Abstract
Objective:
Brain damage within the right middle cerebral artery (MCA) territory is particularly disruptive to mediolateral postural stabilization. The objective of this cross-sectional study was to test the hypothesis that chronic right MCA infarcts (as compared to left) are associated with slower and more bilaterally asymmetrical gait. We further hypothesized that in those with chronic right MCA infarct, locomotor performance is more dependent on gray matter (GM) volumes within noninfarcted regions of the brain that are involved in motor control yet lie outside of the MCA territory.
Methods:
Gait speed was assessed in 19 subjects with right MCA infarct, 20 with left MCA infarct, and 108 controls. Bilateral plantar pressure and temporal symmetry ratios were calculated in a subset of the cohort. GM volumes within 5 regions outside of the MCA territory (superior parietal lobe, precuneus, caudate, putamen, and cerebellum) were quantified from anatomic MRIs.
Results:
Right and left infarct groups had similar poststroke duration (7.6 ± 6.0 years), infarct size, and functional independence. The right infarct group demonstrated slower gait speed and greater asymmetry compared to the left infarct group and controls (p < 0.05). In the right infarct group only, those with larger GM volumes within the cerebellum (r2 = 0.32, p = 0.02) and caudate (r2 = 0.56, p < 0.001) exhibited faster gait speed.
Conclusion:
Individuals with chronic lesions within the right MCA territory, as compared to the left MCA territory, exhibit slower, more asymmetrical gait. For these individuals, larger GM volumes within regions outside of the infarcted vascular territory may help preserve locomotor control.
Although approximately two-thirds of stroke survivors regain the ability to walk,1 many present with diminished locomotor performance characterized by slow walking speed and bilaterally asymmetrical walking patterns.2,3 Lesions within the right hemisphere of the brain, as compared to the left, appear to be more disruptive to both gait and postural control.4–9 More specifically, lesions within the right middle cerebral artery (MCA) territory are particularly disturbing to both the sense of postural verticality and the ability to stabilize the body in the frontal plane.8,10–13 Recent studies, however, have also indicated that the complex motor control system can compensate for impairment to one or more elements of the system by placing increased reliance on remaining intact elements.14 As such, the extent of residual gait abnormality following an infarct may be dependent on not only infarct hemisphere but also on the integrity of noninfarcted brain regions.
The objectives of the study were to investigate the effects of infarct hemisphere, as well as gray matter (GM) volumes within regions outside of the infarcted vascular territory, on locomotor performance in individuals with chronic MCA infarcts. We hypothesized that individuals with right MCA infarct would exhibit worse locomotor performance, characterized by slower gait speed and greater bilateral asymmetry, compared to those with left MCA infarct and controls. We further hypothesized that in only those individuals with right MCA infarct, locomotor performance would be more dependent on specific brain regions outside of the MCA territory with known involvement in motor control.
METHODS
Participants.
We tested our hypotheses by completing a retrospective analysis of data collected from 2005 to 2012. Community-dwelling men and women aged 50–85 years were recruited via advertisement, the stroke registry, and records review at the Beth Israel Deaconess Medical Center. Individuals with stroke were at least 6 months postinfarct and had documented chronic large-vessel hemispheric infarcts affecting less than one-third of the MCA territory,15 as confirmed by examination of radiologic MRI or CT images. The control group consisted of individuals recruited from the community to match the age and sex characteristics of the stroke group.
Exclusion criteria were intracranial or subarachnoid hemorrhage on MRI or CT, bilateral infarction, any unstable medical condition, vertebrobasilar or carotid disease (not associated with stroke), diabetes mellitus, valvular heart disease or clinically significant arrhythmia, inability to walk unassisted, and significant functional impairment as evidenced by a total NIH Stroke Scale (NIHSS) score >20. Additional MRI exclusion criteria included morbid obesity (body mass index >35) or any metallic bioimplants or claustrophobia.
Standard protocol approvals, registrations, and patient consents.
The study protocol was approved by the Committee on Clinical Investigations at the Beth Israel Deaconess Medical Center. All subjects provided written informed consent prior to participation.
Study protocol.
Studies were conducted in the Syncope and Falls in the Elderly Laboratory, the Center for Advanced MRI, and the Clinical Research Center (CRC) at the Beth Israel Deaconess Medical Center. An in-person screening visit was first completed to assess medical history and medication usage, vital signs, resting ECG, and anthropometrics. The NIHSS and modified Rankin Scale (mRS) were also administered to quantify the severity of neurologic and functional stroke outcomes. Eligible subjects were then admitted to the CRC and completed a battery of assessments including a walk test and brain MRI.
