Abstract
Baeyer–Villiger oxidation of racemic bicyclic cyclobutanones with Zr[bis(salicylidene)ethylenediaminato] (salen) complex 1 as catalyst in the presence of a urea-hydrogen peroxide adduct was found to proceed enantiospecifically. The enantiotopos selection in the oxidation was governed primarily by the Zr(salen) catalyst, although migratory aptitude (methine > methylene > methyl) in Baeyer–Villiger oxidation affected the selection to a varied extent, depending on the substrate structures; one enantiomer of cyclobutanones gave exclusively a normal lactone expected from the migratory aptitude, and the other enantiomer gave an abnormal lactone preferentially, the formation of which is counter to the migratory aptitude. Furthermore, the rates of abnormal lactone formation were found to be faster than those of normal lactone formation in most of the oxidations examined. For example, the enantiomer of racemic bicyclo[3.2.0]heptan-6-one giving an abnormal lactone reacted 2.2 times faster than the other enantiomer giving a normal lactone. To our knowledge, this example of chemocatalytic Baeyer–Villiger oxidation giving an abnormal lactone in preference to a normal lactone has been previously unreported. This unusual behavior is likely to be attributable to strict control of stereoelectronic demand in Baeyer–Villiger oxidation and chiral recognition by complex 1.
Baeyer–Villiger (B-V) oxidation, oxidative transformation of carbonyl to ester (or lactone), has high synthetic value and has been widely used in various organic syntheses. In particular, asymmetric B-V oxidation of racemic or prochiral cyclic ketones is a useful tool for the synthesis of optically active lactones. Several enzymes, so-called Baeyer–Villigerase, are known to catalyze highly enantiospecific B-V oxidations (regiodivergent parallel kinetic resolution) (1, 2) of racemic cyclic ketones, although the stereochemistry of the oxidations depends on the enzyme used (3–9). Some of them promote B-V oxidation of only one enantiomer, leaving the other enantiomer intact, whereas some other enzymes promote B-V oxidation of both enantiomers in an enantiospecific and topos-selective manner; that is, one enantiomer is converted to a normal lactone (NL) and the other is converted to an abnormal lactone (AL). [A lactone generated in accord with the migratory aptitude of the carbonyl substituent in B-V oxidation (tertiary > secondary > primary) is called a NL, and a lactone generated in contravention of the aptitude is called an AL (8).] On the other hand, chemical versions of B-V oxidation of racemic ketones are still limited in number and are inferior to biocatalyzed B-V oxidation in terms of enantiospecificity and topos selectivity. In 1994, Bolm et al. (10) reported copper-catalyzed asymmetric B-V oxidation. In the same year, Strukul and coworkers (11) reported platinum-catalyzed asymmetric B-V oxidation. Since then, several catalysts have been introduced for asymmetric B-V oxidation (12–16). Although stoichiometric enantiospecific B-V oxidation has recently been reported (17), molecular catalysts are still difficult to exert a highly sophisticated catalysis comparable with enzymes. It has been reported by Bolm and colleagues (10, 14–16) that chiral copper and aluminum catalysts catalyze B-V oxidation with moderate enantiospecificity and topos selectivity.
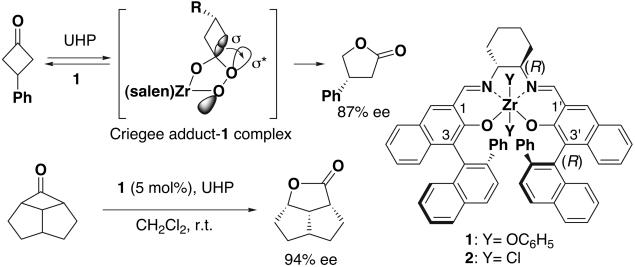
The superior catalytic performance of enzymes is attributed to their efficient chiral recognition of substrates and appropriate control of the stereoelectronic requirement for migration of the Criegee intermediate in B-V oxidation; the stereoelectronic requirement is satisfied by regulating the conformation of the Criegee intermediate suitably through hydrogen bond formation with the enzyme (3–9). Thus, we expected that highly enantioselective B-V oxidation would be realized if the conformation of the Criegee intermediate, a kind of bidentate ligand, is appropriately controlled by its forming a chelate with a chiral molecular catalyst (18–20). In agreement with this expectation, we recently found that, although both cis-β- and trans-Co[bis(salicylidene)ethylenediaminato] (salen) complexes catalyzed B-V oxidation (18, 19), only chiral cis-β-Co(salen) complexes could induce asymmetry in B-V oxidation. On the other hand, it is known that some metallosalen complexes are transformed to the corresponding cis-β-complexes in the presence of a bidentate ligand (21). Thus, we further expected that a metallosalen complex bearing an oxygenophilic metal center and readily exchangeable apical ligands would make a complex with the bidentate Criegee intermediate and, therefore, serve as a good catalyst for asymmetric B-V oxidation (Scheme 1). Indeed, high enantioselectivity of 87% enantiomeric excess (ee) was observed in B-V oxidation of prochiral 3-phenylcyclobutanone with a Zr(salen) complex 1 and urea–hydrogen peroxide adduct (UHP) system (20, 22). Aoki and Seebach (17) have also independently reported stereoelectronic control of the rearrangement of the Criegee intermediate through hydrogen bond formation.
