The “porcine stress syndrome” is a major cause of poor meat quality and death in the pork industry. It is known to be more prevalent in some pig strains than others,1 and susceptible animals can be identified by challenge with halothane, which results in striking elevation in body temperature. This scenario parallels the clinical entity of familial malignant hyperthermia upon exposure to general anesthetics, which was one of the earliest recognized human pharmacogenetic syndromes. We now know that affected pigs and people share the same molecular mechanism, mutations in the sarcoplasmic reticulum (SR) calcium release channel of skeletal muscle encoded by RYR1.2,3 In the pig world, selective breeding programs have been used to develop strains resistant to malignant hyperthermia. In humans, malignant hyperthermia is an anesthetic emergency and is treated by immediate intravenous administration of dantrolene which is effective and thought to be safe. Chronic oral dantrolene is also approved to treat severe muscle spasticity, and in this setting the limiting toxicity is hepatitis which can be fulminant and fatal in up to 1% of exposed subjects.
Leaky RyR channels in skeletal muscle and heart
Abrupt membrane depolarization (excitation) in skeletal or in cardiac muscle results in calcium release from SR stores via ryanodine receptor (RyR) calcium release channels, and the ensuing rise in intracellular calcium then activates the contractile apparatus. The details of the way in which excitation couples to contraction differs somewhat in the two types of muscle (in the heart, but not in skeletal muscle, calcium influx via voltage-gated Cav1.2 channels is required to activate RyR channels, Figure 1) and there are different genes encoding the channels, RYR1 in skeletal muscle and RYR2 in ventricular muscle. Early studies of the mechanism of action of dantrolene highlighted its ability to decouple excitation from contraction in skeletal muscle4 and we now know that in malignant hyperthermia, mutant RyR1 channels become “leaky” on exposure to drugs like halothane, and dantrolene is thought to act by preventing this leak. A body of evidence over the last decade has shown that in heart failure models, RyR2 channels display a baseline leak that is thought to contribute to arrhythmia susceptibility and may also exacerbate contractile dysfunction.5 Further, phosphorylation of the cardiac channel by PKA or CaM kinase II increases calcium release, and RyR2 “hyper-phosphorylation” has been implicated as an exacerbating mechanism in these clinical settings.6,7 While the extent to which RyR2 phosphorylation maintains or exacerbates contractile dysfunction and arrhythmias in human heart failure is controversial,8 molecular genetic studies have left no doubt that leaky RyR2 channels cause arrhythmias since mutations in RYR29 or CASQ210 (encoding the major SR calcium buffering protein) are the predominant cause of the syndrome of catecholaminergic polymorphic ventricular tachycardia (CPVT), a rare disease first described by Coumel and colleagues in the 1970s.11,12
Figure 1.
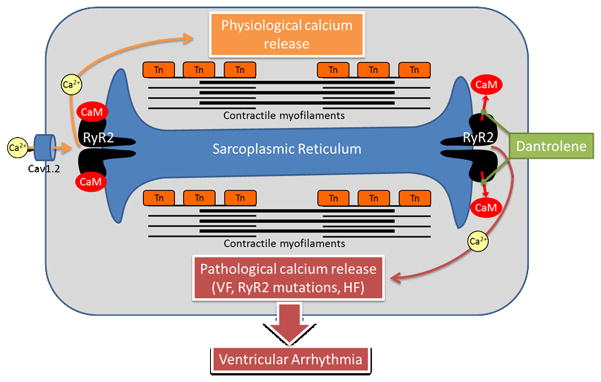
During physiological excitation-contraction coupling (left and top), ryanodine receptor (RyR2) calcium release channels are activated by calcium influx via voltage-gated Cav1.2 channels during the cardiac action potential. In pathological conditions such as heart failure, RyR2 mutations, or ventricular fibrillation (as in the present study), RyR2 channels become hyperactive and can open independent of an action potential (right and bottom); this pathological calcium release can depolarize the cell membrane and trigger ventricular arrhythmias. Dantrolene interrupts this arrhythmogenic chain of events by inhibiting RyR2 channels, likely by stabilizing calmodulin (CaM) binding to abnormal RyR2 channels.
