Abstract
A Cu(I)-catalyzed direct addition of alkynes to imines was developed. The process is simple and provides a diverse range of propargylamines in high enantiomeric excess and good yield both in water and in toluene. The absolute configuration of such addition products has been determined by x-ray crystallography.
As in the case for the addition reaction of carbanions to the carbonyl group of aldehydes and ketones (1–4), the addition of organometallic reagents to the C N bonds of imines or imine derivatives is an important reaction in organic synthesis (5–9). The carbon–carbon bond-forming method, which uses alkynes as a carbon nucleophile source, is a useful method in synthesis (10, 11). The resulting alkynyl amine derivatives can undergo further transformations and are versatile synthetic tools (12–14). However, the reactive alkynilides are usually prepared from terminal alkyne by using highly reactive organometallic reagents such as BuLi (15, 16), EtMgBr (17, 18), or LDA (19, 20) in a separate step, and many metal alkynilides are not easy to handle because the reaction must be carried out under anhydrous solvent, inert atmosphere, and low temperature conditions. Therefore, the development of a method for the direct alkynylation of imines that does not use a stoichiometric amount of highly reactive organometallic reagent and can be performed under mild conditions would be highly desirable. Additionally, there has been greater interest in recent years in developing environmentally friendly reactions in aqueous media (21, 22).
N bonds of imines or imine derivatives is an important reaction in organic synthesis (5–9). The carbon–carbon bond-forming method, which uses alkynes as a carbon nucleophile source, is a useful method in synthesis (10, 11). The resulting alkynyl amine derivatives can undergo further transformations and are versatile synthetic tools (12–14). However, the reactive alkynilides are usually prepared from terminal alkyne by using highly reactive organometallic reagents such as BuLi (15, 16), EtMgBr (17, 18), or LDA (19, 20) in a separate step, and many metal alkynilides are not easy to handle because the reaction must be carried out under anhydrous solvent, inert atmosphere, and low temperature conditions. Therefore, the development of a method for the direct alkynylation of imines that does not use a stoichiometric amount of highly reactive organometallic reagent and can be performed under mild conditions would be highly desirable. Additionally, there has been greater interest in recent years in developing environmentally friendly reactions in aqueous media (21, 22).
Optically active propargyl amines are important synthetic intermediates for the synthesis of various nitrogen compounds and are components of bioactive compounds or natural products (23–25). Recently, great efforts have been made to develop the methodology for generating propargylamines (26–33). Some direct alkynylations of carbonyl compounds with terminal alkynes have been reported (34–37). Miura (38) has reported the addition of acetylene to nitrones in the presence of a catalytic amount of CuI with K2CO3 as the base and phosphine- and nitrogen-containing compounds as the ligands in N,N′-dimethylformamide at 80°C under nitrogen, to give a (2 + 2) coupling product along with a redox product, alkynyl imine. Carreira (39–41) has used a Zn(II)-catalyzed process in CH2Cl2 for the addition of terminal alkynes to nitrones to form propargyl N-hydroxyamine adducts and recently reported (42) the addition of terminal alkynes to imines by an iridium catalyst in toluene or under neat conditions. Ishii (43) reported an “unexpected” case of the addition of alkyne to imine by an Ir(I) catalyst. Jiang (44) has reported the addition of terminal alkynes to imines by using ZnCl2 and Et3N as catalysts and TMSCl as the Lewis acid for the activation of imines. Methods for the catalytic preparation of optically active propargyl amines are still limited (10–14, 30, 31).
One of our ongoing interests is in the development of transition metal-catalyzed carbon–carbon bond formation in water (45–48). We have recently reported the efficient addition reaction of phenylacetylene to aldehydes catalyzed by [Ru]-[In] in a mildly basic aqueous media (49). In extending our addition reaction methodology, we found that phenylacetylene can also react with an imine in the presence of a catalytic amount of Cu(I) and Ru(III) in aqueous media to give the desired adducts (50). This reaction provides a convenient and efficient method for the preparation of propargyl amines. We have subsequently extended this methodology to the enantioselective addition of terminal alkynes to imines catalyzed by a chiral Cu(I)–bis(oxazolinyl)pyridine (pybox) complex in water or toluene (51). In this article, we present a full description of our work.
Experimental Procedures
All experiments were carried out under an inert atmosphere of nitrogen. All solvents and reagents were purchased and used without prior purification. Water was deoxygenated by nitrogen. Flash column chromatography was performed over 30–60 μm of silica gel purchased from Sorbent Technologies (Somerset, NJ). 1H NMR and 13C NMR spectra were recorded at 400 and 100 MHz, respectively, and referenced to the internal solvent signals. IR spectra were recorded by Thermo Nicolet (Madison, WI) Interferometer. Gas chromatography/MS data were obtained by using an HP-5890 Series II Gas Chromatograph and an HP-5989A Mass Spectrometer (Hewlett–Packard). Enantiomeric excess (ee) was determined by using HPLC with a Chiralcel OD (Chiral Technologies, Exton, PA) column and 1/20 hexane/isopropanol as an eluent. High-resolution mass spectra (HRMS) were made by Bin Xin (Institute of Chemistry, Chinese Academy of Sciences, Beijing).
General Procedure for the Nucleophilic Addition of Alkynes to Imines Catalyzed Cu/Ru in Water. The mixture of aldehyde (2 mmol) and aniline (2.4 mmol) was heated at 60°C for ≈2 h. A mixture of ruthenium trichloride (10 mg, 0.06 mmol, 3 mol %) and Cu(I) bromide (85 mg, 0.6 mmol, 30 mol %) was added, then phenylacetylene (250 μl, 2.4 mmol) and water (2 ml) were added into the mixture by a syringe under an inert atmosphere of nitrogen. After stirring at room temperature for 10 min, the mixture was heated at the temperature (shown in Table 1) and stirred overnight. The reaction mixture was then poured into water and extracted with either diethyl ether or methylene chloride. The organic layer was washed with water and dried over anhydrous Mg2SO4. The solvent was removed in vacuo. After flash column chromatography on silica gel with EtOAc/hexane (1/40) as the eluent, the product was isolated as a yellowish oil. Some of products were purified further by recrystallization from methylene chloride/hexane to give pale yellow prisms.
