150 mL). The resulting precipitate was filtered and washed with water (until the washing was neutral). Solids dried under suction to afford white product of 17 (2.23g, 98%): mp 163–164 °C (lit.[21] 161–163 °C); Rf = 0.3 (2% EtOH in MDC); IR (neat) 3353, 1471, 1248, 1094, 832 and 773; 1H NMR (500 MHz, CDCl3) δ 0.010 and 0.016 (s, 6H, Si(CH3)2), 0.701 (s, 3H, 18-CH3), 0.824 (s, 3H, 19-CH3), 0.887 (s, 9H, tert-C4H9), 3.54(m, 1H, 17α-H), 3.59 (m, 1H, 3α-H); 13C NMR (500 MHz, CDCl3) δ 82.04 (C-17), 71.51 (C-3), 54.87 (C-9), 50.90 (C-14), 45.16 (C-13), 43.52 (C-5), 38.42 (C-4), 37.41 (C-12), 37.26 (C-1), 35.80 (2x, C-8 and C10), 31.90 (C-7), 31.73 (C-16), 31.13 (C-2), 28.83 (C-6), 26.06 (3x, CH3)3), 23.74 (C-15), 21.10 (C-11), 18.31 (C(CH3)3), 12.56, 11.60, −4.29 and −4.60 (SiCH3); [α]D28 +92 [1% in CHCl3].
2.6. 17β-[(tert-Butyldimethylsilyl)oxy]-5α-androstan-3-one (18)
Tetrapropylammonium perruthenate (0.16g, 0.458mmol) was added to a solution of 3β-hydroxy-5α-androstan-17β-yl dimethyl-tert-butylsilyl ether (17) (2g, 4.93 mmol), 4-methylmorpholine N-oxide (1.07g, 9.16 mmol) and molecular sieves (0.3g) in MDC (40 mL). After stirring at rt. for 2 h, the reaction mixture was concentrated under reduced pressure. Flash column chromatography (FCC) over short silica column eluting with 1% ethanol in MDC afford off white solid of 18 (1.86g, 93.4%): mp 131–133 °C; Rf = 0.65 (2% EtOH in MDC); IR (neat) 1717, 1250, 1092, 833 and 771; 1H NMR (500 MHz, CDCl3) δ 0.015 and 0.021 (2s, 6H, Si(CH3)2), 0.731 (s, 3H, 18-CH3), 0.891 (s, 9H, tert-C4H9), 1.02 (s, 3H, 19-CH3), 3.54 (m, 1H, 17α-H); 13C NMR (500 MHz, CDCl3) δ 212.29 (C-3), 81.95 (C-17), 54.30 (C-9), 50.70 (C-14), 47.01 (C-5), 44.92 (C-4), 43.52 (C-13), 38.81 (C-1), 38.39 (C-2), 37.29 (C-12), 35.96 (C-8), 35.70 (C-10), 31.53 (C-7), 31.12 (C-16), 29.06 (C-6), 26.05 (3x, CH3)3), 23.74 (C-15), 21.30 (C-11), 18.30 (C(CH3)3), 11.70 (C-18), 11.60 (C-19), −4.29 and −4.61 (SiCH3); [α]D28 +31.48 [1% in CHCl3].
