Abstract
Using the newly introduced designer surfactant polyethyleneglycol ubiquinol sebacate (PQS), as the platform for micellar catalysis, nonracemic BINAP has been covalently attached and rhodium(I) inserted to form PQS-BINAP-Rh. This species, the first example of a nonracemically-ligated transition metal catalyst-tethered amphiphile, can be utilized for Rh-catalyzed asymmetric conjugate addition reactions of arylboronic acids to acyclic and cyclic enones. These are performed in water at room temperature, while the catalyst can be recycled without its removal from water in the reaction vessel.
Keywords: 1,4-additions; Rh-catalyzed 1,4-additions; rhodium-catalyzed 1,4-additions micellar catalysis; catalyst recycling; in-flask recycling; green chemistry; chemistry in water; in water; homogenous; homogenous catalysis; polyethyleneglycol ubiquinol sebacate; PQS
The chemical enterprise is rapidly gaining an awareness of, and an appreciation for, the 12 Principles of Green Chemistry[1] as guidelines in the design of new chemical processes. One component highlights catalysis, and implied in this context is the highly desirable feature of catalyst recycling.[2a] Another important component of green chemistry is the use of “safer solvents”, ideally being “innocuous when used.” Thirdly, “safer chemicals” of minimal toxicity should be utilized, while maintaining functionality. Based on these key teachings, a new amphiphile, polyethyleneglycol ubiquinol sebacate (“PQS”, 2; Scheme 1), was constructed[3] derived from the reduced form of readily available coenzyme Q10; i.e., ubiquinol.[4] This species undergoes spontaneous self-aggregation in water, with the resulting nanomicelles enabling homogeneous catalysis within their lipophilic hydrocarbon cores.[2b] Since this platform is derived from a hydroquinone, one hydroxyl moiety remains available for further derivatization. Previously, we have described opportunities to covalently attach both Grubbs-Hoveyda-1[5] and -2[6] ruthenium carbene catalysts, thereby leading to olefin metathesis reactions within these aqueous nanomicelles. Likewise, we recently disclosed a strategy for effecting organocatalysis in water at room temperature using the related PQS-containing proline,[7] which serves as both the reaction medium and as catalyst. In each type of chemistry, the amphiphile was designed to remain in the water; extraction with minimal organic solvent (e.g., EtOAc) leads to subsequent product isolation without loss of catalyst from the reaction vessel; i.e., the catalyst remains in the aqueous phase within the flask ready for recycling.
Scheme 1.
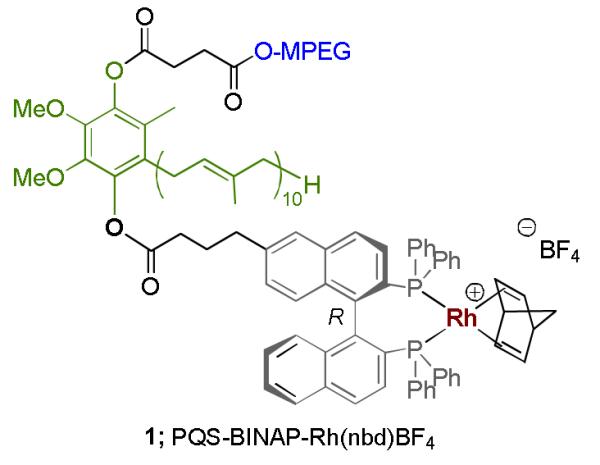
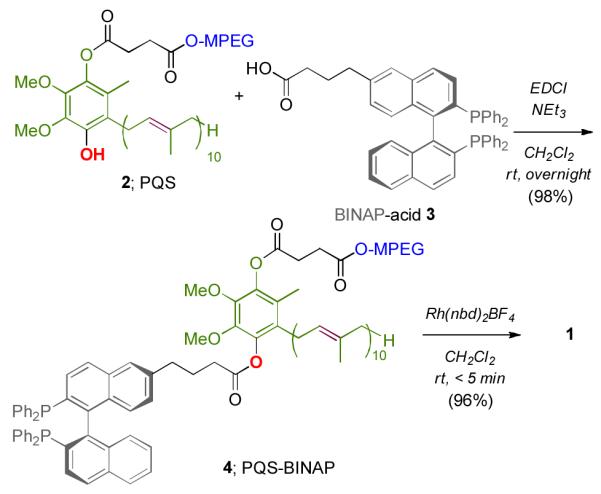
Synthesis of PQS-BINAP-Rh (1).