Table 1. Direct addition of alkynes to imines.
| Entry | Aldehyde | Aniline | Alkyne | Solvent/temperature, °C/time, days | Yield, % |
|---|---|---|---|---|---|
| 1 | PhCHO | PhNH2 | PhCCH | H2O/60/1 | 91 |
| 2 | 4-CH3C6H4CHO | PhNH2 | PhCCH | H2O/60/1 | 87 |
| 3 | 4-C2H5C6H4CHO | PhNH2 | PhCCH | H2O/60/1 | 86 |
| 4 | 4tBuC6H4CHO | PhNH2 | PhCCH | H2O/60/1 | 86 |
| 5 | 4-ClC6H4CHO | PhNH2 | PhCCH | H2O/70/1 | 90 |
| 6 | 3-ClC6H4CHO | PhNH2 | PhCCH | H2O/70/1 | 89 |
| 7 | 4-BrC6H4CHO | PhNH2 | PhCCH | H2O/70/1 | 95 |
| 8 | 3-BrC6H4CHO | PhNH2 | PhCCH | H2O/70/1 | 93 |
| 9 | 1-NaphCHO | PhNH2 | PhCCH | H2O/70/1 | 96 |
| 10 | 2-NaphCHO | PhNH2 | PhCCH | H2O/70/1 | 75 |
| 11 | 4-PhC6H4CHO | PhNH2 | PhCCH | H2O/70/1 | 85 |
| 12 | 4-CH3OC6H4CHO | PhNH2 | PhCCH | H2O/70/1 | 52 |
| 13 | 4-CF3C6H4CHO | PhNH2 | PhCCH | H2O/35/1 | 87 |
| 14 | 4-NCC6H4CHO | PhNH2 | PhCCH | H2O/45/1 | 40 |
| 15 | 4-O2NC6H4CHO | PhNH2 | PhCCH | H2O/45/1 | 27 |
| 16 | (CH3)3CCHO | PhNH2 | PhCCH | H2O/35/1 | 64 |
| 17 | (C2H5)2CHCHO | PhNH2 | PhCCH | H2O/45/1 | 47 |
| 18 | c-C6H11CHO | PhNH2 | PhCCH | H2O/45/1 | 58 |
| 19 | PhCHO | 4-BrC6H4NH2 | PhCCH | Neat/45/1 | 82 |
| 20 | PhCHO | 4-ClC6H4NH2 | PhCCH | Neat/45/1 | 87 |
| 21 | PhCHO | 4-CH3C6H4NH2 | PhCCH | Neat/45/1 | 77 |
| 22 | PhCHO | 4-CH3OC6H4NH2 | PhCCH | Neat/45/1 | 55 |
| 23 | PhCHO | PhNH2 | HCCH | DMF/22/0.5 | 78 |
| 24 | PhCHO | PhNH2 | n-C3H7CCH | Neat/45/1 | 86 |
| 25 | PhCHO | PhNH2 | Et3SiCCH | Toluene/45/1 | 83 |
| 26 | PhCHO | PhNH2 | Cl(CH2)4CCH | H2O/60/1 | 57 |
| 27 | PhCHO | PhNH2 | HO(CH2)4CCH | H2O/60/1 | 80 |
| 28 | PhCHO | PhNH2 | HO(CH2)2CCH | H2O/60/1 | 82 |
| 29 | PhCHO | PhNH2 | A | H2O/60/1 | 75 |
A, 1-Ethynyl-1-cyclohexanol.
General Procedure for the Enantioselective Addition of Alkynes to Imines Catalyzed by Pybox 5-Cu(OTf) Complex in Toluene and Water. The mixture of aldehyde (0.2 mmol) and aniline (0.24 mmol) was heated at 60°C for ≈2 h. Pybox 5 (6 mg, 0.2 mmol, 10 mol %) and Cu(I) triflate toluene complex (10 mg, 0.2mmol, 10 mol %) were added, then alkyne (0.3 mmol) and solvent (0.5 ml) were added to the mixture by a syringe under an inert atmosphere of nitrogen. The mixture was stirred at room temperature or at 35°C for 2–4 days. The mixture in water was extracted with diethyl ether or methylene chloride. The organic layer was washed with water and dried over anhydrous Mg2SO4. The solvent was removed in vacuo. The residue was filtered through silica gel with methylene chloride. The reaction mixture in toluene was filtered through silica gel with methylene chloride directly. After flash column chromatography with EtOAc/hexane (1/40) as the eluent, the product was isolated as a yellowish oil, and some were purified further by recrystallization from ethyl acetate/methylene chloride/hexane to give pale yellow prisms.
rac-N-(1,3-diphenyl-2-propynyl)aniline. HPLC (Daicel Chiralcel OD, hexane/i-PrOH = 95:5, flow rate = 0.5 ml/min) tR = 14.8 min, tR = 18.0 min, ee = 0%; IR (film): νmax 3402 (m), 2225 (w), 1600 (s), 1502 (s), 1316 (s), 1179 (s) cm–1; 1H NMR (CDCl3, 400 MHz, ppm): δ 7.70–7.64 (m, 2H), 7.46–7.18 (m, 10H), 6.83–6.77 (m, 3H), 5.51 (s, 1H), 4.36 (s, 1H); 13C NMR (CDCl3, 100 MHz, ppm): δ 146.7, 139.9, 132.0, 129.4, 129.0, 128.6, 128.5, 128.4, 127.6, 123.0, 118.9, 114.4, 88.6, 85.3, 50.9; HRMS calc. for C21H17N, 283.1361; found, 283.1362.
rac-N-[1-(4-methylphenyl)-3-phenyl-2-propynyl]aniline. HPLC (Daicel Chiralcel OD, hexane/i-PrOH = 95:5, flow rate = 0.5 ml/min) tR = 14.4 min, tR = 16.3 min, ee = 0%; IR (film): νmax 3402 (m), 2225 (w), 1601 (s), 1501 (s), 1316 (s), 1180 (m) cm–1; 1H NMR (CDCl3, 400 MHz, ppm): δ 7.58 (d, J = 8.0 Hz, 2H), 7.48–7.43 (m, 2H), 7.34–7.29 (m, 3H), 7.28–7.22 (m, 4H), 6.85–6.80 (m, 3H), 5.50 (s, 1H), 4.20 (br, 1H), 2.41 (s, 3H); 13C NMR (CDCl3, 100 MHz, ppm): δ 146.8, 138.2, 137.0, 132.0, 129.8, 129.4, 128.5, 128.5, 127.5, 123.1, 118.9, 114.4, 88.9, 85.2, 50.7, 21.5; HRMS calc. for C22H19N, 297.1518; found, 297.1517.
rac-N-[1-(4-ethylphenyl)-3-phenyl-2-propynyl]aniline. HPLC (Daicel Chiralcel OD, hexane/i-PrOH = 95:5, flow rate = 0.5 ml/min) tR = 13.7 min, tR = 14.9 min, ee = 0%; IR (film): νmax 3402 (m), 2207 (w), 1601 (s), 1501 (s), 1316 (s), 1180 (s) cm–1; 1H NMR (CDCl3, 400 MHz, ppm): δ 7.58 (d, J = 7.6 Hz, 2H), 7.45–7.40 (m, 2H), 7.35–7.22 (m, 7H), 6.85–6.80 (m, 3H), 5.49 (s, 1H), 4.25 (br, 1H), 2.70 (q, J = 7.6 Hz, 2H), 1.26 (t, J = 7.6 Hz, 3H); 13C NMR (CDCl3, 100 MHz, ppm): δ 146.8, 144.5, 137.2, 132.0, 129.4, 128.5, 128.5, 128.5, 127.6, 123.1, 118.8, 114.4, 88.9, 85.1, 50.7, 28.8, 15.8; HRMS calc. for C23H22N (M+H), 312.1747; found, 312.1748.