2.7. 17β-Hydroxy-5α-androstan-3-one (1, DHT)
To a stirred solution of 17β-[(tert-butyldimethylsilyl)oxy]-5α-androstan-3-one (18) (1.75, 4.33 mmol) and ethanol (15 mL) was added 20% ethanolic-HCl (5 ml) and stirred at rt. for 3 h. The reaction mixture was concentrated to ~ 5 mL, diluted with water and neutralized with 10% Na2CO3 solution. The resulting off white solid was filtered, washed with water and dried under suction. The crude product was recrystallized by dissolving in minimum quantity of acetone under heat, concentrated to a paste at rt. and finally stirred while slowly adding excess petroleum ether to afford white solid. Finally, filtration and drying under suction yielded pure 1 (1.23g, 97.5%): mp 181–182 °C (lit.[22] 181–183 °C); Rf = 0.32 (2% EtOH in MDC); IR (neat) 3416, 1704, 14450, and 1028; 1H NMR (500 MHz, CDCl3) δ 0.762 (s, 3H, 18-CH3), 1.02 (s, 3H, 19-CH3), 3.65 (m, 1H, 17α-H); 13C NMR (500 MHz, CDCl3) δ 212.35 (C-3), 82.00 (C-17), 54.12 (C-9), 51.03 (C-14), 46.94 (C-5), 44.87 (C-4), 43.18 (C-13), 38.77 (C-1), 38.34 (C-2), 36.85 (C-12), 35.94 (C-10), 35.64 (C-8), 31.45 (C-7), 30.67 (C-16), 28.99 (C-6), 23.58 (C-15), 21.23 (C-11), 11.69 (C-19), 11.35 (C-18); [α]D29 +32 [1% in CHCl3](lit.[12] +31–33[1% in CHCl3]); HRMS calcd 603.4383 (C19H30O2)2.Na+, found 603.4392. The overall yield from the starting material was 82%.
3. Results and Discussion
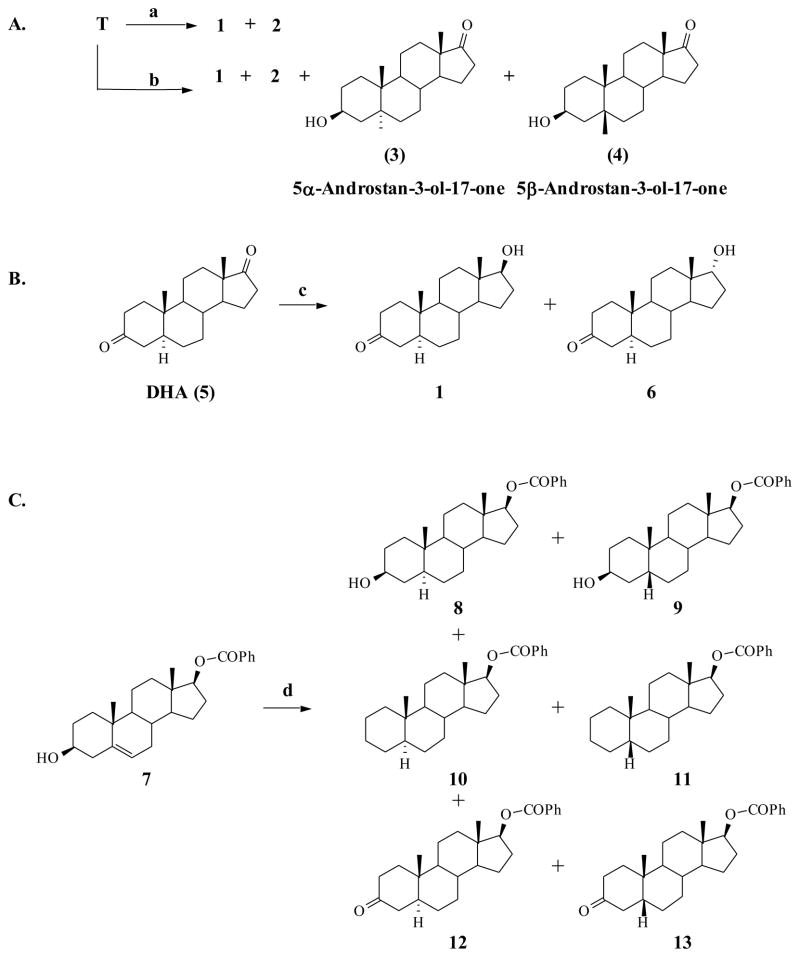
We reviewed numerous synthetic strategies, including a few enzymatic biotransformation reactions [23–25] and several that involve the catalytic hydrogenation of steroidal Δ4-ketone moiety [22, 26–29]. We found that none of these procedures yielded desired pure 5α-hydrogenated product, but instead gave low yields of mixed 5α and 5β hydrogenated products as summarized in Scheme 1A and Table 1: entries 1–4. A procedure that involved reduction of the double bond in an α,β-unsaturated ketone molecule (e.g., T) with lithium-ammonia complex gave the desired 5α-H compound (73%) but was also accompanied by reduction of the 3,17-diketone groups to form considerable quantity of saturated diol (Table 1, entry 5) [27]. Reduction of diketo-steroid (DHA) using LiAlH4 Activated Template Polymers (molecular imprinting technique) was found to selectively reduce the C17-keto group albeit with low stereo-specificity (Scheme 1B) [30]. The procedure involving the catalytic hydrogenation of Δ5-alcohol compound 7 (Scheme 1C) was also not stereospecific [31, 32]. Thus, when androst-5-ene-3β,17β-diol 17-benzoate (7) was hydrogenated in isopropyl alcohol over Pd/C in an autoclave, reduction of the double bond was accompanied by hydrogenolysis/oxidation of the 3-hydroxy group (Scheme 1C), resulting in at least 6 products [12].