Walk test.
All subjects completed a 12-minute walk along a 75-m course on an 80 × 4–m indoor hallway at their preferred speed. To minimize potential effects associated with turning and fatigue, we limited the analysis to the first 75 m of the walk. Instrumented shoe insoles that do not interfere with walking (Pedar-X system, Novel, Munich, Germany) were used to record plantar pressures at 50 Hz.16 Each insole was 2.5 mm thick and contained a matrix of 99 capacitive pressure sensors with a spatial resolution of 1.6–2.2 cm.
MRI studies.
Brain imaging was completed on a 3T GE Signa Vhi scanner with a quadrature and phase array head coil (GE Medical Systems, Milwaukee, WI). To examine GM volumes, high-resolution anatomic images were acquired using a 3D magnetization-prepared rapid gradient echo (MPRAGE) sequence: repetition time (TR)/echo time (TE)/inversion time (TI) = 7.8/3.1/600 ms, 3.0 mm slice thickness, 52 slices, bandwidth = 122 Hz per pixel, flip angle = 10°, 24 × 24 cm field of view (FOV), 256 × 192 matrix size. Fluid-attenuated inversion recovery (FLAIR) sequences were also acquired and used to examine infarct characteristics. Parameters were as follows: TR/TE/TI = 11,000/161/2,250 ms, 5 mm slice thickness, 30 slices, bandwidth = 122 Hz per pixel, flip angle = 90°, 24 × 24 cm FOV, 256 ×160 matrix size. Image data were saved offline on a CD-RW attached to the scanner.
Data analysis.
Walk analysis.
The primary outcome related to locomotor performance was preferred gait speed, which was calculated from the time taken to walk the first 75 m of the 12-minute walk. Secondary outcomes were selected to provide insight into the bilateral symmetry of walking patterns and included the plantar pressure and temporal symmetry ratio.17 These variables were calculated from foot pressure data obtained from the first 50 steps of the trial. For this analysis, data from 46 controls, 12 subjects with left MCA infarct, and 9 subjects with right MCA infarct were included, as technological issues negatively affected data quality in the other subjects. The demographics, stroke characteristics, gait speed, and regional GM volumes of each subgroup were representative of the larger groups.
The plantar pressure symmetry ratio was calculated by (1) determining the maximum plantar pressure experienced by each foot during each step, (2) calculating the average maximum pressure for each foot normalized to body weight (% body weight), and (3) dividing the average maximum pressure of the affected lower limb by that of the unaffected lower limb.18,19 A symmetry ratio less than 1.0 thus indicates smaller values associated with the affected as compared to the unaffected lower limb.
In order to calculate the temporal symmetry ratio, plantar pressure data were first used to quantify single support time of each foot (seconds). This variable was defined as the percentage of time during each stride when only the right or left foot was in contact with the ground. The temporal symmetry ratio was then calculated by dividing single support time of the affected lower limb by that of the unaffected lower limb.
MRI analysis.
Imaging data were analyzed on a Linux workstation using interactive data language tools (Research Systems, Boulder, CO). Stroke volumes were quantified by outlining abnormalities on T2-weighted FLAIR images. Normalized stroke volumes were divided by intracranial cavity volume and then multiplied by 100.
Regional GM tissue volumes were calculated from MPRAGE images using an inherently circular model with spatial normalization within the statistical parametric mapping software package (SPM, University College London, UK). GM volumes were segmented and quantified into anatomic regions of interest using the LONI Probabilistic Brain Atlas. Here, we limited our analysis to regions that are involved in motor control, yet located outside of the MCA territory; namely, the superior parietal lobe (visuospatial attention), precuneus (motor imagery), caudate (balance and muscle coordination), putamen (balance and muscle coordination), and cerebellum (balance and muscle coordination).13,14 Normalized GM volumes in each region were combined bilaterally, divided by intracranial cavity volume, and multiplied by 100.
Statistics.
All analyses were performed using JMP software (SAS Institute, Cary, NC). For the purpose of this study, we separated subjects with MCA infarct into 2 groups based on lesion hemisphere.
Descriptive statistics were generated for all variables. Categorical variables were shown as numbers (percentages). Continuous variables were presented as mean ± SD or median values (interquartile range) as appropriate. Potential group differences in demographic and stroke characteristics were tested with the χ2 test for nominal variables, Mann-Whitney U test for ordinal variables, and Student t tests or one-way analysis of variance (ANOVA) for continuous variables as appropriate. Tukey post hoc analyses were used to examine means of significant models.