rac-N-[1-(4-chlorophenyl)-3-phenyl-2-propynyl]aniline. HPLC (Daicel Chiralcel OD, hexane/i-PrOH = 95:5, flow rate = 0.5 ml/min) tR = 21.6 min, tR = 26.6 min, ee = 0%; IR (film): νmax 3411 (m), 2234 (w), 1602 (s), 1502 (s), 1316 (s), 1180 (m) cm–1; 1H NMR (CDCl3, 400 MHz, ppm): δ 7.64–7.59 (m, 2H), 7.46–7.42 (m, 2H), 7.42–7.37 (m, 2H), 7.35–7.28 (m, 3H), 7.28–7.21 (m, 2H), 6.87–6.75 (m, 3H), 5.50 (s, 1H), 4.23 (br, 1H); 13C NMR (CDCl3, 100 MHz, ppm): δ 146.5, 138.5, 134.1, 132.0, 129.5, 129.2, 128.9, 128.8, 128.6, 122.8, 119.2, 114.5, 88.2, 85.7, 50.4; HRMS calc. for C21H16ClN, 317.0971; found, 317.0966.
rac-N-[1-(4-bromophenyl)-3-phenyl-2-propynyl]aniline. HPLC (Daicel Chiralcel OD, hexane/i-PrOH = 95:5, flow rate = 0.5 ml/min) tR = 22.9 min, tR = 28.6 min, ee = 0%; IR (film): νmax 3375 (m), 2217 (w), 1602 (s), 1502 (s), 1316 (s), 1179 (m) cm–1; 1H NMR (CDCl3, 400 MHz, ppm): δ 7.56–7.51 (m, 4H), 7.43–7.39 (m, 2H), 7.33–7.27 (m, 3H), 7.26 (m, 2H), 6.84–6.72 (m, 3H), 5.47 (s, 1H) 4.25 (br, 1H); 13C NMR (CDCl3, 100 MHz, ppm): δ 146.3, 139.0, 132.1, 132.0, 129.5, 129.3, 128.8, 128.5, 122.7, 122.3, 119.2, 114.5, 88.0, 85.7, 50.5; HRMS calc. for C21H16BrN, 361.0466; found, 361.0453.
rac-N-[1-(4-phenylphenyl)-3-phenyl-2-propynyl]aniline. HPLC (Daicel Chiralcel OD, hexane/i-PrOH = 95:5, flow rate = 0.5 ml/min) tR = 27.7 min, tR = 32.6 min, ee = 0%; IR (film): νmax 3402 (m), 2225 (w), 1601 (s), 1501 (s), 1316 (s), 1180 (m) cm–1; 1H NMR (CDCl3, 400 MHz, ppm): δ 7.88 (d, J = 8.0 Hz, 2H), 7.81–7.73 (m, 4H), 7.63–7.55 (m, 4H), 7.54–7.47 (m, 1H), 7.46–7.39 (m, 3H), 7.38 (d, J = 8.0 Hz, 2H), 7.01–6.92 (m, 3H), 5.70 (s, 1H), 4.35 (br, 1H); 13C NMR (CDCl3, 100 MHz, ppm): δ 147.0, 141.4, 141.0, 139.2, 132.2, 129.7, 129.3, 128.8, 128.7, 128.2, 128.0, 127.9, 127.6, 123.2, 119.1, 114.6, 89.0, 85.6, 50.8; HRMS calc. for C27H21N, 359.1674; found, 359.1672.
rac-N-[1-(2-naphthyl)-3-phenyl-2-propynyl]aniline. HPLC (Daicel Chiralcel OD, hexane/i-PrOH = 95:5, flow rate = 0.5 ml/min) tR = 27.7 min, tR = 30.7 min, ee = 0%; IR (film): νmax 3411 (m), 2277 (m), 1601 (s), 1501 (s), 1316 (s), 1180 (m) cm–1; 1H NMR (CDCl3, 400 MHz, ppm): δ 8.30–8.25 (m, 1H), 8.04–7.94 (m, 3H), 7.90–7.84 (m, 1H), 7.66–7.58 (m, 4H), 7.44–7.33 (m, 5H), 6.99–6.90 (m, 3H), 5.80 (s, 1H), 4.40 (s, 1H); 13C NMR (CDCl3, 100 MHz, ppm): δ 147.0, 137.6, 133.8, 133.6, 132.3, 129.7, 129.2, 128.8, 128.7, 128.6, 128.2, 126.8, 126.7, 126.5, 125.8, 123.2, 119.1, 114.6, 89.0, 85.8, 51.2; HRMS calc. for C25H19N, 333.1518; found, 333.1507.
rac-N-[1-(4-trifluoromethylphenyl)-3-phenyl-2-propynyl]-aniline. HPLC (Daicel Chiralcel OD, hexane/i-PrOH = 95:5, flow rate = 0.5 ml/min) tR = 22.6 min, tR = 30.2 min, ee = 0%; IR (film): νmax 3402 (m), 2199 (w), 1602 (s), 1502 (s), 1320 (s), 1162 (s) cm–1; 1H NMR (CDCl3, 400 MHz, ppm): δ 7.88 (d, J = 8.0 Hz, 2H), 7.76 (d, J = 8.0 Hz, 2H), 7.58–7.53 (m, 2H), 7.43–7.38 (m, 3H), 7.38–7.32 (m, 2H), 6.95 (td, J = 7.6, 0.8 Hz, 1H), 6.86 (d, J = 8.8 Hz, 2H), 5.67 (s, 1H), 4.35 (br, 1H); 13C NMR (CDCl3, 100 MHz, ppm): δ 146.5, 144.2, 132.2, 130.6 (q, J = 32.7 Hz), 129.7, 129.0, 128.7, 128.0, 126.1 (q, J = 3.8 Hz), 124.5 (q, J = 271.1 Hz), 122.7, 119.4, 114.5, 88.0, 86.1, 50.7; HRMS calc. for C22H16F3N, 351.1235; found, 351.1227.
rac-N-(1,3-diphenyl-2-propynyl)-4-bromoaniline. HPLC (Daicel Chiralcel OD, hexane/i-PrOH = 95:5, flow rate = 0.5 ml/min) tR = 19.7 min, tR = 22.0 min, ee = 0%; IR (film): νmax 3403 (m), 2225 (w), 1596 (s), 1492 (s), 1311 (s), 1180 (m) cm–1; 1H NMR (CDCl3, 400 MHz, ppm): δ 7.67 (d, J = 7.6 Hz, 2H), 7.48–7.41 (m, 4H), 7.41–7.35 (m, 1H), 7.35–7.28 (m, 5H), 6.70–6.64 (m, 2H), 5.48 (s, 1H), 4.21 (s, 1H); 13C NMR (CDCl3, 100 MHz, ppm): δ 145.8, 139.5, 132.3, 132.1, 129.2, 128.8, 128.6 (2C), 127.6, 122.8, 116.0, 110.6, 88.2, 85.6, 50.8; HRMS calc. for C21H16BrN, 361.0446; found, 361.0458.
rac-N-(1,3-diphenyl-2-propynyl)-4-chloroaniline. HPLC (Daicel Chiralcel OD, hexane/i-PrOH = 95:5, flow rate = 0.5 ml/min) tR = 17.7 min, tR = 20.4 min, ee = 0%; IR (film):νmax 3408 (m), 2225 (w), 1598 (s), 1495 (s), 1311 (s), 1175 (m) cm–1; 1H NMR (CDCl3, 400 MHz, ppm): δ 7.72 (d, J = 7.6 Hz, 2H), 7.52–7.45 (m, 4H), 7.45–7.38 (m, 1H), 7.38–7.23 (m, 3H), 7.25–7.20 (m, 2H), 6.70–6.64 (m, 2H), 5.48 (s, 1H), 4.21 (s, 1H); 13C NMR (CDCl3, 100 MHz, ppm): δ 145.4, 139.6, 132.1, 129.4, 129.2, 128.8, 128.7, 128.6, 127.7, 123.5, 122.9, 115.6, 88.3, 85.7, 51.0; HRMS calc. for C21H16ClN, 317.0971; found, 317.0967.