Scheme 1.
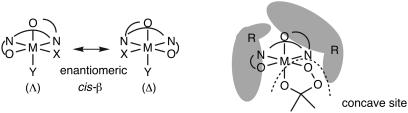
Different from B-V oxidation of prochiral ketones, the stereochemistry of B-V oxidation of racemic ketones is affected not only by stereoelectronic control but also by chiral recognition. Efficient stereoelectronic control and chiral recognition by Baeyer–Villigerase enables regiodivergent parallel kinetic resolution of racemic ketones through B-V oxidation (see above). The first coordination sphere of a cis-β-metallosalen complex is chiral, and the cis-β-complex is expected to provide a concave-type chiral reaction site when an appropriate substituent like a 2-phenylnaphthyl group is introduced at the C3(3′) position of the salen ligand (Fig. 1). An asymmetric reaction site of the concave type is known to be efficient for chiral recognition (23). Thus, it was expected that the Zr(salen) complex 1 would also serve as an efficient catalyst for enantiospecific B-V oxidation of racemic ketones.
Fig. 1.
The first coordination spheres of metallosalen complex and a schematic diagram of substituted cis-β-metallosalen complex chelated by the Criegee intermediate.
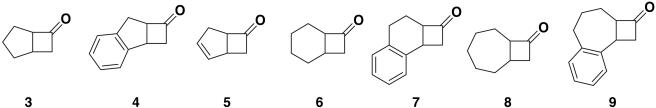
Most biological reactions show strong substrate specificity, referred to as the lock-and-key model, because of their strict molecular recognition. The above-described biological B-V oxidations also undergo substrate specificity to a considerable extent (3–9). The whole mechanism of molecular recognition by molecular catalysts is not completely understood, but some factors participating in interaction between the substrate and the catalyst have become obvious. For example, some attractive interaction(s) such as the CH-π interaction (24) between the substrate and the catalyst participates in asymmetric induction by the catalyst. The salen ligand possessing a binaphthyl unit has been revealed to interact attractively with organic compounds through CH-π interaction (25). Thus, the stereochemistry of B-V oxidation catalyzed by complex 1 bearing a concave reaction site was considered to be strongly affected by the substrate structure. Based on this consideration, we examined B-V oxidation of a series of racemic bicyclic cyclobutanones with complex 1 as the catalyst (Scheme 2).
Scheme 2.
Materials and Methods
1H NMR spectra were recorded at 400 MHz on a JEOL JNM-AL-400 instrument. IR spectra were obtained with a Shimadzu FTIR-8400 instrument. Optical rotations were measured with a P-1020 polarimeter (Jasco, Tokyo). High-resolution fast atom bombardment mass spectra were obtained from a JEOL JMX-SX/SX 102A spectrometer with the m-nitrobenzyl alcohol matrix. Column chromatography was conducted on silica gel 60N (spherical, neutral), 63–210 μm, available from Kanto Chemical (Tokyo), and preparative TLC was performed on a 0.5 mm × 20 cm × 20 cm Merck silica gel plate (60 F-254). Enantiomeric excesses were determined by HPLC analysis by using Shimadzu LC-10AT-VP or by GLC analysis using Shimadzu GC-17A equipped with an appropriate optically active column, as described in Table 1. Solvents were dried and distilled shortly before use. For spectral data of Zr(salen)Cl2 2, which is the synthetic precursor of complex 1, the starting bicyclobutanones and the produced lactones, see Supporting Text, which is published as supporting information on the PNAS web site.
Table 1.