Dantrolene as an antiarrhythmic
The striking mechanistic parallels between RYR1 mutations causing malignant hyperthermia and RYR2 mutations causing CPVT, both due to “leaky” ryanodine release channels, raises the question of whether dantrolene could be effective in CPVT or other settings in which defective RyR2 function leads to arrhythmias. In fact, dantrolene’s effects on cardiac rhythm were first investigated in the 1980s, with somewhat mixed results. Dantrolene significantly reduced the frequency and duration of episodes of ventricular fibrillation after coronary artery ligation in rats13 but another early study suggested that pretreatment with dantrolene actually increased the frequency of ventricular fibrillation induced by coronary artery occlusion in dogs.14 More recent studies demonstrated that dantrolene prevents abnormal calcium leak in both malignant hyperthermia RyR115 and CPVT RyR216 channels. In vitro, a CPVT mutant channel (R2474S) was shown to decrease the threshold at which luminal calcium elicited RyR2 channel opening, and thereby induced calcium “sparks” and delayed afterdepolarizations (DADs);17 in mice with this mutation, dantrolene stabilized the channels and was antiarrhythmic.16 Studies using myocytes derived from induced pluripotent stem cells of a subject with a different CPVT mutation18 as well as myocytes from rabbits with heart failure19 similarly demonstrated that dantrolene decreased calcium leak through abnormal RyR2 channels and increased threshold for spontaneous calcium release, both effects predicted to normalize pathophysiologic RyR2 function and thus be antiarrhythmic without altering, or perhaps even improving, contractile dysfunction.
Interestingly, while effective in failing rabbit myocytes, dantrolene had no effect on SR calcium release in healthy rabbit myocytes,19 and in healthy pigs, dantrolene only inhibited SR calcium release in skeletal but not in cardiac muscle.20 These findings beget the question of how dantrolene acts on RyR channels. Recent work has demonstrated that dantrolene binds to the Leu590-Cys609 region of RyR1 and stabilizes interdomain interactions within the RyR1 channel, which are thought to be disrupted by mutations that cause malignant hyperthermia. The dantrolene binding site has not yet been identified in cardiac RyR2, but dantrolene action in the heart may require altered calmodulin binding to RyR2: calmodulin physiologically bound to RyR2 reduces channel activity.21 Calmodulin binding is reduced either in heart failure22 or by CPVT mutations,23 rendering RyR2 channels hyperactive (Figure 1). Defective calmodulin binding can be restored by dantrolene (Figure 1),22 providing a possible explanation as to why dantrolene apparently affects SR calcium release in diseased but not in healthy hearts.
Use of dantrolene in VF – the present study
Zamiri and colleagues24 report in this issue of Circulation that dantrolene, administered after initiation of ventricular fibrillation in pigs (ironically enough) exerted dramatic beneficial effects on a range of indices of recovery of normal function after CPR and defibrillation: these included a dramatic decrease in the time to return to spontaneous circulation, decreases in the number of shock resistant ventricular fibrillation episodes, and a decrease in re-fibrillation. Dantrolene pre-treatment in isolated perfused rabbit hearts reduced the ability to induce ventricular fibrillation and reduced calcium leak. Interestingly, modeling dantrolene effects in ventricular muscle and in the Purkinje network suggested that while VF or very rapid stimulation promotes calcium-dependent DADs in both cell types, only DADs arising in the Purkinje network propagate to other sites to cause the VF; the extent to which abnormal calcium control and DADs in ventricular muscle serve to create a VF-prone substrate is not addressed. The finding is in keeping with studies suggesting that ablation of the Purkinje network renders ventricular fibrillation much more difficult to elicit and maintain in isolated perfused dog hearts.25 The demonstration of dantrolene efficacy when administered only minutes after the initiation of ventricular fibrillation provides evidence that disordered RyR2 function plays a critical role in determining the lethality of ventricular fibrillation within minutes of its onset. This, of course, makes the assumption that dantrolene lacks effects on other important electrogenic pathways such as ion channels, exchangers, or other signaling pathways affecting cardiac electrogenesis. While studies to date have not been comprehensive, there is no evidence that dantrolene exerts such effects.
Where to next?
In the present study, an old drug with an increasingly well-understood mechanism of action was used as a probe to define the contribution of perturbed RyR2 function early in VF. We have demonstrated that the sodium channel blocker flecainide also inhibits RyR2 channels, is antiarrhythmic in both mouse models of CPVT as well as in humans with the disease,26,27 and a randomized clinical trial comparing flecainide to placebo in patients with CPVT and implanted defibrillators is underway. In addition, new compounds have been reported that target RyR2 channels28,29 and may therefore find a clinical niche. Whether chronic therapy would be feasible would require a lot more work: in the Zamiri experiment, pretreatment was beneficial in isolated rabbit hearts, but pretreatment also exacerbated arrhythmias in earlier dog studies.14
Anesthesiologists have little compunction in reaching for intravenous dantrolene in the occasional patient with malignant hyperthermia. It is therefore possible to envision a similar use of intravenous dantrolene in the setting of ventricular fibrillation. Developing the drug for this indication would be challenging since it is long off patent for arrhythmias and finding a sponsor would be problematic. Nevertheless, the compound could serve as a lead to others with similar actions. The long road from the search for more tender pork to reversal of ventricular fibrillation once again illustrates the importance that understanding underlying mechanisms can contribute to the development or deployment of rational drug therapies.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported in part by grants from the United States Public Health Service (R01 HL049989, R01 HL118952, R01 HL071670, R01 HL108173 and R01 HL088635).