rac-N-(1,3-diphenyl-2-propynyl)-4-methylaniline. HPLC (Daicel Chiralcel OD, hexane/i-PrOH = 95:5, flow rate = 0.5 ml/min) tR = 15.8 min, tR = 16.6 min, ee = 0%; IR (film): νmax 3404 (m), 2225 (w), 1616 (s), 1520 (s), 1300 (s), 1184 (m) cm–1; 1H NMR (CDCl3, 400 MHz, ppm): δ 7.72 (d, J = 7.6 Hz, 2H), 7.50–7.42 (m, 4H), 7.42–7.36 (m, 1H), 7.35–7.30 (m, 3H), 7.08 (d, J = 8.0 Hz, 2H), 6.76 (d, J = 7.6 Hz, 2H), 5.53 (s, 1H), 4.09 (br, 1H), 2.32 (s, 3H); 13C NMR (CDCl3, 100 MHz, ppm): δ 144.6, 140.2, 132.1, 130.0, 129.1, 128.6, 128.5, 128.3, 128.1, 127.6, 123.2, 114.6, 89.0, 85.3, 51.3, 20.8; HRMS calc. for C22H19N, 297.1518; found, 297.1510.
rac-N-[1-(3-bromophenyl)-3-phenyl-2-propynyl]aniline. HPLC (Daicel Chiralcel OD, hexane/i-PrOH = 95:5, flow rate = 0.5 ml/min) tR = 19.3 min, tR = 29.7 min, ee = 0%; IR (film): νmax 3402 (m), 2251 (w), 1602 (s), 1503 (s), 1316 (s), 1179 (s) cm–1; 1H NMR (CDCl3, 400 MHz, ppm): δ 7.86 (s, 1H), 7.62 (d, J = 8.4 Hz, 1H), 7.53–7.43 (m, 3H), 7.36–7.23 (m, 6H), 6.86 (dt, J = 7.2, 1.2 Hz, 1H), 6.79 (dd, J = 8.4, 1.2 Hz), 5.51 (s, 1H), 4.36 (br, 1H); 13C NMR (CDCl3, 100 MHz, ppm): δ 146.4, 142.4, 132.1, 131.5, 130.6, 129.6 (2C), 128.8, 128.6, 126.2, 123.1, 122.7, 119.2, 114.5, 88.0, 85.9, 50.5; HRMS calc. for C21H16BrN, 361.0466; found, 361.0455.
R-N-[1-(3-bromophenyl)-3-phenyl-2-propynyl)aniline. HPLC (Daicel Chiralcel OD, hexane/i-PrOH = 95:5, flow rate = 0.5 ml/min) tR = 19.2 min (R), tR = 29.5 min (S), ee = 95%. Spectroscopic data were identical to those of the racemic compound.
N-[1-(3-chlorophenyl)-3-phenyl-2-propynyl]aniline. IR (film): νmax 3411 (m), 2216 (w), 1602 (s), 1502 (s), 1320 (s), 1188 (m) cm–1; 1H NMR (CDCl3, 400 MHz, ppm): δ 7.72 (s, 1H), 7.62–7.58 (m, 1H), 7.50–7.45 (m, 2H), 7.38–7.31 (m, 5H), 7.30–7.25 (m, 2H), 6.87 (t, J = 7.2 Hz, 1H), 6.80 (d, J = 8.4 Hz, 2H), 5.53 (s, 1H), 4.25 (br, 1H); 13C NMR (CDCl3, 100 MHz, ppm): δ 146.5, 142.2, 134.9, 132.1, 130.4, 129.6, 128.8, 128.6, 128.6, 127.8, 125.7, 122.8, 119.2, 114.5, 88.0, 85.8, 50.5; HRMS calc. for C21H16ClN, 317.0971; found, 317.0968.
N-[1-(4-tert-butylphenyl)-3-phenyl-2-propynyl]aniline. IR (film): νmax 3402 (m), 2207 (m), 1602 (s), 1507 (s), 1316 (s), 1184 (m) cm–1; 1H NMR (CDCl3, 400 MHz, ppm): δ 7.61 (d, J = 8.0 Hz, 2H), 7.48–7.41 (m, 4H), 7.32–7.28 (m, 3H), 7.28–7.22 (m, 3H), 6.85–6.80 (m, 3H), 5.50 (s, 1H), 4.25 (br, 1H), 1.36 (s, 9H); 13C NMR (CDCl3, 100 MHz, ppm): δ 151.4, 146.7, 136.8, 132.0, 129.4, 128.5, 128.5, 127.4, 126.0, 123.1, 118.9, 114.5, 88.8, 85.2, 50.6, 34.9, 31.6; HRMS calc. for C25H25N, 339.1987; found, 339.1988.
N-[1-(4-methoxyphenyl)-3-phenyl-2-propynyl]aniline. IR (film): νmax 3402 (m), 2198 (w), 1601 (s), 1506 (s), 1302 (s), 1171 (s) cm–1; 1H NMR (CDCl3, 400 MHz, ppm): δ 7.60 (d, J = 8.4 Hz, 2H), 7.46–7.41 (m, 2H), 7.32–7.28 (m, 3H), 7.27–7.20 (m, 2H), 6.95 (d, J = 8.4 Hz, 2H), 6.85–6.79 (m, 3H), 5.47 (d, J = 5.6 Hz, 1H), 4.13 (d, J = 5.6 Hz, 1H), 3.82 (s, 3H); 13C NMR (CDCl3, 100 MHz, ppm): δ 159.7, 146.9, 132.1, 132.0, 129.4, 129.0, 128.8, 128.5, 128.5, 123.1, 118.8, 114.3, 89.0, 85.1, 55.6, 50.3; HRMS calc. for C22H19NO, 313.1467; found, 313.1458.
N-[1-(4-cyanophenyl)-3-phenyl-2-propynyl]aniline. IR (film): νmax 3366 (m), 2225 (s), 1602 (s), 1501 (s), 1316 (s), 1193 (m) cm–1; 1H NMR (CDCl3, 400 MHz, ppm): δ 7.78 (d, J = 8.0 Hz, 2H), 7.67 (d, J = 8.0 Hz, 2H), 7.50–7.45 (m, 2H), 7.37–7.31 (m, 3H), 7.30–7.24 (m, 2H), 6.87 (t, J = 7.2 Hz, 1H), 6.77 (d, J = 8.4 Hz, 2H), 5.59 (d, J = 5.6 Hz, 1H), 4.41 (d, J = 5.6 Hz, 1H); 13C NMR (CDCl3, 100 MHz, ppm): δ 146.3, 145.5, 132.9, 132.1, 129.6, 129.1, 128.7, 128.2, 122.5, 119.4, 119.1, 114.5, 112.1, 87.5, 86.3, 50.6; HRMS calc. for C22H16N2, 308.1314; found, 308.1301.