| Ketone
|
NL
|
ent-AL
|
||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Substrate | Conversion, % | ee, % | Time, h | Yield, % | ee, % | Yield, % | ee, % |
| 1 | 3 | 54a | 27a | 2.0 | 21a | 88a,b | 31a | 97a,c |
| 2 | 3 | 61a | 32a | 2.3 | 24a | 90a,b | 34a | 96a,c |
| 3 | 3 | 73a | 41a | 2.7 | 30a | 91a,b | 40a | 96a,c |
| 4 | 3 | 90a | 62a | 3.7 | 41a | 93a,b | 45a | 96a,c |
| 5 | 4 | 54d | 42e | 2.3 | 15d | 91f,g | 34d | 95f,h |
| 6 | 4 | 63d | 52e | 3.0 | 20d | 93f,g | 40d | 95f,h |
| 7 | 4 | 74d | 61e | 3.3 | 27d | 93f,g | 44d | 95f,h |
| 8 | 5 | 50i | 27i | 2.0 | 18j | 89k,l | 31j | >99k,m |
| 9 | 5 | 62i | 38i | 2.8 | 23j | 89k,l | 36j | >99k,m |
| 10 | 5 | 67i | 42i | 3.0 | 23j | 91k,l | 38j | 96k,m |
| 11 | 6 | 71n | 77n | 2.0 | 48n | 85n,o | 21i | >99n,p |
| 12 | 6 | 76n | 86n | 2.5 | 54n | 82n,o | 22i | >99n,p |
| 13 | 6 | 83n | 94n | 3.0 | 55n | 80n,o | 25i | >99n,p |
| 14 | 7 | 57q | 74q | 2.0 | 10q | 58q,r | 47q | 95q,h |
| 15 | 7 | 59q | 80q | 2.3 | 11q | 65q,r | 47q | 95q,h |
| 16 | 7 | 63q | 86q | 2.7 | 11q | 67q,r | 50q | 94q,h |
| 17 | 7 | 68q | 92q | 3.0 | 14q | 76q,r | 54q | 94q,h |
| 18 | 8 | 49s | 30s | 2.0 | 16s | 82s,h | 29p | >99s,h |
| 19 | 8 | 66s | 49s | 2.7 | 24s | 83s,h | 39p | >99s,h |
| 20 | 8 | 75s | 65s | 3.3 | 30s | 86s,h | 44p | >99s,h |
| 21 | 8 | 85s | 79s | 3.7 | 35s | 87s,h | 47p | >99s,h |
| 22 | 9 | 60t | 7u | 2.5 | 36t | 81v,h | 24t | 98v,h |
| 23 | 9 | 96t | 29u | 4.5 | 57t | 76v,h | 39t | 98v,h |
Determined by GLC analysis with optically active column Supelco Beta-Dex-255 [initial column temperature, 115°C for 10 min, heating rate 3.0°C/min (to 165°C)].
Absolute configuration was determined to be (1S, 5S), based on comparison of optical rotation (31).
Absolute configuration was determined to be (1R, 5S), based on comparison of optical rotation (32).
Conversion of racemic ketone and yields of lactones were determined by, 1H NMR (400 MHz) analysis.
Determined by HPLC analysis with Daicel CHIRALCEL OJ-H (hexane/2-propanol 98:2) (Chemical Industries, Osaka).
Determined by GLC analysis with optically active column Supelco Beta-Dex-255 (column temperature 165°C for 70 min).
Absolute configuration was determined to be (1S, 5R), based on comparison of optical rotation (33).
Absolute configuration has not been determined.
Determined by GLC analysis with optically active column Supelco Beta-Dex-255 [initial column temperature, 115°C for 13 min, heating rate 5.0°C/min (to 180°C)].
Yields of lactones were determined by 1H NMR (400 MHz) analysis.
Determined by HPLC analysis with Daicel CHIRALCEL OJ-H (hexane/2-propanol 95:5)
Absolute configuration was determined to be (1R, 5S), based on comparison of optical rotation (34).
Absolute configuration was determined to be (1R, 5S), based on comparison of optical rotation (35).
Determined by GLC analysis with optically active column Supelco Beta-Dex-255 [initial column temperature, 125°C for 14 min, heating rate 40.0°C/min (to 140°C) for 4 min. heating rate 40.0°C/min (to 170°C)].
Absolute configuration was determined to be (1S, 6S), based on comparison of optical rotation (36).
Absolute configuration was determined to be (1S, 6R), based on comparison of optical rotation (32).
Determined by GLC analysis with optically active column Supelco Beta-Dex-255 [initial column temperature, 150°C for 40 min, heating rate 1.0°C/min (to 170°C)].
Absolute configuration was determined to be (1S, 6R), based on comparison of optical rotation (36).
Determined by GLC analysis with optically active column Supelco Beta-Dex-255 [initial column temperature, 115°C for 31 min, heating rate 10.0°C/min (to 170°C)].
Conversion of racemic ketone and yields of lactones were determined by 1H NMR (400 MHz) analysis.
Determined by HPLC analysis with Daicel CHIRALCEL OD-H (hexane/2-propanol 99.9:0.1).
Determined by GLC analysis with optically active column Supelco Beta-Dex-255 (initial column temperature, 165°C for 168 min).