To test the first hypothesis that individuals with right MCA infarct would exhibit worse locomotor performance as compared to those with left MCA infarct and controls, one-way ANOVAs were used to examine the effects of group on walking outcomes; i.e., preferred gait speed and the plantar pressure and temporal symmetry ratio. Tukey post hoc analyses were used to examine differences in group means within significant models. The potential effects of covariates related to age, body mass index, and sex were also explored.
To test the second hypothesis that the locomotor performance would be more dependent on GM volumes within specific brain regions located outside of the MCA territory (and thus not affected by the infarct) in the right MCA infarct group as compared to the other 2 groups, mixed models were used to examine the effects of group on the relationships between gait speed and regional GM tissue volume. Each regional volume was analyzed in a separate model. The potential effects of covariates related to age, body mass index, and sex were also explored. We did not examine either symmetry ratio within this analysis due to the relatively small sample size with available data.
A significance level of α = 0.05 was used for all analyses except mixed models. As 5 brain regions were examined, the significant level was adjusted with a Bonferroni correction (α = 0.01) to reduce the possibility of statistical error.
RESULTS
Group characteristics.
Twenty subjects with left MCA infarct, 19 subjects with right MCA infarct, and 108 controls were examined. Compared to controls, both right and left MCA infarct groups had greater body mass (p = 0.022) and body mass index (p = 0.015) and were more likely to have hypertension (p < 0.001) (table 1). Other demographic characteristics were similar between groups. Leukoaraiosis burden was relatively low20 and the global volume of white matter hyperintensities was similar between groups.
Table 1.
Group demographics, stroke characteristics, and walking outcomes
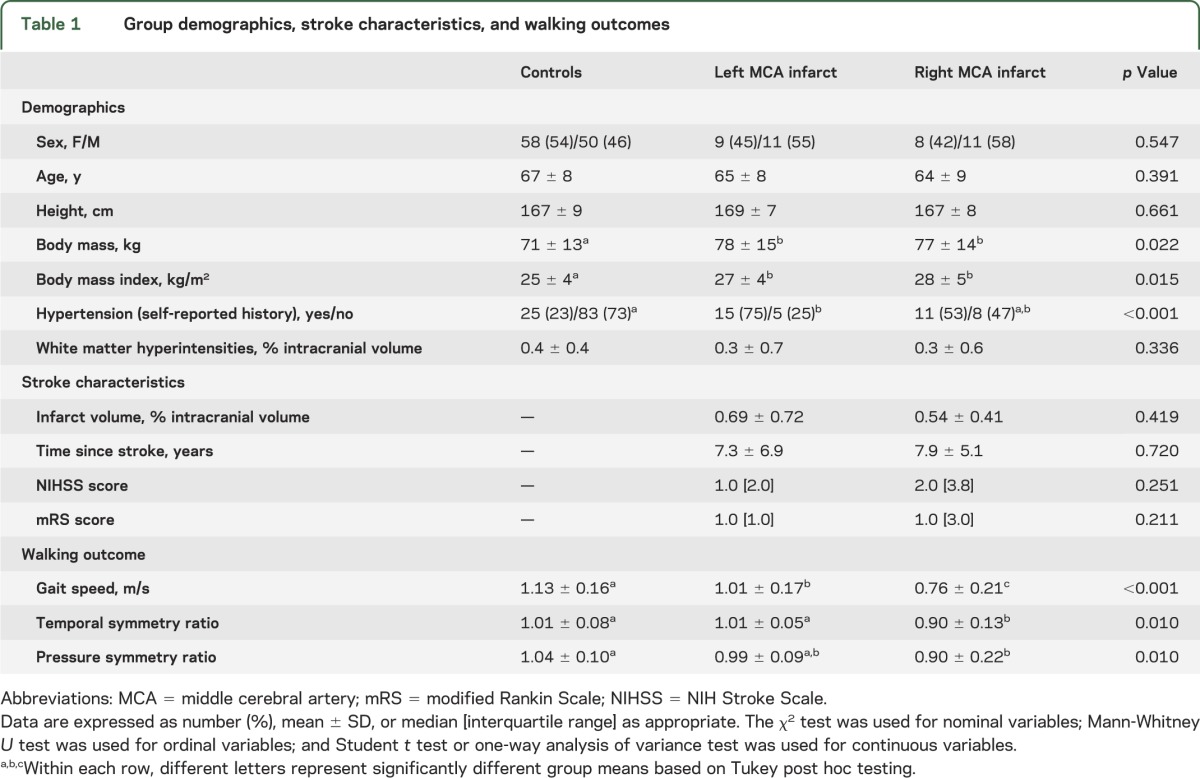
Right and left MCA infarct groups had similar infarct volumes, duration of time since infarct, level of stroke symptoms (NIHSS score 0–10), and level of functional independence (mRS score 0–3). The level of disability in these participants ranged from no symptoms to moderate disability, and all were able to walk without assistance.
