Abstract
The reconstruction of musculoskeletal defects is a constant challenge for orthopaedic surgeons. Musculoskeletal injuries such as fractures, chondral lesions, infections and tumor debulking can often lead to large tissue voids requiring reconstruction with tissue grafts. Autografts are currently the gold standard in orthopaedic tissue reconstruction; however, there is a limit to the amount of tissue that can be harvested before compromising the donor site. Tissue engineering strategies using allogeneic or xenogeneic decellularized bone, cartilage, skeletal muscle, tendon and ligament have emerged as promising potential alternative treatment. The extracellular matrix provides a natural scaffold for cell attachment, proliferation and differentiation. Decellularization of in vitro cell-derived matrices can also enable the generation of autologous constructs from tissue specific cells or progenitor cells. Although decellularized bone tissue is widely used clinically in orthopaedic applications, the exciting potential of decellularized cartilage, skeletal muscle, tendon and ligament cell-derived matrices has only recently begun to be explored for ultimate translation to the orthopaedic clinic.
Keywords: Tissue engineering, extracellular matrix, decellularized matrix, tissue scaffolds, bone, skeletal muscle, articular cartilage, tendons, ligaments
1. Introduction
Orthopaedic injuries and degenerative diseases are common reasons for emergency room and office visits in the United States. There are more than 33 million orthopaedic injuries each year [1]. In the United States alone the estimated incidence of long bone fractures is about 1,500,000 per year [2], anterior cruciate ligament (ACL) injuries affect more than 120,000 athletes every year [3] and worldwide estimates for symptomatic osteoarthritis are 9.6% of men and 18% of women greater than 60 years old of age [4]. Loss of musculoskeletal tissue and function can occur as a result of athletic or traumatic injuries, degenerative changes, infections or tumor resection in bone, cartilage, skeletal muscle or tendon and ligament. Occasionally these injuries may result in large boney non-unions or large tissue voids requiring repair with either autologous grafts or allografts. However, autologous and allogeneic grafting techniques each have their own benefits and disadvantages. Autologous grafts have a low risk of transmitting diseases, good histocompatibility and are non immunogenic [5]. Unfortunately, there is a limit to the quantity of autologous graft tissue that can be harvested before compromising the donor-site. Although allografts may eliminate donor-site morbidity and decrease operating time, they are associated with the risk of severe immune response, disease transmission and slower integration with native tissue compared to autologous grafts [5]. For these reasons, there is a growing interest in engineering musculoskeletal tissues that can avoid donor site complications, are available in large quantities and have good histocompatibility.
During the past decade, there has been increased interest in creating biological scaffolds composed of extracellular matrix (ECM) derived from the decellularization of tissues or organs. The use of decellularized ECM from donor tissue has been utilized in the repair of skin [6], bladder [7], heart valve [8] and small intestinal submucosa [9]. Furthermore, several commercialized decellularized scaffolds have received FDA approval for use in humans, including dermis tissue (Alloderm®; LifeCell), porcine heart valves (Synergraft®; Cryolife) and porcine urinary bladder (Urinary bladder matrix; ACell) [10, 11]. In preclinical trials, decellularized scaffolds made from porcine small intestinal submucosa (SIS) have been used in orthopaedic surgical applications for repair of rotator cuff [12], anterior cruciate ligament (ACL) [13, 14] and Achilles tendon [15]. Although tissue-derived biologic scaffolds are commonly used to repair non-homologous anatomic sites, studies of skeletal muscle and liver tissue engineering have suggested that biologic scaffolds derived from site-specific homologous tissues may be better suited for constructive tissue remodeling than non-site specific tissue sources [16, 17]. This has motivated the development of orthopaedic tissue engineering strategies that utilize biologic scaffolds derived from specific homologous orthopaedic tissues.
ECM components differ between bone, cartilage, skeletal muscle, ligament and tendon. The use of homologous orthopaedic tissues as scaffolds for tissue engineering would provide tissue-specific ECM compositions, which can influence the behavior of resident and/or transplanted cells. ECM is a product of cells that functions to maintain tissue and organ structure, organization and function. It is a complex network of proteins and polysaccharides forming an intricate meshwork within tissue that interacts with the resident cells to regulate cell behavior, such as migration, proliferation and differentiation. The ECM exists in a state of dynamic equilibrium with its resident cells and is constantly being built, reshaped and degraded in response to changing environmental conditions and to cellular, tissue and organ demands [18]. Musculoskeletal tissues require proper organization of resident cells and ECM to withstand loads and produce adequate forces for everyday activities. Decellularized tissue explants may provide a naturally occurring three-dimensional scaffold with tissue-specific orientations of ECM molecules that are not easily created synthetically in the laboratory. This manuscript provides an overview of biological scaffolds created from decellularized ECM of musculoskeletal tissues and in vitro cell-derived matrices and their use in in vitro and in vivo applications of tissue engineering.
2. ECM immunogenicity
The decellularization process is crucial for eliminating cellular components and antigenicity from tissue explants in order to avoid disease transmission, reduce inflammatory and immune responses toward the scaffold and decrease the risk of rejection after implantation, particularly with xenogeneic or allogeneic donor tissues [9]. Unlike cellular material, ECM components are predominantly conserved among species and are therefore well tolerated when used as allografts or xenografts [19–21]. The ideal decellularization technique would remove cellular remnants without the destruction of the original tissue architecture or the removal of ECM components, and thus maintaining the mechanical properties of the natural ECM.
DNA and the cell surface oligosaccharide molecule α-Gal (Galα1,3-Galβ1-4GlcNAc-R) also known as “Gal epitope” are two common antigens known to trigger an inflammatory response against biological scaffolds [22]. In most tissues, cells are embedded within a dense ECM making it difficult for complete removal of cellular material. In fact, most commercially available decellularized biological scaffold material, such as Restore™, GraftJacket™, and TissueMend™, contain trace amount of remnant DNA that are less than 300 bp in length [23–25]. Although the majority of the commercially available biologic scaffolds contain DNA remnants, the clinical efficacy of these scaffolds has been largely positive [22]. Therefore, the small amount of DNA remaining may not be enough to elicit an immune response or adversely affect the remodeling process. There may be a threshold amount of cellular material that is required to trigger a severe immune response, and further studies are needed to determine this threshold.
Gal epitopes are cell surface molecules that are commonly found in most species except humans and Old World monkeys due to mutations in the α1,3-galactosyl-transferase gene [22]. As a result of the lack of Gal epitopes, humans produce a large amount of anti-Gal antibodies due to constant exposure to intestinal bacteria carrying Gal epitopes [22]. This is particularly important when creating decellularized biological scaffolds using xenografts for human implantation. Gal epitopes have been found in porcine ACL [26], cartilage [27], SIS-ECM [28] and bioprosthetic heart valves [29]. Konakci et al. demonstrated that patients receiving porcine bioprosthetic heart valves have a xenograft-specific immune response with elevated levels of cytotoxic IgM antibodies directed against α-Gal. The authors speculate that this may contribute to the failure of the tissue in some patients [29]. Treatment of xenogeneic tissues with α-galactosidase to remove Gal epitopes has been shown to minimize adverse immune responses to biologic scaffolds [26, 27]. Stone et al. implanted α-galactosidase treated porcine meniscus and articular cartilage into the suprapatellar pouch of cynomolgus monkeys and found a significant reduction in T lymphocytes at the site of remodeling compared to untreated grafts [27]. Similarly, α-galactosidase treated porcine patellar tendon grafts, untreated porcine tendon grafts or allografts were used for ACL reconstruction in rhesus monkeys. Untreated porcine grafts were resorbed and rejected while treated porcine grafts and allografts were incorporated by the hosts with gradual host cell infiltration and remodeling [30].
Decellularized allogeneic and xenogeneic biological scaffolds are commonly used in tissue engineering and regenerative medicine. However, research looking at the host immune response towards biological scaffolds is limited and further studies are necessary to improve the safety and efficacy of decellularized biological scaffolds.
3. Bone
Bone is a dynamic tissue that is constantly changing in response to daily mechanical loads. Fractures of normal, healthy bone with good anatomical alignment usually heal well. Fracture healing requires an intricate and well-organized series of cellular and molecular events. It involves interactions between cortical bone, the periosteum, undifferentiated fascial tissue surrounding the fracture and the bone marrow. Fracture healing is divided into three stages: inflammation, repair and remodeling [31]. After an injury, there is initial bleeding from the damaged bone ends and surrounding tissue resulting in the formation of a hematoma, which provides a source of hematopoietic cells capable of secreting growth factors. The invasion of inflammatory cells, fibroblasts, mesenchymal cells, and osteoprogenitor cells at the fracture site forms granulation tissue around the fracture ends. Fractures that are anatomically aligned with absolute stability, such as those surgically repaired with compression plates, undergo primary bone healing or Haversian remodeling, in which there is direct osteonal healing within the cortex by intramembranous ossification [32]. More commonly, in closed reduced fractures, secondary bone healing occurs with the formation of a bridging soft callus consisting of cartilage tissue connecting the fracture ends. Over time, bone formation occurs under the periosteum and calcification of cartilage results in the formation of hard callus or woven bone by endochondral ossification [33]. The remodeling phase begins during the middle of the repair phase and may continue for up to 7 years even after the fracture is clinically healed. Over time, woven bone is replaced by lamellar bone and there is repopulation of the marrow space.
Frequently, there are instances when skeletal defects are unable to heal on their own, either due to large boney voids secondary to bone tumor excisions, large osteotomies, traumatic injuries or impaired bone healing due to age, chronic diseases or lifestyle resulting in non-unions or delayed unions. In these situations bone grafting is commonly used to reconstruct skeletal defects, enhance and accelerate fracture repair and fill boney defects after tumor excisions, fracture non-unions, or osteotomy healing [5, 34]. Bone transplant is the second most common tissue transplant, with about 2.2 million bone grafting surgeries performed annually worldwide [35]. Autologous bone is considered to be the preferred bone grafting material, specifically autologous cortical and cancellous bone harvested from the iliac crest, because it has osteoconductive, osteoinductive and osteogenic properties [5, 36]. However, it has been well documented in the literature that autologous bone grafting is not without complications, which include but are not limited to infections, prolonged wound drainage, increased post operative pain, large hematomas, neurovascular injuries and increased operative blood loss [37, 38]. Alternatives to autologous bone grafts include allograft bone, ceramics, demineralized bone matrix, autologous bone marrow and composite grafts [5, 36].
3.1 General ECM of bone
Mature bone consists of a central area of marrow surrounded by bone tissue and periosteum. Normal bone tissue is made up of both cortical (compact) bone and cancellous (trabecular) bone [39]. Cortical bone makes up about 80% of the mature skeleton and surrounds the cancellous bone and marrow. It is made up of tightly packed osteons connected by Haversian canals containing arterioles, venules, capillaries and nerves [40]. It has a slow turnover rate and high resistance to torsion and bending. Cancellous bone, on the other hand, is porous and undergoes more remodeling with a higher turnover rate [39].
Bone matrix is composed of organic compounds (about 20% of the wet weight of bone), inorganic compounds (70–80% of the wet weight of bone), and water (8–10% of the wet weight of bone) [41]. Organic components provide the bone with form and contribute to its ability to resist tension. Inorganic components surround and impregnate collagen fibers endowing bone with the ability to resist compressive loads [42]. The organic components are predominantly collagen type I with small amounts of collagen type V and XII, which together make up about 90% of the organic matter [40]. The other 10% is made up of proteoglycans, glycoproteins and bone matrix proteins such as osteocalcin, osteonectin and bone sialoproteins [43]. Bone matrix also contains growth factors, such as isoforms of transforming growth factor-β (TGF-β), insulin-like growth factor-1 (IGF-1), bone morphogenetic proteins (BMPs), platelet-derived growth factors (PDGFs), and cytokines such as interleukin-1 (IL-1), IL-6 and colony-stimulating factors [39]. Hydroxyapatite makes up the inorganic phase of bone and together with the organic matrix create a rigid material with the mechanical properties necessary to withstand the forces of normal daily activities [44].
Cells that reside in bone originate from two cell lineages: the mesenchymal stem cell lineage, which includes preosteoblasts, osteoblasts and osteocytes, and the hematopoietic stem cell lineage, which includes monocytes, preosteoclasts and osteoclasts [39]. Preosteoblasts are undifferentiated mesenchymal cells that can differentiate into osteoblasts when stimulated, such as during bone repair [39]. Osteoblasts are responsible for the synthesis and secretion of organic matrix as well as matrix mineralization [39]. Osteocytes make up 90% of cells in the mature skeleton and are derived from osteoblasts. Osteoclasts function in bone resorption and are important in bone turnover and remodeling [39].
3.2 Tissue-derived decellularized bone ECM
Decellularized bone has been widely used as a scaffold for bone tissue engineering due to its three-dimensional (3D) structure and similarity to the native bone matrix as well as its osteoinductive and biomechanical properties. Various 3D scaffolds made from synthetic polymers, metals and ceramics have been shown to promote osteogenic differentiation of mesenchymal stem cells (MSC) [45–47] and embryonic stem cells (ESC) [48, 49], however, these synthetic scaffolds do not contain the ECM components and organization found naturally in bone matrix. On the other hand, decellularized bone contains natural ECM which provides cells with structural support, cell-matrix interactions and exposure to growth factors and cytokines that are naturally stored within bone matrix to guide bone tissue formation [50, 51].
Demineralized bone matrix (DBM) is an allogeneic bone graft material commonly used in orthopaedic surgery for filling in boney defects after fractures or tumor debulking, strengthening of arthrodesis and spinal fusion due to its compositional, structural and functional similarities to autologous bone [52]. Currently there are at least eight manufacturers with approximately 25 different DBM products on the market. DBM products are available in different forms, such as powder, putty, chips or gel-filled syringes [52]. The processes utilized for demineralizing allogeneic bone also simultaneously decellularize the bone. The general DBM preparation involves removal of soft tissue, blood and lipids, follow by antibiotic soaks, acid demineralization and several rounds of freeze-drying [53]. The end product is a composite of collagens type I, IV and X, as well as non-collagenous proteins which provide an osteoconductive matrix. The presence of growth factors such as bone morphogenetic proteins (BMPs) and transforming growth factor-beta 1, 2, and 3 endows it with osteoinductive properties [53].
A number of in vitro studies have demonstrated the potential of DBM to stimulate osteogenesis in human MSC [54], adipose derived stem cells (ASC) [55] and periosteal cells [56, 57]. Clinically, DBM constructs have been successfully used in reconstruction of phalangeal and metacarpal defects following enchondroma and congenital hand deformities [58]. The use of DBM, as opposed to autografted tissue, reduced operative time, donor site morbidity and tourniquet time. Similarly, Michelson et al. demonstrated that using DBM for subtalar fusions and triple arthrodesis resulted in similar outcomes as iliac crest bone graft without the increased blood loss, cost and postoperative pain associated with iliac crest bone harvest [59]. Although DBM generally appears to be an attractive bone graft material, the amount of osteogenic activity of a particular DBM preparation is dependent upon specific donor characteristics such as age, gender and lifestyle habits [60]. Additionally, the osteogenic activity of commercially available DBM depends on the method of its preparation and the carrier in which the DBM is mixed [61]. The preparation of DBM particles may also affect host cell viability. For instance, DBM particles mixed with a glycerol carrier are very acidic and can be detrimental to host cells, while DBM particles mixed with hyaluronic acid are less harmful to host cells [62].
Similar to DBM, decellularized bone matrixes that are not demineralized have the potential to be used as bone grafts. Studies suggest that these bone matrixes can also have osteogenic effects on progenitor cells. Hashimoto et al. compared MSC differentiation on 3D decellularized bone tissue to that on a two-dimensional tissue culture polystyrene (TCPS) dish and found that alkaline phosphatase (ALP) activity, an early marker for osteogenesis, was significantly elevated in the MSC grown in the decellularized bone matrix [63]. Even in the absence of dexamethasone, a common osteogenic factor, MSC in decellularized bone had more ALP activity as determined biochemically and via staining than cells grown on TCPS, suggesting that decellularized bone matrix favorably promotes early osteogenic differentiation of MSC. Additionally, subcutaneous implantation of decellularized bone matrix into rats demonstrated cell infiltration with neovascularization after 6 months. Growth factors and various ECM components such as fibronectin, heparin sulfate, dermatan sulfate, chondroitin sulfate and hyaluronic acid, are believed to still be present and remain at least partially active even after decellularization and could contribute to the osteogenic differentiation of MSC [63–65].
In addition to ECM components, the internal architecture of decellularized bone, such as scaffold porosity and pore size can affect cell growth, signaling and differentiation [66]. In an interesting study by Marcos-Campos et al. the authors compared osteogenesis of mesenchymal progenitors derived from human ESC in decellularized bone of different densities [67]. The density of each cylindrical-shaped scaffold was calculated based on the measured weight and the volume of each scaffold. The decellularized bone scaffolds were divided into three different groups: low density (0.281 ± 0.018 mg/mm3), medium density (0.434 ± 0.015 mg/mm3) and high density (0.618 ± 0.027 mg/mm3). Pore size and porosity decreased with increasing bone density while compressive elastic modulus increased with increasing bone density. After five weeks in culture, the medium density group cultured with mesenchymal progenitor cells showed the highest concentration of live cells and bone matrix formation in the inner regions of the constructs [66]. These findings suggest that decellularized bone scaffolds of medium density (0.434 ± 0.015 mg/mm3) may be best suited for bone tissue engineering due to a balance in nutrient transport, cell attachment, cell infiltration and proliferation, matrix production, and scaffold mechanical strength.
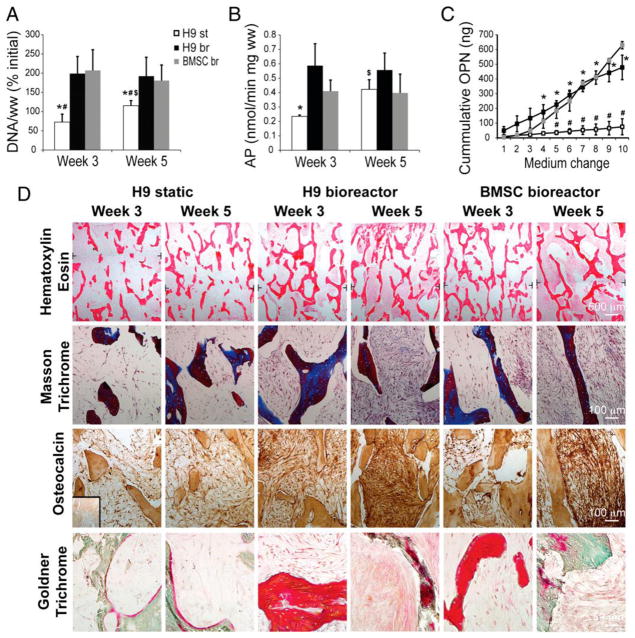
Decellularized bone matrix has been found to support and guide the osteogenic differentiation of MSC [63], ESC [67, 68], induced pluripotent stem cells [69] and ASC [35]. In past studies, use of human ESC yielded limited bone formation and was usually accompanied by the formation of teratomas in two dimensional cultures [70]. Marolt et al. demonstrated that human ESC-derived mesenchymal progenitor cells cultured in decellularized trabecular bone in a perfusion bioreactor lead to bone formation with significantly elevated ALP activity and osteopontin release compared to static cultures. Nevertheless, they were comparable to human bone marrow-derived MSC cultured in decellularized trabecular bone in a perfusion bioreactor (Figure 1A, B, C) [68]. Additionally, histological findings further corroborated the biochemical assays, demonstrating denser tissue deposition in constructs incubated in a bioreactor compared to static cultures after 5 weeks of culture with positive staining for osteocalcin, collagen (Masson’s Trichrome stain), and osteoid (Goldner’s Trichrome stain) (Figure 1D) [68]. Furthermore, there was no sign of teratoma formation in any of the constructs.
Figure 1.
Bone tissue engineering with decellularized trabecular bone cultured in a bioreactor. (a) DNA content per wet weight (ww) of tissue constructs seeded with human embryonic stem cell H9 cell line after 3 and 5 weeks of culture in a bioreactor (br) compared to static (st) cultures. Both cultures were also compared to tissue constructs seeded with bone marrow-derived MSC (BMSC) cultured in a bioreactor. Values are expressed as percent of the initial value. (b) Alkaline phosphatase (AP) activity measured after 3 and 5 weeks of culture. (c) Cumulative osteopontin (OPN) content in culture medium after 2 weeks of culture. Data for DNA content, AP activity and OPN are expressed as averages ± standard deviation (n = 3–5). Statistical significance when P <0.05. Statistical significance between H9 static and H9 bioreactor at the same time point indicated by “*” and between H9 static and BMSC bioreactor group at the same time point indicated by “#”. Statistically significant difference within the same group but at different time points indicated by “$”. (d) Histological staining with hematoxylin and eosin to visualize tissue morphology, Masson’s trichrome and Goldner’s trichrome to visualize bone matrix deposition and osteocalcin immunohistochemistry on tissue constructs seeded with H9 human embryonic stem cell line cultured statically or in a bioreactor and on tissue constructs seeded with bone marrow-derived stem cells cultured in bioreactor for 3 and 5 weeks. Reprinted from Marolt et al. 2012 with permission from Proceedings of the National Academy of Science.
Decellularized bone may perhaps direct the osteogenic differentiation of mesenchymal progenitor cells and prevent their differentiation into other cell types. Frohlich et al. induced human ASC to undergo osteogenesis when cultured in decellularized trabecular bone from bovine carpometacarpal joints [35]. After 5 weeks of culture, the constructs stained positive for bone sialoprotein and osteopontin by immunohistochemistry (IHC) and total collagen by Masson’s Trichrome; all of these proteins are commonly found in mature bone ECM [35].