Zr(salen)(OPh)2 1. Complex 2 (333.8 mg, 0.338 mmol) was dissolved in THF (10 ml) under nitrogen. Then, a THF solution of lithium phenoxide (1.0 M, 680 μl) was added to this solution and stirred for 6 h. The solution was concentrated on a rotary evaporator, and the resulting residue was redissolved in toluene. The mixture was filtered through a pad of Celite to remove lithium chloride. Toluene was evaporated and the residue was purified by recrystallization from a mixture of dichloromethane and heptane (2:1). Complex 1 was obtained as yellow crystals in 83% yield. 1H NMR (400 MHz): δ 8.53 (s, 2H), 7.92 (m, 4H), 7.84 (d, J = 8.0 Hz, 2H), 7.68 (m, 2H), 7.53 (d, J = 8.5 Hz, 2H), 7.42 (d, J = 8.5 Hz, 2H), 7.18 (m, 2H), 7.13–6.95 (m, 12H), 6.87 (m, 4H), 6.74 (m, 2H), 6.67–6.53 (m, 6H), 6.07 (dd, J = 8.4, 1.0, 4H), 3.42 (br-d, 2H), 2.37 (br-d, 2H), 1.93 (br-d, 2H), 1.50–1.37 (m, 2H), 1.30–1.20 (m, 2H). IR (KBr): 3053, 2928, 1611, 1585, 1479, 1356, 1275, 1186, 1151, 1124, 1024, 957, 864, 760, 696, 609, 515 cm–1. Anal. calcd. for C72H54N2O4Zr·2H2O: C, 75.96, H, 5.14, N, 2.46. Found: C, 76.14, H, 5.24, N, 2.37.
General Procedure for B-V Oxidation of Racemic Bicyclic Cyclobutanone Derivatives in the Presence of Zr(salen)(OPh)2 1 as a Catalyst. Racemic bicyclic cyclobutanone (0.1 mmol) was dissolved in chlorobenzene (1 ml). To this solution, bicyclohexyl or 1-bromonaphthalene was added as an internal standard where needed. Then, complex 1 (8.8 mg, 8 μmol) and UHP (11.3 mg, 0.12 mmol) were added successively, and the resulting mixture was stirred for the time specified in Table 1. Yields and enantiomeric excesses of the unreacted substrate and the resulting NLs and ALs were determined as described in Table 1.
Determination of the Relative Reaction Ratio of Enantiomers of Racemic Ketones and the Ratio of Normal and Abnormal Lactones from Each Enantiomer. The relative reaction ratio of the enantiomers of the starting racemic ketones was determined by using Kagan's equation [krel = kfast/kslow = ln{(1 – c)(1 – ee)/ln(1 – c)(1 + ee)}], where c stands for the conversion of the starting ketone and ee stands for the ee of unreacted ketone (26). Conversion of ketones was determined by using GLC or NMR analysis, and the ee was determined as described in Table 1.
The ratio of NLs and ALs (RK = FNL:FAL and ReK = FeNL:FeAL) from each enantiomer (K or eK) of the starting ketone was determined by GLC or HPLC analysis, where F, NL, AL, eNL, and eAL stand for the amount of an enantiomer, NL, AL, enantiomeric NL, and enantiomeric AL, respectively (see Scheme 2). Retention times of the enantiomers of NLs and ALs were confirmed by the analysis of racemic lactones that were prepared by conventional methods. If no side reaction occurs, the following equation should be hold: FA + FNL + FAL = FB + FeNL + FeAL, where A and B stand for the enantiomers of the unreacted ketone. We did not observe any reaction other than B-V oxidation and the this equation was satisfied within a margin of error of 4%, when the observed F values were applied to the equation.
X-Ray Crystallographic Data for 1·(CH2Cl2)2. Recrystallization from dichloromethane and heptane, C74H60N2O5Cl4Zr, M = orthorhombic space group P212121, a = 13.0741(2), b = 18.6919(3), c = 25.9780(4), V = 6348.5(2), T = –90°C, Z = 4, Dc = 1.35, μ(Mo-Kα) = 3.95 cm–1, R = 0.064 [I > 2σ(I)], Rw = 0.152 (all data) for 8,124 reflections and 812 variables, GOF = 0.87, residual electron density 0.53/–0.53 eÅ–3; programs, sir97 (27), dirdif94 (28), and shelxl-97 (29) linked to texsan (30) crystallographic software package. Experimental detail is summarized in Supporting Text.
Results and Discussion
Bicyclo[3.2.0]heptan-6-one 3, 2,3-benzobicyclo[3.2.0]heptan-6-one 4, bicyclo[3.2.0]hept-2-en-6-one 5, bicyclo[4.2.0]octan-7-one 6, 2,3-benzobicyclo[4.2.0]octan-7-one 7, bicyclo[5.2.0]nonan-8-one 8, and 2,3-benzobicyclo[5.2.0]nonan-8-one 9 were chosen as substrates to explore the influence of substrate structure and olefinic or aromatic functionality on the stereochemistry of their B-V oxidation with complex 1. The results obtained are summarized in Table 1.