Effect of infarct hemisphere on walking outcomes.
Subjects with right MCA infarct walked slower than subjects with left MCA infarct, and both infarct groups walked slower than controls (p < 0.001) (table 1).
Forty-six controls, 12 subjects with left MCA infarct, and 9 subjects with right MCA infarct had available right and left foot pressure data recorded during the walk. Figure 1, A–C, illustrates the average maximum pressures experienced beneath the feet of a representative subject from each group. Both the pressure symmetry ratio and the temporal symmetry ratio were lower in the right MCA infarct group compared to the other 2 groups, which did not differ from one another (p < 0.02, table 1). In other words, in those with right MCA infarct, the average maximum plantar pressure experienced by the affected lower limb was less than that of the unaffected lower limb. Similarly, the time spent with the affected lower limb in single support was less than that of the unaffected lower limb. In controls and those with left MCA infarct, the pressure symmetry ratio and the temporal symmetry ratio were not significantly different from zero, indicating that the average maximum plantar pressures and single support times experienced by each lower limb were bilaterally symmetrical.
Figure 1. Effects of middle cerebral artery infarct on plantar pressure symmetry when walking.
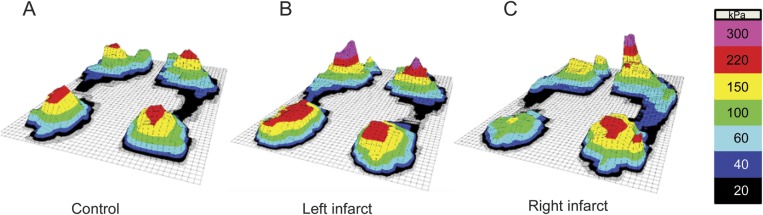
Plantar pressures of a representative control (A) and subjects with left (B) and right (C) middle cerebral artery (MCA) infarct. Values within each sensor region reflect the average maximum pressure (kPa) achieved over 50 consecutive steps. In the control group and left MCA infarct group, the average maximum pressure experienced during the stance phase of walking was bilaterally symmetrical. In those with right MCA infarct, however, the average maximum pressure experienced by the affected lower limb was lower than that of the unaffected lower limb.
GM volumes within noninfarcted brain regions.
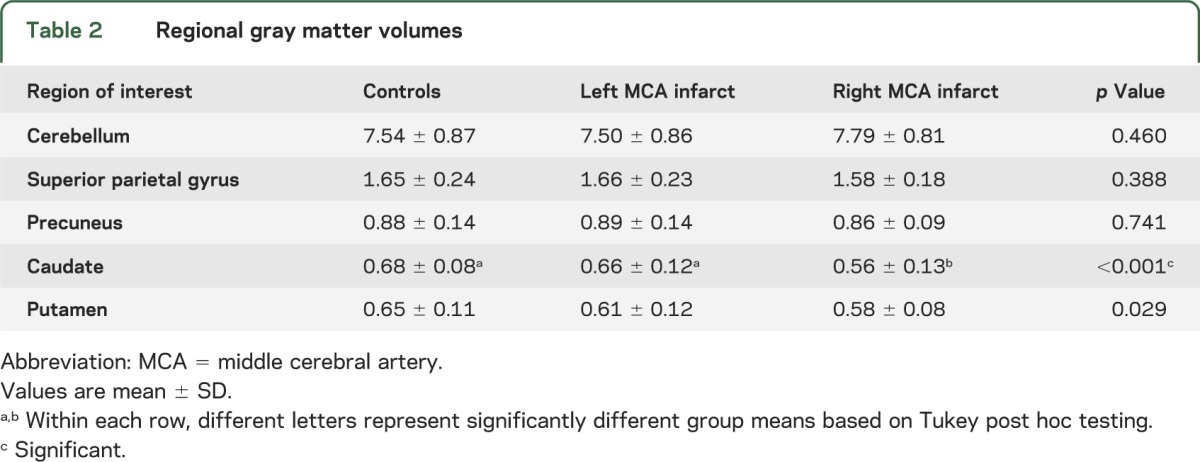
Subjects with right MCA infarct had less GM volume within the caudate compared to subjects with left MCA infarct (p = 0.003) and controls (p < 0.001) (table 2). Regional GM volumes within the cerebellum, superior parietal gyrus, precuneus, and putamen did not differ among the 3 groups. These results were independent of covariance-associated age, body mass index, and sex.