An important, alternative application of PQS technology yet to be documented focuses on asymmetric catalysis. In this regard, we are now pleased to report the first example of a recyclable, amphiphile-bound, nonracemic transition metal catalyst that functions in water at room temperature. Given the rich history of BINAP in asymmetric catalysis,[8] this ligand was selected as a “proof of principle” example for covalent attachment to the PQS skeleton 2 in the form of catalyst 1. Complexation of nonracemic BINAP to rhodium(I) is well known from the work of Miyaura.[9] Hayashi,[10] and Carreira[11] to mediate 1,4-addition reactions of arylboronic acids to enones with high levels of enantiocontrol.[12, 13] Catalyst 1 was prepared via (R)-BINAP derivative 4 by esterification of PQS with known BINAP acid 3 (Scheme 1).[14] Subsequent addition of [Rh(nbd)2]BF4 to 4 leads to complex 1, which readily dissolves and self-assembles in water forming 43 nm nanomicelles as shown by dynamic light scattering.[15] As illustrated in Figure 1, water-soluble, highly colored catalyst 1 is untouched upon attempted extraction into solvents such as Et2O.
Figure 1.

Aqueous solution of PQS-BINAP-Rh complex 1 in water in the presence of Et2O.
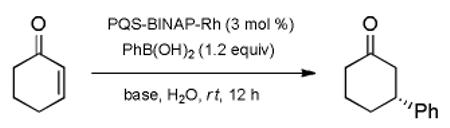
Conditions most amenable to micellar catalysis using model system 2-cyclohexenone and PhB(OH)2 focused on the selection of the base, which can be a major factor in such Rh-catalyzed conjugate additions.[16] On the one hand, use of KOH would be expected to accelerate both the transmetalation event from an ate salt of the boronic acid, as well as arrive at a more reactive RhOH-containing species.[17] Unfortunately, given the diester nature of the PQS platform, presumed saponification of PQS led to little-to-none of the desired 1,4-adduct (Table 1, entry 1). While Hunig’s base was far more effective (entry 2), Et3N (3.0 equiv) provided the best balance of basicity and lipophilicity, leading to the product of 1,4-addition in 95% yield and >99% ee (entry 3), results that compare quite favorably with those using alternative ligand and catalyst arrays.[18,19] Only three mole percent of the catalyst PQS-BINAP-Rh (1) is needed, and that only 1.2 equivalents of boronic acid suffice may reflect the high concentrations of reactants normally present within the limited amounts of surfactant in the aqueous medium.[20b] Less base (entry 4) led to essentially no reaction. While dilution of the reaction by an equal volume had little impact (entry 5), dropping the catalyst loading from three to one percent slowed the 1,4-addition considerably (entry 6).
Table 1.
Screening for the optimal base.a)
| entry | base | yieldb)[%] | eec)[%] |
|---|---|---|---|
| 1 | KOH | <5 | - |
| 2 | DIPEA | 61 | 98 (R) |
| 3 | NEt3 | 95 | 99 (R) |
| 4d) | NEt3 | <5 | - |
| 5e) | NEt3 | 92 | 00 (R) |
| 6f) | NEt3 | 58 | 99 (R) |
General procedure: 0.20 mmol cyclohexenone, 0.24 mmol PhB(OH)2, 0.006 mmol PQS-BINAP-Rh, 0.60 mmol base, and 0.4 mL H2O (0.5 M).
Yield of isolated product.
Determined by HPLC on chiral stationary phase.
0.5 equiv base.
Reaction run in 0.8 mL H2O (0.25 M).
1 mol % of PQS-BINAP-Rh.
Representative examples of both cyclic and acyclic enones are illustrated in Scheme 2. Arylboronic acids bearing either electron-donating or -withdrawing groups participated in good to high yields with excellent enantioselectivities. The more hindered case of ortho-methylboronic acid reacted smoothly as well, affording the conjugate adduct in close to 98% ee. Furthermore, asymmetric additions of phenylboronic acid to five- and seven-membered cyclic enones were both quite successful, affording good yields with excellent levels of asymmetric induction.
Scheme 2.