N-[1-(4-nitrophenyl)-3-phenyl-2-propynyl]aniline. IR (film): νmax 3405 (m), 2207 (m), 1599 (s), 1520 (s), 1347 (s), 1180 (m) cm–1; 1H NMR (CDCl3, 400 MHz, ppm): δ 8.22 (d, J = 8.8 Hz, 2H), 7.80 (d, J = 8.8 Hz, 2H), 7.42–7.36 (m, 2H), 7.31–7.26 (m, 3H), 7.22–7.16 (m, 2H), 6.80 (t, J = 7.2 Hz, 1H), 6.69 (d, J = 8.0 Hz, 2H), 5.57 (d, J = 5.6 Hz, 1H), 4.30 (d, J = 5.6 Hz, 1H); 13C NMR (CDCl3, 100 MHz, ppm): δ 147.9, 147.4, 146.1, 132.0, 129.6, 129.0, 128.6, 128.3, 124.3, 122.3, 119.5, 114.4, 87.1, 86.4, 50.5; HRMS calc. for C21H16N2O2, 328.1212; found, 328.1194.
N-[1-(1,1-dimethylethyl)-3-phenyl-2-propynyl]aniline. IR (film): νmax 3411 (m), 1602 (s), 1501 (s), 1311 (s), 1225 (m) cm–1; 1H NMR (CDCl3, 400 MHz, ppm): δ 7.40–7.33 (m, 2H), 7.30–7.20 (m, 5H), 6.81–6.77 (m, 3H), 4.03 (s, 1H), 3.80 (br, 1H), 1.18 (s, 9H); 13C NMR (CDCl3, 100 MHz, ppm): δ 147.8, 131.9, 129.5, 128.4, 128.2, 123.5, 118.5, 114.4, 89.4, 84.0, 56.8, 35.9, 26.8; HRMS calc. for C19H21N, 263.1674; found, 263.1674.
Crystal Data of R-N-[1-(3-bromophenyl)-3-phenyl-2-propynyl)aniline. C21H16NBr, mol wt. = 362.27, colorless, prism, orthorhombic, space group P212121 (no. 19), a = 5.3510(4), b = 8.39105, c = 37.579(2) Å, volume (V) = 1687.3(2) Å3, Z = 4, calculated density = 1.12 g·cm–3, F(000) = 736, μ(MoKα) = 24.42 cm–1. For detailed x-ray analysis, please contact the corresponding author.
Supporting Information. For additional characterization of products, see the supporting information, which is published on the PNAS web site.
Results and Discussion
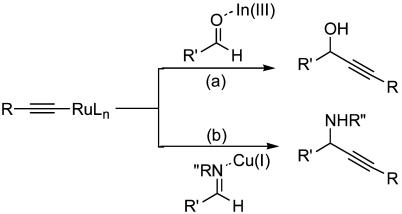
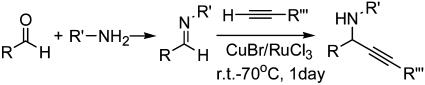
Initially, we examined the reaction of an imine, which was readily accessible via the in situ condensation of benzaldehyde with aniline, with phenylacetylene by using the [Ru]/[In] catalytic system in water (48). However, it did not provide any of the desired imine addition product, although this catalyst was very effective for alkyne-aldehyde addition. The failure of the reaction was attributed to the inability of indium(III), although effective in activating aldehyde (52–53) (Fig. 1, route a), to coordinate and activate the imine effectively in water. It was speculated that copper ions (soft Lewis acids) (54–56) may be effective in activating the imine (Fig. 1, route b). Subsequently, we found that the desired addition product was formed in a moderate yield by using copper catalysts in water (Scheme 1). Among the catalysts tested, copper salts such as CuCl, CuCl2, CuBr, and CuI all showed moderate catalytic activities, with CuSO4, Cu(OAc)2, and CuCN being only slightly active. CuBr was found to be the best catalyst among the copper salts. By using 3 mol % RuCl3 as a co-catalyst, the yield of the desired addition product was dramatically increased, but no imine addition product was observed when RuCl3 was used as the catalyst alone. The reaction of 1.5 eq of phenylacetylene with imine in water in the presence of 30 mol % Cu(I) bromine and 3 mol % RuCl3 at 70°C for 15 h afforded the adduct in 90% yield. Further addition of various ligands did not improve the yield.
Fig. 1.
Two routes for the addition of metal alkynilide to an activating aldehyde or an activating imine.
Scheme 1.
[Adapted with permission from ref. 50 (Copyright 2002, The Royal Society of Chemistry).]
A variety of substrates were then examined in the reaction both in water, in organic solvent, and under neat conditions, and the results are summarized in Table 1. As shown in Table 1, this catalytic system could be applied to a broad range of substituted aromatic imines and aliphatic imines to afford the corresponding phenylalkynylamines. In many examples, when a mixture of phenylacetylene and an imine was treated with the RuCl3/CuBr catalytic system in water, the addition reaction took place smoothly giving a propargylamine in good yield. In most cases, we carried out the reaction directly with a mixture of aldehyde, aniline, alkyne, and CuBr/RuCl3 in water, without the need to synthesize the imines in a separate step. However, some imines were easily hydrolyzed in water, resulting in a mixture of aldehyde, imine, and propargylamine.
To minimize the hydrolysis of these imines, we examined the addition reaction in toluene and under neat conditions. We found that the reactions were highly effective under neat conditions: aromatic imines with electron-withdrawing groups were more reactive, and the reaction was carried out at lower temperatures. The imines with cyano and nitro substituents on the aromatic aldehyde gave lower yields accompanied by the formation of some unidentified byproducts (the major product was the addition of aldehyde to triple bond in alkyne to form an α,β-unsaturated carbonyl compound). This catalytic system could also be applied to aliphatic alkynes to afford the corresponding propargylamine. The results are shown as entries 23–29 in Table 1.
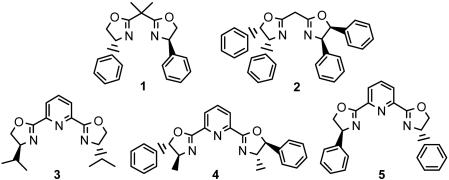
With this simple and effective method in hand, our next step was to develop an efficient enantioselective alkyne-imine addition. During our investigation, it was observed that CuBr alone could provide the desired product, albeit in low conversions. It was postulated that the low catalytic activity when using Cu(I) alone could be due to the strong bonding and thus low reactivity of the C—Cu bond of copper acetylides (57–58). We conceived that the addition of a strongly coordinating and electron-rich ligand may weaken the strong C—Cu bond. This postulate also provides opportunities for developing asymmetric alkyne-imine additions. Following this hypothesis, we examined a variety of chiral compounds as ligands in the addition reaction of phenylacetylene with N-benzylideneaniline with 10% CuBr as the catalyst. However, it was both encouraging and disappointing to find that although the reactivity was indeed enhanced significantly, no enantioselectivity was observed with most of these chiral catalysts. On the other hand, numerous enantioselective addition reactions with chiral copper–bis(oxazoline) box complexes have been described during the past two decades (59–61). Therefore, we decided to try chiral bis(oxazolinyl) ligands in our reaction and found that the desired enantioselective addition product was formed with a low enantioselectivity by using bidentate box 1 and 2 with CuBr complex as catalysts in water (Fig. 2). On the other hand, tridentate bis(oxazolinyl)pyridines (pybox) such as 3, 4, and 5 showed more enantioselective catalytic activity. The optimal enantioselectivity of addition adducts was observed with 5–CuBr complex (90% conversion and 24% ee in water). The use of 5-Cu(OTf) complex instead of 5-CuBr complex afforded the product with both high reactivity and enantioselectivity (90% conversion and 83% ee in water). However, the corresponding 5–CuSbF6 complex was much less reactive but had similar enantioselectivity (20% conversion and 78% ee in water). The reaction of phenylacetylene and the imine catalyzed by 5–Cu(OTf) proceeded with excellent enantioselectivity and in good yield in toluene (78% isolated yield and 96% ee). The conversion of the addition reaction increased effectively in the presence of a slight excess of aniline.