Scheme 1.
Reported methods for the synthesis of 5α-DHT.[22, 26, 28, 29, 31, 32]
a) Table 1 entry 1–3;[22, 28, 29] b) Table 1 entry 4;[26] c) LiAlH4 activated template polymer;[30] d) Pd/C, IPA, Autoclave[31, 32]
Table 1.
Reported Catalysts and reagents used in the synthesis of 5α-DHT from testosterone
| Entry | Reagent | catalyst | % conversion | Products % | Ref | |||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||
| 1 | (EtO)3SiH | Rh/DIOP | 14 | 36 | 64 | - | - | (27) |
| 2 | Na2H2PO2 | 10% Pd/C | 60 | 24 | - | - | (23) | |
| 3 | Sodium Octacarbonyl hydridodiferrate | 10 | NR | NR | - | - | (26) | |
| 4 | H2 | Cu/Al2O3 | 60 | 17% (14:86) | 43% (23:77) | (24) | ||
| 5 | Lithium-NH3 complex | 73% | Saturated 3,17-diol | (25) | ||||
T – Testosterone, NR- not reported
We wanted to develop a more efficient procedure for the synthesis of 5α-DHT by minimizing the number of steps, minimizing the chromatographic purification steps, and employing mild reagents/reaction conditions. Indeed, we have successfully devised a simple high yield synthetic route (Scheme 2) for the 5α-DHT, starting from commercially available 3β-hydroxy-5α-androstan-17-one. The selection of this starting material avoids the difficulties of formation of 5α-H steroidal scaffold which is usually obtained from either Δ4 or Δ5 steroids as discussed above. Menshova et al. previously reported the synthesis of 5α-DHT using this material with 73% overall yield [12]. This reported synthetic route begins with 17-O-benzoylation followed by deprotection of 3-acetoxy group in alkaline condition which results in contamination with debenzoylated and unreacted materials. The method also requires the use of corrosive and carcinogenic chromate containing oxidizing agent (Jones reagent) for the introduction of 3-keto group. Our synthetic route addresses these two issues by modifying the 17-hydroxy protecting group and using a mild oxidizing agent. Synthesis began with protection of the hydroxyl group of 3β-hydroxy-5α-androstan-17-one using acetic anhydride in pyridine with excellent yield (14). Reduction of 17-keto (14) with reported NaBH4 in methanol method, lead to the preferential formation of the thermodynamically more stable equatorial alcohol (17β-hydroxyl) 15 [12, 20]. Formation of 17β-hydroxyl was confirmed by the appearance of C-18 peak at δ 11.34 (absence of C-18 