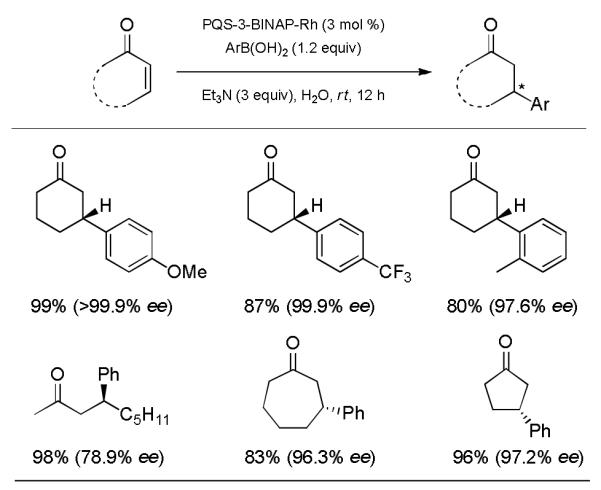
Representative examples using PQS-BINAP-Rh as the catalyst for 1,4-addition reactions.a)
a) General procedure: 0.20 mmol enone, 0.24 mmol ArB(OH)2, 0.006 mmol PQS-BINAP-Rh, 0.60 mmol Et3N, and 0.4 mL H2O (0.5 M).
Comparison cases with literature examples using the identical catalyst, albeit in organic media, document the potential improvements that may be realized, perhaps in a general sense, based on the hydrophobic effect.[20] Thus, cases in both the 2-cyclohexenone and 2-cycloheptenone series afford yields and ee’s of conjugate adducts that are comparable to, or better than, those previously described (Figure 2).
Figure 2.
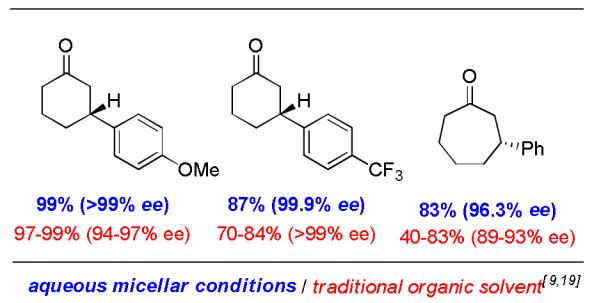
Comparisons with literature results.
The inherent water solubility of PQS-BINAP-Rh is conducive to in-flask catalyst recycling. Hence, upon completion of the 1,4-addition, the product was extracted from the reaction mixture with a single, suitable organic solvent (e.g., EtOAc). Reintroduction of fresh substrate, base, and boronic acid only (i.e., no additional catalyst, solvent, or cosolvent) resulted in product, likewise, of excellent enantioselectivity, as well as in good isolated yield (Scheme 3). Different substrates could be used, added to the same reaction mixture sequentially, and in each case the anticipated product was obtained. While the integrity of the PQS-BINAP-Rh system appears to remain intact judging from the consistently high ee’s obtained, the extent of conversion, and hence, isolated yield from cycle 4 was only 50%. This suggests that the PQS-BINAP-Rh catalyst may have a fundamentally limited lifetime under such conditions, or perhaps eventual oxidation of the phosphines is responsible for drop in activity. Since the starting enone could be easily recovered from the reaction mixture, it was initially thought that potential buildup of boronic acid(s) might be hindering reactivity. However, a control experiment conducted in the presence of three equivalents of boronic acid (rather than the usual 1.2 equivalents) had no influence on reaction rate.
Scheme 3.
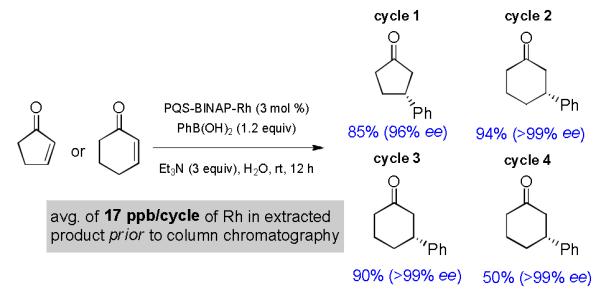
In-flask recycling of aqueous PQS-BINAP-Rh (1).