Fig. 2.
Some chiral bis(oxazolinyl) ligands used in our reaction. [Adapted with permission from ref. 51 (Copyright 2002, American Chemical Society).]
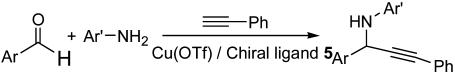
Subsequently, the scope of the asymmetric alkyne-imine addition was investigated (Scheme 2). As shown in Tables 2 and 3, this catalytic system could be applied to a broad range of substituted aromatic imines to afford the corresponding (+)-propargyl amine in high enantioselectivity and good yields. In all cases, when a mixture of phenylacetylene and imine was treated with pybox 5 complexed with Cu(OTf) in toluene, the addition reaction took place smoothly, giving a (+)-propargyl amine. The reaction in toluene provided slightly higher yields and enantioselectivities than in water.
Scheme 2.
Table 2. Enantioselective addition of phenylacetylene to aromatic imines in toluene.
| Entry | Aldehyde | Aniline | Temperature, °C/time, days | Yield, % | OR/ee % |
|---|---|---|---|---|---|
| 1 | PhCHO | PhNH2 | 22/4 | 78 | (+) 96 |
| 2 | PhCHO | PhNH2 | 35/2 | 83 | (+) 93 |
| 3 | 4-CH3C6H4CHO | PhNH2 | 35/2 | 85 | (+) 92 |
| 4 | 4-C2H5C6H4CHO | PhNH2 | 22/4 | 70 | (+) 96 |
| 5 | 4-C2H5C6H4CHO | PhNH2 | 35/2 | 73 | (+) 95 |
| 6 | 4-ClC6H4CHO | PhNH2 | 22/4 | 85 | (+) 94 |
| 7 | 4-ClC6H4CHO | PhNH2 | 35/2 | 90 | (+) 92 |
| 8 | 4-BrC6H4CHO | PhNH2 | 22/4 | 87 | (+) 94 |
| 9 | 4-BrC6H4CHO | PhNH2 | 35/2 | 90 | (+) 92 |
| 10 | 3-BrC6H4CHO | PhNH2 | 22/4 | 85 | (+) 95 |
| 11 | 3-BrC6H4CHO | PhNH2 | 35/2 | 88 | (+) 92 |
| 12 | 4-PhC6H4CHO | PhNH2 | 22/4 | 81 | (+) 94 |
| 13 | 4-PhC6H4CHO | PhNH2 | 35/2 | 85 | (+) 90 |
| 14 | 2-NaphCHO | PhNH2 | 22/4 | 63 | (+) 88 |
| 15 | 2-NaphCHO | PhNH2 | 35/2 | 67 | (+) 82 |
| 16 | 4-CF3C6H4CHO | PhNH2 | 22/4 | 71 | (+) 93 |
| 17 | PhCHO | 4-BrC6H4NH2 | 35/2 | 93 | (+) 91 |
| 18 | PhCHO | 4-ClC6H4NH2 | 35/2 | 92 | (+) 91 |
| 19 | PhCHO | 4-CH3C6H4NH2 | 35/2 | 93 | (+) 94 |
OR, optical rotation.
Table 3. Enantioselective addition of phenylacetylene to aromatic imines in water.
| Entry | Aldehyde | Aniline | Temperature, °C/time, days | Yield, % | OR/ee % |
|---|---|---|---|---|---|
| 1 | PhCHO | PhNH2 | 22/4 | 71 | (+) 84 |
| 2 | PhCHO | PhNH2 | 35/2 | 77 | (+) 80 |
| 3 | 4-CH3C6H4CHO | PhNH2 | 35/2 | 86 | (+) 81 |
| 4 | 4-C2H5C6H4CHO | PhNH2 | 22/4 | 68 | (+) 89 |
| 5 | 4-C2H5C6H4CHO | PhNH2 | 35/2 | 68 | (+) 78 |
| 6 | 4-ClC6H4CHO | PhNH2 | 22/4 | 70 | (+) 87 |
| 7 | 4-ClC6H4CHO | PhNH2 | 35/2 | 74 | (+) 85 |
| 8 | 4-BrC6H4CHO | PhNH2 | 22/4 | 63 | (+) 87 |
| 9 | 4-BrC6H4CHO | PhNH2 | 35/2 | 74 | (+) 85 |
| 10 | 3-BrC6H4CHO | PhNH2 | 22/4 | 69 | (+) 85 |
| 11 | 3-BrC6H4CHO | PhNH2 | 35/2 | 72 | (+) 82 |
| 12 | 4-PhC6H4CHO | PhNH2 | 22/4 | 48 | (+) 84 |
| 13 | 4-PhC6H4CHO | PhNH2 | 35/2 | 56 | (+) 82 |
| 14 | 2-NaphCHO | PhNH2 | 22/4 | 57 | (+) 86 |
| 15 | 2-NaphCHO | PhNH2 | 35/2 | 65 | (+) 78 |
| 16 | 4-CF3C6H4CHO | PhNH2 | 22/4 | 56 | (+) 87 |
| 17 | PhCHO | 4-BrC6H4NH2 | 35/2 | 82 | (+) 83 |
| 18 | PhCHO | 4-ClC6H4NH2 | 35/2 | 77 | (+) 84 |
| 19 | PhCHO | 4-CH3C6H4NH2 | 35/2 | 68 | (+) 91 |
This catalytic system could be extended to aliphatic alkynes to afford the corresponding propargyl amines. As shown in Table 4, various alkynes add to imines in toluene with good yields and enantioselectivity. Phenylacetylene afforded better yields and enantioselectivity than other aliphatic alkynes. The effect of solvent on the yield and enantioselectivity of the addition of phenylacetylene to N-benzylideneaniline was examined (Table 5). Both the yield and ee % are strongly affected by the reaction solvent.