peak at δ 17.05 for 17α-hydroxyl derivative) in 13C NMR and appearance of 17α-H peak at δ 3.63 (absence proton peak at δ 3.74 for 17β-H) in 1HNMR spectroscopic data [24]. The 17β-hydroxyl group of 15 was protected as silyl ether (16) by reacting with TBDMSCl, in DMF using imidazole as proton abstractor. Unlike reported 17-benzoyl derivative [12], silyl ether function is stable under alkaline reaction conditions during deprotection of 3-acetoxy group. This obviates the observed difficulties of controlling 17-debenzoylation which is sensitive to amount of alkali, purity of substrate, and the temperature of the reaction which is reported to produce 5α-androstane-diol (0.5%) and unreacted substrate [12]. We observed that in the presence of 17β-silyl ether, the acetyl group of 16 was smoothly hydrolyzed (10 % ethanolic-KOH solution) to obtain the corresponding 3β-OH product 17. We have replaced harsh Jones reagent with NMO/TPAP method for oxidation of 3-hydroxy of 17 to 3-keto (18) which is environment friendly, non-hazardous and provided higher yield of the desired product than previously reported. Finally, compound 18 was desilylated using 20% ethanolic-HCl to accomplish synthesis of DHT (1). Final product and intermediates were fully characterized by physical, spectral methods and are in agreement with literature data. The overall yield from the readily available 3β-hydroxy-5α-androstan-17-one through six highly efficient steps was 82% and was reproducible (repeated two times with standard deviation of ~2%). The advantage of our process is its mild reaction conditions, easy workup, no hazardous waste, does not require chromatographic purification in most of steps and it gives high yield than any previously reported procedures.
Scheme 2.
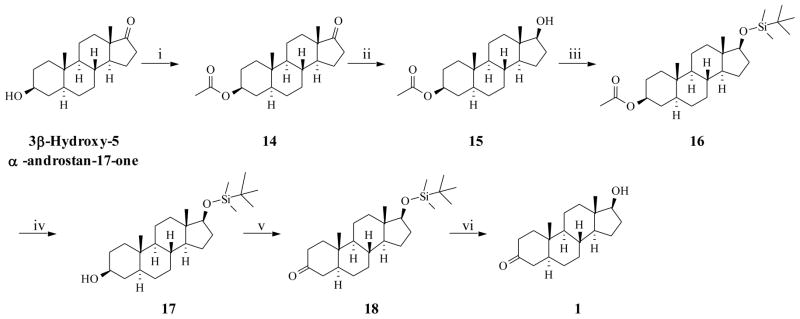
New synthetic route of 5α-DHT
i) Pyridine, (CH3CO)2O, 0 °C, rt, 12 hr; ii) MeOH, NABH4, 0 °C, 1.5 hr iii) DMF, Imidazole, TBDMSCl, rt, Ar, 1.5 hr; iv) MeOH, 10% MeOH-KOH, reflux, 2 hr; v) MDC, NMO, TPAP, mol sieves, rt, 2 hr; vi) EtOH, 20% EtOH-HCl, rt, 3 hr.
Research Highlights.
Discovered unprecedented synthesis of 5α-dihydroandrosterone (DHT) in excellent yield.
Novel procedure will provide easy access to DHT and important starting material for synthesis of novel drug-like compounds.
Synthetic procedure may be suitable for large-scale industrial production.