Table 2.
Regional gray matter volumes
Relationship between gait speed and regional GM volumes.
The relationship between gait speed and GM volume within noninfarcted brain regions was dependent on group, specifically with respect to the cerebellum (F = 5.01, p = 0.008) and caudate (F = 5.67, p = 0.004) (figure 2). These relationships were independent of covariance-associated age, body mass index, and sex. In patients with right MCA infarct, those with greater GM volume within the cerebellum (R = 0.57, p = 0.02) or caudate (R = 0.75, p < 0.001) walked faster. In patients with left MCA infarct and controls, however, gait speed was not correlated with GM volumes within these regions. No significant relationships or interactions were observed between gait speed and regional GM volume within the superior parietal gyrus, precuneus, or putamen.
Figure 2. Relationships between gait speed and gray matter tissue volume within the caudate and cerebellum.
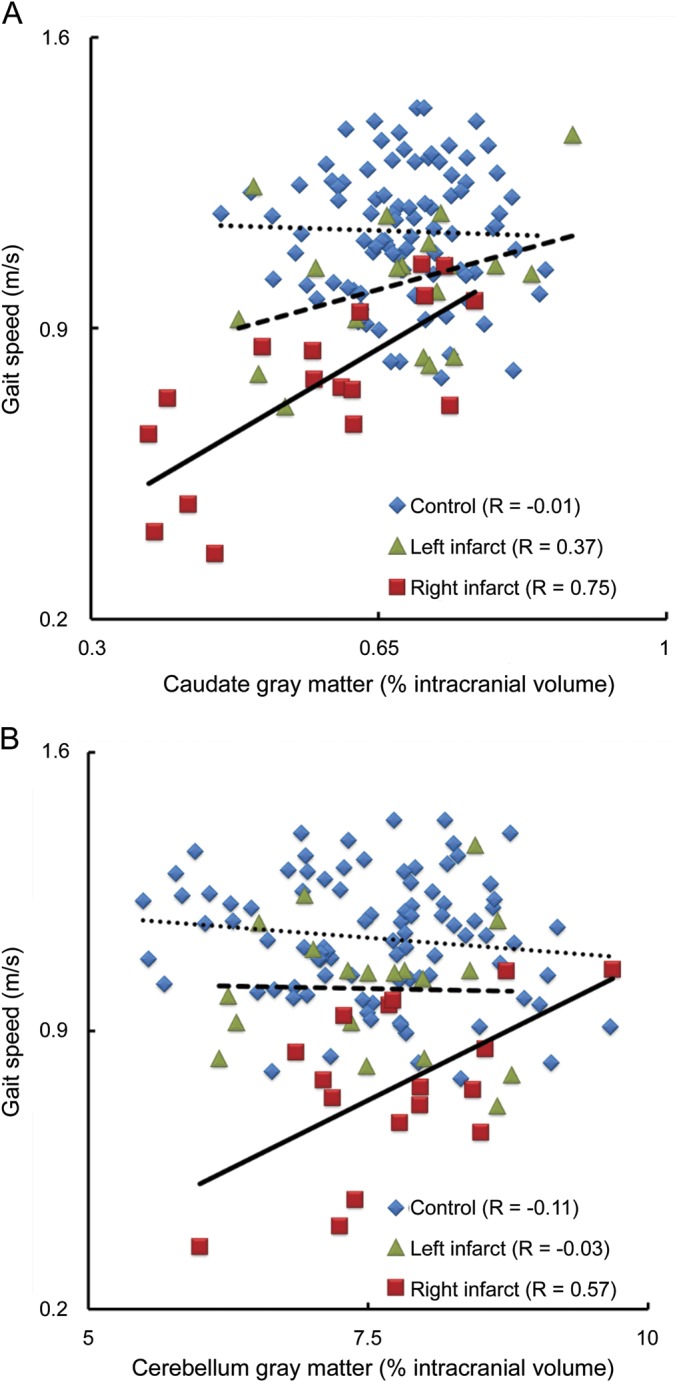
Within the right middle cerebral artery (MCA) infarct group, subjects with larger gray matter volumes within the caudate (A) or cerebellum (B) had faster preferred walking speeds. This relationship was independent of age, sex, and body mass index. In controls and those with left MCA infarct, gait speed did not correlate with gray matter volume within either region.