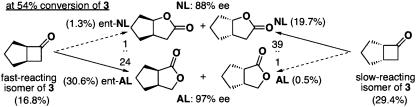
The B-V oxidation of racemic 3 was found to be highly topos-selective (entries 1–4). The topos selection by Zr(salen) 1 overwhelmingly overrode the migratory aptitude in B-V oxidation. It is noteworthy that the fast-reacting isomer gave the AL [enantiomeric (ent)-AL] preferentially in ≈27:1 ratio of ent-AL and ent-NL, despite the fact that topos selection and migratory aptitude were canceled. On the other hand, the slow-reacting isomer gave the NL exclusively in ≈40:1 ratio of NL and AL, suggesting that topos selection and migratory aptitude acted synergetically to give the NL. The relative reaction ratio (26) of the fast- to the slow-reacting isomers was ≈2.0. The stereochemistry of the oxidation of racemic 4 was similar to that of racemic 3, although the relative reaction ratio (≈2.9) was somewhat improved (entries 5–7); the fast-reacting isomer gave ent-AL exclusively in an ≈51:1 ratio of ent-AL/ent-NL, whereas the slow-reacting isomer gave NL preferentially in ≈22:1 ratio of NL/AL. The stereochemistry of the oxidation of racemic 5 was also similar to those of 3 and 4 (entries 8–10); the fast-reacting isomer gave ent-AL preferentially in an ≈35:1 ratio of ent-AL and ent-NL, whereas the slow-reacting isomer gave NL selectively in a ≈40:1 ratio of NL and AL. The relative reaction ratio of the fast- to the slow-reacting isomers was ≈2.2. In these reactions, the ees of ALs were much better than the ees of the corresponding NLs, especially at the early stage of the reaction, since the enantiomers giving a NL showed high topos selection and reacted slower than the enantiomers giving an AL. Scheme 3 explains this stereochemistry with the reaction of 3 as a typical example; the supply of the ent-NL was faster than the supply of the AL at the early stage because of the relative reaction ratio and the topos selectivity. Scheme 3 also shows that the reaction is almost a regiodivergent parallel kinetic resolution. The ees of the NLs improved and the ees of the ALs decreased gradually as the reaction proceeded. This change in ees with time reflects the above-described NL/AL ratio and the relative reaction ratio. These results indicate that the transition state for the oxidation of the fast-reacting isomer, leading to AL, is favored probably because of some attractive interaction between the salen ligand and the fast-reacting isomer, or the transition state for the oxidation of the slow-reacting isomer is disfavored because of some repulsive interaction between the salen ligand and the slow-reacting isomer. At this moment, however, our knowledge about the transition state is too naive to determine which is the case. The presence of an olefin or aromatic ring affects stereoselectivity in the reactions of 3–5 to a small extent. To our knowledge, this example of chemocatalytic B-V oxidation whereby a fast-reacting isomer gives AL stereospecifically is previously unreported.
Scheme 3.
The stereochemistry of the reaction of racemic bicyclo [4.2.0]octan-7-one 6 was different from that observed in the reactions of 3, 4, and 5 (entries 11–13); the reaction of the fast-reacting isomer gave NL exclusively, whereas topos selection in the reaction of the slow-reacting isomer was moderate (ent-AL/ent-NL 5.1:1). The fast-reacting isomer was consumed about four times faster than the slow-reacting isomer. A part of this chemistry has been reported (20). The stereochemistry of the reaction of racemic 2,3-benzobicyclo[4.2.0]octan-7-one 7 seemed similar to that of the reaction of 6 (entries 14–17). However, the sense of topos selection observed in the reaction of 7 was opposite to that observed in the reaction of 6; the oxidation of the fast-reacting isomer gave AL preferentially in ≈30:1 ratio of AL and NL, whereas the reaction of the slow-reacting isomer gave ent-NL preferentially in ≈9:1 ratio of ent-NL and ent-AL. The fast-reacting isomer was consumed approximately eight times faster than the slow-reacting isomer. Different from the reactions of bicyclo[3.2.0]heptan-6-one derivatives, the presence of a benzene ring in bicyclo[4.2.0]octan-7-one derivatives had a strong influence on the topos-selection by 1. The relative reaction ratio between the enantiomers observed in the oxidation of 6 reflected the matching or mis-matching of the migratory aptitude and the topos selection.
Intrigued with these results, we further examined the oxidation of bicyclo[5.2.0]nonan-8-one derivatives. Bicyclo[5.2.0]-nonan-8-one 8 behaved similarly to compounds 3-5 (entries 18–21); the slow-reacting isomer gave NL selectively, whereas the fast-reacting isomer gave ent-AL preferentially in ≈22:1 ratio of ent-AL and ent-NL. The relative reaction ratio was ≈2.6. On the other hand, 2,3-benzobicyclo[5.2.0]nonan-8-one 9 behaved similarly to 6; the fast-reacting isomer gave NL exclusively and the slow-reacting isomer gave AL in preference to NL (AL/NL lactone =≈6.5:1), albeit with small relative reaction ratio (1.2). Thus, bicyclo[5.2.0]nonan-8-one derivatives were found to behave differently from both bicyclo[3.2.0]heptan-6-one derivatives and bicyclo[4.2.0]octan-7-one derivatives, but the sense of the topos selection in the oxidation of bicyclo[5.2.0]nonan-8-one derivatives is unclear, because the absolute configuration of the products obtained from compounds 8 and 9 could not be determined.