Table 4. Enantioselective addition of alkynes to N-benzylideneaniline.
| Entry | Alkyne | Solvent/temperature °C/time, days | Yield, % | OR/ee % |
|---|---|---|---|---|
| 1 | n-C3H7CCH | Neat/22/4 | 55 | (+) 71 |
| 2 | Et3SiCCH | Toluene/22/4 | 53 | (+) 60 |
| 3 | Cl(CH2)4CCH | Toluene/22/4 | 48 | (+) 65 |
| 4 | HO(CH2)4CCH | Toluene/22/4 | 62 | (+) 85 |
| 5 | HO(CH2)2CCH | Toluene/22/4 | 58 | (-) 82 |
Table 5. Enantioselective addition of phenylacetylene to N-benzylideneaniline in various solvents.
| Entry | Solvent | Yield, % | ee % |
|---|---|---|---|
| 1 | Toluene | 78 | 96 |
| 2 | Benzene | 74 | 97 |
| 3 | Heptane | 80 | 99 |
| 4 | Dichloromethane | 79 | 98 |
| 5 | Chloroform | 81 | 97 |
| 6 | 1,2-Dichloroethane | 71 | 99.5 |
| 7 | Carbon tetrachloride | 25 | 99.4 |
| 8 | 1,4-Dioxane | 82 | 98 |
| 9 | Tetrahydrofuran | 65 | 96 |
| 10 | Ethyl acetate | 53 | 98 |
| 11 | Acetone | 80 | 99 |
| 12 | N,N-dimethylformamide | 80 | 97 |
| 13 | Acetonitrile | 82 | 99 |
| 14 | Methanol | 85 | 88 |
| 15 | Triethylamine | 48 | 97 |
| 16 | Water | 71 | 84 |
To determine our product with greater certainty, we wanted to ascertain the absolute configuration of the propargylamine. We tried to correlate our products with known compounds in which the absolute configuration was determined, but only racemic amines were formed by following the literature procedures (62, 63).
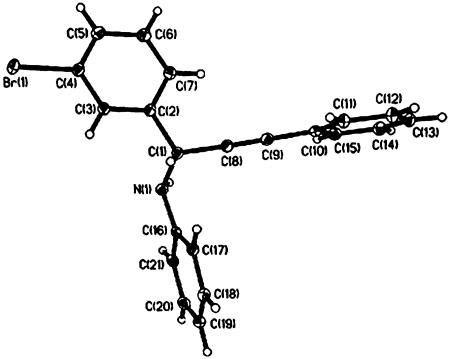
Fortunately, three of the addition products could be grown into single crystals from ethyl acetate/dichloromethane/hexane. With a bromo-atom in the molecule serving as a structure marker, the absolute configuration of one of our products was conveniently established as (R) by x-ray crystallographic analysis (Fig. 3). The structure was solved and refined by using the shelxtl (Version 6.1, Bruker, Billerica, MA) software package, and the absolute structure was unambiguously determined [Flack parameter –0.009 (7)]. The absolute configuration of other products of the enantioselective addition can also be correlated with this structure from their same optical rotation direction and similar order of elution.
Fig. 3.
The x-ray structure of R-N-[1-(3-bromophenyl)-3-phenyl-2-propynyl]aniline.
In conclusion, we have developed a Cu(I) complex-catalyzed direct addition of alkynes to imines. The process is simple and provides a diverse range of propargylamines in high ee and good yield both in water and in organic solvents. The absolute configuration of such addition products has also been determined by x-ray crystallography. The scope, mechanism, and synthetic application of this enantioselective reaction, along with other C—C bond formation reactions via C—H reactivity in water or in various solvents, are under investigation.
Supplementary Material
Acknowledgments
We are grateful to the National Science Foundation–Environmental Protection Agency joint program for a Sustainable Environment and the National Science Foundation for the support of this research.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ee, enantiomeric excess; HRMS, high-resolution MS; pybox, bis(oxazolinyl)pyridine.
References
- 1.McMurry, J. (2000) Organic Chemistry (Brooks/Cole, Pacific Grove, CA), pp. 768–769.
- 2.Carey, F. A. & Sundberg, R. J. (2000) Advanced Organic Chemistry (Kluwer Academic/Plenum, New York), pp. 462–470.
- 3.Evans, D. A., Rovis, T. & Johnson, J. S. (1999) Pure Appl. Chem. 71, 1407–1415. [Google Scholar]
- 4.Goldfuss, B. (2003) in Topics in Organometallic Chemistry, eds. Brown, J. M., Dixneuf, P., Fürstner, A., Hegedus, L. S., Hofmann, P., Knochel, P., Koten, G. V., Murai, S. & Reetz, M. (Springer, Heidelberg), Vol. 5, pp. 21–35. [Google Scholar]
- 5.Bloch, R. (1998) Chem. Rev. 98, 1407–1438. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi, S. & Ishitani, H. (1999) Chem. Rev. 99, 1069–1094. [DOI] [PubMed] [Google Scholar]
- 7.Ellman, J. A., Owens, T. D. & Tang, T. P. (2002) Acc. Chem. Res. 35, 984–995. [DOI] [PubMed] [Google Scholar]
- 8.Legros, J., Meyer, F., Coliboeuf, M., Crousse, B., Bonnet-Delpon, D. & Begue, J.-P. (2003) J. Org. Chem. 68, 6444–6446. [DOI] [PubMed] [Google Scholar]
- 9.Akullian, L. C., Snapper, M. L. & Hoveyda, A. H. (2003) Angew. Chem. Int. Ed. Engl. 42, 4244–4247. [DOI] [PubMed] [Google Scholar]
- 10.Traverse, J. F., Hoveyda, A. H. & Snapper, M. L. (2003) Org. Lett. 5, 3273–3275. [DOI] [PubMed] [Google Scholar]
- 11.Prajapati, D., Laskar, D. D., Gogoi, B. J. & Devi, G. (2003) Tetrahedron Lett. 44, 6755–6757. [Google Scholar]
- 12.Mori, Y. & Hayashi, H. (2002) Tetrahedron 58, 1789–1797. [Google Scholar]
- 13.Galliford, C. V., Beenen, M. A., Nguyen, S. T. & Scheidt, K. A. (2003) Org. Lett. 5, 3487–3490. [DOI] [PubMed] [Google Scholar]
- 14.Garcia, C. I., Tillack, A., Hartung, C. G. & Beller, M. (2003) Tetrahedron Lett. 44, 3217–3221. [Google Scholar]
- 15.Takahashi, T., Bao, F., Gao, G. & Ogasawara, M. (2003) Org. Lett. 5, 3479–3481. [DOI] [PubMed] [Google Scholar]
- 16.Qian, M. & Negishi, E. (2003) Org. Proc. Res. Dev. 7, 412–417. [Google Scholar]
- 17.Umeno, M. & Suzuki, A. (1996) in Handbook of Grignard Reagent, eds. Silverman, G. S. & Rakita, P. E. (Dekker, New York), Vol. 64, pp. 645–666. [Google Scholar]