Over the past decade, the use of bioreactors has become popular in tissue engineering, and may prove particularly useful for culture of tissue engineered bone constructs. Perfusion bioreactors can allow for medium perfusion through constructs that can enhance bone tissue formation by improving nutrient transfer and creating intrinsic shear stresses associated with medium flow [71–73]. The presence of shear forces have been shown to specifically influence the induction of osteoblastic differentiation in MSC [74]. Grayson et al. looked at human bone marrow-derived MSC growth and differentiation patterns in decellularized bovine trabecular bone with different medium perfusion rates (100 μms−1 and 400 μms−1) and found that higher rates led to more uniform cell distribution in the constructs with increased production of bone ECM proteins [75]. In a similar study, human ASC seeded in decellularized trabecular bone and cultured in perfusion bioreactors in the presence of osteogenic supplements formed viable bone tissue constructs with improved cell and bone matrix distribution throughout the constructs compared to similar constructs grown under static conditions [35]. In future studies, bioreactors may play an essential role in generating large bone constructs with uniform distribution of cells and matrix proteins.
Decellularized bone ECM scaffolds may be advantageous for bone reconstruction in clinical settings. There is often a need for anatomically shaped bone grafts, particularly in craniofacial reconstruction. Decellularized bone can be machined to form anatomically shaped bone scaffolds based on digitized medical images. Grayson et al. created decellularized bovine trabecular bone scaffolds resembling a temporomandibular joint (TMJ) condyle and seeded the scaffolds with human MSC in a bioreactor [76]. After 5 weeks in culture, there was evidence of lamellar bone formation observed on scanning electron microscopy photomicrographs, increased mineralized matrix volume as determined by microcomputer tomography and formation of osteoid with Masson’s Trichrome staining [76]. Using this technology, viable bone grafts can be customized to treat specific patients and defects without the risk of donor site morbidity associated with autografts or disease transmission associated with allografts.
In a recently published case report, decellularized bovine trabecular bone discs cultured with patient-specific MSC in a perfusion culture chamber were used to treat a non-healing distal tibia fracture [77]. Callus formation around the graft and native bone was evident at six weeks after surgery. Histology from biopsies taken at six months showed active remodeling of the callus but not the graft. However, bone remodeling measurements based on radiodensity evaluated by PET/CT imaging suggested the presence of active bone formation in both the callus and the graft [77]. These studies demonstrate that cell-loaded decellularized bone grafts have promise for orthopaedic surgery applications. However, more animal and clinical studies are required to further assess the osteogenic activity and integration of cell-loaded decellularized bone grafts with the native bone in an in vivo environment, particularly with decellularized allografts or xenografts.
3.3 In vitro cell-derived decellularized bone ECM
Decellularized ECM derived from in vitro cultured cell constructs offers an alternative to decellularized whole-bone tissues for creating tissue engineered scaffolds. This technique enables the potential for the formation of autologous grafts without donor site morbidity and of predefined shapes and sizes. Cell-derived osteogenic ECM can be created in vitro using patient-specific cells such as MSC [79–84], fibroblasts [85], chondrocytes [86] or osteoblasts [85] cultured in the presence of osteogenic media. Cell-derived ECM, in the absence of osteogenic growth factors or supplements, has been shown to facilitate expansion of mesenchymal colony forming units in vitro as well as preserve their stem cell properties [78]. Additionally, matrices derived from MSC have been shown to be osteoconductive for calvarial bone healing. Zeitouni et al. repaired mouse calvaria defects with decellularized human MSC-derived ECM reseeded with human MSC pretreated with GW9662, a small molecule that directs stem cells towards osteogenesis [81]. Cell-seeded decellularized ECM resulted in 80–100% bone healing after 3 weeks compared to 30% and 60% healing with decellularized ECM only and MSC only treatment groups, respectively. Enhanced bone healing was attributed to increase cell retention at the defect site possibly due to the presence of cell binding sites on the MSC-derived matrix.
Studies have also demonstrated that in vitro cell-derived ECM may retain biological factors that contribute to the differentiation of reseeded cells. Thibault et al. created rat MSC-derived mineralized ECM by seeding rat bone marrow-derived MSC onto electrospun poly (ε-caprolactone) (PCL) fiber mesh scaffolds. Rat MSC were reseeded on to decellularized MSC-derived mineralized ECM/PCL scaffolds and cultured with or without dexamethasone [82]. Calcium deposition, which is indicative of late stage differentiation of osteoblasts [72], was found to be similar between ECM/PCL constructs cultured with MSC in the presence and absence of dexamethasone and significantly higher than groups with PCL scaffold alone, suggesting that the sustained osteogenic differentiation of MSC may be due to retention of osteogenic factors secreted during the generation of the ECM construct or the retention of dexamethasone during the generation of PCL/ECM scaffolds, which was absent in PCL only scaffolds [82]. As cell-derived ECM presents a level of biological activity sufficient to sustain the osteogenic differentiation of progenitor cells in the absence of exogenous osteogenic supplementation, this may provide a novel method for the delivery of biologic factors for bone tissue engineering.
To enhance the osteoinductive properties of cell-derived ECM, different groups have incorporated a variety of inorganic materials into decellularized cell-derived ECM scaffolds. It has previously been shown that materials commonly used to produce orthopaedic implants, such as hydroxyapatite, glass-ceramics and titanium have the ability to drive the differentiation of rat bone marrow stromal cells into osteoblasts and induce the formation of mineralized tissue [87–89]. Datta et al. cultured rat bone marrow-derived MSC onto decellularized rat MSC-derived ECM incorporated with titanium fiber mesh with or without osteogenic supplementation [79]. ALP activity was significantly elevated in cell-ECM-titanium constructs with osteogenic supplementation compared to constructs without osteogenic supplementation, however, calcium content was similar in cell-ECM-titanium constructs with and without osteogenic supplementation compared to groups without ECM after 12 days [79]. In a follow up study looking at gene expression of rat bone marrow-derived MSC cultured in decellularized rat MSC-derived ECM with titanium mesh, the presence of ECM and titanium resulted in upregulation of osteogenic markers such as ALP, osteocalcin, osteomodulin, osteopontin and runx2 compared to cells seeded on titanium mesh alone [50]. Instead of titanium, Tour et al. incorporated hydroxyapatite with ECM derived in vitro from either rat calvarial osteoblasts or dermal fibroblasts and implanted decellularized scaffolds into rat calvarial defects [85]. They demonstrated that ECM incorporated with hydroxyapatite induced significantly more new bone formation based on histomorphometric analysis compared to hydroxyapatite alone, even in the absence of progenitor stem cells or growth factors. However, there was a significant increase in inflammatory cells in constructs incorporated with hydroxyapatite, which may be beneficial in bone repair through their ability to recruit mesenchymal stem cells and osteoprogenitor cells to initiate the formation of granulation tissue and callus formation for bone healing [85]. These studies suggest that osteoprogenitor cells cultured in the presence of inorganic material combined with ECM can enhance osteogenic differentiation and bone repair.
Rather than culturing inorganic material with cells to form cell-derived matrices as mentioned in previous examples, decellularized osteogenic cell-derived matrices without inorganic material can be formed separately and transferred onto the surfaces of inorganic materials without losing their osteogenic potential. A recent study demonstrated that decellularized osteogenic cell-derived matrices, created by culturing human bone marrow-derived MSC in monolayer on tissue culture plastic, can retain their composition and efficacy to accelerate osteogenesis even after collection and transfer to another plastic tissue culture plate [84]. Human bone marrow-derived MSC were reseeded onto the transferred decellularized ECM and were found to have similar levels of ALP activity after 7 days and Alizarin red staining after 2–3 weeks compared to MSC seeded on non-transferred decellularized ECM. Decaris et al. collected the decellularized cell-derived matrices by scraping the decellularized ECM off plates in the presence of acetic acid, transferring to microcentrifuge tubes and sonicating the ECM to mechanically homogenize the ECM contents. The contents were then pipetted onto standard tissue culture plates and allowed to dry in a sterile biosafety cabinet before reseeding with MSC. In a follow-up study, Decaris et al. removed decellularized ECM as a whole sheet from a culture plate and used it to coat three-dimensional poly(lactide-co-glycolide) scaffolds. They verified that the ECM sheet continues to retain its ability to drive osteogenic differentiation of MSC when cultured in vitro under osteogenic culture conditions and after ectopic implantation into the back of nude mice [90]. Translational applications of this technology may involve coating biomaterials with cell-derived ECM for bone repair or commonly used orthopaedic implants with cell-derived decellularized ECM produced in vitro or cell-derived ECM loaded with progenitor cells to enhance osteoinduction and osseointegration.
3.4 Decellularization of bone tissue and in vitro cell-derived extracellular matrix
Of the two types of bone matrix (cortical and trabecular), trabecular bone is commonly used as a decellularized scaffold due to its high porosity and pore interconnectivity [91], which may enable simpler cell removal and potentially improve cell seeding and infiltration. Decellularization of bone generally involves a combination of chemical and enzymatic treatments, but there is currently no standard method for bone decellularization. Vunjak-Novakovic and colleagues have published several studies using decellularized trabecular bone matrices as scaffolds. In these studies, trabecular bone is initially washed with a high velocity stream of water to remove marrow from the pore spaces, followed by treatment with ethylenediaminetetraacetic acid (EDTA) for cell dissociation and then treatment with sodium dodecyl sulfate (SDS), a detergent which solubilizes the cell membranes. The tissue then undergoes enzymatic treatments with immersion in RNase and DNase solutions to remove nucleic acid material [35, 68, 75]. Interestingly, osteocytes trapped in the mineralized portion of the bone were also removed as demonstrated on histology [75]. Decellularization with detergents such as SDS and Triton X-100 has also been shown to remove ECM components including glycosaminoglycans in cartilage tissue, which is undesirable. However, this has not been examined in bone tissue [92–94]. Hashimoto et al. developed a novel method of bone decellularization without the use of detergents. Using a cold isostatic pressurization machine, compact and cancellous bone from porcine femur and costal bones, respectively, were hydrostatically pressurized at 980 MPa at 30°C for 10 minutes followed by DNase treatment [63]. Through hematoxylin and eosin (H&E) staining they demonstrated complete removal of cellular material and a significant decrease in DNA content compared to untreated bone tissue by DNA quantification. Using this method, the collagen network within the bone was preserved [63].
Unlike bone tissue, the composition of cell-derived bone ECMs engineered by different groups varies depending on the various materials incorporated in their constructs such as hydroxyapatite [85], titanium [79] or PCL fiber mesh [82]. Tour et al. cultured rat calvarial osteoblasts with hydroxyapatite particles and compared decellularization by 3 freeze-thaw cycles versus treatment with 0.5% Triton X-100 buffer containing 20 mM NH4OH in PBS for 3 minutes at 37°C. SEM analysis revealed that Triton X-100 treatment preserved the in vitro generated ECM architecture by maintaining fibrillar networks compared to the freeze-thaw treatment, which resulted in disorganized matrix with loss of fibrillar structures [85]. Thibault et al. and Datta et al. incorporated PCL and titanium fiber meshes in their cell-derived ECM, respectively, and reported success with decellularization by 3 cycles of freeze-thaw treatment only [79, 82]. There is no standard method of decellularizing cell-derived bone ECMs, and the method chosen for decellularization appears to be based on the preference and experience of each investigating group. The different techniques of bone tissue and cell-derived bone tissue decellularization are summarized in Table 1.
Table 1.
Methods for tissue-derived and cell-derived bone ECM
| Tissue Type | Decellularization Method | Sterilization | Cell Repopulation | References |
|---|---|---|---|---|
| Bone Tissue | ||||
| Bovine trabecular bone from carpometacarpal joint |
|
70% Ethanol | Human ASC | [35] |
| Porcine femur (Compact bone) & costa (Cancellous bone) |
|
80% Ethanol | Rat bone marrow-derived MSC | [63] |
| Bovine trabecular bone from wrists |
|
Ethanol | Human ESC (Cell line H9) | [67] |
| Bovine trabecular bone from carpometacarpal joint |
|
70% Ethanol | Human ESC (Cell line H9) | [68] [75] |
| Cell-Derived Bone Matrix | ||||
| Rat femoral and tibial bone marrow-derived MSC cultured on titanium fiber mesh scaffolds | Three freeze/thaw cycles | Unspecified | Rat bone marrow-derived MSC | [50] [79] |
| Rat osteoblasts and dermal fibroblasts cultured on synthetic hydroxapatite microparticles | 0.5% Triton X-100 for 3 min at 37°C | Unspecified | None | [85] |
| Rat bone marrow-derived MSC cultured on PCL scaffold |
|
Ethylene oxide | Rat bone marrow-derived MSC | [82] |
| Mouse bone marrow-derived MSC | 0.5% Triton X-100 for 5 min at 37°C | Unspecified | Mouse bone marrow-derived MSC | [83] |
Key: MSC=Mesenchymal stem cell, ASC= Adipose derived stem cell, ESC= Embryonic stem cell, SDS=Sodium dodecyl sulfate, PCL = Polycaprolactone
4. Articular cartilage
Articular cartilage lines the surface of diarthroidal joints. It functions to protect the subchondral bone from forces associated with high mechanical loads and creates a low-friction gliding surface that helps distribute load evenly across the underlying bone [95]. Defects in articular cartilage, either as a result of traumatic injury, degenerative changes or congenital anomalies, result in debilitating joint pain and can severely affect the quality of life of individuals in all age groups [96–98]. Once damaged, cartilage tissue has a limited capacity to repair itself due to its low cellularity and avascular and aneural properties [10, 99, 100]. Chondral lesions that do not involve the subchondral bone generally do not heal [101, 102] and injuries that penetrate the subchondral bone often result in the formation of fibrocartilage which is biomechanically insufficient compared to hyaline cartilage, resulting in further damage over time [103–105]. If cartilage lesions are left untreated, they can lead to debilitating joint pain, joint dysfunction and osteoarthritis (OA). Currently there is no single, consistently effective treatment for OA. The common first-line treatment includes nonsurgical management with pharmaceuticals, such as nonsteroidal anti-inflammatory drugs, corticosteroid injections or viscosupplementation injections, along with lifestyle modifications and physical therapy [106].
Although nonsurgical management methods may temporarily reduce joint pain and inflammation they do not provide permanent relief for some patients [106]. Therefore, surgical treatments have been developed with the goal of restoring the cartilage surface. Current cartilage repair strategies include bone marrow stimulation (microfracture), osteochondral autograft transfer system (OATS) or mosaicplasty, and autologous chondrocyte transplantation (ACT) [96, 107, 108]. Bone marrow stimulation is frequently used for treating small symptomatic articular cartilage lesions and involves penetrating the subchondral plate in order to release MSC and blood from the bone marrow to fill the cartilage defect. However, this repair can result in the formation of fibrocartilage [109]. OATS or mosaicplasty involves the transplantation of multiple small cylindrical autologous osteochondral plugs harvested from low weight-bearing areas of the knee into an area of full-thickness cartilage defects [107]. Although this method can create a smooth surface in the defect, limitations including donor site morbidity, limited availability of graft tissue, graft subsidence at the surface with postoperative weight bearing and the absence of repair within the dead space between cylindrical grafts, affect the quality of repair [96]. ACT is another surgical procedure that involves removing healthy cartilage from low weight bearing regions of the articular cartilage, isolating and expanding the chondrocytes in culture, and then transplanting the chondrocytes into the cartilage defect under a periosteal flap or collagen membrane [108]. Although there can be improvements in joint pain and function, this procedure is associated with donor site morbidity and variable patient outcomes and histology of repair tissue after one year has revealed a mix of hyaline-like and fibrocartilage tissue [110, 111]. Due to the frequent lack of hyaline cartilage repair in these surgical managements, there is a continued interest in tissue engineering to develop strategies for the repair or regeneration of new cartilage with similar biomechanical properties and biological composition and organization as native articular cartilage.
4.1 General ECM of cartilage
The ECM of cartilage is made up of a complex mixture of structural and bioactive molecules secreted by resident chondrocytes that help regulate cell adhesion, survival, proliferation and differentiation. Cartilage tissue is rich in ECM and water, with water making up 70–85% of whole cartilage tissue weight. The ECM of cartilage is composed primarily of proteoglycans, specifically aggrecan, and collagen, which make up approximately 30% and 60–70% of the dry weight of articular cartilage, respectively [95]. Collagen type II accounts for approximately 95% of total collagen in articular cartilage while the other 5% is made up of collagens type V, VI, IX and XI, which are involved in the intermolecular interactions and the modulation of collagen type II [112]. Intermolecular cross-links that form between collagen type II molecules create collagen fibrils which join with other collagen fibrils to form collagen fibers. The intramolecular bonds that form within collagen fibers create a stiff tropocollagen molecule to create a strong collagen lattice, lending cartilage its tensile properties [113]. Interlaced throughout the collagen lattice are proteoglycans. Proteoglycans are made up of a protein core bound to multiple chains of glycosaminoglycans (GAG), such as chondroitin sulfate or keratan sulfate, which have a strong negative charge. Proteoglycans can further bind or aggregate to a backbone of hyaluronic acid forming larger macromolecules. The strong negative charge of the proteoglycans attracts cations which results in water being pulled into and trapped within the matrix due to osmosis. This, combined with the repulsive forces present due to the close proximity of negatively charged GAG aggregates within the collagen network, creates a swelling pressure that provides cartilage with the ability to withstand compressive forces [113, 114].
4.2 Tissue-derived decellularized cartilage ECM
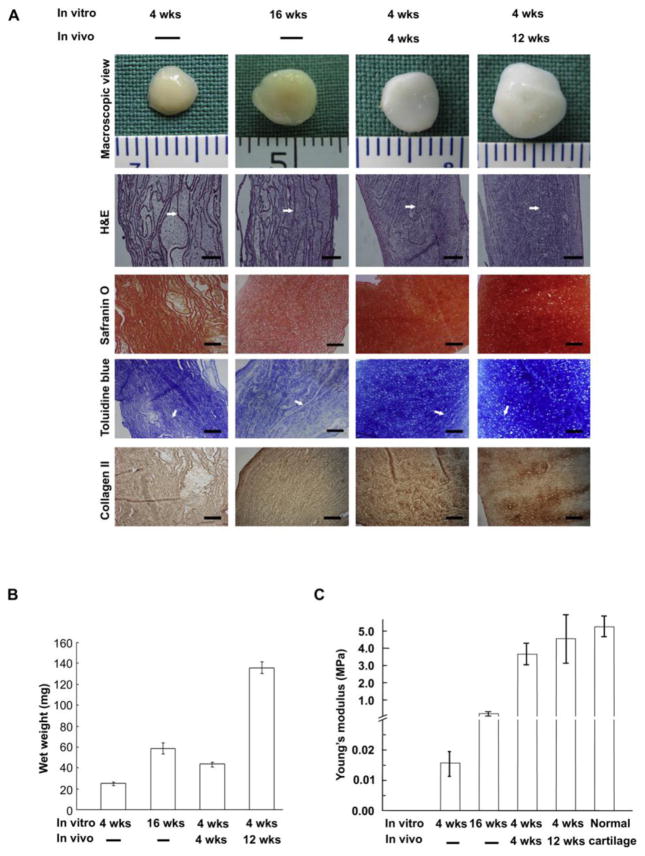
Decellularized ECM derived from cartilage tissue has been used to create 3D scaffolds for tissue engineering of articular cartilage [99], menisci [115], tracheal cartilage [116], facet joint cartilage [117] and the temporomandibular joint disc (TMJ) [118]. Decellularized cartilage matrix has demonstrated potential as a scaffold for tissue engineering of menisci [115, 119] and trachea [116, 120]. As described previously, cartilage tissue has a dense ECM, which makes it difficult for cell infiltration into decellularized cartilage tissue. Results from various studies suggest that the thickness of decellularized cartilage tissue can affect cell infiltration. For the development of tissue engineered menisci, Stapleton et al. cultured human dermal fibroblasts and porcine medial meniscal cells at a high seeding density on to 2–3 mm thick decellularized meniscal tissue. As observed via scanning electron microscopy (SEM), the cells formed a monolayer-like structure across the surface of the acellular porcine meniscus with no infiltration into the tissue scaffold [119]. Similar findings of low cell infiltration were seen in repopulation of decellularized porcine trachea cartilage with newborn porcine auricular chondrocytes [116]. For articular cartilage tissue engineering, Peretti et al. used 1 mm thick slices of decellularized ovine donor cartilage cultured with ovine chondrocytes [121]. Three pieces of the chondrocyte-seeded decellularized cartilage sheets were glued together with fibrin glue and implanted into the subcutaneous tissue of nude mice for 42 days. At 28 days, under histological evaluation the fibrin glue was replaced by new cartilage tissue and by 42 days, there was growth of the donor chondrocytes along the exposed cartilage surfaces, but not within the construct. To address this problem, Gong et al. showed that infiltration of decellularized cartilage tissue can be improved by using thinner cartilage tissue. Gong’s group developed a “sandwich model”, in which adult porcine auricular cartilage cut into 6 mm-diameter cylinders were frozen and sectioned into 10 μm and 30 μm thick sheets [99]. Twenty of these sheets were stacked with 5 μl of chondrocyte suspension (100 × 106 cells/ml), isolated from newborn porcine auricular cartilage, between each sheet and cultured for 16 weeks in vitro or 4 weeks in vitro with an additional 4 or 12 weeks of subcutaneous implantation in nude mice. The 10 μm thick sheets had superior cartilage formation compared to 30 μm thick sheets after implantation in vivo. This may be attributed to the formation of thicker constructs when using 30 μm thick sheets, which can impede the diffusion of nutrients through the construct. Histological analysis of in vivo constructs demonstrated formation of cartilage tissue with an abundance of newly formed matrix. The wet weight of their cartilage constructs that were implanted in vivo was significantly higher than constructs cultured in vitro (Figure 2B). Safranin O and Toluidine blue staining demonstrated the presence of proteoglycan, and IHC demonstrated the presence of type II collagen (Figure 2A). As determined by loading in confined compression, the compressive Young’s modulus of the cell sheets was about 87% of that of normal pig ear cartilage after 12 weeks implantation (Figure 2C). After 7 days in culture, SEM of the constructs showed a uniform distribution of cells in both the 10 and 30 μm thick sheets, which may be attributed to the thin nature of the sheets with cells seeded between each layer, allowing cells to infiltrate from both the top and bottom of each sheet within each construct.