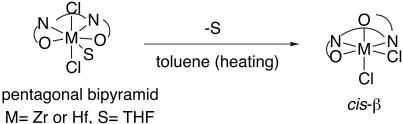
Metallosalen complexes usually take octahedral configuration, but Zr- and Hf(salen) complexes have been reported to adopt unique pentagonal bipyramidal configuration (37). Structurally, related Zr- and Hf-N,N′-ethylenebis(acetylacetoneiminate) (acen) complexes have also been reported to adopt pentagonal bipyramidal configuration (Scheme 4). These Zr- and Hf-complexes are equatorially coordinated by a solvent. Furthermore, these pentagonal bipyramidal Zr- and Hf(acen) complexes have been reported to change into octahedral cis-β-Zr- and Hf(acen) complexes, respectively, with loss of the solvent ligand, when they were heated in toluene. This study indicates that octahedral Zr- and Hf(acen) complexes and probably also octahedral Zr- and Hf(salen) complexes prefer cis-β-configuration to trans-configuration. To see if complex 1 behaves similarly to the reported simple Zr(salen) and Zr(acen) complexes, we studied the x-ray structure of complex 1. In accord with the reported Zr(salen) complex, it was demonstrated that 1 also adopted pentagonal bipyramidal configuration, where the basal salen ligand takes an umbrella conformation and one water molecule coordinated with the zirconium ion in its equatorial position (Fig. 2). The presence of the equatorial aqua ligand should be of advantage for B-V reaction, because aqua and alkoxide ligand exchange is usually fast. We next carried out 1H NMR studies of complex 1, a B-V reaction mixture, and the mixture of 1 and 1,3-propanediol in CDCl3. 1H NMR analysis of 1 in CDCl3 showed the signals for two imino protons (CH N) and two methine protons at the carbons adjacent to the imino group (CH—N) in the cyclohexane moiety at 8.53 and 3.42 ppm, respectively, suggesting that the configuration of 1 in solution is not cis-β. The B-V oxidation of 3 in the presence of stoichiometric 1 and UHP was traced by 1H NMR, but no change in the NMR spectra of 3 was observed during the reaction, although 3 was converted into the corresponding lactones. Thus, to see if complex 1 adopts cis-β-configuration at the reaction temperature in the presence of a bidentate ligand that can replace a phenoxide and an aqua ligand, a 1:1 mixture of 1 and 1,3-propanediol was analyzed by 1H NMR. The NMR spectra indicated that all the 1,3-propanediol added was coordinated to the zirconium ion, and the resulting solution contained two new complexes (C and D) in a ratio of 20:1. The spectrum of C was almost the same as that of 1 except for the signals of the 1,3-propanediol moiety; the signals of two imino protons and two methine protons at the carbons adjacent to the imino group appeared at 8.52 and 3.42 ppm, respectively. This finding suggested that the 1,3-propanediol coordinated with the zirconium ion at the equatorial or apical position as a monodentate ligand, and the structure of complex C is similar to that of 1. In contrast, the spectrum of D showed the signals of two imino protons and two methine protons at the carbons adjacent to the imino group at 8.45 and 8.58 ppm and 3.90 and 4.40 ppm, respectively, indicating that complex D adopted a cis-β-structure. These spectroscopic and the experimental data above support our proposal that the B-V oxidation using 1 proceeds through a cis-β-complex bearing the Criegee intermediate as the bidentate ligand, and the concave-type reaction site on the cis-β-complex can recognize well the structure of the substrate. The chelation of the Criegee intermediate to 1 should be easier than the chelation of externally added 1,3-diol, because the hydroperoxy group is more nucleophilic than the hydroxy group, and five-membered chelate formation is more favored than six-membered chelate formation (Scheme 5).
N) and two methine protons at the carbons adjacent to the imino group (CH—N) in the cyclohexane moiety at 8.53 and 3.42 ppm, respectively, suggesting that the configuration of 1 in solution is not cis-β. The B-V oxidation of 3 in the presence of stoichiometric 1 and UHP was traced by 1H NMR, but no change in the NMR spectra of 3 was observed during the reaction, although 3 was converted into the corresponding lactones. Thus, to see if complex 1 adopts cis-β-configuration at the reaction temperature in the presence of a bidentate ligand that can replace a phenoxide and an aqua ligand, a 1:1 mixture of 1 and 1,3-propanediol was analyzed by 1H NMR. The NMR spectra indicated that all the 1,3-propanediol added was coordinated to the zirconium ion, and the resulting solution contained two new complexes (C and D) in a ratio of 20:1. The spectrum of C was almost the same as that of 1 except for the signals of the 1,3-propanediol moiety; the signals of two imino protons and two methine protons at the carbons adjacent to the imino group appeared at 8.52 and 3.42 ppm, respectively. This finding suggested that the 1,3-propanediol coordinated with the zirconium ion at the equatorial or apical position as a monodentate ligand, and the structure of complex C is similar to that of 1. In contrast, the spectrum of D showed the signals of two imino protons and two methine protons at the carbons adjacent to the imino group at 8.45 and 8.58 ppm and 3.90 and 4.40 ppm, respectively, indicating that complex D adopted a cis-β-structure. These spectroscopic and the experimental data above support our proposal that the B-V oxidation using 1 proceeds through a cis-β-complex bearing the Criegee intermediate as the bidentate ligand, and the concave-type reaction site on the cis-β-complex can recognize well the structure of the substrate. The chelation of the Criegee intermediate to 1 should be easier than the chelation of externally added 1,3-diol, because the hydroperoxy group is more nucleophilic than the hydroxy group, and five-membered chelate formation is more favored than six-membered chelate formation (Scheme 5).