Abbreviations
- AR
Androgen receptor
- BPH
benign prostate hyperplasia
- DHT
5α-dihydrotestosterone
- NMO
4-methylmorpholine N-oxide
- PC
prostate cancer
- T
testosterone
- TPA
tetrapropylammonium perruthenate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–74. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 2.Russell DW, Wilson JD. Steroid 5 alpha-reductase: two genes/two enzymes. Annu Rev Biochem. 1994;63:25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- 3.Cilotti A, Danza G, Serio M. Clinical application of 5alpha-reductase inhibitors. J Endocrinol Invest. 2001;24:199–203. doi: 10.1007/BF03343844. [DOI] [PubMed] [Google Scholar]
- 4.Saartok T, Dahlberg E, Gustafsson JA. Relative binding affinity of anabolic-androgenic steroids: comparison of the binding to the androgen receptors in skeletal muscle and in prostate, as well as to sex hormone-binding globulin. Endocrinology. 1984;114:2100–6. doi: 10.1210/endo-114-6-2100. [DOI] [PubMed] [Google Scholar]
- 5.Donike M, Ueki M, Kuroda Y, Geyer H, Nolteernsting E, Rauth S, et al. Detection of dihydrotestosterone (DHT) doping: alterations in the steroid profile and reference ranges for DHT and its 5 alpha-metabolites. J Sports Med Phys Fitness. 1995;35:235–50. [PubMed] [Google Scholar]
- 6.Salvador JP, Sanchez-Baeza F, Marco MP. A high-throughput screening (HTS) immunochemical method for the analysis of stanozolol metabolites in cattle urine samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:243–52. doi: 10.1016/j.jchromb.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 7.Itoh Y, Kitaguchi R, Ishikawa M, Naito M, Hashimoto Y. Design, synthesis and biological evaluation of nuclear receptor-degradation inducers. Bioorg Med Chem. 2011;19:6768–78. doi: 10.1016/j.bmc.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 8.Roy J, Breton R, Martel C, Labrie F, Poirier D. Chemical synthesis and biological activities of 16α-derivatives of 5α-androstane-3α,17β-diol as antiandrogens. Bioorg Med Chem. 2007;15:3003–18. doi: 10.1016/j.bmc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Roy J, DeRoy P, Poirier D. 2β-(N-substituted piperazino)-5α-androstane-3α,17β-diols: parallel solid-phase synthesis and antiproliferative activity on human leukemia HL-60 cells. J Comb Chem. 2007;9:347–58. doi: 10.1021/cc060098z. [DOI] [PubMed] [Google Scholar]
- 10.Schobert R, Seibt S, Effenberger-Neidnicht K, Underhill C, Biersack B, Hammond GL. (Arene)Cl(2)Ru(II) complexes with N-coordinated estrogen and androgen isonicotinates: interaction with sex hormone binding globulin and anticancer activity. Steroids. 2011;76:393–9. doi: 10.1016/j.steroids.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Thibeault D, Roy J, DeRoy P, Poirier D. Chemical synthesis of 2beta-amino-5alpha-androstane-3alpha,17beta-diol N-derivatives and their antiproliferative effect on HL-60 human leukemia cells. Bioorg Med Chem. 2008;16:5062–77. doi: 10.1016/j.bmc.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 12.Men’shova NI, Grinenko GS, Korzinkina NA, PFE Synthesis of 5α-dihydrotestosterone from an intermediate in tigogenin degradation. Pharm Chem J. 1977;11:1533–5. [Google Scholar]
- 13.Ralf W, Sven R, Peter D, Alexander H, Walter E, Birgitt S, et al. Steroid prodrugs with androgenic action. 2005. [Google Scholar]
- 14.Alberto C, Giuseppe B, Giorgio F, Patrizia F, Mauro G. Aminooxime derivatives of 2- AND/OR 4-substituted androstanes and androstenes as medicaments for cardiovascular disorders. 2009. [Google Scholar]
- 15.Mohamed NR, Elmegeed GA, Younis M. Studies on organophosphorus compounds VII1,2: Transformation of steroidal ketones with Lawesson’s reagent into thioxo and heterofused steroids. results of antimicrobial and antifungal activity. Phosphorus, Sulfur Silicon Relat Elem. 2003;178:2017–03. [Google Scholar]