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Kukoyi EA, Addis PB, McGrath CJ, Rempel WE, Martin FB. Porcine stress syndrome and postmortem muscle characteristics of two purebreds and three specific terminal crosses. J Anim Sci. 1981;52:278–284. doi: 10.2527/jas1981.522278x. [DOI] [PubMed] [Google Scholar]
- 2.MacLennan DH, Duff C, Zorzato F, Fujii J, Phillips M, Korneluk RG, Frodis W, Britt BA, Wortont RG. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature. 1990;343:559–561. doi: 10.1038/343559a0. [DOI] [PubMed] [Google Scholar]
- 3.Fujii J, Otsu K, Zorzato F, de Leon S, Khanna VK, Weiler JE, O’Brien PJ, MacLennan DH. Identification of a mutation in porcine ryanodine receptor associated with malignant hyperthermia. Science. 1991;253:448–451. doi: 10.1126/science.1862346. [DOI] [PubMed] [Google Scholar]
- 4.Ellis KO, Bryant SH. Excitation-contraction uncoupling in skeletal muscle by dantrolene sodium. Naunyn Schmiedebergs Arch Pharmacol. 1972;274:107–109. doi: 10.1007/BF00501011. [DOI] [PubMed] [Google Scholar]
- 5.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 6.Marks AR, Reiken S, Marx SO. Progression of Heart Failure: Is Protein Kinase A Hyperphosphorylation of the Ryanodine Receptor a Contributing Factor? Circulation. 2002;105:272–275. [PubMed] [Google Scholar]
- 7.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 8.McCauley MD, Wehrens XH. Ryanodine receptor phosphorylation, calcium/calmodulin-dependent protein kinase II, and life-threatening ventricular arrhythmias. Trends Cardiovasc Med. 2011;21:48–51. doi: 10.1016/j.tcm.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli GA. Mutations in the Cardiac Ryanodine Receptor Gene (hRyR2) Underlie Catecholaminergic Polymorphic Ventricular Tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 10.Lahat H, Pras E, Olender T, Avidan N, Ben Asher E, Man O, Levy-Nissenbaum E, Khoury A, Lorber A, Goldman B, Lancet D, Eldar M. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet. 2001;69:1378–1384. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995;91:1512–1519. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- 12.Coumel P, Fidelle J, Lucet V, Attuel P, Bouvrain Y. Catecholamine-induced severe ventricular arrhythmias with Adams-Stokes syndrome in children: report of four cases. Brit Heart J. 1978;40 (suppl):28–37. [Google Scholar]
- 13.Brooks RR, Carpenter JF, Jones SM, Gregory CM. Effects of dantrolene sodium in rodent models of cardiac arrhythmia. Eur J Pharmacol. 1989;164:521–530. doi: 10.1016/0014-2999(89)90260-4. [DOI] [PubMed] [Google Scholar]
- 14.Pelleg A, Roth A, Shargordsky B, Belhassen B, Chagnac A, Laniado S. Effects of dantrolene sodium on occlusion and reperfusion arrhythmias in the canine heart. Methods Find Exp Clin Pharmacol. 1985;7:239–243. [PubMed] [Google Scholar]
- 15.Kobayashi S, Bannister ML, Gangopadhyay JP, Hamada T, Parness J, Ikemoto N. Dantrolene stabilizes domain interactions within the ryanodine receptor. J Biol Chem. 2005;280:6580–6587. doi: 10.1074/jbc.M408375200. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi S, Yano M, Uchinoumi H, Suetomi T, Susa T, Ono M, Xu X, Tateishi H, Oda T, Okuda S, Doi M, Yamamoto T, Matsuzaki M. Dantrolene, a therapeutic agent for malignant hyperthermia, inhibits catecholaminergic polymorphic ventricular tachycardia in a RyR2(R2474S/+) knock-in mouse model. Circulation J. 2010;74:2579–2584. doi: 10.1253/circj.cj-10-0680. [DOI] [PubMed] [Google Scholar]