Tests for loss of metal from catalyst 1 were conducted to assess the level of anticipated bleed under our micellar catalysis conditions, an important factor for future applications to the potential syntheses of pharmacologically active targets. Analysis of the toluene/Et2O extract containing the desired 1,4-addition aduct by induced coupled plasma mass spectrometry (ICP-MS) revealed that each extract contained rhodium in a range of 1–40 ppb, with an average of 17 ppb (see Supporting Information). Thus, over four recycles essentially none of the metal was lost in workup, sugesting that the decrease in catalyst activity is due to factors other than removal of 1 from the reaction mixture (e.g., ligand oxidation).
In summary, a new catalyst system, PQS-BINAP-Rh, has been constructed that enables homogeneous catalysis in the form of asymmetric conjugate additions of arylboronic acids to enones to be conducted in water at ambient temperatures. Yields are high given the mild reaction conditions, and the extent of enantiocontrol is found to be comparable to that previously seen for related methodologies. No organic solvents are needed as reaction media, and neither heating nor cooling is required, a phenomenon that takes advantage of the high concentrations associated with the micellar effect.[20b] Most noteworthy is the opportunity to recycle the water-soluble, covalently bound catalyst without its removal from the reaction flask. Finally, this study should highlight the many alternatives available for attaching and thereby recycling other ligand arrays to the PQS platform, especially those that are lengthy to prepare, and that ultimately involve investment of precious metals.
Experimental Section
Representative Procedure for 1,4-Addition Reactions: (Scheme 2)
Under an atmosphere of argon a 1 mL screwcap vial was charged with PQS-BINAP-Rh precatalyst (30 mg, 0.006 mmol), boronic acid (0.24 mmol), and removed from the glovebox. Water (0.4 mL) and Et3N (84 μL, 0.6 mmol) were added to the vial and stirred for 15 min. When the solution became homogeneous, enone (0.2 mmol) was added via syringe and the mixture was stirred at rt for 12 h. The homogeneous reaction mixture was then diluted with EtOAc (0.5 mL), filtered through a bed of silica gel, and the bed further washed (3 × 2 mL) with EtOAc to collect all of the coupled material. The volatiles were removed in vacuo to afford the crude product which was subsequently purified by flash chromatography on silica gel using EtOAc/hexanes.
Supplementary Material
Acknowledgements
Financial support provided by the NIH (GM 86485) is warmly acknowledged with thanks. Additionally, we acknowledge Sean Duncan of Scripps Institute of Oceanography for his ICP analytical work. Lastly, Wendy Leong for her assistance synthesizing PQS-BINAP-Rh used to complete the presented work.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/adsc.201######.
References
- [1].Anastas PT, Warner JC, editors. Green Chemistry: Theory and Practice. Oxford University Press; New York: 2000. [Google Scholar]
- [2]a).Sheldon RA, Arends I, Hanefeld U, editors. Green Chemistry and Catalysis. Wiley-VCH; Weinhem, Germany: 2007. for examples of homogeneous catalysis in water, see: Leseurre L, Genêt J-P, Michelet V. Coupling Reactions in Water. In: Anastas P, Li C–J, editors. Handbook of Green Chemistry Series: Water as a Green Solvent. John Wiley & Sons; 2010.
- [3].Moser R, Ghorai S, Lipshutz BH. J. Org. Chem. 2012;77:3143. doi: 10.1021/jo202564b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Morgan AC, Graves SS, Woodhouse CS, Sikorska M, Walker R, Wilbur DS, Borowy-Borowski H. Water Soluble Ubiquinone Compositions, Prodrugs, and Methods Relating Thereto. 1996. PCT, CAN 125:123707. [Google Scholar]