Scheme 4.
THF, tetrahydrofuran.
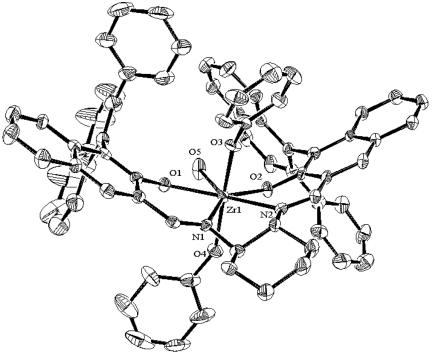
Fig. 2.
An ortep diagram‡ for Zr–salen complex 1 with 30% probability. All hydrogen atoms and the solvent molecules are omitted for clarity. Selected bond lengths and angles are as follows: Zr—O1 = 2.081(4), Zr—O2 = 2.011(4), Zr—O3 = 1.999(3), Zr—O4 = 2.071(4), Zr—O5 = 2.334(4), Zr—N1 = 2.348(5), Zr—N2 = 2.372(4), ∠O1—Zr—O5 = 67.4(2), ∠O1—Zr—N1 = 77.1(1), ∠\O2—Zr—O3 = 169.7(1), ∠O4—Zr—O5 = 70.7(2), ∠O4—Zr1—N2 = 76.9(1), and ∠N1—Zr1—N2 = 69.9(2).
Scheme 5.
In conclusion, we have demonstrated that Zr(salen) complex 1 exhibits asymmetric catalytic activity similar to the catalytic performance of some Baeyer–Villigerases. In B-V oxidation of racemic bicyclo[3.2.0]alkan-5-ones, one enantiomer led to a NL and the other enantiomer led to an AL. This unique catalytic performance of 1 may be explained by formation of a cis-β-Zr(salen) complex chelated by the Criegee intermediate, the conformation of which is regulated by the salen ligand of concave structure to enable topos-selective σ–σ* interaction necessary for enantiospecific B-V oxidation. The NMR study supported that a bidentate ligand coordinates with 1 and forces it to adopt cis-β-configuration. However, B-V oxidation with 1 showed unique substrate specificity, suggesting that a reaction site of the concave type recognizes well the substrate structure and a structural change is reflected in the stereochemistry of the reaction.
Supplementary Material
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: salen, bis(salicylidene)ethylenediaminato; B-V, Baeyer–Villiger; UHP, urea–hydrogen peroxide adduct; AL, abnormal lactone; NL, normal lactone; ent, enantiomeric; ee, enantiomeric excess; acen, N,N′-ethylenebis(acetylacetoneiminate).
Data deposition: Crystallographic data for the structure of 1·(CH2Cl2)2 have been deposited in the Cambridge Structural Database, Cambridge Crystallographic Data Centre, Cambridge CB2 1EZ, United Kingdom (CSD reference no. 222736).
Footnotes
Farrugia, L. J. (1997) J. Appl. Crystallogr. 30, 565 (abstr.).
References
- 1.Bertozzi, F., Crotti, P., Macchia, F., Pineshi, M. & Feringa, B. L. (2001) Angew. Chem. Int. Ed. Engl. 40, 930–932. [PubMed] [Google Scholar]
- 2.Dehli, J. R. & Gotor, V. (2002) Chem. Soc. Rev. 31, 365–370. [DOI] [PubMed] [Google Scholar]
- 3.Alphand, V. & Furstoss, R. (1995) in Handbook of Enzyme Catalysis in Organic Synthesis, eds. Drauz, K. & Waldmann, H. (VCH, Weinheim, Germany), pp. 744–772.
- 4.Stewart, J. D. (1997) Curr. Org. Chem. 2, 211–232. [Google Scholar]
- 5.Roberts, S. M. & Wan, P. W. H. (1998) J. Mol. Catal. B, 111–136.
- 6.Kayser, M., Chen, G. & Stewart, J. (1999) Synlett, 153–158.