DISCUSSION
In this cross-sectional study, subjects with chronic right MCA infarct demonstrated slower gait speed and more bilateral asymmetry in the amount of plantar pressure and single limb support time compared to those with left MCA infarct and controls. In the right MCA infarct group only, gait speed was also correlated with the amount of GM tissue volume within the cerebellum and caudate—2 noninfarcted brain regions located outside of the MCA territory with known contribution to motor control. These results suggest that chronic damage to the right MCA territory is particularly disruptive to locomotor performance. In the presence of chronic damage within this region, however, the control of walking may become more dependent on specific brain regions distant to the infarct site.
In addition to walking more slowly, individuals with right MCA infarctions walked with greater frontal plane asymmetry. These patients walked with smaller plantar pressures and shorter periods of single leg support on the affected lower limb relative to the unaffected lower limb. The observed asymmetry between each lower limb during walking in the presence of chronic right-hemisphere lesion may stem in part from the inability to control the center of pressure over the affected lower limb.21 This notion is supported by previous research indicating that compared to lesions within the left MCA territory, those within the right MCA territory—and particularly to regions such as the insula and temporoparietal junction—often result in more severe distortion of spatial postural representation, as well as the visual and nonvisual subjective perception of the body's verticality.22–24 Previous work by our group has also suggested that right hemisphere infarcts are more disruptive to the ability to regulate postural sway when standing, but only in the frontal plane.14,23–25 Future research is therefore warranted to study the effects of MCA infarct on the relationship between residual impairments in the subjective perception of one's posture and gait symmetry in this vulnerable population.
Human locomotion is controlled by a complex system comprising numerous peripheral, spinal, and supraspinal elements.26–29 This system also possesses the capacity to adapt to chronic impairments to one or more of its elements.14,30 Within the right MCA infarct group only, we observed a strong positive correlation between gait speed and GM volumes within the caudate and cerebellum—2 brain regions located outside of the MCA territory. This observation suggests that the “brain reserve” hypothesis may also apply to the locomotor control system. Brain reserve has been defined as the capacity to tolerate age- or disease-related changes within the brain without developing clinical signs or symptoms.31 When applied to the current results, this notion suggests that individuals may be better able to tolerate or compensate for damage to critical brain structures within the right MCA territory, provided that they have relatively larger GM volumes in areas distant to the infarct site.
Both the caudate and cerebellum are involved in numerous aspects of motor control that are important for locomotion. The caudate is located within the cortico-basal ganglia-thalamocortical circuit and is involved in movement accuracy and motor planning.32,33 Increased brain activity within this region during active ankle movements has been linked to better walking performance in stroke subjects.34 The cerebellum is critical for intralimb and interlimb coordination of cyclic movements, along with the dynamic regulation of balance.35,36 Atrophy of this region has been linked to poor walking outcomes in community-dwelling older adults.37 In addition, both the cerebellum and the basal ganglia are part of a perceptual timing network within the brain.38 Larger GM tissue volumes within these 2 regions may therefore be reflected in better temporospatial control of lower-extremity movements, particularly in those with chronic lesions due to right MCA infarcts. Since a number of rehabilitation strategies are pursuing top-down approaches, such as the use of noninvasive brain stimulation to modulate brain activity following stroke,39 our results suggest that such therapies may enhance gait recovery poststroke by first considering the different effects of right and left hemisphere brain damage, and subsequently targeting the potential compensatory processes that may emerge from noninfarcted elements involved in the control of locomotion.
This study has several limitations. Only patients with unilateral infarcts affecting less than one-third of the MCA territory were studied. Our results may therefore not generalize to individuals with larger lesions, or lesions within other vascular territories. Due to technical issues, we were only able to include plantar pressure data from a relatively small subgroup of the entire cohort. Caution should therefore be taken when generalizing related results and conclusions. Finally, we only examined relationships between walking outcomes and volumetric properties of the brain. Future research should thus utilize a combination of anatomical and functional neuroimaging techniques throughout the recovery process to determine whether infarcts result in chronically altered brain activity or neural pathways within and between noninfarcted brain regions.
Supplementary Material
GLOSSARY
- ANOVA
analysis of variance
- CRC
Clinical Research Center
- FLAIR
fluid-attenuated inversion recovery
- FOV
field of view
- GM
gray matter
- MCA
middle cerebral artery
- MPRAGE
magnetization-prepared rapid gradient echo
- mRS
modified Rankin Scale
- NIHSS
NIH Stroke Scale
- TE
echo time
- TI
inversion time
- TR
repetition time
Footnotes
Editorial, page 822
AUTHOR CONTRIBUTIONS
Dr. I-Hsuan Chen: statistical analysis and manuscript preparation. Dr. Vera Novak: study concept and design, acquisition of data, MRI analysis. Dr. Brad Manor: study concept and design, statistical analysis and interpretation, manuscript preparation.