- [5].Lipshutz BH, Ghorai S. Org. Lett. 2009;11:705. doi: 10.1021/ol8027829. [DOI] [PubMed] [Google Scholar]
- [6].Lipshutz BH, Ghorai S. Tetrahedron. 2010;66:1057. [Google Scholar]
- [7].Lipshutz BH, Ghorai S. Org. Lett. 2012;14:422. doi: 10.1021/ol203242r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Noyori R. Adv. Synth. Catal. 2003;345:15. [Google Scholar]
- [9]a).Sakai M, Hayashi H, Miyaura N. Organometallics. 1997;16:4229. [Google Scholar]; b) Takaya Y, Ogasawara M, Hayashi T, Sakai M, Miyaura N. J. Am. Chem. Soc. 1998;120:5579. [Google Scholar]
- [10]a).Takaya Y, Ogasawara M, Hayashi T. Tetrahedron Lett. 1999;40:6957. [Google Scholar]; b) Hayashi T, Takahashi M, Takaya Y, Ogasawara M. J. Am. Chem. Soc. 2002;124:5052. doi: 10.1021/ja012711i. [DOI] [PubMed] [Google Scholar]; c) Kina A, Iwamura H, Hayashi T. J. Am. Chem. Soc. 2006;128:3904. doi: 10.1021/ja060225v. [DOI] [PubMed] [Google Scholar]; d) Kina A, Yasuhara Y, Nishimura T, Iwamura H, Hayashi T. Chem. Asian J. 2010;5:707. doi: 10.1002/asia.200600184. [DOI] [PubMed] [Google Scholar]
- [11].Fischer C, Defieber C, Suzuki T, Carreira EM. J. Am. Chem. Soc. 2004;126:1628. doi: 10.1021/ja0390707. [DOI] [PubMed] [Google Scholar]
- [12].For recent reviews on rhodium-catalyzed asymmetric 1,4-additions, see: Tian P, Dong H-Q, Lin G-Q. ACS Catal. 2012;2:95. Edwards HJ, Hargrave JD, Penrose SD, Frost CG. Chem. Soc. Rev. 2010;39:2093. doi: 10.1039/b919762c. Shintani R, Hayashi T. Aldrichimica Acta. 2009;42:31. Defieber C, Grützmacher H, Carreira EM. Angew. Chem., Int. Ed. 2008;47:4482. doi: 10.1002/anie.200703612. Johnson JB, Rovis T. Angew. Chem., Int. Ed. 2008;47:840. doi: 10.1002/anie.200700278.
- [13]a).Berthon G, Hayashi T. chap. 1. In: Córdova A, editor. Catalytic Asymmetric Conjugate Reactions. Wiley-VCH; Weinheim: 2010. [Google Scholar]; b) Shintani R, Hayashi T, Mikami K, Lautens M. New Frontiers in Asymmetric Catalysis. Wiley; Hoboken: 2007. chap. 3. [Google Scholar]; c) Yoshida K, Hayashi T. chap. 4. In: Hall DG, editor. Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine. Wiley-VCH; Weinheim: 2005. [Google Scholar]; d) Yoshida K, Hayashi T. chap. 3. In: Evans PA, editor. Modern Rhodium-Catalyzed Organic Reactions. Wiley-VCH; Weinheim: 2005. [Google Scholar]
- [14].Synthesized through a known and modified procedure: Bayston DJ, Fraser JL, Ashton MR, Baxter AD, Polywka MEC. J. Org. Chem. 1998;63:3137. (see supporting information)
- [15].Borkovec M, editor. Handbook of Applied Surface and Colloid Chemistry. John Wiley & Sons Ltd.; Chichester, U.K.: 2002. pp. 357–370. [Google Scholar]
- [16].Miyaura N. Bull. Chem. Soc. Jpn. 2008;81:1535. [Google Scholar]
- [17].Hayashi T, Yamasaki K. Chem. Rev. 2003;103:2829. doi: 10.1021/cr020022z. [DOI] [PubMed] [Google Scholar]
- [18]a).Martina SLX, Minnaard AJ, Hessen B, Feringa BL. Tetrahedron Lett. 2005;46:7159. [Google Scholar]; b) Berhal F, Esseiva O, Martin C-H, Tone H, Genêt JP, Ayad T, Ratovelomanana-Vidal V. Org. Lett. 2011;13:2806. doi: 10.1021/ol200495a. [DOI] [PubMed] [Google Scholar]
- [19]a).Yuan W-C, Cun L-F, Mi A-Q, Jiang Y-Z, Gong L-Z. Tetrahedron. 2009;65:4130. Amengual R, Michelet V, Genêt J-P. Synlett. 2002;11:1791. for catalyst recycling of chiral modified BINAP ligand, see: Otomaru Y, Senda T, Hayashi T. Org. Lett. 2004;6:3357. doi: 10.1021/ol048695m. for catalyst recycling with achiral water soluble ligand, see: Amengual R, Michelet V, Genêt J-P. Tetrahedron Lett. 2002;43:5905.
- [20]a).Malmsten M, editor. Surfactants and Polymer in Drug Delivery. Marcel Dekker; New York: 2002. [Google Scholar]; b) Fendler JH, Fendler EJ, editors. Catalysis in Micellar and Macromolecular Systems. Academic Press; New York: 1975. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