- 7.Kelly, D. R. (2000) Chim. Oggi 18, 33–39, 52–56. [Google Scholar]
- 8.Alphand, V. & Furstoss, R. (2001) in Asymmetric Oxidation Reactions: A Practical Approach, ed. Katsuki, T. (Oxford Univ. Press, Oxford), pp. 214–226.
- 9.Mihovilovic, M. D., Muller, B. & Stanetty, P. (2002) Eur. J. Org. Chem., 3711–3730.
- 10.Bolm, C., Schlingloff, G. & Weickhardt, K. (1994) Angew. Chem. Int. Ed. Engl. 33, 1848–1849. [Google Scholar]
- 11.Gusso, A., Baccin, C., Pinna, F. & Strukul, G. (1994) Organometallics 13, 3442–3451. [Google Scholar]
- 12.Bolm, C. & Beckmann, O. (1999) in Comprehensive Asymmetric Catalysis, eds. Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. (Springer, Berlin), Vol. 2, pp. 803–810. [Google Scholar]
- 13.Strukul, G. (1998) Angew. Chem. Int. Ed. Engl. 37, 1198–1209. [DOI] [PubMed] [Google Scholar]
- 14.Bolm, C. & Schlingloff, G. (1995) Chem. Commun., 1247–1248.
- 15.Kagan, H. B. (1996) Croat. Chem. Acta 69, 669–680. [Google Scholar]
- 16.Bolm, C., Beckmann, O. & Palazzi, C. (2001) Can. J. Chem. 79, 1593–1597. [Google Scholar]
- 17.Aoki, M. & Seebach, D. (2001) Helv. Chim. Acta 84, 187–207. [Google Scholar]
- 18.Uchida, T. & Katsuki, T. (2001) Tetrahedron Lett. 42, 6911–6914. [Google Scholar]
- 19.Uchida, T., Katsuki, T., Ito, K., Akashi, S., Ishii, A. & Kuroda, T. (2002) Helv. Chim. Acta 85, 3078–3089. [Google Scholar]
- 20.Watanabe, A., Uchida, T., Ito, K. & Katsuki, T. (2002) Tetrahedron Lett. 43, 4481–4485. [Google Scholar]
- 21.Yamada, S. (1999) Coord. Chem. Rev. 190–192, 537–555. [Google Scholar]
- 22.Bolm, C. & Beckmann, O. (2000) Chirality 12, 523–525. [DOI] [PubMed] [Google Scholar]
- 23.Helmchen, G. & Roland, S. (1981) Angew. Chem. 93, 208–209. [Google Scholar]
- 24.Nishio, M. & Hirota, M. (1989) Tetrahedron 45, 7201–7245. [Google Scholar]
- 25.Hashihayata, T., Punniyamurthy, T., Irie, R., Katsuki, T., Akita, M. & Moro-oka, Y. (1999) Tetrahedron 55, 14599–14610. [Google Scholar]
- 26.Kagan, H. B. & Fiaud, J. C. (1988) Top. Stereochem. 18, 249–330. [Google Scholar]
- 27.Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A, Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999) J. Appl. Crystallogr. 32, 115–119. [Google Scholar]
- 28.Beurskens, P. T., Admiraal, G., Beurskens, G., Bosman, W. P., Garcia-Granda, S., Gould, R. O., Smits, J. M. M. & Smykalla, C. (1992) The dirdif Program System, Technical Report of the Crystallography Laboratory (University of Nijmegen, Nijmegen, The Netherlands).
- 29.Sheldrick, G. M. (1997) SHELXL-97, Program for Crystal Structure Refinement (University of Göttingen, Göttingen, Germany).
- 30.Rigaku (2000) texsan, Crystal Structure Analysis Package (Rigaku, Tokyo), Version 1.11.
- 31.Irwin, A. J. & Bryan Jones, J. (1977) J. Am. Chem. Soc. 99, 1625–1630. [DOI] [PubMed] [Google Scholar]
- 32.Jakovac, I. J., Goodbrand, H. B., Lok, K. P. & Bryan Jones, J. (1982) J. Am. Chem. Soc. 104, 4659–4665. [Google Scholar]
- 33.Smith, A. B., III, Cantin, L.-D., Pasternak, A., Guise-Zawacki, L., Yao, W., Charnley, A. K., Barbosa, J., Sprengeler, P. A., Hirschmann, R., Munsei, S., et al. (2003) J. Med. Chem. 46, 1831–1844. [DOI] [PubMed] [Google Scholar]
- 34.Nara, M., Terashima, S. & Yamada, S. (1980) Tetrahedron 36, 3161–3170. [Google Scholar]
- 35.Alphand, V. & Furstoss, S. V. (1992) J. Org. Chem. 57, 1306–1309. [Google Scholar]
- 36.Pirkle, W. H. & Adams, P. E. (1980) J. Org. Chem. 45, 4111–4117. [Google Scholar]
- 37.Corazza, F., Solari, E., Floriani, C., Chiesi-Villa, A. & Guastini, C. (1990) J. Chem. Soc. Dalton Trans., 1335–1344.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.