STUDY FUNDING
Funded by the Center for Dynamical Biomarkers and Translational Medicine, National Central University, Taiwan (NSC 101-2911-I-008-001), a KL2 Medical Research Investigator Training (MeRIT) award (1KL2RR025757-04) from Harvard Catalyst/The Harvard Clinical and Translational Science Center (NIH Award KL2 RR 025757), and grants from the NIH (R01-NS045745, AG023480) and the American Diabetes Association (1-06-CR-25). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the NIH.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil 1995;76:27–32 [DOI] [PubMed] [Google Scholar]
- 2.Patterson KK, Parafianowicz I, Danells CJ, et al. Gait asymmetry in community-ambulating stroke survivors. Arch Phys Med Rehabil 2008;89:304–310 [DOI] [PubMed] [Google Scholar]
- 3.Chen CY, Hong PW, Chen CL, et al. Ground reaction force patterns in stroke patients with various degrees of motor recovery determined by plantar dynamic analysis. Chang Gung Med J 2007;30:62–72 [PubMed] [Google Scholar]
- 4.Titianova EB, Tarkka IM. Asymmetry in walking performance and postural sway in patients with chronic unilateral cerebral infarction. J Rehabil Res Dev 1995;32:236–244 [PubMed] [Google Scholar]
- 5.Cassvan A, Ross PL, Dyer PR, Zane L. Lateralization in stroke syndromes as a factor in ambulation. Arch Phys Med Rehabil 1976;57:583–587 [PubMed] [Google Scholar]
- 6.Perennou D, Benaim C, Rouget E, Rousseaux M, Blard JM, Pelissier J. Postural balance following stroke: towards a disadvantage of the right brain-damaged hemisphere. Rev Neurol 1999;155:281–290 [PubMed] [Google Scholar]
- 7.Perennou D. Postural disorders and spatial neglect in stroke patients: a strong association. Restor Neurol Neurosci 2006;24:319–334 [PubMed] [Google Scholar]
- 8.Perennou DA, Mazibrada G, Chauvineau V, et al. Lateropulsion, pushing and verticality perception in hemisphere stroke: a causal relationship? Brain 2008;131:2401–2413 [DOI] [PubMed] [Google Scholar]
- 9.Genthon N, Rougier P, Gissot AS, Froger J, Pelissier J, Perennou D. Contribution of each lower limb to upright standing in stroke patients. Stroke 2008;39:1793–1799 [DOI] [PubMed] [Google Scholar]
- 10.Brandt T, Dieterich M, Danek A. Vestibular cortex lesions affect the perception of verticality. Ann Neurol 1994;35:403–412 [DOI] [PubMed] [Google Scholar]
- 11.Baier B, Suchan J, Karnath HO, Dieterich M. Neural correlates of disturbed perception of verticality. Neurology 2012;78:728–735 [DOI] [PubMed] [Google Scholar]
- 12.Perennou DA, Leblond C, Amblard B, Micallef JP, Rouget E, Pelissier J. The polymodal sensory cortex is crucial for controlling lateral postural stability: evidence from stroke patients. Brain Res Bull 2000;53:359–365 [DOI] [PubMed] [Google Scholar]
- 13.Miyai I, Mauricio RLR, Reding MJ. Parietal-insular strokes are associated with impaired standing balance as assessed by computerized dynamic posturography. Neurorehabil Neural Repair 1997;11:35–40 [Google Scholar]
- 14.Manor B, Hu K, Zhao P, et al. Altered control of postural sway following cerebral infarction: a cross-sectional analysis. Neurology 2010;74:458–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalafut MA, Schriger DL, Saver JL, Starkman S. Detection of early CT signs of >1/3 middle cerebral artery infarctions: interrater reliability and sensitivity of CT interpretation by physicians involved in acute stroke care. Stroke 2000;31:1667–1671 [DOI] [PubMed] [Google Scholar]
- 16.Hessert MJ, Vyas M, Leach J, Hu K, Lipsitz LA, Novak V. Foot pressure distribution during walking in young and old adults. BMC Geriatr 2005;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen IH, Yang YR, Chan RC, Wang RY. Turning-based treadmill training improves turning performance and gait symmetry after stroke. Neurorehabil Neural Repair 2014;28:45–55 [DOI] [PubMed] [Google Scholar]
- 18.Potdevin FJ, Femery VG, Decatoire A, Bosquet L, Coello Y, Moretto P. Using effect size to quantify plantar pressure asymmetry of gait of nondisabled adults and patients with hemiparesis. J Rehabil Res Dev 2007;44:347–354 [DOI] [PubMed] [Google Scholar]