Figure 2.
Tissue engineered cartilage after in vitro culture (4 and 16 weeks) or after in vitro culture for 4 weeks follow by in vivo implantation (4 and 12 weeks). (a) Macroscopic view and histology of tissue engineered cartilage stained with hematoxylin and eosin, Safranin O, Toluidine blue and type II collagen immunohistochemistry. Arrows show non-degraded acellular cartilage sheets. Scale bars: 100 mm. (b) Wet weight of tissue engineered cartilage at different time points expressed in milligram (mg). (c)Young’s modulus of tissue engineered cartilage at different time points compared with normal porcine auricular cartilage. Reprinted from Gong Yi Yi et al. 2011 with permission from Elsevier Publisher, Ltd.
In addition to using cartilage sheets, cartilage particles can also be used in cartilage tissue engineering [10, 104, 122, 123]. Peretti et al., while using non-decellularized ovine articular cartilage chips, created constructs made from fibrin glue, ovine articular chondrocytes and allogeneic cartilage chips. These constructs, along with control samples made from either fibrin glue alone or fibrin glue with chondrocytes and/or cartilage chips, were implanted subcutaneously into nude mice for 6, 9, or 12 weeks [104]. Histology demonstrated that constructs containing fibrin glue, chondrocytes and cartilage chips produced new cartilage-like matrix between the cartilage chips, while fibrin glue with cartilage chips produced fibrous tissue. Control samples containing fibrin glue and chondrocytes also produced cartilaginous matrix but had substantial loss of mass after in vivo culture for 12 weeks. With gross mechanical probing using forceps, constructs containing fibrin glue and chondrocytes with or without cartilage chips demonstrated greater stiffness than control groups (fibrin glue alone or fibrin glue with cartilage chips). In later studies, decellularized cartilage particles were incorporated into tissue engineered cartilage constructs [10]. Using acellular human articular cartilage particles with diameters ranging from 500 nm to 5 μm, Yang et al. created 3D porous acellular scaffolds by crosslinking the ECM particles and assessed the growth of chondrogenically-induced canine bone marrow-derived MSC in the scaffolds both in vitro and in vivo [10]. The decellularized ECM particles were frozen and lyophilized prior to cross-linking with dehydrothermal treatment and carbodiimide solution, which forms collagen-collagen and collagen-aggrecan cross-links. SEM of the constructs revealed adhesion of MSC displaying chondrocyte-like morphology adhering to the surrounding matrix. To identify transplanted cells, chondrogenically-induced canine bone marrow-derived MSC were labeled with PKH26, a fluorescent membrane dye. Histologic sections of in vivo samples exhibited PKH26 labeled cells surrounded by positive type II collagen stained matrix, suggesting that the implanted bone marrow-derived MSC were the source of collagen matrix. The ECM of cartilage can also bind to and sequester growth factors such as TGF-β1, IGF-1 and BMP-2, that are released by resident chondrocytes and continue to remain in the ECM even after decellularization [125]. The presence of growth factors in decellularized cartilage particles could play a role in the preservation or promotion of a chondrogenic phenotype.
4.3 In vitro cell-derived decellularized cartilage ECM
Although native tissue-derived cartilage ECM may serve as a scaffold material for cartilage engineering, there are challenges with using allogenic or xenogeneic tissues as previously mentioned, including the risk of pathogen transfer and induction of adverse host immune response. Similar to decellularized in vitro cell-derived bone matrix, there is an interest in developing decellularized cartilage ECM derived from cultured cells. Autologous ECM scaffolds can be engineered by isolating and expanding a patient’s own cells ex vivo, thus preventing host immune reactions.
Various methods have been developed to engineer in vitro cell-derived ECM. The majority of them involve using a polymer, such as collagen [126], poly(lactic-co-glycolic acid) (PLGA) [127, 128], or alginate [86, 129], as a template. Depending on the formulation, the polymer may either remain in the scaffold or can be degraded, leaving behind only the cell-deposited ECM. Lu et al. created cell-ECM-PLGA complexes, using PLGA as a template for the matrix architecture [128]. Human bone marrow-derived MSC were seeded onto both sides of a PLGA knitted mesh disc and cultured for 5 days. The constructs were decellularized by various methods before PLGA was removed by incubating with 0.5M Na3PO4 aqueous solution at 37° C for 48 hours. The remaining ECM was freeze dried before seeding with human bone marrow-derived MSC. Culturing cells in 2 or 3D cultures without the use of polymer has also been successful in forming ECM for cartilage engineering [130–132].
Different cell types have been studied as a source for ECM, including chondrocytes [86, 126, 130, 131, 133], MSC [127, 128, 130, 134] and fibroblasts [127, 130]. Hoshiba et al. compared decellularized ECM derived from cultured human articular chondrocytes, human MSC or human dermal fibroblasts and compared their effects on the adhesion, proliferation and differentiation of human articular chondrocytes [130]. When human articular chondrocytes were cultured on decellularized ECM derived from chondrocytes, MSC or fibroblasts, there was a significant increase in chondrocyte adhesion in chondrocyte-derived ECM compared to that from MSC or fibroblasts, while proliferation was suppressed in the chondrocyte-derived ECM compared to MSC or fibroblast-derived ECM. ECM obtained from the same cell source as the reseeded cells may be a more favorable microenvironment for cell adhesion as different cell populations can synthesize ECM containing distinctive molecules that have various effects on cell behavior. Kwon et al. showed that human MSC grown in decellularized rat calvaria osteoblast-derived ECM behaved differently compared to when they were grown in rabbit articular chondrocyte-derived ECM in the presence of BMP-2 (100 ng/ml) [132]. MSC in osteoblast-derived ECM were driven towards an osteogenic lineage with increased collagen type I production and positive von Kossa staining, which stains for calcium deposition. Additionally, there was a significant increase in expression of the osteogenic markers Runx2 and Osteocalcin. Human MSC cultured in decellularized chondrocyte-derived ECM were driven towards a chondrogenic phenotype with increased collagen type II production and positive Alcian blue staining, which stains for GAG. There was also significantly increased expression of the chondrogenic markers Sox9 and Aggrecan. This suggests that cell-specific ECMs can provide microenvironments with unique signals that can modulate MSC behavior and differentiation. Interestingly, Kwon’s group also looked at human MSC seeded on 2D plates coated with either fibronectin, collagen type I or collagen type II in the presence of 100ng/ml of BMP-2 and found that the gene expression of osteogenic markers was significantly lower in these MSC compared to MSC grown in the cell-derived ECM, suggesting that the differentiation effect of ECM may involve a complex interaction and organization of ECM molecules with MSC. Furthermore, culture in the 3D environment provided by cell-derived ECM may also contribute to MSC chondrogenesis compared to the 2D plates.
Three-dimensional pellet culture has been a standard method for driving MSC chondrogenesis. Pellet cultures provide an environment that allows for cell-cell interactions similar to pre-cartilage condensation during embryonic development [135]. When comparing MSC cultured in pellets to MSC cultured in MSC-derived or chondrocyte-derived decellularized matrix with chondrogenic induction media, cells grown in decellularized matrix produced significantly more GAG, collagen type II and aggrecan [127]. Furthermore, when human MSC were grown in collagen type I hydrogel microspheres or chondrocyte-derived decellularized ECM in the absence of chondrogenic medium, the latter group showed more positive immunohistochemical staining for Sox9 and Alcian blue-staining around newly proliferated cell clusters [126]. This suggests that MSC seeded in an acellular matrix can differentiate towards a chondrogenic lineage in the absence of chondrogenic differentiation medium.
4.4 Decellularization of cartilage tissue and in vitro cell derived extracellular matrix
The dense, compact ECM of cartilage can complicate the process of decellularization due to inefficient penetration of decellularization solutions and can act as a barrier for cells to repopulate the matrix. As a result, the process of decellularization of cartilage tissue is much more difficult than that of vascularized tissue, where the vascular system can be exploited for infusion of decellularization solutions [9, 136]. Currently, there is no standard method of decellularizing cartilage. Various methods have been cited in the literature, including physical and chemical treatments. Physical decellularization treatments include freeze-thaw cycles and pulverization of cartilage tissue to increase surface area for chemical treatments [10]. Chemical treatments include hypotonic buffers or detergents, such as SDS or Triton X-100 [136]. Freeze-thaw cycling causes cryoinjuries to cells by extracellular and intracellular ice formation and dehydration [137]. Hypotonic buffer aids in cell lysis and detergent helps to solubilize the cell membrane by disturbing lipid-lipid and lipid-protein interactions while leaving protein-protein interactions intact [138]. SDS is an ionic detergent that is generally effective in solubilizing both nuclear and cytoplasmic cellular membranes while Triton X-100 is a non-ionic surfactant commonly used in tissue decellularization due to its relatively mild effects on tissue structure [139, 140]. Both SDS and Triton X-100 have been cited in the literature to effectively remove immunogenic cellular material but they both appear to change the biological activity and mechanical integrity of the tissue ECM [141].
Multiple techniques have been explored for the decellularization of articular cartilage [99, 117, 121], meniscus [115, 142, 143] and tracheal cartilage [92, 144, 145]. Peretti et al. provided the initial concept of decellularized cartilage when they demonstrated removal of resident chondrocytes by preparing 1mm × 3mm × 5mm slices of ovine articular cartilage and exposing them to 5 cycles of freeze/thawing [121]. In addition to freeze/thawing, Gong et al. incorporated SDS in the decellularization process of frozen sections of 10 μm and 30 μm thick cartilage pieces from porcine ears. The cartilage sheets showed no positive cell nuclei with DAPI staining and empty lacunar structures on SEM [99]. SDS has been shown to be successful in decellularization of cartilage tissues, although the duration of exposure can alter the mechanical properties of the ECM. Elder et al. compared the mechanical properties of articular cartilage isolated from distal femurs of calves after 2, 4 or 8 hours of treatment with 2% SDS. They found that with increased exposure time, there was a decrease in thickness of the decellularized tissue and loss of GAG staining after 4 and 8 hours. With longer exposure to SDS, there was elimination of all nuclei by 4 hours based on H&E staining and a significant decrease in DNA content at 8 hours. Collagen staining with Picrosirius red stain and total collagen content measurements showed no change at all time points compared to untreated cartilage tissue. In terms of mechanical properties, there were no significant differences in Poisson’s ratio among the groups at any time point. As determined by creep indentation testing, the aggregate compressive modulus, which was similar to untreated controls after 2 hours of SDS treatment, was significantly reduced after 4 and 8 hours [117]. The decrease in aggregate modulus correlated with the biochemical findings, which showed an accompanying decrease in GAG content. When the same constructs were tested under tensile loading, the tensile Young’s modulus after 2, 4 and 8 hours of 2% SDS treatment remained similar compared to untreated constructs [117]. This study suggested that increased incubation time with SDS improved the removal of cell components at the cost of lost ECM proteins that can be associated with changes in mechanical properties. Similarly, for menisci, Stapleton et al. adapted a decellularization protocol that was initially used in producing acellular cardiac valves [146]. Ovine menisci were treated with 3 freeze/thaw cycles, incubated in hypotonic tris buffers to lyse the cells, treated with 0.1% SDS with protease inhibitors and 0.1% EDTA, rinsed in buffer containing RNase and DNase, and then extensively washed with hypertonic buffer and PBS to remove cellular remnants. Using this method, H&E staining showed absence of cells and retention of collagen types I, II and III by IHC. However, there was a significant loss of GAG following treatment based on Alcian blue staining, as also observed in the study by Elder et al. Tensile testing showed no significant difference between untreated menisci and decellularized meniscal tissue, but compressive properties were not evaluated [115].
When comparing SDS and Triton X-100 for decellularization of TMJ discs, Lumpkins et al. showed that both detergents preserved the general morphology of the discs as well as collagen fiber orientation, although the fibers appeared to be compressed into larger bundles not evident in untreated TMJ discs [118]. Since TMJ discs undergo cyclic compressive loading during chewing, TMJ discs decellularized by SDS or Triton X-100 were loaded with 15 compression cycles, with a compressive strain of 10% and four different loading frequencies (0.1, 0.5, 1 and 1.5 Hz). Under cyclic compressive loading, the compressive moduli values at each of the 4 loading frequencies were similar between native tissue and SDS treated tissue. On the other hand, the tangent moduli of Triton X-100 treated tissues were significantly lower than that of native tissue. Based on mechanical test findings, this study suggests SDS may be more suitable for decellularization of TMJ discs due to conservation of mechanical properties compared to native tissues; however, GAG and collagen content were not evaluated.
Similar methods of decellularization of cartilage tissue have also been applied to in vitro cell-derived cartilage matrices [128, 130, 133, 134, 141]. Elder et al. created constructs using chondrocytes grown in agarose scaffolds and compared five decellularization treatments (1% SDS, 2% SDS, 2% tributyl phosphate (TnBP), 2% Triton X-100 and a combination of hypotonic/hypertonic solutions) for different durations and assessed cellularity and biochemical and biomechanical properties [141]. Treatment with 2% SDS for 1–2 hours was most effective in removing cellular content and preserving ECM components. DNA content decreased by 33% with similar GAG content compared to undecellularized control groups based on quantitative assays. Preservation of collagen content was observed with Picrosirius red staining. In terms of mechanical properties, in vitro cell-derived cartilage matrices treated with 2% SDS for 1–2 hours displayed similar aggregate compressive modulus values under creep indentation testing and increased tensile Young’s modulus relative to control undecellularized matrices. Increasing incubation time in 2% SDS treatment to 8 hours resulted in a 46% decrease in DNA content but caused a significant loss of GAG content and reduction in aggregate and tensile Young’s modulus [141].
Enzymatic decellularization of native cartilage tissue has not been presented in the literature, however, it has been used for decellularization of in vitro cell-derived ECM [130]. Hoshiba et al. created cell-derived ECM from human dermal fibroblasts, articular chondrocytes and MSC and treated the engineered cartilage by either enzymatic decellularization (0.025% trypsin and 0.002% EDTA in phosphate-buffered saline) or chemical decellularization (hypotonic solution containing 0.1% Triton X-100). Both methods were successful in removing cellular components from the cell-derived ECM. However, when evaluating human articular chondrocyte adhesion to the decellularized ECM, it was found that there was a greater percentage of adherent cells on ECM decellularized by the chemical method compared to the enzymatic method [130]. Further studies would need to evaluate the effects of different decellularization methods on ECM components and organization that would result in differences in cell adhesion. The different techniques of cartilage tissue and cell-derived cartilage tissue decellularization are summarized in Table 2.
Table 2.
Methods for tissue-derived and cell-derived cartilage ECM
| Tissue Type | Decellularization Method | Sterilization | Cell Repopulation | References |
|---|---|---|---|---|
| Cartilage Tissue | ||||
| Porcine auricular cartilage | 1% SDS for 24 hrs or 7 days | Unspecified | Chondrocytes from porcine auricular cartilage | [99] [125] |
| Porcine tracheal cartilage |
|
Unspecified | Chondrocytes from porcine auricular cartilage | [116] |
| Rat tracheal cartilage |
|
Unspecified | Rat bone marrow-derived MSC | [92] |
| Human cadaveric joint cartilage |
|
60Co γ irradiation (5 Mrad) | Canine bone marrow-derived MSC | [10] |
| Cell-Derived Cartilage Matrix | ||||
| Human Dermal Fibroblasts Human Articular Chondrocytes Human bone marrow-derived MSC |
|
Unspecified | Human articular chondrocytes | [130] |
| PLGA mesh cultured with either human articular chondrocytes or human bone marrow-derived MSC |
|
Ethylene oxide | Human bone marrow-derived MSC | [128] |
| Porcine chondrocytes microencapsulated in type I collagen | 2% Sodium deoxycholate for 1 hr at room temperature | Unspecified | Human bone marrow-derived MSC | [126] |
| Bovine articular chondrocytes cultured in PCL mats | Three freeze/thaw cycles | Ethylene oxide | Laprine bone marrow-derived MSC | [133] |
| Porcine articular chondrocytes | Three freeze/thaw cycles | 70% Ethanol | Laprine stifles articular chondrocytes | [131] |
| Bovine articular chondrocytes |
|
Unspecified | None | [141] |
| Human bone marrow-derived MSC cultured in PLGA template |
|
Unspecified | None | [127] |
Key: MSC= Mesenchymal stem cell, SDS= Sodium dodecyl sulfate, PLGA = poly(lactic-co-glycolic acid), PCL = Polycaprolactone
5. Skeletal muscle
Skeletal muscle makes up 38% and 42% of the average female and male body mass, respectively [147], and allows for movements, posturing and support. Skeletal muscles are connected to bones by tendons, which transmit forces created during muscle contraction to the skeletal structure, allowing for movement. Any impairment in muscle function can be debilitating and severely affect one’s quality of life. Muscle injury can be the result of trauma such as lacerations, ruptures or contusions, muscle disease such as the various forms of muscular dystrophy, ischemia, exposure to myotoxic agents, or exercise-induced muscle damage. Unlike cartilage tissue, however, skeletal muscle has a remarkable ability to regenerate and heal after an injury.
Skeletal muscle regeneration is a highly synchronized process that requires coordination between the degeneration and regeneration processes. The degeneration process occurs immediately after the injury when there is an influx of inflammatory cells in response to biologically active molecules that are released either by necrotic myofibers or nearby inflammatory cells [148]. Initially, neutrophils enter the site of injury within 1–6 hours followed by macrophages about 48 hours after injury [148]. These cells are necessary for the removal of cellular debris and damaged myofilaments that result from muscle injury. Macrophages also play a role in activating the myogenic cells necessary for muscle regeneration [149]. During the regeneration phase, there is proliferation and differentiation of myogenic cells followed by fusion of these cells to damaged muscle fibers for repair or to each other to form new myofibers [150]. There are several other factors that contribute to the repair process, such as satellite cells, stem cells and trophic factors, as well as the ECM, which plays a significant role in the reconstruction of myofibers [151]. One of the main reasons for skeletal muscle’s ability to regenerate is the presence of satellite cells, which are undifferentiated mononuclear myogenic cells that are normally quiescent and arrested at an early stage of the myogenic program. They are located at the periphery of mature skeletal myofibers within the basal lamina that sits between the plasma membrane of the muscle fiber and the basement membrane [152]. Following muscle injury, satellite cells are activated to proliferate and differentiate into myoblasts and fuse to form myofibers. These cells are present in all skeletal muscles; however the number of satellite cells differs among different muscles. It has also been found that the number of satellite cells decreases with age perhaps due to restricted self-renewal capacity [153]. This may contribute to the poor healing capacity seen in the elderly population.
Although skeletal muscles have a robust regenerative capacity, there are instances when skeletal muscle regeneration is challenging, such as injuries involving large volumetric muscle loss. Such extensive muscle loss is commonly seen in military personnel who are wounded in combat secondary to gunshots and blasts injuries [154] or with aggressive tumor ablations or functional damage due to myopathies [155]. Self regeneration is limited in these cases because the remaining myofibers are unable to bridge the gap created by the injury. Any attempt in regeneration results in scar tissue formation in the area of injury or the muscle remodels such that the area of injury becomes permanently devoid of tissue [156, 157]. When evaluating muscle healing capacity in rats, Merritt et al. created 0.5 cm × 1 cm or 1 cm × 1 cm full thickness defects in the rat gastrocnemius and found that smaller defects healed by 14 days while the larger defects exhibited no functional recovery after 28 days [157]. Similarly, Terada et al. looked at muscle regeneration in rats in which the ends of lacerated muscle fibers were kept either 1 mm or 4 mm apart using silicone tubes [156]. It was found that muscle fibers were able to bridge the 1 mm gaps with well-aligned collagen fibers. On the other hand, muscle fibers were unable to bridge 4 mm gaps, and instead the muscle ends were covered with randomly aligned collagen fibers [156].
Clinically, restoration of large volumetric muscle loss involves transfer of autologous muscle tissue or muscle flaps from local or distant sites to the area of injury [155, 158]. Functional free muscle transplantations have been reported in the forearm [159] and elbow [160], however they have not been successfully applied to weight-bearing muscles of the lower extremities. These procedures are associated with donor site morbidity resulting in functional loss and volume deficiency at the donor site. Therefore, the ability to engineer skeletal muscle tissue may be a potential solution for replacement of damaged and large volumetric muscle loss and prevent the morbidity associated with autologous tissue transfer. Nevertheless, there are challenges associated with skeletal tissue engineering, such as creating or maintaining the complex hierarchical 3D organization of muscle fibrils and the surrounding ECM. To support muscle tissue growth and function, engineered skeletal tissue needs to be vascularized, innervated and form tendinous connections with bone allowing for mechanical musculoskeletal interaction.