- 16.Bruno RD, Vasaitis TS, Gediya LK, Purushottamachar P, Godbole AM, Ates-Alagoz Z, et al. Synthesis and biological evaluations of putative metabolically stable analogs of VN/124-1 (TOK-001): head to head anti-tumor efficacy evaluation of VN/124-1 (TOK-001) and abiraterone in LAPC-4 human prostate cancer xenograft model. Steroids. 2011;76:1268–79. doi: 10.1016/j.steroids.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartig PC, Bobseine KL, Britt BH, Cardon MC, Lambright CR, Wilson VS, et al. Development of two androgen receptor assays using adenoviral transduction of MMTV-luc reporter and/or hAR for endocrine screening. Toxicol Sci. 2002;66:82–90. doi: 10.1093/toxsci/66.1.82. [DOI] [PubMed] [Google Scholar]
- 18.Purushottamachar P, Khandelwal A, Vasaitis TS, Bruno RD, Gediya LK, Njar VC. Potent anti-prostate cancer agents derived from a novel androgen receptor down-regulating agent. Bioorg Med Chem. 2008;16:3519–29. doi: 10.1016/j.bmc.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 19.Purushottamachar P, Khandelwal A, Chopra P, Maheshwari N, Gediya LK, Vasaitis TS, et al. First pharmacophore-based identification of androgen receptor down-regulating agents: discovery of potent anti-prostate cancer agents. Bioorg Med Chem. 2007;15:3413–21. doi: 10.1016/j.bmc.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merlani MI, Amiranashvili LS, Men’shova NI, Kemertelidze EP. Synthesis of 5 α-androstan-3β,17β-diol from tigogenin. Chem Nat Compd. 2007;43:97–9. [Google Scholar]
- 21.Hosoda H, Yamashita K, Sagae H, Nambara T. Studies on dimethyl-tert-butylsilyl ethers of steroids. Chem Pharm Bull. 1975;23:2118–22. [Google Scholar]
- 22.Spyriounis DM, Ikonomidis G, Demopoulos VJ. A convenient “hydrogen transfer” hydrogenation of testosterone. Org Prep Proced Int. 1989;21:515–7. [Google Scholar]
- 23.Cabeza MS, Gutierrez EB, Garcia GA, Avalos AH, Hernandez MA. Microbial transformations of testosterone to 5alpha-dihydrotestosterone by two species of Penicillium: P. chrysogenum and P. crustosum. Steroids. 1999;64:379–84. doi: 10.1016/s0039-128x(98)00115-9. [DOI] [PubMed] [Google Scholar]
- 24.Parr MK, Zapp J, Becker M, Opfermann G, Bartz U, Schanzer W. Steroidal isomers with uniform mass spectra of their per-TMS derivatives: synthesis of 17-hydroxyandrostan-3-ones, androst-1-, and -4-ene-3,17-diols. Steroids. 2007;72:545–51. doi: 10.1016/j.steroids.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Peart PC, McCook KP, Russell FA, Reynolds WF, Reese PB. Hydroxylation of steroids by Fusarium oxysporum, Exophiala jeanselmei and Ceratocystis paradoxa. Steroids. 2011;76:1317–30. doi: 10.1016/j.steroids.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Ravasio N, Gargano M. Selective hydrogenation promoted by copper catalyst. 2. Hydrogen-transfer reactions leading to stereoselective hydrogination of Δ5-3β-sterols to 5β-derivatives1. J Org Chem. 1993;58:1259–61. [Google Scholar]
- 27.Mel’nikova VI, Pivnitskii KK. Selective reduction of unsaturated ketones by a lithium-ammonia complex. Zh Org Khim. 1972;8:2090–8. [Google Scholar]
- 28.Hossain MM, Saha AK. Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons, Ltd; 2001. Sodium Octacarbonylhydridodiferrate. [Google Scholar]
- 29.Göndös G, McGirr LG, Jablonski CR, Snedden W, Orr JC. The reduction of steroid 2α– fluoro 4-en-3-ones. J Org Chem. 1988;53:3057–9. [Google Scholar]
- 30.Byström SE, Börje A, Akermark B. Selective reduction of steroid 3- and 17-ketones using LiAlH4 activated template polymer. J Am Chem Soc. 1993;115:2081–3. [Google Scholar]
- 31.Volovel’skii LN, Kogan LM, Yakovleva MY, Popova NV. Δ5-Androstene-3β,17β-diol 17-benzoate hydrogenation products. Khim Farm Zh. 1971;5:12–4. [Google Scholar]
- 32.Volovel’skii LN, Knorozova GV. Synthesis of alkyl derivatives of the androstane series, 2hydroxymethylenedihydrotestosterone and 2α-methyldihydrotestosterone. Zh Prikl Khim. 1962;35:2580–2. [Google Scholar]