- 18.Tuulmets, A., Pallin, V., Tammiku-Taul, J., Burk, P. & Raie, K. (2002) J. Phys. Org. Chem. 15, 701–705. [Google Scholar]
- 19.Harada, T., Fujiwara, T., Iwazaki, K. & Oku, A. (2000) Org. Lett. 2, 1855–1857. [DOI] [PubMed] [Google Scholar]
- 20.Rosas, N., Sharma, P., Alvarez, C., Gomez, E., Gutierrez, Y., Mendez, M., Toscano, R. A. & Maldonado, L. A. (2003) Tetrahedron Lett. 44, 8019–8022. [Google Scholar]
- 21.Miyamoto, H., Daikawa, N. & Tanaka, K. (2003) Tetrahedron Lett. 44, 6963–6964. [Google Scholar]
- 22.Manabe, K. & Kobayashi, S. (2002) Chem. Eur. J. 8, 4094–4101. [DOI] [PubMed] [Google Scholar]
- 23.Kauffman, G. S., Harris, G. D., Dorow, R. L., Stone, B. R. P., Parsons, R. L., Jr., Pesti, J. A., Magnus, N. A., Fortunak, J. M., Confalone, P. N. & Nugent, W. A. (2000) Org. Lett. 2, 3119–3121. [DOI] [PubMed] [Google Scholar]
- 24.Huffman, M. A., Yasuda, N., DeCamp, A. E. & Grabowski, E. J. J. (1995) J. Org. Chem. 60, 1590–1594. [Google Scholar]
- 25.Enders, D. & Reinhold, U. (1997) Tetrahedron Asymmetry 8, 1895–1946. [Google Scholar]
- 26.Imada, Y., Yuassa, M., Nakamura, I. & Murahashi, S.-I. (1994) J. Org. Chem. 59, 2282–2284. [Google Scholar]
- 27.Mahrwald, R. & Quint, S. (2001) Tetrahedron Lett. 42, 1655–1656. [Google Scholar]
- 28.Caporusso, A. M., Geri, R., Polizzi, C. & Lardicci, L. (1991) Tetrahedron Lett. 32, 7471–7472. [Google Scholar]
- 29.Koradin, C., Gommermann, N., Polborn, K. & Knochel, P. (2003) Chem. Eur. J. 9, 2797–2811. [DOI] [PubMed] [Google Scholar]
- 30.Koradin, C., Polborn, K. & Knochel, P. (2002) Angew. Chem. Int. Ed. Engl. 41, 2535–2538. [DOI] [PubMed] [Google Scholar]
- 31.Gommermann, N., Koradin, C., Polborn, K. & Knochel, P. (2003) Angew. Chem. Int. Ed. Engl. 42, 5763–5766. [DOI] [PubMed] [Google Scholar]
- 32.Sakai, N., Hirasawa, M. & Konakahara, T. (2003) Tetrahedron Lett. 44, 4171–4174. [Google Scholar]
- 33.Traverse, J. F., Hoveyda, A. H. & Snapper, M. L. (2003) Angew. Chem. Int. Ed. Engl. 42, 4244–4247. [DOI] [PubMed] [Google Scholar]
- 34.Tzalis, D. & Knochel, P. (1999) Angew. Chen. Int. Ed. Engl. 38, 1463–1465. [DOI] [PubMed] [Google Scholar]
- 35.Frantz, D. E., Fässler, R. & Carreira, E. M. (2000) J. Am. Chem. Soc. 122, 1806–1807. [Google Scholar]
- 36.Anand, N. K. & Carreira, E. M. (2001) J. Am. Chem. Soc. 123, 9687–9688. [DOI] [PubMed] [Google Scholar]
- 37.Lu, G., Li, X., Jia, X., Chan, W. L. & Chan, A. S. C. (2003) Angew. Chem. Int. Ed. Engl. 42, 5057–5058. [DOI] [PubMed] [Google Scholar]
- 38.Miura, M., Enna, M., Okuro, K. & Nomura, M. (1995) J. Org. Chem. 60, 4999–5004. [Google Scholar]
- 39.Frantz, D. E., Fässler, R. & Carreira, E. M. (1999) J. Am. Chem. Soc. 121, 11245–11246. [Google Scholar]
- 40.Carreira, E. M. (2001) Chimia 55, 818–820. [Google Scholar]
- 41.Carreira, E. M., Frantz, D. E., Fässler, R. & Tomooka, C. S. (2000) Acc. Chem. Res. 33, 373–381. [DOI] [PubMed] [Google Scholar]
- 42.Carreira, E. M. & Fischer, C. (2001) Org. Lett. 3, 4319–4321. [DOI] [PubMed] [Google Scholar]
- 43.Sakaguchi, S., Kubo, T. & Ishii, Y. (2001) Angew. Chem. Int. Ed. Engl. 40, 2534–2536. [DOI] [PubMed] [Google Scholar]
- 44.Si, Y.-G. & Jiang, B. (2003) Tetrahedron Lett. 44, 6767–6768. [Google Scholar]
- 45.Venkatraman, S., Huang, T. & Li, C. J. (2002) Adv. Synth. Catal. 344, 399–405. [Google Scholar]
- 46.Huang, T., Venkatraman, S., Meng, Y., Nguyen, T. V., Kort, D., Wang, D., Ding, R. & Li, C. J. (2001) Pure Appl. Chem. 73, 1315–1318. [Google Scholar]
- 47.Li, C. J. (2002) Acc. Chem. Res. 35, 533–538. [DOI] [PubMed] [Google Scholar]
- 48.Li, C. J. & Chan, T. H. (1999) Tetrahedron 55, 11149–11176. [Google Scholar]
- 49.Wei, C. & Li, C. J. (2002) Green Chem. 4, 39–41. [Google Scholar]
- 50.Li, C. J. & Wei, C. (2002) Chem. Commun. 268–269. [DOI] [PubMed]
- 51.Wei, C. & Li, C. J. (2002) J. Am. Chem. Soc. 124, 5638–5639. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi, S., Nagayama, S. & Busujima, T. (1998) J. Am. Chem. Soc. 120, 8287–8288. [Google Scholar]
- 53.Onishi, Y., Ito, T., Yasuda, M. & Baba, A. (2002) Eur. J. Org. Chem. 1578–1581.
- 54.Woodward, S. (2000) Curr. Sci. 78, 1314–1317. [Google Scholar]
- 55.Mishra, K. & Saxena, M. C. (1986) J. Indian Chem. Soc. 63, 288–290. [Google Scholar]
- 56.Muroi, M., Kamiki, T. & Sekido, E. (1989) Bull. Chem. Soc. Jpn. 62, 1797–1801. [Google Scholar]
- 57.Eriksson, M., Iliefski, T., Nilsson, M. & Olsson, T. (1997) J. Org. Chem. 62, 182–187. [DOI] [PubMed] [Google Scholar]
- 58.Olbrich, F., Kopf, J. & Weiss, E. (1993) Angew. Chem. Int. Ed. Engl. 32, 1077–1079. [Google Scholar]
- 59.Evans, D. A., Kozlowski, M. C., Murry, J. A., Burgey, C. S., Campos, K. R., Connell, B. T. & Staples, R. J. (1999) J. Am. Chem. Soc. 121, 669–685. [Google Scholar]
- 60.Fache, F., Schulz, E., Tommasino, M. L. & Lemaire, M. (2000) Chem. Rev. 100, 2159–2232. [DOI] [PubMed] [Google Scholar]
- 61.Ghosh, A. K., Mathivanan, P. & Cappiello, J. (1998) Tetrahedron Asymmetry 9, 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marshall, J. A. & Wolf, M. A. (1996) J. Org. Chem. 61, 3238–3239. [Google Scholar]
- 63.Barmettler, P. & Hansen, H.-J. (1990) Helv. Chim. Acta 73, 1515–1573. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.