5.1 General ECM of skeletal muscle
Skeletal muscle is a highly organized and complex tissue. It is not only rich in contractile elements and connective tissue but is also highly vascularized and innervated to provide essential nutrients for muscle cell growth and contractile functions, respectively. The basic units of skeletal muscle are the muscle fibers, which are individual contractile units allowing for muscle contraction. Muscle fibers are formed from the fusion of multiple mononucleated myocytes to each other creating a multinucleated syncytium. Multiple muscle fibers are then closely packed together in an extracellular 3D matrix, forming an organized tissue with high cell density and cellular orientation called a muscle fascicle which allows for longitudinal contraction [155]. A collection of muscle fascicles ultimately makes up the muscle body.
The ECM of skeletal muscle is organized in a hierarchical fashion in which the endomysium surrounds each muscle fiber, the perimysium surrounds each muscle fascicle, and the epimysium surrounds the entire muscle body [161]. The major structural protein in skeletal muscle ECM is collagen, which accounts for 1–10% of muscle mass dry weight [162, 163]. Various types of collagen are expressed during skeletal muscle development, including collagen types I, III, IV, V, VI, XI, XII, XIV, XV and XVIII [164–167]. However, collagen types I and III predominate in the adult epi-, peri- and endomysium [168]. Like other ECM of mesenchymal tissues, proteoglycans are also present in the ECM of skeletal muscle. Many of the proteoglycans in skeletal muscle ECM belong to the family of small leucine-rich proteoglycans (SLRPs), which include decorin, biglycan, fibromodulin and lumican. The majority of these proteoglycans have chondroitin sulfate and dermatan sulfate GAG chains. About 30% of the proteoglycans found in skeletal muscle ECM have heparan sulfate GAG, which are known to bind growth factors [169].
The ECM of skeletal muscle may play an intricate role in muscle development and regeneration. Skeletal muscle ECM is involved in the regulation of growth factors during regeneration by acting as a reservoir for them. Molecular studies have shown that certain growth factors bind to negatively charged GAGs, particularly heparan sulfate in the ECM. For instance, TGF-β1 and -β2 have been shown to bind to the heparan sulfate GAG chains on decorin proteoglycans [170], and fibroblastic growth factor (FGF) -1,-2 and -5 binds to the heparan sulfate GAG chains on various proteoglycans [171]. Enzymes in the ECM, such as matrix metalloproteinases, collagenase and stromelysin, can cleave growth factor-associated GAG molecules, allowing the release of growth factors necessary for cell signaling [172, 173]. The remodeling of ECM during regeneration can release trophic factors that can aid in healing. Furthermore, ECM may also participate in cell migration, particularly, the migration of satellite cells to site of injury. Cell migration involves a complex interaction between cells and the matrix through binding various integrins in the ECM. Once at the site of injury, the ECM directs cells to their proper alignment allowing for transmission of forces.
In addition to the repair of muscle fibers during skeletal muscle injuries, the repairs of peripheral nerves are also crucial. Studies have suggested that the interaction between Schwann cells and the ECM are essential for nerve growth [174]. In a wound chamber study by Liu et al., silicone tubes were sutured to the ends of transected rat sciatic nerves and the contents of the tubes were removed at interval times and assessed [174]. The chamber was initially filled with fluid similar to serum, followed by the migration of Schwann cells, endothelial cells and fibroblasts into the chamber mediated by cell-fibrin interactions. After one week, the fibrin matrix was replaced by collagen matrix prior to Schwann cell myelination and nerve fascicle formation [174]. The order of events leading to nerve fascicle formation suggests that there may be a possible role for ECM in the reinnervation of injured skeletal muscle.
5.2 Tissue-derived decellularized skeletal muscle ECM
Although there is a vast amount of work on decellularization of cardiac [175–177] and smooth muscles [178–181] and their use as biologic scaffolds for reconstruction of their respective tissues, studies on decellularized skeletal muscle have only recently begun to emerge. Besides studying the decellularization of skeletal muscle for repair of tissue involved in locomotion and movements, there is also a growing interest in engineering skeletal muscle of the abdominal wall for repair of congenital defects, such as omphalocele and gastroschisis [182–184]. Unlike cardiac and smooth muscles, skeletal muscles are larger in diameter, which is essential to generate strong forces for mechanical functions. With a larger diameter, there is a need for more extensive vascular and neural supply making it a challenging tissue to engineer [185]. The use of acellular skeletal muscle ECM as scaffolds for tissue engineering has been shown to be promising. As mentioned previously, skeletal muscle tissue relies heavily upon ECM for organization, structural support and mechanical function. Additional roles for ECM include myoblast proliferation and differentiation. Stern et al. showed that culture plates coated with ECM derived from skeletal muscle increased proliferation of C2C12 cells (a mouse myoblast cell line) as well as rat and human muscle progenitor cells (MPC) or satellite cells compared to rat tail collagen type I coated surfaces and uncoated tissue culture plastic [186]. Furthermore, C2C12 cells cultured on surfaces coated with skeletal muscle ECM extracts differentiated faster and produced more mature myotubes in comparison to cells cultured on the uncoated surfaces [186].
These results suggest that skeletal muscle ECM creates an environment that is myogenic for progenitor cells. Similar findings have also been demonstrated in in vivo studies [157, 187]. Perniconi et al. replaced normal tibialis anterior (TA) muscle in mice with acellular ECM scaffolds derived from mouse TA and found that at two weeks post-implantation there was infiltration of both inflammatory cells and cells with positive markers for muscle interstitial stem cells. These are similar cell populations to those seen during skeletal muscle degeneration and regeneration [187]. As implanted ECM remodels, it releases stored biologic factors that attract myogenic progenitor cells and stimulate their proliferation and differentiation [186, 188, 189]. Similarly, to enhance recovery of gastrocnemius defects in rats, Merritt et al. implanted decellularized rat gastrocnemius muscle ECM into 1 cm × 1 cm defects and after 42 days found that there were significantly higher numbers of desmin-positive myofibers and vWF-positive endothelial cells within the ECM graft, suggesting the formation of new myofibers and blood vessels, respectively. However, these were prevalent at the periphery of the scaffolds, while there was no evidence of regenerating fibers or blood vessels within the central region of the ECM [157]. In a follow up study by Merritt et al., acellular rat gastrocnemius muscle ECM scaffolds were similarly implanted into rat gastrocnemius defects and rat bone marrow-derived MSC were injected into multiple locations within the scaffolds seven days after scaffold implantation. With injection of rat bone marrow-derived MSC, the repair tissue after 42 days demonstrated significantly higher isometric force compared to ECM only controls and there was more intense staining for desmin-positive myofibers and vWF-positive endotheial cells [158]. Although the number of myofibers and blood vessels in the central region was significantly lower than that in the proximal or distal regions of the scaffold with the injected MSC group, it was generally higher than the corresponding ECM scaffold only group [158]. The presence of increased myofibers and blood vessels in the proximal and distal ends of the implanted ECM may be due to regeneration of myofibers transected during implantation. In a similar study, Conconi et al. created abdominal wall defects in rats, treated them with either acellular abdominal muscle ECM only or acellular abdominal muscle ECM pre-incubated with autologous myoblasts and examined the defects at various time points up to 90 days after implantation [184]. The defects treated with acellular ECM alone were completely replaced by fibrous tissue with no detection of electrical activity. In contrast, implants containing myoblasts showed well preserved myoblasts with integration into the host tissue as well as an increased number of blood vessels and myoblasts within the implants at 30 days post surgery. Interestingly, electrical activity increased from sporadic motor-unit potentials with short discharges at 12 days after surgery, to single motor-unit potentials ranging from 150–300 μV at 30 days. These continued until 90 days, after which there was general worsening of electromyographic activity with potentials between 25–30 μV [184].
As tissue engineered skeletal muscle constructs grow, there is an increased demand for nutrients which may not be met due to limited vascular supply, which could explain the worsening of electrical activity potentially indicating cell death overtime [184]. Similarly, the lack of myofibers in the central region of Merritt’s cellular construct could also be secondary to the lack of vascular supply in the middle region of the scaffold which would be necessary to support the proliferation of myofibers [157, 158]. The presence of native vascular supply along the proximal and distal ends of the scaffold supports the nutritional demands of myoblasts in these regions allowing for robust growth. Therefore, improving the vascular supply could potentially improve the function of the engineered tissue. Methods to improve vasculature could include the use of angiogenic factors [190], co-culturing myoblasts or stem cells with endothelial cells [191], transfecting cells to upregulate expression of genes associated with vascular tissue formation [192] or creating an arteriovenous unit within the scaffold [193]. Overall, use of acellular skeletal muscle ECM as scaffolds is promising but further work is necessary to improve on current methods. Unlike tissue engineering of bone and cartilage, there are currently no published studies on cell-derived skeletal muscle ECM. Future studies could involve co-culturing myoblasts or satellite cells with endothelial cells and neural cells to create a cell-derived skeletal muscle ECM with properties that may promote vascular and neural tissue formation within regenerating functional muscle tissue.
5.3 Decellularization of skeletal muscle
Decellularization of skeletal muscle is a relatively new method of creating scaffolds for skeletal muscle engineering. One of the earlier studies that looked at decellularization of skeletal muscle was by Carlson et al., in which they developed a multi step method to prepare acellular muscle. Rat extensor digitorum longus muscles were isolated and treated with both X-radiation and Marcaine and the treated tissue was grafted back into rats for 7–14 days. Afterward the tissues were explanted and subjected sequentially to EDTA, Triton X-100, DNase and sodium deoxycholate to remove cells. Using this method they were able to obtain acellular muscle grafts [194]. Later studies reported decellularization methods that did not require the use of radiation or reimplanting the graft back into the donor. Many of the later attempts to decellularize skeletal muscles have incorporated freeze-thawing methods [195] or detergent and enzymatic treatments, with either SDS, Triton X-100, sodium deoxycholate, trypsin or a combination thereof to remove cellular components [93, 157, 158, 186, 187, 196, 197]. Additional treatments with chloroform showed improvement of lipid extraction from muscle grafts [157, 158, 197]. Qing et al. treated skeletal muscle from rats with different concentrations of SDS for various amounts of time to determine the optimal decellularization conditions and found that tissue treated with 1% SDS for 72 hours at 4°C was the best condition tested, as treatment did not result in significant differences in ultimate tensile strength or hydroxyproline content compared to native tissue [93]. Longer treatment times and higher SDS concentrations led to significant decrease in hydroxyproline content compared to normal tissue [93]. Instead of using detergents, Gillies et al. developed a decellularization method that prevents extensive disruption in the ECM by utilizing latrunculin B to disrupt the actin cytoskeleton, osmotic shock to induce cell lysis, high ionic strength salt solution to depolymerize myosin and DNase I to remove residual DNA [198]. When comparing grafts decellularized with latrunculin B to grafts treated with trypsin and Triton X-100 according a protocol used by Stern et al.,[186] it was found that more DNA was removed with a 40% reduction in GAG compared to 75% reduction in GAG using the method established by Stern et al. Collagen content remained relatively similar in the grafts treated with osmotic shock along with actin and myosin depolymerization compared to those treated by the method used by Stern et al.[198]. Like cartilage tissue, there is currently no ideal method to decellularize skeletal muscle. A balance must be achieved to remove as much of the cellular component as possible without compromising the mechanical and biochemical properties of the ECM. The different techniques of skeletal muscle tissue decellularization are summarized in Table 3.
Table 3.
Methods for tissue-derived skeletal muscle ECM
| Tissue Type | Decellularization Method | Sterilization | Cell Repopulation | References |
|---|---|---|---|---|
| Skeletal Muscle Tissue | ||||
| Rat gastrocnemius muscles |
|
UV Light | Rat bone marrow-derived MSC | [157] [158] |
| Mouse extensor digitorum longus muscles |
|
UV Light | C2C12 cell line (myoblasts derived from mouse lineage) | [196] |
| Canine quadriceps and hamstring muscles |
|
Ethylene Oxide |
|
[197] |
| Mouse tibialis anterior muscles |
|
Unspecified | C2C12 cell line | [198] |
| Rat quadriceps and hamstring muscles |
|
Unspecified | None | [186] |
| Mouse tibialis anterior and extensor digitorum longus muscles |
|
Unspecified | None | [187] |
| Rat abdominal muscles |
|
Unspecified | Rat myoblasts | [182] [183] [184] |
Key: MSC= Mesenchymal stem cell, SDS= Sodium dodecyl sulfate, w/v =weight/volume, v/v =volume/volume, EDTA=Ethylenediaminetetraacetic acid
6. Tendons and ligaments
Tendons and ligaments are specialized connective tissues that are responsible for transferring tensile forces from skeletal muscle to bone or from bone to bone, respectively, allowing for locomotion, movement and joint stability. For tendons and ligaments to function effectively, they respond to changes in mechanical forces by modifying their cellular metabolic rates as well as structural and mechanical properties [199–201]. Different tendons and ligaments in the body are subjected to different levels of mechanical loads. In humans, the peak force transmitted through the Achilles tendon while running is 9 kN, which is about 12.5 times the body weight [202, 203], while flexors of the wrist experience forces between 1 and 6 N during passive movements [204]. Therefore, when engineering tendons or ligaments it is important that the grafts are constructed to withstand the specific forces they will experience in vivo.
Tendons and ligaments exhibit anisotropic mechanical behavior and are relatively compliant under slow loading conditions due to their viscoelastic behavior and their ability to recruit “crimped” collagen fibers [205]. Crimped collagen fibers are unloaded collagen fibers that have a wavy pattern; with increased tensile loads these collagen fibers straighten out and exhibit increased stiffness, allowing tendons and ligaments to withstand higher forces [206]. With increasing tensile loads, collagen fibers exhibit maximal stiffness and are more effective in withstanding large loads [205]. Both of these connective tissues also share similar viscoelastic properties, aiding in the maintenance of joint homeostasis. A property that is specific to ligaments is their role in joint proprioception, which is the ability to perceive the position of the limb in space. This perception is based on the strains experienced by the ligament, invoking neurological feedback signals that activate muscular contraction in response to limb positioning [206]. These properties are important in maintaining joint stability in response to their functional demands.
Traumatic injuries to tendons and ligaments often result in partial or complete tears. Injuries to these tissues can induce abnormal joint motions and alter joint loading patterns, resulting in degenerative joint disease over time [207]. Tendons and ligaments share a similar healing process that follows three phases: inflammatory phase, proliferation phase and remodeling phase [205, 206]. The inflammatory phase involves the migration of inflammatory cells to the site of injury, where necrotic materials are removed by phagocytosis. Chemotactic factors are released by the inflammatory cells, which recruit tendon and ligament fibroblasts for the proliferation phase. Tendon and ligament fibroblasts then synthesize abundant collagen and other ECM components such as proteoglycan and deposit them at the wound site. This results in the production of scar tissue, which forms a dense, cellular, collagenous connective tissue that bridges the torn ends. During the remodeling phase, there is decreased cellularity as well as decreased collagen and GAG synthesis. The scar tissue is remodeled, forming a fibrous tissue.
Clinically, there are two main management strategies for injured tendons and ligaments: either they can be left untreated and allowed to heal naturally or they may require surgical intervention depending on the patient’s activity requirements and comorbidities. Surgical procedures typically involve primary repair, use of autografts or allografts, or use of biological grafts such as porcine small intestinal submucosa [12, 14, 208] or acellular dermal matrix grafts [207, 209]. However, whether they heal on their own or are surgically repaired, the biochemical and mechanical properties of the healed tendon or ligament are typically inferior to those of native tissue resulting in potential risks for re-rupture [210, 211].
6.1 General ECM of tendons and ligaments
Since tendons and ligaments share similar mechanical properties, it follows that they would also share similar architecture and ECM components. Both of these connective tissues are characterized by sparse cellularity distributed within an ECM, similar to cartilage tissue. Fibroblasts are the cells that reside within tendons and ligaments. Both tissues have a multi-unit hierarchical structure consisting of collagen molecules that come together to form collagen fibrils, fiber bundles, fascicles and tendon or ligament units aligned parallel to the long axis of the tissue [205]. The fibril is the smallest structural unit, made up of collagen molecules that are aligned end-to-end. Collagen fibers are composed of collagen fibrils bound by endotenons, which are thin layers of connective tissue containing vessels, lymphatics and nerves. Fascicles are composed of collagen fibers that are bound by the epitenon [212].
Tendon ECM consists of collagens, proteoglycans, glycoproteins, water, and tendon cells (tenocytes and tenoblasts). Type I collagen is the most abundant component, making up 60% of the dry mass and 95% of total collagen [213]. The remaining 5% of collagen are types III and V [214]. Type III collagen is typically located in the endotenon and epitenon, but its synthesis is upregulated in degenerative or highly stressed tendons [214, 215]. Type V collagen is intercalated into the type I collagen and regulates fibril growth [216]. Types II, VI, IX, X and XI are found in trace quantities mainly in fibrocartilage of the bone insertion point [217]. Proteoglycan content varies with the site of the tendon and depends on the specific mechanical loading conditions typically experienced at that location [218]. In compressive load-bearing regions, there is a higher content of proteoglycan compared to regions experiencing tensile loads [219, 220]. Similar to cartilage, the proteoglycans hold water and are necessary for resisting compressive loads. Glycoproteins present in tendon ECM include tenascin-C, which interacts with collagen fibrils to provide mechanical stability of the ECM, and fibronectin, which is located on the surface of collagen and plays a role in wound healing [221, 222]. The ECM is also comprised of elastin which makes up about 2% of the dry weight of tendons and drives recovery of the crimped collagen fibers after stretching [223, 224]. Within the tendon ECM, tenocytes are aligned in rows and situated between collagen fiber bundles [205]. They are responsible for synthesizing ECM proteins, organizing the collagen matrix and remodeling the ECM in response to environmental factors.
The extracellular tissue of ligaments is made up of about one-thirds ECM components and two-third water, with the water providing ligaments with their viscoelastic behavior [206]. Similar to tendon ECM, type I collagen is the most abundant collagen in ligament ECM, making up 85% of total collagen and accounting for 75% of the dry weight [206]. Other collagens in ligament include types III, VI, V, XI and XIV. Ligament ECM also includes proteoglycan, elastin, and laminin [206]. Unlike tendons, the composition of ligament’s ECM is not as well characterized and is still an area of ongoing research.
6.2 Tissue-derived decellularized tendons and ligaments ECM
As shown in tissue engineering of skeletal muscle, re-seeding decellularized scaffolds with cells can improve their mechanical and biochemical properties compared to acellular scaffolds [158, 184]. However, one of the major hurdles to overcome when using acellular scaffolds for tissue engineering is the ability to repopulate decellularized scaffold with cells. Poor recellularization of decellularized tendons and ligaments has been attributed to changes in matrix architecture after decellularization which can inhibit repopulation and remodeling of the graft [225] or residual detergent components that can decrease proliferation [226]. Several studies have indicated that tendon grafts that were decellularized with SDS were more resistant to cellular in-growth compared to other detergents [227, 228]. Harrison et al. compared cellular ingrowths into porcine ACL decellularized with either SDS, TnBP or Triton X-100 and found that SDS-treated grafts had the lowest cell infiltration, followed by Triton X-100-treated grafts, and then TnBP-treated grafts, which exhibited the most central cellular ingrowths [228]. Different approaches have been developed to repopulate scaffolds used in tissue engineering, such as static cultures [229], pulsatile perfusion [230], and centrifugal force [231]; however, the resulting cell distribution is usually not homogenous, and the process may require a large number of cells. Re-seeding grafts in static cultures commonly resulted in clusters of cells on the scaffold surface without central infiltration [232]. Harrison et al. also found that the addition of epidermal growth factor (EGF) to culture medium did not significantly increase fibroblast ingrowths into decellularized porcine ACL [228]. Other biologic factors, such as FGF-2 and IGF-1, have been shown to regulate fibroblast behavior and their benefit in stimulating cellular ingrowths will need to be further investigated [233]. Ingram et al. developed a novel use of ultrasonication to stimulate human tenocyte infiltration into acellular porcine patellar tendon [234]. Various ultrasonication intensities were tested. At 90 W, there was no change in the scaffold architecture, while at 360 W there was significant gapping between collagen bundles and the surrounding endotenon. With ultrasonication intensities above 360 W, there was visible damage to the collagen bundles, such as large holes within the fascicles with complete removal of the endotenon [234]. Therefore, ultrasonication at 360 W with pulse application time of 1 sec “on” and 1 sec “off” for a total of 1 minute worked best in opening collagen bundles, resulting in a more porous scaffold and allowing for cell infiltration without extensive collagen degradation. After 7 days in culture, live tenocytes were found within the scaffold, but by 21 days in culture tenocytes in the central area of the scaffold were 50% viable [234]. This was attributed to scaffold thickness and limited nutrient supply. In further studies using ultrasonicated 500 μm thick tendon fascicles, cell viability throughout the scaffold was maintained for up to 3 weeks in culture [234].