- 17.Uchinoumi H, Yano M, Suetomi T, Ono M, Xu X, Tateishi H, Oda T, Okuda S, Doi M, Kobayashi S, Yamamoto T, Ikeda Y, Ohkusa T, Ikemoto N, Matsuzaki M. Catecholaminergic polymorphic ventricular tachycardia is caused by mutation-linked defective conformational regulation of the ryanodine receptor. Circ Res. 2010;106:1413–1424. doi: 10.1161/CIRCRESAHA.109.209312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung CB, Moretti A, Mederos y Schnitzler M, Iop L, Storch U, Bellin M, Dorn T, Ruppenthal S, Pfeiffer S, Goedel A, Dirschinger RJ, Seyfarth M, Lam JT, Sinnecker D, Gudermann T, Lipp P, Laugwitz K-L. Dantrolene rescues arrhythmogenic RYR2 defect in a patient-specific stem cell model of catecholaminergic polymorphic ventricular tachycardia. EMBO Mol Med. 2012;4:180–191. doi: 10.1002/emmm.201100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maxwell JT, Domeier TL, Blatter LA. Dantrolene prevents arrhythmogenic Ca2+ release in heart failure. Am J Physiol Heart Circ Physiol. 2012;302:H953–H963. doi: 10.1152/ajpheart.00936.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fruen BR, Mickelson JR, Louis CF. Dantrolene inhibition of sarcoplasmic reticulum Ca2+ release by direct and specific action at skeletal muscle ryanodine receptors. J Biol Chem. 1997;272:26965–26971. doi: 10.1074/jbc.272.43.26965. [DOI] [PubMed] [Google Scholar]
- 21.Smith JS, Rousseau E, Meissner G. Calmodulin modulation of single sarcoplasmic reticulum Ca2+-release channels from cardiac and skeletal muscle. Circ Res. 1989;64:352–359. doi: 10.1161/01.res.64.2.352. [DOI] [PubMed] [Google Scholar]
- 22.Ono M, Yano M, Hino A, Suetomi T, Xu X, Susa T, Uchinoumi H, Tateishi H, Oda T, Okuda S, Doi M, Kobayashi S, Yamamoto T, Koseki N, Kyushiki H, Ikemoto N, Matsuzaki M. Dissociation of calmodulin from cardiac ryanodine receptor causes aberrant Ca(2+) release in heart failure. Cardiovasc Res. 2010;87:609–617. doi: 10.1093/cvr/cvq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu X, Yano M, Uchinoumi H, Hino A, Suetomi T, Ono M, Tateishi H, Oda T, Okuda S, Doi M, Kobayashi S, Yamamoto T, Ikeda Y, Ikemoto N, Matsuzaki M. Defective calmodulin binding to the cardiac ryanodine receptor plays a key role in CPVT-associated channel dysfunction. Biochem Biophys Res Commun. 2010;394:660–666. doi: 10.1016/j.bbrc.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zamiri N, Massé S, Ramadeen A, Kusha M, Hu X, Azam M, Liu J, Lai PFH, Vigmond EJ, MBP, Behradfar E, Al-Hesayen A, Waxman M, Backx P, Dorian P, Nanthakumar K. Dantrolene Improves Survival Following Ventricular Fibrillation by Mitigating Impaired Calcium Handling in Animal Models. Circulation. 2014;129:XX–XXX. doi: 10.1161/CIRCULATIONAHA.113.005443. [DOI] [PubMed] [Google Scholar]
- 25.Dosdall DJ, Tabereaux PB, Kim JJ, Walcott GP, Rogers JM, Killingsworth CR, Huang J, Robertson PG, Smith WM, Ideker RE. Chemical ablation of the Purkinje system causes early termination and activation rate slowing of long-duration ventricular fibrillation in dogs. Am J Physiol Heart Circ Physiol. 2008;295:H883–889. doi: 10.1152/ajpheart.00466.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe H, Chopra N, Laver D, Hwang HS, Davies SS, Roach DE, Duff HJ, Roden DM, Wilde AA, Knollmann BC. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009;15:380–383. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Werf C, Kannankeril PJ, Sacher F, Krahn AD, Viskin S, Leenhardt A, Shimizu W, Sumitomo N, Fish FA, Bhuiyan ZA, Willems AR, van der Veen MJ, Watanabe H, Laborderie J, Haïssaguerre M, Knollmann BC, Wilde AAM. Flecainide Therapy Reduces Exercise-Induced Ventricular Arrhythmias in Patients With Catecholaminergic Polymorphic Ventricular Tachycardia. J Am Coll Cardiol. 2011;57:2244–2254. doi: 10.1016/j.jacc.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunt DJ, Jones PP, Wang R, Chen W, Bolstad J, Chen K, Shimoni Y, Chen SR. K201 (JTV519) suppresses spontaneous Ca2+ release and [3H]ryanodine binding to RyR2 irrespective of FKBP12.6 association. Biochem J. 2007;404:431–438. doi: 10.1042/BJ20070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Q, Xiao J, Jiang D, Wang R, Vembaiyan K, Wang A, Smith CD, Xie C, Chen W, Zhang J, Tian X, Jones PP, Zhong X, Guo A, Chen H, Zhang L, Zhu W, Yang D, Li X, Chen J, Gillis AM, Duff HJ, Cheng H, Feldman AM, Song L-S, Fill M, Back TG, Chen SRW. Carvedilol and its new analogs suppress arrhythmogenic store overload-induced Ca2+ release. Nat Med. 2011;17:1003–1009. doi: 10.1038/nm.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