- 19.Seliktar R, Mizrahi J. Some gait characteristics of below-knee amputees and their reflection on the ground reaction forces. Eng Med 1986;15:27–34 [DOI] [PubMed] [Google Scholar]
- 20.Wolfson L, Wakefield DB, Moscufo N, et al. Rapid buildup of brain white matter hyperintensities over 4 years linked to ambulatory blood pressure, mobility, cognition, and depression in old persons. J Gerontol A Biol Sci Med Sci 2013;68:1387–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiriyama K, Warabi T, Kato M, Yoshida T, Kokayashi N. Medial-lateral balance during stance phase of straight and circular walking of human subjects. Neurosci Lett 2005;388:91–95 [DOI] [PubMed] [Google Scholar]
- 22.Barra J, Marquer A, Joassin R, et al. Humans use internal models to construct and update a sense of verticality. Brain 2010;133:3552–3563 [DOI] [PubMed] [Google Scholar]
- 23.Bonan IV, Hubeaux K, Gellez-Leman MC, Guichard JP, Vicaut E, Yelnik AP. Influence of subjective visual vertical misperception on balance recovery after stroke. J Neurol Neurosurg Psychiatry 2007;78:49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spinazzola L, Cubelli R, Della Sala S. Impairments of trunk movements following left or right hemisphere lesions: dissociation between apraxic errors and postural instability. Brain 2003;126:2656–2666 [DOI] [PubMed] [Google Scholar]
- 25.Ishii F, Matsukawa N, Horiba M, et al. Impaired ability to shift weight onto the non-paretic leg in right-cortical brain-damaged patients. Clin Neurol Neurosurg 2010;112:406–412 [DOI] [PubMed] [Google Scholar]
- 26.Yeo SS, Ahn SH, Choi BY, Chang CH, Lee J, Jang SH. Contribution of the pedunculopontine nucleus on walking in stroke patients. Eur Neurol 2011;65:332–337 [DOI] [PubMed] [Google Scholar]
- 27.Hausdorff JM, Peng CK, Ladin Z, Wei JY, Goldberger AL. Is walking a random walk? Evidence for long-range correlations in stride interval of human gait. J Appl Physiol 1995;78:349–358 [DOI] [PubMed] [Google Scholar]
- 28.Jordan K, Challis JH, Newell KM. Long range correlations in the stride interval of running. Gait Posture 2006;24:120–125 [DOI] [PubMed] [Google Scholar]
- 29.Manor B, Lipsitz LA. Physiologic complexity and aging: Implications for physical function and rehabilitation. Prog Neuropsychopharmacol Biol Psychiatry 2013;45:287–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsao H, Galea MP, Hodges PW. Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain. Brain 2008;131:2161–2171 [DOI] [PubMed] [Google Scholar]
- 31.Fratiglioni L, Wang HX. Brain reserve hypothesis in dementia. J Alzheimers Dis 2007;12:11–22 [DOI] [PubMed] [Google Scholar]
- 32.Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst 2002;18:386–404 [DOI] [PubMed] [Google Scholar]
- 33.Chang WH, Kim YH, Yoo WK, et al. rTMS with motor training modulates cortico-basal ganglia-thalamocortical circuits in stroke patients. Restor Neurol Neurosci 2012;30:179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Enzinger C, Dawes H, Johansen-Berg H, et al. Brain activity changes associated with treadmill training after stroke. Stroke 2009;40:2460–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bracewell RM, Balasubramaniam R, Wing AM. Interlimb coordination deficits during cyclic movements in cerebellar hemiataxia. Neurology 2005;64:751–752 [DOI] [PubMed] [Google Scholar]
- 36.Morton SM, Bastian AJ. Cerebellar control of balance and locomotion. Neuroscientist 2004;10:247–259 [DOI] [PubMed] [Google Scholar]
- 37.Rosano C, Aizenstein H, Brach J, Longenberger A, Studenski S, Newman AB. Special article: gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J Gerontol A Biol Sci Med Sci 2008;63:1380–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teki S, Grube M, Kumar S, Griffiths TD. Distinct neural substrates of duration-based and beat-based auditory timing. J Neurosci 2011;31:3805–3812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belda-Lois JM, Mena-del Horno S, Bermejo-Bosch I, et al. Rehabilitation of gait after stroke: a review towards a top-down approach. J Neuroeng Rehabil 2011;8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