Acellular ECM of tendons and ligaments has supported growth of tenocytes and fibroblasts [226, 228, 232, 234], bone marrow-derived MSC [235], adipose-derived MSC [236] and tendon progenitor cells [237], all of which can be potential candidates for the tissue engineering of functional tendon or ligament. However, tenocytes have limited proliferative potential and can quickly lose their phenotype in culture [238] and bone marrow-derived MSC have formed ectopic bone in rabbit tendons [239]. In recent years, tendon stem/progenitor cells (TSCs) have been identified in humans, mice, rabbits and rats [240–242]. TSCs differ from tenocytes in that they posses clonogencity, self-renewal and multi-differentiation potential [241]. Tendon stem cells are programmed to differentiate into tenocytes and have been found to form tendon-like tissue in vivo [240, 241]. However, TSCs make up only 5% of total tendon cells and when they are expanded on plastic tissue culture plates, they typically lose their stemness after several passages [240]. Zhang et al. demonstrated that tendon matrix from decellularized rabbit patellar tendon tissue was able to stimulate rabbit patellar tendon-derived TSC proliferation and preserve their phenotype in vitro [237]. TSCs grown on tissue culture plates coated with rabbit decellularized tendon ECM had formed large colonies with significant upregulation of stem cell genes (oct-4 and nanog) and tenocyte-related genes (collagen type I, III, and tenomodulin) compared to TSCs cultured on uncoated plastic surfaces [237]. Subcutaneous implantation of acellular rabbit patellar tendon ECM seeded with human patellar tendon-derived TSCs in the backs of nude rats promoted tendon-like tissue formation compared to TSCs alone [237]. Further studies will need to compare the mechanical and biochemical properties of these scaffolds cultured with different cell types.
Another hurdle in tendon and ligament tissue engineering is creating tendon to bone and ligament to bone interfaces. As shown in ACL repair, it is often difficult to obtain sufficient integration and stable fixation between tendon and bone, resulting in postoperative knee laxity and ultimately symptomatic instability [243, 244]. Studies have shown that bone to bone healing typically creates a stronger fixation than tendon to bone healing. Bone to bone healing occurs by endochondral ossification at the healing surface, while tendon to bone healing involves extensive scar tissue formation that remodels over time [245]. The use of acellular tissue derived ECM technology can potentially create constructs analogous to bone-patellar tendon-bone grafts without the associated morbidity. Woods et al. was able to demonstrate effective decellularization of porcine bone-ACL-bone constructs (Figure 3) [94]. These constructs can potentially be reseeded with cells and used in ACL repair. Endress et al. created bone-collateral ligament-bone composites grafts from human finger proximal interphalangeal joints as a substitute ligament for scapholunate ligament repair [246]. Complete decellularization of bone and ligament was demonstrated without adversely affecting the scaffold integrity, ultimate load and displacement to failure and stiffness. Furthermore, they were able to reestablish cellularity after reseeding their scaffold with green fluorescent protein labeled cells [246]. Experimentally, they also demonstrated fixation of their composite graft on to cadaver scapholunate joints by creating scaphoid and lunate troughs and inserting bone plugs from the bone-collateral ligament-bone graft into the carpal troughs [246]. This approach can potentially be applied to various ligaments or tendon injuries in the body; however, further in vivo studies are needed to evaluate integration and the ability for the graft to be retained and functional in vivo.
Figure 3.
Decellularized and untreated ACL and its tibial insertion site in porcine samples stained with hematoxylin and eosin at 20× magnification. (a) Untreated ACL with collagen fibers (F) passing through fibrocartilage (FC), mineralized cartilage (MC) and bone (B). (b) ACL treated with Triton–SDS. (c) ACL treated with Triton–Triton. (d) ACL treated with Triton–TnBP. Scale bars: 100 μm. Reprinted from Woods et al. 2005 with permission from Elsevier Publisher, Ltd.
Porcine tibial insertion of decellularized and untreated Anterior Cruciated Ligaments.
6.3 Decellularization of tendons and ligaments
The ideal decellularization method would be one by which cellular components are completely removed and the structural architecture of the natural ECM is preserved. Different studies have compared various methods of decellularization of tendons and ligaments. Although there is no consensus on which method is superior, the use of detergents seems to be preferred among many different studies. Cartmell et al. compared Triton X-100, sodium dodecyl sulfate (SDS) and tri(n-butyl)phosphate (TnBP) in the decellularization of tendon tissue and found that 1% SDS treatment resulted in complete decellularization with preservation of tendon mechanical and structural properties [247]. TnBP, an organic solvent, was successful in removing cellular components, but did not preserve tissue mechanical properties. Triton X-100 failed at removing cells and disrupted collagen structure with increased collagen denaturation [247]. Similarly Hu et al., demonstrated that treatment with Triton X-100 resulted in a 50% decrease in tensile strength and stiffness compared to fresh rat tail tendon [248]. In terms of ligament decellularization, Woods et al. compared decellularization of porcine bone-anterior cruciate ligament-bone constructs using SDS, TnBP and Triton X-100 [94]. Tissue samples were divided into three sections, soft tissue at the tibial end, mid substance and soft tissue at the femoral end, and decellularization of each section was compared. SDS and TnBP were effective in removal of cellular nuclei with removal of about 90–97% of cell nuclei from the soft tissue regions adjacent to the tibial and femoral ends; however, SDS affected both the GAG and collagen content and increased the stiffness of the ligament. Alternatively, Deeken et al. compared decellularization of central tendon in porcine diaphragm using peracetic acid, Triton X-100, SDS and TnBP and found that 1% TnBP was most effective [139]. Although both TnBP and SDS treated tissues lacked cell nuclei, SDS treated tissue contained residual cellular components. The authors suggested that the cellular components could be removed with more vigorous rinse treatments [139]. Similar comparisons have been studied using human flexor tendons by Pridgen et al., finding SDS to be more effective than Triton X-100 and TnBP for removing cellular debris [249]. In all treatment groups there was no reduction in GAG or collagen and no significant difference in elastic modulus and ultimate tensile strength between the different treatment groups and native tissue [249]. Overall, the results of various studies suggest that treatment with SDS appears to be the most effective in removing cellular components from these tissue types. However, there are inconsistent reports of its effect on maintaining collagen architecture after decellularization.
Although various studies have shown effective decellularization with H&E staining or quantification of DNA content of treated tendons, there is no evidence that the decellularization process also eliminates major histocompatibility complexes (MHC), which are immunogenic. The removal of MHC is crucial when using xenografted or allografted tissue. Raghavan et al. determined the immunogenicity of their decellularized human flexor tendon both in vitro and in vivo [250]. Human flexor tendons were treated with EDTA, SDS and peracetic acid and demonstrated effective removal of cells and MHC-1 complexes with IHC. Decellularized tendons were implanted into immunocompetent Wistar Rats for 2 and 4 weeks, and IHC showed increased infiltration of B-cells in control untreated tendons compared to acellular tissue in both 2 and 4 week samples, suggesting that the decellularization method resulted in removal of cellular antigens and decreased immune response in Wistar rats [250].
Although decellularization can remove immunogenic donor cells from tissues, there are still a small number of cellular components that may induce immunogenic reactions found in xenografts. One such cellular component is the alpha-gal epitope, which is a carbohydrate structure that is absent in humans but present in non-primate mammals, such as porcine and bovine animals [251]. Interaction between human anti-alpha-gal antibodies and alpha-gal epitopes is an important obstacle for using xenografts in humans [252]. This issue can be avoided by treating xenografts with alpha-galactosidase to remove the epitope [26]. Yoshida et al. evaluated the response of human peripheral blood mononuclear cells (PBMCs) to decellularied bovine ACL fascicles compared to untreated ACL fascicles and decellularized ACL fascicles treated with alpha-galactosidase [253]. They found that there was increased migration of PBMCs towards untreated ACL fascicles and increased secretion of IL-6 compared to decellularized ACL fascicles and alpha-galactosidase treated decellularized ACL fascicles [253]. On IHC using anti-alpha-gal antibody, epitopes were present throughout the untreated ACL, but not seen in the decellularized ACL either treated or untreated with alpha-galactosidase. These results suggest that decellularization alone may be enough to remove alpha-gal epitopes and minimize immunogenic reactions of human PBMCS to some xenografts [253]. The different techniques of tendon and ligament tissue decellularization are summarized in Table 4.
Table 4.
Methods for tissue-derived tendon and ligament ECM
| Tissue Type | Decellularization Method | Sterilization | Cell Repopulation | References |
|---|---|---|---|---|
| Tendon & Ligament Tissue | ||||
| Rat tail tendons |
|
70% Ethanol | None | [247] |
| Central tendons of porcine diaphragms | 1% (v/v) TnBP for 24 hrs at room temperature | 70% Ethanol | L929 mouse fibroblast cells | [139] |
| Porcine patella tendons |
|
0.1% Peracetic acid | Human tenocytes | [234] |
| Canine infraspinatus tendons |
|
Unspecified | Canine bone marrow-derived MSC | [235] |
| Human flexor digitorum superficialis and flexor digitorum profundus tendons | 0.1% SDS for 24 hrs | Unspecified | Human dermal fibroblasts | [249] |
| Human flexor digitorum profundus tendons |
|
Unspecified | None | [250] |
| Laprine semitendinosus tendons | 1% SDS for 24 hrs | 70% Ethanol | Laprine dermal fibroblasts | [226] |
| Porcine anterior cruciate ligaments |
|
Unspecified | Human anterior cruciate ligament fibroblasts | [232] |
| Porcine bone-anterior cruciate ligament-bone | 1% Triton X-100 | Unspecified | None | [94] [228] |
| Bovine anterior cruciate ligaments | 0.25% Triton X-100 + 0.25% Sodium deoxycholate for 24 hrs at 37°C | Unspecified | None | [253] |
Key: MSC=Mensenchymal stem cell, SDS=Sodium dodecyl sulfate, TnBP=Tri(n-butyl)phosphate, v/v= volume/volume,
7. Conclusions and future outlook
Although autografts are the gold standard method for the repair of orthopaedic tissues, they are associated with donor site morbidity and are limited in supply [5]. Allografts and xenografts are potential tissue sources; however, they pose the risk of generating a severe immune response and rejection due to the presence of cellular components and the expression of major histocompatibility complexes [251]. Using the novel technique of decellularization, cellular components that can elicit an immune response can be removed from allografts and xenografts, improving their potential as alternative tissue sources. Decellularized allografts and xenografts can serve as scaffolds for patient-specific cells and composite constructs can be used in surgical repair of damaged tissues. This has been well documented in the treatment of both non orthopaedic applications and bone repair [10, 11]. However, the utilization of this technology with cartilage, skeletal muscle, tendons and ligaments is just beginning.
The use of decellularized tissue is promising for engineering orthopaedic tissues. It may function as a scaffold to support the proliferation of differentiated primary cells as well as the differentiation of progenitor cells. Even in the absence of specific differentiation media progenitor cells are capable of undergoing differentiation towards specific cell lineages based on interactions with a particular ECM as demonstrated in both in vivo and in vitro studies [82].
ECM has been shown to act as reservoirs for tissue-specific bioactive factors that play a role in cell proliferation, differentiation and maintenance of cell-specific phenotypes [125]. These bioactive factors are present even after decellularization treatments and can interact with reseeded cells [125]. Although only a few growth factors have been identified in decellularized ECM, other bioactive factors are likely present and it would be of interest to identify them in future studies.
Orthopaedic tissues rely on their ECM to provide organization, structural support and mechanical function. The ECM of different musculoskeletal tissues is arranged to support and respond to the mechanical loads they experience. Since acellular ECM maintains most of the natural architecture of its native tissue, the organization of ECM may serve as a guide for cell arrangement within reseeded tissue matrices. This is especially important for skeletal muscles since myofibers need to be arranged parallel to the orientation of muscle contraction in order to generate enough force to power movements. However, one of the hurdles in using acellular ECM scaffolds is maintaining uniform infiltration of cells throughout scaffolds of a clinically relevant size. The use of bioreactors for culturing tissue engineered constructs has shown promise in generating decellularized scaffolds with uniform distributions of cells, as demonstrated in reseeding decellularized bone tissue [68, 76]. Bioreactors can help provide adequate perfusion of nutrients to support the growing tissue allowing the formation of engineered tissue that are of clinically relevant size [75].
Another hurdle in tissue engineering of musculoskeletal tissues is creating scaffolds to accommodate tissue interfaces, such as osteochondral interfaces [254], musculotendinous junctions [255] and tendon-bone or ligament-bone connections [94, 246]. The use of decellularized tissue interfaces can provide potential scaffolds as demonstrated with decellularized bone-ACL-bone constructs [94] and bone-collateral ligament-bone constructs [246]. Although cell components are removed at the bone-ligament junction, there is still sufficient integration of the bone and ligament matrix, which can be reseeded with patient-specific osteoblasts or fibroblasts. This matrix is potentially superior to the fibrous repair that forms between bone and tendon during ACL repair surgery.
Decellularized cell-derived matrices offer an attractive potential alternative to native tissue-derived ECM. For example, compared to cartilage tissue, cartilage progenitor cells are easily available. Autologous ECM scaffolds can be engineered by isolating and expanding a patient’s cells in vitro, thus preventing host immune reactions. Currently, published studies on MSC-derived [81, 82, 127], osteoblast-derived [85] and chondrocyte-derived ECM [127, 132] have shown promising results for engineering bone and cartilage tissue. Decellularized in vitro cell-derived ECM scaffolds have enhanced the osteogenesis and chondrogenesis of MSC [132], which can have potential application in bone and cartilage tissue repair, respectively. Integration of inorganic material with cell-derived ECM has also been shown to drive osteogenesis of MSC [79, 85]. Translational applications of this technology may involve coating orthopaedic implants with in vitro derived decellularized ECM with or without progenitor cells in order to enhance integration of implants with native bone. Presently there are no published studies on cell-derived skeletal muscle, tendon or ligament tissues, but these have potential for future exploration.
Tissue decellularization is a novel technique in generating scaffolds for orthopaedic tissue engineering. It allows for the creation of a graft that shares similar structural and mechanical properties with native tissue without the risk of undesirable immunological reactions and without the complications of donor site morbidity. However, before decellularized tissues and cell-derived ECM can be used in clinical settings, protocols for decellularization of the various tissues need to be optimized and standardized. Since allogeneic tissues from different donors may not behave the same due to gender, age, ethnicity and lifestyle habits [60], additional studies should determine the ideal donor for different tissues. Once decellularized constructs are available for clinical use they will provide orthopaedic surgeons with an additional tool in their arsenal for the repair of musculoskeletal tissue.
Acknowledgments
The authors wish to thank Lauren Philips and Anna Dikina for their contributions and acknowledge funding from the National Institutes of Health (AR007505, AR063194, DE022376, AR061265), the Department of Defense Congressionally Directed Medical Research Programs (OR110196), the AO Foundation, a New Scholar in Aging grant from the Ellison Medical Foundation and an Orthopaedic Research and Education Foundation resident research grant.
Biographies
 Christina W. Cheng is an Orthopaedic Resident at University Hospitals Case Medical Center. She obtained a Bachelor of Science with distinction in research in Biology with emphasis in Molecular and Cellular Biology from Cornell University and a Doctor of Medicine from University at Buffalo School of Medicine and Biomedical Sciences. Her prior research includes genetic engineering using viral vectors in mesenchymal stem cells to induce chondrogenesis and characterization of chondrocyte subpopulations in articular cartilage. Currently she is completing her Allen Research Fellowship studying cartilage tissue engineering under the guidance of Dr. Eben Alsberg at Case Western Reserve University.
Christina W. Cheng is an Orthopaedic Resident at University Hospitals Case Medical Center. She obtained a Bachelor of Science with distinction in research in Biology with emphasis in Molecular and Cellular Biology from Cornell University and a Doctor of Medicine from University at Buffalo School of Medicine and Biomedical Sciences. Her prior research includes genetic engineering using viral vectors in mesenchymal stem cells to induce chondrogenesis and characterization of chondrocyte subpopulations in articular cartilage. Currently she is completing her Allen Research Fellowship studying cartilage tissue engineering under the guidance of Dr. Eben Alsberg at Case Western Reserve University.
 Loran D. Solorio is a postdoctoral researcher in the Department of Biomedical Engineering at Case Western Reserve University (CWRU), where she is developing orthopaedic tissue engineering strategies utilizing biopolymer systems for the controlled delivery of growth factors to direct stem cell differentiation. She received a BS in Biomedical Engineering with a minor in Engineering Mathematics from Saint Louis University and a PhD in Biomedical Engineering from CWRU. Her main research interests include tissue engineering, regenerative medicine, and biomaterial-mediated drug delivery.
Loran D. Solorio is a postdoctoral researcher in the Department of Biomedical Engineering at Case Western Reserve University (CWRU), where she is developing orthopaedic tissue engineering strategies utilizing biopolymer systems for the controlled delivery of growth factors to direct stem cell differentiation. She received a BS in Biomedical Engineering with a minor in Engineering Mathematics from Saint Louis University and a PhD in Biomedical Engineering from CWRU. Her main research interests include tissue engineering, regenerative medicine, and biomaterial-mediated drug delivery.
 Eben Alsberg is an Associate Professor in the Department of Biomedical Engineering, the Department of Orthopaedic Surgery, and the National Center for Regenerative Medicine of the Division of General Medicine Sciences at Case Western Reserve University (CWRU). He obtained a BSE (Cum Laude) in Biomedical Engineering and Mechanical Engineering and Materials Science from Duke University, and an MSE in Biomedical Engineering, an MSE in Mechanical Engineering and a PhD in Biomedical Engineering from the University of Michigan after completing his doctoral research as an NIH-NIDCR graduate fellow. He trained as a postdoctoral fellow in the Vascular Biology Program at Harvard Medical School prior to joining the faculty at CWRU in 2005. His research group focuses on tissue engineering and regenerative medicine, advanced biomaterials and bioactive factor delivery vehicles, control of stem cell fate decision, mechanotransduction and biomechanics, and therapeutic angiogenesis. Alsberg’s prior honors include the Crain’s Cleveland Business Forty Under 40 Award, the Ellison Medical Foundation New Scholar in Aging Award, the AADR William J. Gies Award, the IADR/Lion Dental Research Award, 1st Place in AADR/Warner-Lambert Hatton Award Competition, 2nd Place in IADR/Warner-Lambert Hatton Award Competition, and the Biovalley Young Investigator Award from the Tissue Engineering Society International.
Eben Alsberg is an Associate Professor in the Department of Biomedical Engineering, the Department of Orthopaedic Surgery, and the National Center for Regenerative Medicine of the Division of General Medicine Sciences at Case Western Reserve University (CWRU). He obtained a BSE (Cum Laude) in Biomedical Engineering and Mechanical Engineering and Materials Science from Duke University, and an MSE in Biomedical Engineering, an MSE in Mechanical Engineering and a PhD in Biomedical Engineering from the University of Michigan after completing his doctoral research as an NIH-NIDCR graduate fellow. He trained as a postdoctoral fellow in the Vascular Biology Program at Harvard Medical School prior to joining the faculty at CWRU in 2005. His research group focuses on tissue engineering and regenerative medicine, advanced biomaterials and bioactive factor delivery vehicles, control of stem cell fate decision, mechanotransduction and biomechanics, and therapeutic angiogenesis. Alsberg’s prior honors include the Crain’s Cleveland Business Forty Under 40 Award, the Ellison Medical Foundation New Scholar in Aging Award, the AADR William J. Gies Award, the IADR/Lion Dental Research Award, 1st Place in AADR/Warner-Lambert Hatton Award Competition, 2nd Place in IADR/Warner-Lambert Hatton Award Competition, and the Biovalley Young Investigator Award from the Tissue Engineering Society International.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christina W. Cheng, Email: chengcw@gmail.com.
Loran D. Solorio, Email: loran.solorio@gmail.com.
Eben Alsberg, Email: eben.alsberg@case.edu.
References
- 1.Mamaril ME, Childs SG, Sortman S. Care of the orthopaedic trauma patient. Journal of PeriAnesthesia Nursing. 2007;22:184–194. doi: 10.1016/j.jopan.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, Laforte AJ, Yin S. Osteogenic Protein-1 (Bone Morphogenetic Protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83-A:S151–S158. [PMC free article] [PubMed] [Google Scholar]
- 3.Hewett TE, Di Stasi SL, Myer GD. Current concepts for injury prevention in athletes after anterior cruciated ligament reconstruction. Am J Sports Med. 2013;41:216–224. doi: 10.1177/0363546512459638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646–656. [PMC free article] [PubMed] [Google Scholar]
- 5.Gazdag AR, Lane JM, Glaser D, Forster RA. Alternatives to autogenous bone graft: efficacy and indications. J Am Acad Orthop Surg. 1995;3:1–8. doi: 10.5435/00124635-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Livesey SA, Herndon DN, Hollyoak MA, Atkinson YH, Naq A. Transplanted acellular allograft dermal matrix. Potential as a template for the reconstruction of viable dermis. Transplantation. 1995;60:1–9. [PubMed] [Google Scholar]
- 7.Sutherland RS, Baskin LS, Hayward SW, Cunha GR. Regeneration of bladder urothelium, smooth muscle, blood vessels and nerves into an acellular tissue matrix. J Urol. 1996;156:571–577. doi: 10.1097/00005392-199608001-00002. [DOI] [PubMed] [Google Scholar]
- 8.Dohmen PM, Lembcke A, Holinski S, Pruss A, Konertz W. Ten Years of Clinical Results With a Tissue-Engineered Pulmonary Valve. Ann Thorac Surg. 2011;92:1308–1314. doi: 10.1016/j.athoracsur.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Badylak SF, Taylor D, Uygun K. Whole-Organ Tissue Engineering: Decellularization and Recellularization of Three-Dimensional Matrix Scaffolds. Annu Rev Biomed Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Q, Peng J, Guo Q, Huang J, Zhang L, Yao J, Yang F, Wang S, Xu W, Wang A, Lu S. A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials. 2008;29:2378–2387. doi: 10.1016/j.biomaterials.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Dejardin LM, Arnoczky SP, Ewers BJ, Haut RC, Clarke RB. Tissue-engineered rotator cuff tendon using porcine small intestine submucosa. Histologic and mechanical evaluation in dogs. Am J Sports Med. 2001;29:175–184. doi: 10.1177/03635465010290021001. [DOI] [PubMed] [Google Scholar]
- 13.Badylak SF, Arnoczky S, Plouhar P, Haut R, Mendenhall V, Clarke R, Horvath C. Naturally occurring extracellular matrix as a scaffold for musculoskeletal repair. Clin Orthop Relat Res. 1999;367(Suppl):S333–343. doi: 10.1097/00003086-199910001-00032. [DOI] [PubMed] [Google Scholar]
- 14.Badylak SF, Toombs JP, Shelbourne KD, Hiles MC, Lantz GC, Van Sickle D. Small intestinal submucosa as an intra-articular ligamentous graft material: a pilot study in dogs. Vet Comp Orthop Traumatol. 1994;7 [Google Scholar]
- 15.Badylak SF, Tullius R, Kokini K, Shelbourne KD, Klootwyk T, Voytik SL, Kraine MR, Simmons C. The use of xenogeneic small intestinal submucosa as a biomaterial for Achilles tendon repair in a dog model. J Biomed Mater Res. 1995;29:977–985. doi: 10.1002/jbm.820290809. [DOI] [PubMed] [Google Scholar]
- 16.Sellaro TL, Ravindra AK, Stolz DB, Badylak SF. Maintenance of hepatic sinusoidal endothelial cell phenotype in vitro using organ-specific extracellular matrix scaffolds. Tissue Eng. 2007;13:2301–2310. doi: 10.1089/ten.2006.0437. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, He Y, Bharadwaj S, Hammam N, Carnagey K, Myers R, Atala A, Van Dyke M. Tissue-specific extracellular matrix coatings for the promotion of cell proliferation and maintenance of cell phenotype. Biomaterials. 2009;30:4021–4028. doi: 10.1016/j.biomaterials.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bissell DM, Stamatoglou SC, Nermut MV, Hughes RC. Interactions of rat hepatocytes with type IV collagen, fibronectin and laminin matrices: distinct matrix-controlled modes of attachment and spreading. Eur J Cell Biol. 1986;40:72–78. [PubMed] [Google Scholar]
- 19.Bernard MP, Chu ML, Myers JC, Ramirez F, Eikenberry EF, Prockop DJ. Nucleotide sequences of complementary deoxyribonucleic acids for the pro alpha 1 chain of human type I procollagen. Statistical evaluation of structures that are conserved during evolution. Biochemistry. 1983;22:5213–5223. doi: 10.1021/bi00291a023. [DOI] [PubMed] [Google Scholar]
- 20.Constantinou CD, Jimenez SA. Structure of cDNAs encoding the triple-helical domain of murine alpha 2 (VI) collagen chain and comparison to human and chick homologues. Use of polymerase chain reaction and partially degenerate oligonucleotide for generation of novel cDNA clones. Matrix. 1991;11:1–9. doi: 10.1016/s0934-8832(11)80221-0. [DOI] [PubMed] [Google Scholar]
- 21.Exposito JY, D’Alessio M, Solursh M, Ramirez F. Sea urchin collagen evolutionarily homologous to vertebrate pro-alpha 2(I) collagen. J Biol Chem. 1992;267:15559–15562. [PubMed] [Google Scholar]
- 22.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109–116. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng MH, Chen J, Kirilak Y, Willers C, Xu J, Wood D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J Biomed Mater Res B Appl Biomater. 2005;73:61–67. doi: 10.1002/jbm.b.30170. [DOI] [PubMed] [Google Scholar]
- 24.Derwin KA, Baker AR, Spragg RK, Leigh DR, Iannotti JP. Commercial extracellular matrix scaffolds for rotator cuff tendon repair. Biomechanical, biochemical, and cellular properties. J Bone Joint Surg Am. 2006;88:2665–2672. doi: 10.2106/JBJS.E.01307. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert TW, Freund JM, Badylak SF. Quantification of DNA in biologic scaffold materials. J Surg Res. 2009;152:135–139. doi: 10.1016/j.jss.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stone KR, Abdel-Motal UM, Walgenbach AW, Turek TJ, Galili U. Replacement of human anterior cruciate ligaments with pig ligaments: a model for anti-non-gal antibody response in long-term xenotransplantation. Transplantation. 2007;83:211–219. doi: 10.1097/01.tp.0000250598.29377.13. [DOI] [PubMed] [Google Scholar]
- 27.Stone KR, Ayala G, Goldstein J, Hurst R, Walgenbach A, Galili U. Porcine cartilage transplants in the cynomolgus monkey. III. Transplantation of alpha-galactosidase-treated porcine cartilage. Transplantation. 1998;65:1577–1583. doi: 10.1097/00007890-199806270-00007. [DOI] [PubMed] [Google Scholar]
- 28.McPherson TB, Liang H, Record RD, Badylak SF. Galalpha(1,3)Gal epitope in porcine small intestinal submucosa. Tissue Eng. 2000;6:233–239. doi: 10.1089/10763270050044416. [DOI] [PubMed] [Google Scholar]
- 29.Konakci KZ, Bohle B, Blumer R, Hoetzenecker W, Roth G, Moser B, Boltz-Nitulescu G, Gorlitzer M, Klepetko W, Wolner E, Ankersmit HJ. Alpha-Gal on bioprostheses: xenograft immune response in cardiac surgery. Eur J Clin Invest. 2005;35:17–23. doi: 10.1111/j.1365-2362.2005.01441.x. [DOI] [PubMed] [Google Scholar]
- 30.Stone KR, Walgenbach AW, Turek TJ, Somers DL, Wicomb W, Galili U. Anterior cruciate ligament reconstruction with a porcine xenograft: a serologic, histologic, and biomechanical study in primates. Arthroscopy. 2007;23:411–419. doi: 10.1016/j.arthro.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 31.McKibbin B. The biology of fracture healing in long bones. J Bone Joint Surg Br. 1978;60B:150–162. doi: 10.1302/0301-620X.60B2.350882. [DOI] [PubMed] [Google Scholar]
- 32.Phillips AM. Overview of the fracture healing cascade. Injury. 2005;36(Suppl):S5–S7. doi: 10.1016/j.injury.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 33.Einhorn TA. The science of fracture healing. J Orthop Trauma. 2005;19(Suppl):S4–S6. doi: 10.1097/00005131-200511101-00002. [DOI] [PubMed] [Google Scholar]
- 34.Friedlaender GE, Goldberg VM. Bone and Cartilage Allografts: Biology and Clinical Applications. American Academy of Orthopaedic Surgeons; Park Ridge, Ill: 1991. [Google Scholar]
- 35.Frohlich M, Grayson WL, Marolt D, Gimble JM, Kregar-Velikonja N, Vunjak-Novakovic G. Bone grafts engineered from human adipose-derived stem cells in perfusion bioreactor culture. Tissue Eng Part A. 2010;16:179–189. doi: 10.1089/ten.tea.2009.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finkemeier CG. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am. 2002;84-A:454–464. doi: 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 37.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Summers BN, Eisenstein SM. Donor Site pain from the ilium. J Bone Joint Surg Br. 1989;71:677–680. doi: 10.1302/0301-620X.71B4.2768321. [DOI] [PubMed] [Google Scholar]
- 39.Buckwalter JA, Glimcher MJ, Cooper RR, Recker R. Bone Biology Part I: Structure, blood supply, cells, matrix and mineralization. J Bone Joint Surg Am. 1995;77:1256–1272. [PubMed] [Google Scholar]
- 40.Triffit JT. The organic matrix of bone tissue. J.B. Lippincott; Philadelphia: 1980. [Google Scholar]
- 41.Buckwalter JA. Musculoskeletal tissues and the musculoskeletal system. In: Weinstein BJ, Turek’s SL, editors. Orthopaedics Principles and Their Application. Lippincott Williams & Wilkins; Philadelphia, Pennsylvania: 2005. pp. 3–56. [Google Scholar]
- 42.Weatherholt AM, Fuchs RK, Warden SJ. Specialized connective tissue: bone, the structural framework of the upper extremity. J Hand Ther. 2012;25:123–132. doi: 10.1016/j.jht.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boskey AL. Noncollagenous matrix proteins and their role in mineralization. Bone Miner. 1989;6:111–123. doi: 10.1016/0169-6009(89)90044-5. [DOI] [PubMed] [Google Scholar]
- 44.Glimcher MJ. The nature of the mineral component of bone and the mechanisms of calcification. Raven Press; New York: 1992. [PubMed] [Google Scholar]
- 45.Ivirico JL, Salmeron-Sanchz M, Ribelles JL, Pradas M, Soria JM, Gomes ME, Reis Rl, Mano JF. Proliferation and differentiation of goat bone marrow stromal cells in 3D scaffolds with tunable hydrophilicity. J Biomed Mater Res B Appl Biomater. 2009;1:277–286. doi: 10.1002/jbm.b.31400. [DOI] [PubMed] [Google Scholar]
- 46.Mygind T, Stiehler M, Baatrup A, Li H, Zou X, Flyvbjerg A, Kassem M, Bunger C. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials. 2007;28:1036–1047. doi: 10.1016/j.biomaterials.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Schantz JT, Brandwood A, Hutmacher DW, Khor HL, Bittner K. Osteogenic differentiation of mesenchymal progenitor cells in computer designed fibrin-polymer-ceramic scaffolds manufactured by fused deposition modeling. J Mater Sci Mater Med. 2005;16:807–819. doi: 10.1007/s10856-005-3584-3. [DOI] [PubMed] [Google Scholar]
- 48.Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovits-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci USA. 2003;100:12741–12746. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Battista S, Guarnieri D, Borselli C, Zeppetelli S, Borzacchiello A, Mayol L, Gerbasio D, Keene DR, Ambrosio L, Netti PA. The effect of matrix composition of 3D constructs on embryonic stem cell differentiation. Biomaterials. 2005;26:6194–6207. doi: 10.1016/j.biomaterials.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Pham QP, Kasper FK, Baggett LS, Raphael RM, Jansen JA, Mikos AG. The influence of an in vitro generated bone-like extracellular matrix on osteoblastic gene expression of marrow stromal cells. Biomaterials. 2008;29:2729–2739. doi: 10.1016/j.biomaterials.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 51.Gomes ME, Bossano CM, Johnston CM, Reis RL, Mikos AG. In vitro localization of bone growth factors in constructs of biodegradable scaffolds seeded with marrow stromal cells and cultured in a flow perfusion bioreactor. Tissue Eng. 2006;12:177–188. doi: 10.1089/ten.2006.12.177. [DOI] [PubMed] [Google Scholar]
- 52.Drosos GI, Kazakos KI, Kouzoumpasis P, Verettas DA. Safety and efficacy of commercially available demineralised bone matrix preparations: A critical review of clinical studies. Injury. 2007;38S4:S13–S21. doi: 10.1016/s0020-1383(08)70005-6. [DOI] [PubMed] [Google Scholar]
- 53.Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO. Demineralized bone matrix in bone repair: History and use. Advance Drug Delivery Reviews. 2012;64:1063–1077. doi: 10.1016/j.addr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park BW, Kang EJ, Byun JH, Son MG, Kim HJ, Hah YS, Kim TH, Mohana Kumar B, Ock SA, Rho GJ. In vitro and in vivo osteogenesis of human mesenchymal stem cells derived from skin, bone marrow and dental follicle tissues. Differentiation. 2012;83:249–259. doi: 10.1016/j.diff.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 55.Shi Y, Niedzinski JR, Samaniego A, Bogdansky S, Atkinson BL. Adipose-derived stem cells combined with a demineralized cancellous bone substrate for bone regeneration. Tissue Eng Part A. 2012;18:1313–1321. doi: 10.1089/ten.TEA.2011.0357. [DOI] [PubMed] [Google Scholar]
- 56.Honsawek S, Bumrungpanichthaworn P, Thitiset T, Wolfinbarger L. Gene expression analysis of demineralized bone matrix-induced osteogenesis in human periosteal cells using cDNA array technology. Genet Mol Res. 2011;10:2093–2103. doi: 10.4238/vol10-3gmr1329. [DOI] [PubMed] [Google Scholar]
- 57.Zhang M, Powers RM, Wolfinbarger L. A quantitative assessment of osteoinductivity of human demineralized bone matrix. J Periodontol. 1997;68:1076–1084. doi: 10.1902/jop.1997.68.11.1076. [DOI] [PubMed] [Google Scholar]
- 58.Upton J, Glowacki J. Hand reconstruction with allograft demineralized bone: twenty-six implants in twelve patients. J Hand Surg. 1992;17:704–713. doi: 10.1016/0363-5023(92)90321-f. [DOI] [PubMed] [Google Scholar]
- 59.Michelson JJ, Curl LA. Use of demineralized bone in hindfoot arthrodesis. Clin Orthop. 1996;325:203–208. doi: 10.1097/00003086-199604000-00024. [DOI] [PubMed] [Google Scholar]
- 60.Russell JL, Block JE. Clinical utility of demineralized bone matrix for osseous defects, arthrodesis, and reconstruction: impact of processing techniques and study methodology. Orthopedics. 2004;22:524–531. [PubMed] [Google Scholar]
- 61.Berven S, Tay BKB, Kleinstueck FS, Bradford DS. Clinical applications of bone graft substitutes in spine surgery: consideration of mineralized and demineralized preparations and growth factor supplementation. Eur Spine J. 2001;10:S169–S177. doi: 10.1007/s005860100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee KJH, Roper JG, Wang JC. Demineralized bone matrix and spinal arthrodesis. The Spine Journal. 2005;5:S217–S223. doi: 10.1016/j.spinee.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 63.Hashimoto Y, Funamoto S, Kimura T, Nam K, Fujisato T, Kishida A. The effect of decellularized bone/bone marrow produced by high-hydrostatic pressurization on the osteogenic differentiation of mesenchymal stem cells. Biomaterials. 2011;32:7060–7067. doi: 10.1016/j.biomaterials.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 64.Harakas NK. Demineralized bone-matrix-induced osteogenesis. Clin Orthop Relat Res. 1984;188:239–251. [PubMed] [Google Scholar]
- 65.Becerra J, Andrades JA, Ertl DC, Sorgente N, Nimni ME. Demineralized bone matrix mediates differentiation of bone marrow stromal cells in vitro: effect of age of cell donor. J Bone Miner Res. 1996;11:1703–1714. doi: 10.1002/jbmr.5650111114. [DOI] [PubMed] [Google Scholar]
- 66.Zeltinger J, Sherwood JK, Graham DA, Mueller R, Griffith LG. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 2001;7:557–572. doi: 10.1089/107632701753213183. [DOI] [PubMed] [Google Scholar]
- 67.Marcos-Campos I, Marolt D, Petridis P, Bhumiratana S, Schmidt D, Vunjak-Novakovic G. Bone scaffold architecture modulates the development of mineralized bone matrix by human embryonic stem cells. Biomaterials. 2012;33:8329–8342. doi: 10.1016/j.biomaterials.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marolt D, Marcos-Campos I, Bhumiratana S, Koren A, Petridis P, Zhang G, Spitalnik PF, Grayson WL, Vunjak-Novakovic G. Engineering bone tissue from human embryonic stem cells. Proc Natl Acad Sci USA. 2012;109:8705–8709. doi: 10.1073/pnas.1201830109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Peppo GM, Marcos-Campos I, Kahler DJ, Alsalman D, Shang L, Vunjak-Novakovic G, Marolt D. Engineering bone tissue substitutes from human induced pluripotent stem cells. Proc Natl Acad Sci USA. 2013;110:8680–8685. doi: 10.1073/pnas.1301190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuznetsov SA, Cherman N, Robey PG. In vivo bone formation by progency of human embryonic stem cells. Stem Cells Dev. 2011;20:269–287. doi: 10.1089/scd.2009.0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sikavitsas VI, Bancroft GN, Holtorf HL, Jansen JA, Mikos AG. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc Natl Acad Sci USA. 2003;100:14683–14688. doi: 10.1073/pnas.2434367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bancroft GN, Sikavitsast VI, van den Dolder J, Sheffield TL, Ambrose CG, Jansen JA, Mikos AG. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc Natl Acad Sci USA. 2002;99:12600–12605. doi: 10.1073/pnas.202296599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Botchwey EA, Dupree MA, Pollack SR, Levine EM, Laurencin CT. Tissue engineered bone: measurement of nutrient transport in three-dimensional matrices. J Biomed Mater Res A. 2003;67:357–367. doi: 10.1002/jbm.a.10111. [DOI] [PubMed] [Google Scholar]
- 74.Holtorf HL, Jansen JA, Mikos AG. Flow perfusion culture induces the osteoblastic differentiation of marrow stroma cell-scaffold constructs in the absence of dexamethasone. J Biomed Mater Res A. 2005;72:326–334. doi: 10.1002/jbm.a.30251. [DOI] [PubMed] [Google Scholar]
- 75.Grayson WL, Bhumiratana S, Cannizzaro C, Chao PHG, Lennon DP, Caplan AI, Vunjak-Novakovic V. Effects of initial seeding density and fluid perfusion rate on formation of tissue-engineered bone. Tissue Eng Part A. 2008;14:1809–1820. doi: 10.1089/ten.tea.2007.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grayson WL, Frohlich M, Yeager K, Bhumiratana S, Chan ME, Cannizzaro C, Wan LQ, Liu XS, Guo E, Vunjak-Novakovic G. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci USA. 2010;107:3299–3304. doi: 10.1073/pnas.0905439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hesse E, Kluge G, Atfi A, Correa D, Haasper C, Berding G, Shin HO, Viering J, Langer F, Vogt PM, Krettek C, Jagodzinski M. Repair of a segmental long bone defect in human by implantation of a novel multiple disc graft. Bone. 2010;46:1457–1463. doi: 10.1016/j.bone.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 78.Chen XD, Dusevich V, Feng JQ, Manolagas SC, Jilka RL. Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J Bone Miner Res. 2007;22:1943–1956. doi: 10.1359/jbmr.070725. [DOI] [PubMed] [Google Scholar]
- 79.Datta N, Holtorf HL, Sikavitsas VI, Jansen JA, Mikos AG. Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells. Biomaterials. 2005;26:971–977. doi: 10.1016/j.biomaterials.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 80.Datta N, Pham QP, Sharma U, Sikavitsas VI, Jansen JA, Mikos AG. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc Natl Acad Sci USA. 2006;103:2488–2493. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zeitouni S, Krause U, Clough BH, Halderman H, Falster A, Blalock DT, Chaput CD, Sampson HW, Gregory CA. Human mesenchymal stem cell-derived matrices for enhanced osteoregeneration. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thibault RA, Baggett LS, Mikos AG, Kasper FK. Osteogenic differentiation of mesenchymal stem cells on pregenerated extracellular matrix scaffolds in the absence of osteogenic cell culture supplements. Tissue Eng Part A. 2010;16:431–440. doi: 10.1089/ten.tea.2009.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun Y, Li W, Lu Z, Chen R, Ling J, Ran Q, Jilka RL, Chen XD. Rescuing replication and osteogenesis of aged mesenchymal stem cells by exposure to a young extracellular matrix. FASEB J. 2011;25:1474–1485. doi: 10.1096/fj.10-161497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Decaris ML, Mojadedi A, Bhat A, Leach JK. Transferable cell-secreted extracellular matrices enhance osteogenic differentiation. Acta Biomater. 2012;8:744–752. doi: 10.1016/j.actbio.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 85.Tour G, Wendel M, Tcacencu I. Cell-derived matrix enhances osteogenic properties of hydroxyapatite. Tissue Eng Part A. 2011;17:127–137. doi: 10.1089/ten.TEA.2010.0175. [DOI] [PubMed] [Google Scholar]
- 86.Lau TT, Lee LQP, Vo BN, Su K, Wang DA. Inducing ossification in an engineered 3D scaffold-free living cartilage template. Biomaterials. 2012;33:8406–8417. doi: 10.1016/j.biomaterials.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 87.Ozawa S, Kasugai S. Evaluation of implant materials (hydroxyapatite, glass-ceramics, titanium) in rat bone marrow stromal cell culture. Biomaterials. 1996;17:23–29. doi: 10.1016/0142-9612(96)80751-4. [DOI] [PubMed] [Google Scholar]
- 88.Fujibayashi S, Neo M, Kim HM, Kokubo T, Nakamura T. Osteoinduction of porous bioactive titanium metal. Biomaterials. 2004;25:443–450. doi: 10.1016/s0142-9612(03)00551-9. [DOI] [PubMed] [Google Scholar]
- 89.Barradas AMC, Yuan H, van Blitterswijk CA, Habibovic P. Osteoinductive biomaterials: current knowledge of properties, experimental models and biological mechanisms. Eur Cell Mater. 2011;21:407–429. doi: 10.22203/ecm.v021a31. [DOI] [PubMed] [Google Scholar]
- 90.Decaris ML, Binder BY, Soicher MA, Bhat A, Leach JK. Cell-derived matrix coatings for polymeric scaffolds. Tissue Eng Part A. 2012;18:2148–2157. doi: 10.1089/ten.TEA.2011.0677. [DOI] [PubMed] [Google Scholar]
- 91.Abbott LC, Schottstaedt ER, De JB, Saunders CM, Bost FC. The evaluation of cortical and cancellous bone as grafting material. A clinical and experimental study. J Bone Joint Surg Am. 1947;29:381–414. [PubMed] [Google Scholar]
- 92.Zang M, Zhang Q, Chang EI, Mathur AB, Yu P. Decellularized tracheal matrix scaffold for tissue engineering. Plast Reconstr Surg. 2012;130:532–540. doi: 10.1097/PRS.0b013e31825dc084. [DOI] [PubMed] [Google Scholar]
- 93.Qing Q, Qin T. Optimal method for rat skeletal muscle decellularization. Zhonggui Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23:836–839. [PubMed] [Google Scholar]
- 94.Woods T, Gratzer PF. Effectiveness of three extraction techniques in the development of a decellularized bone-anterior cruciated ligament-bone graft. Biomaterials. 2005;26:7339–7349. doi: 10.1016/j.biomaterials.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 95.Mansour JM. Biomechanics of Cartilage, Kinesiology: the Mechanics and Pathomechanics of Human Movement. Lippincott Williams and Wilkins; Philadelphia: 2003. pp. 66–79. [Google Scholar]
- 96.Bedi A, Feeley BT, Williams RJ. Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:994–1009. doi: 10.2106/JBJS.I.00895. [DOI] [PubMed] [Google Scholar]
- 97.Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. [PubMed] [Google Scholar]
- 98.Widuchowski W, Lukasik P, Kwiatkowski G, Faltus R, Szyluk K, Widuchowski J, Koczy B. Isolated full thickness chondral injuries. Prevalance and outcome of treatment. A retrospective study of 5233 knee arthroscopies. Acta Chir Orthop Traumatol Chech. 2008;75:382–386. [PubMed] [Google Scholar]
- 99.Gong YY, Xue JX, Zhang WJ, Zhou GD, Liu W, Cao Y. A sandwich model for engineering cartilage with acellular cartilage sheets and chondrocytes. Biomaterials. 2011;32:2265–2273. doi: 10.1016/j.biomaterials.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 100.Hunziker EB. Articular cartilage repair: are the intrinsic biological constraints undermining this process insuperable? Osteoarthritis and Cartilage. 1999;7:15–28. doi: 10.1053/joca.1998.0159. [DOI] [PubMed] [Google Scholar]
- 101.Ghadially FN, Ailsby RL, Oryschak AF. Scanning electron microscopy of superficial defects in articular cartilage. Ann Rheum Dis. 1974;33:327–332. doi: 10.1136/ard.33.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ghadially FN, Thomas I, Oryschak AF, Lalonde JM. Long-term results of superficial defects in articular cartilage: a scanning electron-microscope study. J Pathol. 1977;121:213–217. doi: 10.1002/path.1711210404. [DOI] [PubMed] [Google Scholar]
- 103.Mitchell N, Shepard N. The resurfacing of adult rabbit articular cartilage by multiple perforations through the subchondral bone. J Bone Joint Surg Am. 1976;58:230–233. [PubMed] [Google Scholar]
- 104.Peretti GM, Randolph MA, Villa MT, Buragas MS, Yaremchuk MJ. Cell-based tissue-engineered allogeneic implant for cartilage repair. Tissue Eng. 2000;6 doi: 10.1089/107632700750022206. [DOI] [PubMed] [Google Scholar]
- 105.Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76-A:579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 106.Manek NJ, Lane NE. Osteoarthritis: current concepts in diagnosis and management. Am Fam Physician. 2000;61:1795–1804. [PubMed] [Google Scholar]
- 107.Redman SN, Oldfield SF, Archer CW. Current strategies for articular cartilage repair. Eur Cell Mater. 2005;9:23–32. doi: 10.22203/ecm.v009a04. [DOI] [PubMed] [Google Scholar]
- 108.Strauss EJ, Fonseca LE, Shah MR, Youm T. Management of focal cartilage defects in the knee is ACI the answer? Bull NYU Hosp Jt Dis. 2011;69:63–72. [PubMed] [Google Scholar]
- 109.Smith GD, Knutsen G, Richardson JB. A clinical review of cartilage repair techniques. J Bone Joint Surg Br. 2005;87:445–449. doi: 10.1302/0301-620X.87B4.15971. [DOI] [PubMed] [Google Scholar]
- 110.Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, Bentley G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87:640–645. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 111.Bentley G, Biant LC, Carrington RW, Akmal M, Goldberg A, Williams AM, Skinner JA, Pringle J. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223–230. doi: 10.1302/0301-620x.85b2.13543. [DOI] [PubMed] [Google Scholar]
- 112.Eyre DR, Wu JJ. Collagen structure and cartilage matrix integrity. J Rheumatol Suppl. 1995;43:82–85. [PubMed] [Google Scholar]
- 113.Han L, Grodzinsky AJ, Ortiz C. Nanomechanics of the Cartilage Extracellular Matrix. Annu Rev Mater Res. 2011;41:133–168. doi: 10.1146/annurev-matsci-062910-100431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kelly DJ, Crawford A, Dickinson SC, Sims TJ, Mundy J, Hollander AP, Prendergast PJ, Hatton PV. Biochemical markers of the mechanical quality of engineered hyaline cartilage. J Mater Sci Mater Med. 2007;18:273–281. doi: 10.1007/s10856-006-0689-2. [DOI] [PubMed] [Google Scholar]
- 115.Stapleton TW, Ingram J, Katta J, Knight R, Korossis S, Fisher J, Ingham E. Development and characterization of an acellular porcine medial meniscus for use in tissue engineering. Tissue Eng Part A. 2008;14:505–518. doi: 10.1089/tea.2007.0233. [DOI] [PubMed] [Google Scholar]
- 116.Conconi MT, De Coppi P, Di Liddo R, Vigolo S, Zanon GF, Parnigotto PP, Nussdorfer GG. Tracheal matrices, obtained by a detergent-enzymatic method, support in vitro the adhesion of chondrocytes and tracheal epithelial cells. Transplant International. 2005;18:727–734. doi: 10.1111/j.1432-2277.2005.00082.x. [DOI] [PubMed] [Google Scholar]
- 117.Elder BD, Kim DH, Athanasiou KA. Developing an articular cartilage decellularization process toward facet joint cartilage replacement. Neurosurgery. 2010;66:722–727. doi: 10.1227/01.NEU.0000367616.49291.9F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lumpkins SB, Pierre N, McFetridge PS. A mechanical evaluation of three decellularization methods in the design of a xenogeneic scaffold for tissue engineering the temporomandibular joint disc. Acta Biomater. 2008;4:808–816. doi: 10.1016/j.actbio.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 119.Stapleton TW, Ingram J, Fisher J, Ingham E. Investigation of the regenerative capacity of an acellular procine medial meniscus for tissue engineering applications. Tissue Eng Part A. 2011;17:231–242. doi: 10.1089/ten.tea.2009.0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kelley TF, Sutton FM, Wallace VP, Wong BJF. Chondrocyte repopulation of allograft cartilage: A preliminary investigation and strategy for developing cartilage matrices for reconstruction. Otolaryngol Head Neck Surg. 2002;127:265–270. doi: 10.1067/mhn.2002.128344. [DOI] [PubMed] [Google Scholar]
- 121.Peretti GM, Randolph MA, Caruso EM, Rossetti F, Zaleske DJ. Bonding of cartilage matrices with cultured chondrocytes: an experimental model. J Orthop Res. 1998;16:89–95. doi: 10.1002/jor.1100160115. [DOI] [PubMed] [Google Scholar]
- 122.Cheng NC, Estes BT, Awad HA, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng Part A. 2009;15:231–241. doi: 10.1089/ten.tea.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Diekman BO, Rowland CR, Lennon DP, Caplan AI, Guilak F. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: Induction by growth factors and cartilage derived matrix. Tissue Eng Part A. 2010;16:523–533. doi: 10.1089/ten.tea.2009.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ghanavi P, Kabiri M, Doran MR. The rationale for using microscopic units of a donor matrix in cartilage defect repair. Cell Tissue Res. 2012;347:643–648. doi: 10.1007/s00441-012-1323-x. [DOI] [PubMed] [Google Scholar]
- 125.Xue JX, Gong YY, Zhou GD, Liu W, Cao Y, Zhang WJ. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells induced by acellular cartilage sheets. Biomaterials. 2012;33:5832–5840. doi: 10.1016/j.biomaterials.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 126.Cheng HW, Eng B, Tsui YK, Phil M, Cheung KMC, Chan D, Chan BP. Decellularization of chondrocyte-encapsulated collagen microspheres: A three-dimensional model to study the effects of acellular matrix on stem cell fate. Tissue Eng Part C. 2009;15:697–706. doi: 10.1089/ten.TEC.2008.0635. [DOI] [PubMed] [Google Scholar]
- 127.Lu H, Hoshiba T, Kawazoe N, Koda I, Song M, Chen G. Cultured cell-derived extracellular matrix scaffolds for tissue engineering. Biomaterials. 2011;32:9658–9666. doi: 10.1016/j.biomaterials.2011.08.091. [DOI] [PubMed] [Google Scholar]
- 128.Lu H, Hoshiba T, Kawazoe N, Chen G. Comparison of decellularization techniques for preparation of extracellular matrix scaffolds derived from three-dimensional cell culture. J Biomed Mater Res A. 2012;100A:2507–2516. doi: 10.1002/jbm.a.34150. [DOI] [PubMed] [Google Scholar]
- 129.Su K, Lau TT, Leong W, Gong Y, Wang DA. Creating a living hyaline cartilage graft free from non-cartilaginous constituents: An intermediate role of a biomaterial scaffold. Adv Funct Mater. 2012;22:972–978. [Google Scholar]
- 130.Hoshiba T, Lu H, Yamada T, Kawazoe N, Tateishi T, Chen G. Effects of extracellular matrices derived from different cell sources on chondrocyte functions. Biotechnol Prog. 2011;27:788–795. doi: 10.1002/btpr.592. [DOI] [PubMed] [Google Scholar]
- 131.Jin CZ, Choi BH, Park SR, Min BH. Cartilage engineering using cell-derived extracellular matrix scaffold in vitro. J Biomed Mater Res A. 2009:1567–1577. doi: 10.1002/jbm.a.32419. [DOI] [PubMed] [Google Scholar]
- 132.Kwon SH, Lee TJ, Park J, Hwang JE, Jin M, Jang HK, Hwang NS, Kim BS. Modulation of BMP-2-induced chondrogenic versus osteogenic differentiation of human mesenchymal stem cells by cell specific extracellular matrices. Tissue Eng Part A. 2012;19:49–58. doi: 10.1089/ten.tea.2012.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liao J, Guo X, Grande-Allen KJ, Kasper FK, Mikos AG. Bioactive polymer/extracellular matrix scaffolds fabricated with a flow perfusion bioreactor for cartilage tissue engineering. Biomaterials. 2010;31:8911–8920. doi: 10.1016/j.biomaterials.2010.07.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Choi JS, Kim BS, Kim JD, Choi YC, Lee HY, Cho YW. In vitro cartilage tissue engineering using adipose-derived extracellular matrix scaffolds seeded with adipose-derived stem cells. Tissue Eng Part A. 2012;18:80–92. doi: 10.1089/ten.tea.2011.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang L, Su P, Xu C, Yang J, Yu W, Huang D. Chondrogenic differentiation of human mesenchymal stem cells: a comparison between micromass and pellet culture systems. Biotechnol Lett. 2010;32:1339–1346. doi: 10.1007/s10529-010-0293-x. [DOI] [PubMed] [Google Scholar]
- 136.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mazur P. Freezing of living cells: Mechanisms and implications. Am J Physiol. 1984;247:C125–C142. doi: 10.1152/ajpcell.1984.247.3.C125. [DOI] [PubMed] [Google Scholar]
- 138.Seddon AM, Curnow P, Booth PJ. Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta. 2004;1666:105–17. doi: 10.1016/j.bbamem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 139.Deeken CR, White AK, Bachman SL, Ramshaw BJ, Cleveland DS, Loy TS, Grant SA. Method of preparing a decellularized porcine tendon using tributyl phosphate. J Biomed mater Res B Appl Biomater. 2011;96:199–206. doi: 10.1002/jbm.b.31753. [DOI] [PubMed] [Google Scholar]
- 140.Gilbert TW. Strategies for Tissue and Organ Decellularization. J Cell Biochem. 2012;113:2217–2222. doi: 10.1002/jcb.24130. [DOI] [PubMed] [Google Scholar]
- 141.Elder BD, Eleswarapu SV, Athanasiou KA. Extraction Techniques for the Decellularization of Tissue Engineered Articular Cartilage Constructs. Biomaterials. 2009;30:3749–3756. doi: 10.1016/j.biomaterials.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Maier D, Braeun K, Steinhauser E, Ueblacker P, Oberst M, Kreuz PC, Roos N, Martinek V, Imhoff AB. In vitro analysis of an allogenic scaffold for tissue-engineered meniscus replacement. J Orthop Res. 2007;25:1598–1608. doi: 10.1002/jor.20405. [DOI] [PubMed] [Google Scholar]
- 143.Stabile KJ, Odom D, Smith TL, Northam C, Whitlock PW, Smith BP, Van Dyke ME, Ferguson CM. An acellular, allograft-derived meniscus scaffold in an ovine model. Arthroscopy. 2010;26:936–948. doi: 10.1016/j.arthro.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 144.Haykal S, Soleas JP, Salna M, Hofer SO, Waddell TK. Evaluation of the structural integrity and extracellular matrix components of tracheal allografts following cyclical decellularization techniques: comparison of three protocols. Tissue Eng Part C Methods. 2012;18:614–623. doi: 10.1089/ten.TEC.2011.0579. [DOI] [PubMed] [Google Scholar]
- 145.Partington L, Mordan NJ, Mason C, Knowles JC, Kim HW, Lowdell MW, Birchall MA, Wall IB. Biochemical changes caused by decellularization may compromise mechanical integrity of tracheal scaffolds. Acta Biomater. 2013;9:5251–5261. doi: 10.1016/j.actbio.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 146.Booth C, Korossis SA, Wilcox HE, Watterson KG, Kearney JN, Fisher J, Ingham E. Tissue engineering of cardiac valve prostheses I: development and histological characterization of an acellular porcine scaffold. J Heart Valve Dis. 2002;11:457–462. [PubMed] [Google Scholar]
- 147.Marieb EN, Hoehn K. Human Anatomy & Physiology. 8. Benjamin Cummings; San Francisco: 2010. [Google Scholar]
- 148.McClung JM, Davis JM, Carson JA. Oavrian hormone status and skeletal muscle inflammation during recovery from disuse in rats. Exp Physiol. 2007;92:219–232. doi: 10.1113/expphysiol.2006.035071. [DOI] [PubMed] [Google Scholar]
- 149.Lescaudron L, Peltekian E, Fontaine-Perus J, Paulin D, Zampieri M, Garcia L, Parrish E. Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant. Neuromuscul Disord. 1999;9:72–80. doi: 10.1016/s0960-8966(98)00111-4. [DOI] [PubMed] [Google Scholar]
- 150.Snow MH. Myogenic cell formation in regenerating rat skeletal muscle injured by mincing. II. An autoradiographic study. Anat Rec. 1977;188:201–217. doi: 10.1002/ar.1091880206. [DOI] [PubMed] [Google Scholar]
- 151.Karalaki M, Fili S, Philippou A, Koutsilieris M. Muscle regeneration: cellular and molecular events. In Vivo. 2009;23:779–796. [PubMed] [Google Scholar]
- 152.Goetsch SC, Hawke TJ, Gallardo TD, Richardson JA, Garry DJ. Transcriptional profiling and regulation of the extracellular matrix during muscle regeneration. Physiol Genomics. 2003;14:261–271. doi: 10.1152/physiolgenomics.00056.2003. [DOI] [PubMed] [Google Scholar]
- 153.Chen JC, Goldhamer DJ. Skeletal muscle stem cells. Reprod Biol Endocrinol. 2003;I:101. doi: 10.1186/1477-7827-1-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bartlett CS, Helfet DL, Hausman MR, Strauss E. Ballistics and gunshot wounds: effects on musculoskeletal tissues. J Am Acad Orthop Surg. 2000;8:21–36. doi: 10.5435/00124635-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 155.Bach AD, Beier JP, Stern-Staeter J, Horch RE. Skeletal muscle tissue engineering. J Cell Mol Med. 2004;8:413–422. doi: 10.1111/j.1582-4934.2004.tb00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Terada N, Takayama S, Yamada H, Seki T. Muscle repair after a transsection injury with development of a gap: an experimental study in rats. Scand J Plast Reconstr Surg Hand Surg. 2001;35:233–238. doi: 10.1080/028443101750523131. [DOI] [PubMed] [Google Scholar]
- 157.Merritt EK, Hammers DW, Tierney M, Suggs LJ, Walters TJ, Farrar RP. Functional assessment of skeletal muscle regeneration utilizing homologous extracellular matrix as scaffolding. Tissue Eng Part A. 2010;16:1395–1405. doi: 10.1089/ten.TEA.2009.0226. [DOI] [PubMed] [Google Scholar]
- 158.Merritt EK, Cannon MV, Hammers DW, Le LN, Gokhale R, Sarathy A, Song TJ, Tierney MT, Suggs LJ, Walters TJ, Farrar RP. Repair of traumatic skeletal muscle injury with bone-marrow-derived mesenchymal stem cells seeded on extracellular matrix. Tissue Eng Part A. 2010;16:2871–2881. doi: 10.1089/ten.TEA.2009.0826. [DOI] [PubMed] [Google Scholar]
- 159.Fan C, Jiang P, Fu L, Cai P, Sun L, Zeng B. Functional reconstruction of traumatic loss of flexors in forearm with gastrocnemius myocutaneous flap transfer. Microsurgery. 2008;28:71–75. doi: 10.1002/micr.20449. [DOI] [PubMed] [Google Scholar]
- 160.Vekris MD, Beris AE, Lykissas MG, Korompilias AV, Vekris AD, Soucacos PN. Restoration of elblow function in severe brachial plexus paralysis via muscle transfers. Injury. 2008;39(Suppl 3):S15–22. doi: 10.1016/j.injury.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 161.Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011;44:318–331. doi: 10.1002/mus.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bendall JR. Elastin content of various muscles of beef animals. J Sci Food Agr. 1967;18:553–558. [Google Scholar]
- 163.Dransfield E. Intramuscular composition and texture of beef muscles. J Sci Food Agr. 1977;28:833–842. [Google Scholar]
- 164.Listrat A, Lethias C, Hocquette JF, Renand G, Menissier F, Geay Y, Picard B. Age-related changes and location of type I, III, XII and XIV collagen during development of skeletal muscles from genetically different animals. Histochem J. 2000;32:349–356. doi: 10.1023/a:1004013613793. [DOI] [PubMed] [Google Scholar]
- 165.Marvulli D, Volpin D, Bressan GM. Spatial and temporal changes of type VI collagen expression during mouse development. Dev Dyn. 1996;206:447–454. doi: 10.1002/(SICI)1097-0177(199608)206:4<447::AID-AJA10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 166.Myers JC, Dion AS, Abraham V, Amenta PS. Type XV collagen exhibits a widespread distribution in human tissues but a distinct localization in basement membrane zones. Cell Tissue Res. 1996;286:493–505. doi: 10.1007/s004410050719. [DOI] [PubMed] [Google Scholar]
- 167.Nishimura T, Ojima K, Hattori A, Takahashi K. Developmental expression of extracellular matrix components in intramuscular connective tissue of bovine semitendinosus muscle. Histochem Cell Biol. 1997;107:215–221. doi: 10.1007/s004180050106. [DOI] [PubMed] [Google Scholar]
- 168.Light N, Champion AE. Characterization of muscle epimysium, perimysium and endomysium collagens. Biochem J. 1984;219:1017–1026. doi: 10.1042/bj2191017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brandan E, Inestrosa NC. Isolation of the heparan sulfate proteoglycans from the extracellular matrix of rat skeletal muscle. J Neurobiol. 1987;18:271–282. doi: 10.1002/neu.480180303. [DOI] [PubMed] [Google Scholar]
- 170.Schonherr E, Broszat M, Brandan E, Bruckner P, Kresse H. Decorin core protein fragment Leu155-Val260 interacts with TGF-beta but does not compete for decorin binding to type I collagen. Arch Biochem Biophys. 1998;355:241–248. doi: 10.1006/abbi.1998.0720. [DOI] [PubMed] [Google Scholar]
- 171.Matsuo I, Kimura-Yoshida C. Extracellular modulation of fibroblast growth factor signaling through heparan sulfate proteoglycans in mammalian development. Curr Opin Genet Dev. 2013 doi: 10.1016/j.gde.2013.02.004. in press. [DOI] [PubMed] [Google Scholar]
- 172.Imai K, Hiramatsu A, Fukushima D, Pierschbacher MD, Okada Y. Degradation of decorin by matrix metalloproteinases: identification of the cleavage sites, kinetic analyses and transforming growth factor-beta1 release. Biochem J. 1997;322:809–814. doi: 10.1042/bj3220809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Whitelock JM, Murdoch AD, Iozzo RV, Underwood PA. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J Biol Chem. 1996;271:10079–10086. doi: 10.1074/jbc.271.17.10079. [DOI] [PubMed] [Google Scholar]
- 174.Liu HM. The role of extracellular matrix in peripheral nerve regeneration: a wound chamber study. Acta Neuropathol. 1992;83:469–474. doi: 10.1007/BF00310022. [DOI] [PubMed] [Google Scholar]
- 175.Akhyari P, Kamiya H, Haverich A, Karck M, Lichtenberg A. Myocardial tissue engineering: the extracellular matrix. Eur J Cardiothorac Surg. 2008;34:229–241. doi: 10.1016/j.ejcts.2008.03.062. [DOI] [PubMed] [Google Scholar]
- 176.Badylak SF, Kochupura PV, Cohen IS, Doronin SV, Saltman AE, Gilbert TW, Kelly DJ, Ignotz RA, Gaudette GR. The use of extracellular matrix as an inductive scaffold for the partial replacement of functional myocardium. Cell Transplant. 2006;15(Suppl 1):S29–40. doi: 10.3727/000000006783982368. [DOI] [PubMed] [Google Scholar]
- 177.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nature. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 178.Reddy PP, Barrieras DJ, Wilson G, Bagli DJ, McLorie GA, Khoury AE, Merguerian PA. Regeneration of functional bladder substitutes using large segment acellular matrix allografts in a porcine model. J Urol. 2000;164(3 Pt2):936–941. doi: 10.1097/00005392-200009020-00005. [DOI] [PubMed] [Google Scholar]
- 179.Valentin JE, Turner NJ, Gilbert TW, Badylak SF. Functional skeletal muscle formation with a biologic scaffold. Biomaterials. 2010;31:7475–7484. doi: 10.1016/j.biomaterials.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Badylak SF, Vorp DA, Spievack AR, Simmons-Byrd A, Hanke J, Freytes DO, Thapa A, Gilbert TW, Nieponice A. Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res. 2005;128:87–97. doi: 10.1016/j.jss.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 181.Parnigotto PP, Marzaro M. Short bowel syndrome: experimental approach to increase intestinal surface in rats by gastric homologous acellular matrix. J Pediatr Surg. 2000;35:1304–1308. doi: 10.1053/jpsu.2000.9309. [DOI] [PubMed] [Google Scholar]
- 182.Gamba PG, Conconi MT, Lo Piccolo R, Zara G, Spinazzi R, Parnigotto PP. Experimental abdominal wall defect repaired with acellular matrix. Pediatr Surg Int. 2002;18:327–331. doi: 10.1007/s00383-002-0849-5. [DOI] [PubMed] [Google Scholar]
- 183.De Coppi P, Bellini S, Conconi MT, Sabatti M, Simonato E, Gamba PG, Nussdorfer GG, Parnigotto PP. Myoblast-acellular skeletal muscle matrix constructs guarantee a long-term repair of experimental full-thickness abdominal wall defects. Tissue Eng. 2006;12:1929–1936. doi: 10.1089/ten.2006.12.1929. [DOI] [PubMed] [Google Scholar]
- 184.Conconi MT, De Coppi P, Bellini S, Zara G, Sabatti M, Marzaro M, Zanon GF, Gamba PG, Parnigotto PP, Nussdorfer GG. Homologous muscle acellular matrix seeded with autologous myoblasts as a tissue-engineering approach to abdominal wall-defect repair. Biomaterials. 2005;26:2567–2574. doi: 10.1016/j.biomaterials.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 185.Faulkner JA, Brooks SV, Dennis RG. Measurement of recovery of function following whole muscle transfer, myoblast transfer, and gene therapy. Methods Mol Med. 1999;18:155–172. doi: 10.1385/0-89603-516-6:155. [DOI] [PubMed] [Google Scholar]
- 186.Stern MM, Myers RL, Hammam N, Stern KA, Eberli D, Kritchevsky SB, Soker S, Van Dyke M. The influence of extracellular matrix derived from skeletal muscle tissue on the proliferation and differentiation of myogenic progenitor cells ex vivio. Biomaterials. 2009;30:2393–2399. doi: 10.1016/j.biomaterials.2008.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Perniconi B, Costa A, Aulino P, Teodori L, Adamo S, Coletti D. The pro-myogenic environment provided by whole organ scale acellular scaffolds from skeletal muscle. Biomaterials. 2011;32:7870–7882. doi: 10.1016/j.biomaterials.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 188.Beattie AJ, Gilbert TW, Guyot JP, Yates AJ, Badylak SF. Chemoattraction of progenitor cells by remodeling extracellular matrix scaffolds. Tissue Eng Part A. 2009;15:1119–1125. doi: 10.1089/ten.tea.2008.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Reing JE, Zhang L, Myers-Irvin J, Cordero KE, Freytes DO, Heber-Katz E, Bedelbaeva K, McIntosh D, Dewilde A, Braunhut SJ, Badylak SF. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Part A. 2009;15:605–615. doi: 10.1089/ten.tea.2007.0425. [DOI] [PubMed] [Google Scholar]
- 190.Zisch AH, Lutolf MP, Hubbell JA. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc Pathol. 2003;12:295–310. doi: 10.1016/s1054-8807(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 191.Conconi MT, Lora S, Baiguera S, Boscolo E, Folin M, Scienza R, Rebuffat P, Parnigotto PP, Nussdorfer GG. In vitro culture of rate neuromicrovascular endothelial cells on polymeric scaffolds. J Biomed Mater Res. 2004;71A:669–674. doi: 10.1002/jbm.a.30198. [DOI] [PubMed] [Google Scholar]
- 192.Ajioka I, Nishio R, Ikekita M, Akaike T, Sasaki M, Enami J, Watanabe Y. Establishment of heterotropic liver tissue mass with direct link to the host liver following implantation of hepatocytes transfected with vascular endothelial growth factor gene in mice. Tissue Eng. 2001;7:335–344. doi: 10.1089/10763270152044198. [DOI] [PubMed] [Google Scholar]
- 193.Chung S, Hazen A, Levine JP, Baux G, Olivier WA, Yee HT, Margiotta MS, Karp NS, Gurtner GC. Vascularized acellular dermal matrix island flaps for the repair of abdominal muscle defects. Plast Reconstr Surg. 2003;111:225–232. doi: 10.1097/01.PRS.0000034934.05304.ED. [DOI] [PubMed] [Google Scholar]
- 194.Carlson EC, Carlson BM. A method for preparing skeletal muscle fiber basal laminae. Anat Rec. 1991;230:325–331. doi: 10.1002/ar.1092300305. [DOI] [PubMed] [Google Scholar]
- 195.Ide C. Nerve regeneration through the basal lamina scaffold of the skeletal muscle. Neurosci Res. 1984;1:379–391. [Google Scholar]
- 196.Borschel GH, Dennis RG, Kuzon WM. Contractile skeletal muscle tissue-engineered on an acellular scaffold. Plast Reconstr Surg. 2004;113:595–602. doi: 10.1097/01.PRS.0000101064.62289.2F. [DOI] [PubMed] [Google Scholar]
- 197.Wolf MT, Daly KA, Reing JE, Badylak SF. Biologic scaffold composed of skeletal muscle extracellular matrix. Biomaterials. 2012;33:2916–2925. doi: 10.1016/j.biomaterials.2011.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gillies AR, Smith LR, Lieber RL, Varghese S. Method for decellularizing skeletal muscle without detergents or proteolytic enzymes. Tissue Eng Part C Methods. 2011;17:383–389. doi: 10.1089/ten.tec.2010.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Langberg H, Rosendal L, Kjaer M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J Physiol. 2001;534:297–302. doi: 10.1111/j.1469-7793.2001.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Suominen H, Kiiskinen A, Heikkinen E. Effects of physical training on metabolism of connective tissues in young mice. Acta Physiol Scand. 1980;108:17–22. doi: 10.1111/j.1748-1716.1980.tb06495.x. [DOI] [PubMed] [Google Scholar]
- 201.Heinemeier KM, Kjaer M. In vivo investigation of tendon responses to mechanical loading. J Musculoskel Neuron Interact. 2011;11:115–123. [PubMed] [Google Scholar]
- 202.Komi PV. Relevance of in vivo force measurements to human biomechanics. Journal of Biomechanics. 1990;23(Suppl 1):23–34. doi: 10.1016/0021-9290(90)90038-5. [DOI] [PubMed] [Google Scholar]
- 203.Komi PV, Fukashiro S, Jarvinen M. Biomechanical loading of Achilles tendon during normal locomotion. Clin Sports Med. 1992;11:521–531. [PubMed] [Google Scholar]
- 204.Schuind F, Garcia-Elias M, Cooney WP, 3rd, An KN. Flexor tendon forces: in vivo measurements. J Hand Surg Am. 1992;17:291–298. doi: 10.1016/0363-5023(92)90408-h. [DOI] [PubMed] [Google Scholar]
- 205.Wang JH. Mechanobiology of tendon. J Biomech. 2006;39:1563–1582. doi: 10.1016/j.jbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 206.Frank CB. Ligament structure, physiology and function. J Musculoskel Neuron Interact. 2004;4:199–201. [PubMed] [Google Scholar]
- 207.Sakabe T, Sakai T. Musculoskeletal disease-tendon. Br Med Bull. 2011;99:211–225. doi: 10.1093/bmb/ldr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gilbert TW, Stewart-Akers AM, Simmons-Byrd A, Badylak SF. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J Bone Joint Surg Am. 2007;89-A:621–630. doi: 10.2106/JBJS.E.00742. [DOI] [PubMed] [Google Scholar]
- 209.Adams JE, Zobitz ME, Reach JS, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22:700–709. doi: 10.1016/j.arthro.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 210.Huang D, Balian G, Chhabra AB. Tendon tissue engineering and gene transfer: the future of surgical treatment. J Hand Surg Am. 2006;31:693–704. doi: 10.1016/j.jhsa.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 211.DeFranco MJ, Derwin K, Iannotti JP. New therapies in tendon reconstruction. J Am Acad Orthop Surg. 2004;12:298–304. doi: 10.5435/00124635-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 212.Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect Tissue Res. 1978;6:11–23. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- 213.Evans JH, Barbenel JC. Structural and mechanical properties of tendon related to function. Equine Vet J. 1975;7:1–8. doi: 10.1111/j.2042-3306.1975.tb03221.x. [DOI] [PubMed] [Google Scholar]
- 214.Duance VC, Restall DJ, Beard H, Bourne FJ, Bailey AJ. The location of three collagen types in skeletal muscle. FEBS Lett. 1977;79:344–358. doi: 10.1016/0014-5793(77)80797-7. [DOI] [PubMed] [Google Scholar]
- 215.Fan L, Sarkar K, Franks DJ, Uhthoff HK. Estimation of total collagen and types I and III collagen in canine rotator cuff tendons. Calcif Tissue Int. 1997;61:223–229. doi: 10.1007/s002239900327. [DOI] [PubMed] [Google Scholar]
- 216.Birk DE, Fitch JM, Babiarz JP, Doane KJ, Linsenmayer TF. Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci. 1990;95(Part 4):649–657. doi: 10.1242/jcs.95.4.649. [DOI] [PubMed] [Google Scholar]
- 217.Fukuta S, Oyama M, Kavalkovich K, Fu FH, Niyibizi C. Identification of types II, IX and X collagens at the insertion site of the bovine achilles tendon. Matrix Biol. 1998;17:65–73. doi: 10.1016/s0945-053x(98)90125-1. [DOI] [PubMed] [Google Scholar]
- 218.Berenson MC, Blevins FT, Plaas AH, Vogel KG. Proteoglycans of human rotator cuff tendons. J Orthop Res. 1996;14:518–525. doi: 10.1002/jor.1100140404. [DOI] [PubMed] [Google Scholar]
- 219.Koob TJ, Vogel KG. Site-related variations in glycosaminoglycan content and swelling properties of bovine flexor tendon. J Orthop Res. 1987;5:414–424. doi: 10.1002/jor.1100050314. [DOI] [PubMed] [Google Scholar]
- 220.Vogel KG, Koob TJ. Structural specialization in tendons under compression. Int Rev Cytol. 1989;115:267–293. doi: 10.1016/s0074-7696(08)60632-4. [DOI] [PubMed] [Google Scholar]
- 221.Elefteriou F, Exposito JY, Garrone R, Lethias C. Binding of tenascin-X to decorin. FEBS Lett. 2001;495:44–47. doi: 10.1016/s0014-5793(01)02361-4. [DOI] [PubMed] [Google Scholar]
- 222.Jozsa L, Lehto M, Kannus P, Kvist M, Reffy A, Vieno T, Jarvinen M, Demel S, Elek E. Fibronectin and laminin in Achilles tendon. Acta Orthop Scand. 1989a;60:469–471. doi: 10.3109/17453678909149322. [DOI] [PubMed] [Google Scholar]
- 223.Jozsa L, Lehto M, Kvist M, Balint JB, Reffy A. Alterations in dry mass content of collage fibers in degenerative tendinopathy and tendon-rupture. Matrix. 1989b;9:140–146. doi: 10.1016/s0934-8832(89)80032-0. [DOI] [PubMed] [Google Scholar]
- 224.Butler DL, Grood ES, Noyes FR, Zernicke RF. Biomechanics of ligaments and tendons. Exerc Sport Sci Rev. 1978;6:125–181. [PubMed] [Google Scholar]
- 225.Gratzer PF, Harrison RD, Woods T. Matrix alteration and not residual sodium dodecyl sulfate cytotoxicity affects the cellular repopulation of a decellularized matrix. Tissue Eng. 2006;12:2975–2983. doi: 10.1089/ten.2006.12.2975. [DOI] [PubMed] [Google Scholar]
- 226.Tischer T, Vogt S, Aryee S, Steinhauser E, Adamczyk C, Milz S, Martinek V, Imhoff AB. Tissue engineering of the anterior cruciate ligament: a new method using acellularized tendon allografts and autologous fibroblasts. Arch Orthop Trauma Surg. 2007;127:735–741. doi: 10.1007/s00402-007-0320-0. [DOI] [PubMed] [Google Scholar]
- 227.Cartmell JS, Dunn MG. Development of cell-seeded patellar tendon allografts for anterior cruciate ligament reconstruction. Tissue Eng. 2004;10:1065–1075. doi: 10.1089/ten.2004.10.1065. [DOI] [PubMed] [Google Scholar]
- 228.Harrison RD, Gratzer PF. Effect of extraction protocols and epidermal growth factor on the cellular repopulation of decellularized anterior cruciate ligament allografts. J Biomed Mater Res. 2005;75A:841–854. doi: 10.1002/jbm.a.30486. [DOI] [PubMed] [Google Scholar]
- 229.Oberpinning F, Meng J, Yoo JJ, Atala A. De novo reconstitution of a functional mammalian bladder by tissue engineering. Nat Biotechnol. 1999;17:149–155. doi: 10.1038/6146. [DOI] [PubMed] [Google Scholar]
- 230.Sodian R, Lemke T, Loebe M, Hoerstrup SP, Potapov EV, Hausmann H, Meyer R, Hetzer R. New pulsatile bioreactor for fabrication of tissue-engineered patches. J Biomed Mater Res. 2001;58:401–405. doi: 10.1002/jbm.1034. [DOI] [PubMed] [Google Scholar]
- 231.Godbey WT, Hindy SB, Sherman ME, Atala A. A novel use of centrifugal force for cell seeding into porous scaffolds. Biomaterials. 2004;25:2799–2805. doi: 10.1016/j.biomaterials.2003.09.056. [DOI] [PubMed] [Google Scholar]
- 232.Vavken P, Joshi S, Murray MM. TRITON-X is most effective among three decellularization agents for ACL tissue engineering. J Orthop Res. 2009;27:1612–1618. doi: 10.1002/jor.20932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Schmidt CC, Georgescu HI, Kwoh CK, Blomstrom GL, Engle CP, Larkin LA, Evans CH, Woo SL. Effect of growth factors on the proliferation of fibroblasts from the medial collateral and anterior cruciated ligaments. J Orthop Res. 1995;13:184–190. doi: 10.1002/jor.1100130206. [DOI] [PubMed] [Google Scholar]
- 234.Ingram JH, Korossis S, Howling G, Fisher J, Ingham E. The use of ultrasonication to aid recellularization of acellular natural tissue scaffolds for use in anterior cruciate ligament reconstruction. Tissue Eng. 2007;13:1561–1572. doi: 10.1089/ten.2006.0362. [DOI] [PubMed] [Google Scholar]
- 235.Omae H, Zhao C, Sun YL, An KN, Amadio PC. Multilayer tendon slices seeded with bone marrow stromal cells: a novel composite for tendon engineering. J Orthop Res. 2009;27:937–942. doi: 10.1002/jor.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Little D, Guilak F, Ruch DS. Ligament-derived matrix stimulates a ligamentous phenotype in human adipose-derived stem cells. Tissue Eng Part A. 2010;16:2307–2319. doi: 10.1089/ten.tea.2009.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhang JY, Li B, Wang JHC. The role of engineered tendon matrix in the stemness of tendon stem cells in vitro and the promotion of tendon-like tissue formation in vivo. Biomaterials. 2011;32:6972–6981. doi: 10.1016/j.biomaterials.2011.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yao L, Bestwick CS, Bestwick LA, Maffulli N, Aspden RM. Phenotypic drift in human tenocyte culture. Tissue Eng. 2006;12:1843–1849. doi: 10.1089/ten.2006.12.1843. [DOI] [PubMed] [Google Scholar]
- 239.Harris MT, Butler DL, Boivin GP, Florer JB, Schantz EJ, Wenstrup RJ. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. 2004;22:998–1003. doi: 10.1016/j.orthres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 240.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 241.Zhang J, Wang JH. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet Disord. 2010;11:10. doi: 10.1186/1471-2474-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rui YF, Lui PP, Li G, Fu SC, Lee YW, Chan KM. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng Part A. 2010;16:1549–1558. doi: 10.1089/ten.TEA.2009.0529. [DOI] [PubMed] [Google Scholar]
- 243.Beynnon BD, Johnson RJ, Fleming BC, Kannus P, Kaplan M, Samani J, Renstrom P. Anterior cruciate ligament replacement: comparison of bone-patellar tendon-bone grafts with two-strand hamstring grafts. A prospective, randomized study. J Bone Joint Surg Am. 2002;84-A:1503–1513. doi: 10.2106/00004623-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 244.Barber-Westin SD, Noyes FR, Heckmann TP, Shaffer BL. The effect of exercise and rehabilitation on anterior-posterior knee displacements after anterior cruciate ligament autograft reconstruction. Am J Sports Med. 1999;27:84–93. doi: 10.1177/03635465990270012201. [DOI] [PubMed] [Google Scholar]
- 245.Leung KS, Qin L, Fu LK, Chan CW. A comparative study of bone to bone repair and bone to tendon healing in patella-patellar tendon complex in rabbits. Clin Biomech. 2002;17:594–602. doi: 10.1016/s0268-0033(02)00075-x. [DOI] [PubMed] [Google Scholar]
- 246.Endress R, Woon CYL, Farnebo SJ, Behn A, Bronstein J, Pham H, Yan X, Gambhir SS, Chang J. Tissue-engineered collateral ligament composite allografts for scapholunate ligament reconstruction: an experimental study. J Hand Surg. 2012;37A:1529–1537. doi: 10.1016/j.jhsa.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 247.Cartmell JS, Dunn MG. Effect of chemical treatments on tendon cellularity and mechanical properties. J Biomed Mater Res. 2000;49:134–140. doi: 10.1002/(sici)1097-4636(200001)49:1<134::aid-jbm17>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 248.Hu XW, Knight DP, Chapman JA. The effect of non-polar liquids and non-ionic detergents on the ultrastructure and assembly of rat tail tendon collagen fibrils in vitro. Biochem Biophys Acta. 1997;1334:327–337. doi: 10.1016/s0304-4165(96)00112-2. [DOI] [PubMed] [Google Scholar]
- 249.Pridgen BC, Woon CYL, Kim M, Thorfinn J, Lindsey D, Pham H, Chang J. Flexor tendon tissue engineering: acellularization of human flexor tendons with preservation of biomechanical properties and biocompatibility. Tissue Eng Part C. 2011;17:819–828. doi: 10.1089/ten.tec.2010.0457. [DOI] [PubMed] [Google Scholar]
- 250.Raghavan SS, Woon CYL, Kraus A, Megerle K, Choi MSS, Pridgen BC, Pham H, Chang J. Human flexor tendon tissue engineering: decellularization of human flexor tendons reduces immunogenicity in vivo. Tissue Eng Part A. 2012;18:796–805. doi: 10.1089/ten.TEA.2011.0422. [DOI] [PubMed] [Google Scholar]
- 251.Galili U. The alpha-gal epitope (Gal alpha 1-3Gal beta 1-4GlcNAc-R) in xenotransplantation. Biochimie. 2001;83:557–563. doi: 10.1016/s0300-9084(01)01294-9. [DOI] [PubMed] [Google Scholar]
- 252.Galili U. Interaction of the natural anti-Gal antibody with alpha-galactosylepitopes: a major obstacle for xenotransplantation in humans. Immunol Today. 1993;14:480. doi: 10.1016/0167-5699(93)90261-i. [DOI] [PubMed] [Google Scholar]
- 253.Yoshida R, Vavken P, Murray MM. Decellularization of bovine anterior cruciate ligament tissues minimizes immunogenic reactions to alpha-gal epitopes by human peripheral blood mononuclear cells. Knee. 2012;19:672–675. doi: 10.1016/j.knee.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Rodrigues MT, Lee SJ, Gomes ME, Reis RL, Atala A, Yoo JJ. Bilayered constructs aimed at osteochondral strategies: the influence of medium supplements in the osteogenic and chondrogenic differentiation of amniotic fluid-derived stem cells. Acta Biomater. 2012;8:2795–2806. doi: 10.1016/j.actbio.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 255.Turner NJ, Yates AJ, Jr, Weber DJ, Qureshi IR, Stolz DB, Gilbert TW, Badylak SF. Xenogeneic extracellular matrix as an inductive scaffold for regeneration of a functioning musculotendinous junction. Tissue Eng Part A. 2010;16:3309–3317. doi: 10.1089/ten.TEA.2010.0169. [DOI] [PubMed] [Google Scholar]