Abstract
Recently, synthetic cannabinoids have been sprayed onto plant material, which is subsequently packaged and sold as “Spice” or “K2” to mimic the effects of marijuana. A recent report identified several synthetic additives in samples of “Spice/K2”, including JWH-081, a synthetic ligand for the cannabinoid receptor 1 (CB1). The deleterious effects of JWH-081 on brain function are not known, particularly on CB1 signaling, synaptic plasticity, learning and memory. Here, we evaluated the effects of JWH-081 on pCaMKIV, pCREB and pERK1/2 signaling events followed by long-term potentiation (LTP), hippocampal-dependent learning and memory tasks using CB1 receptor wild type (WT) and knockout (KO) mice. Acute administration of JWH-081 impaired CaMKIV phosphorylation in a dose-dependent manner, whereas inhibition of CREB phosphorylation in CB1 receptor WT mice was observed only at higher dose of JWH-081 (1.25 mg/kg). JWH-081 at higher dose impaired CaMKIV and CREB phosphorylation in a time –dependent manner in CB1 receptor WT mice but not in KO mice and failed to alter ERK1/2 phosphorylation. In addition, SR treated or CB1 receptor KO mice have a lower pCaMKIV/CaMKIV ratio and higher pCREB/CREB ratio compared to vehicle or WT littermates. In hippocampal slices, JWH-081 impaired LTP in CB1 receptor WT but not in KO littermates. Furthermore, JWH-081 at higher dose impaired object recognition, spontaneous alternation and spatial memory on the Y-maze in CB1 receptor WT mice but not in KO mice. Collectively our findings suggest that deleterious effects of JWH-081 on hippocampal function involves CB1 receptor mediated impairments in CaMKIV and CREB phosphorylation, LTP, learning and memory in mice.
Keywords: LTP; Phosphorylation; CaMKIV; MAPK, CREB; Cannabinoids; CB1 receptor null mice
INTRODUCTION
Cannabis sativa (cannabis, marijuana or hashish) is widely used to treat nausea, pain, seizures, ischemia, cerebral trauma and tumors in humans (Robson, 2001). However, the potential therapeutic use of cannabis is limited by well-known psychoactivity (Pacher et al., 2006). Δ9-tetrahydrocannabinol (Δ9-THC) has been identified as the major psychoactive component out of several bioactive phytocannabinoids found in the C. sativa plant (Taura et al., 2007). Δ9-THC has been well characterized for its several physiological and behavioral effects (Pertwee, 2005). Most Δ9-THC effects are mediated through the cannabinoid receptor type 1 (CB1) (Monory et al., 2007). The CB1 receptor is predominately expressed in the brain, particularly in areas such as the hippocampus, basal ganglia, cortex, amygdala and cerebellum – areas linked to the behavioral effects of Δ9-THC (Herkenham et al., 1991). The CB1 receptor is a G protein coupled receptor (GPCR) that couples to Gi/o class G proteins and is primarily located on presynaptic terminals, a prime location to control neurotransmitter release (Yoshida et al., 2006). Agonist-induced activation of CB1 receptors leads to an inhibition of adenylyl cyclase and a subsequent decrease in cellular cAMP levels. CB1 receptor activation also mediates a wide range of effects on ion channels, including voltage-dependent calcium and potassium channels (Deadwyler et al., 1995; Mackie and Hille, 1992; Mackie et al., 1995). Together, CB1 receptor-mediated intracellular signaling results in reduced cellular excitability and reduced neurotransmitter release (Shen et al., 1996). Because CB1 receptors are located on both GABAergic and glutamatergic terminals, their activation leads to the suppression of both inhibitory and excitatory synaptic transmission in the brain (Basavarajappa et al., 2008; Kellogg et al., 2009; Ohno-Shosaku et al., 2001; Subbanna et al., 2013; Wilson and Nicoll, 2001). The CB1 receptor’s ability to suppress neurotransmission allows both exogenous cannabinoids (such as Δ9-THC) and endogenous cannabinoids (endocannabinoids, ECs) to have a profound impact on neuronal communication, including learning and memory (Egashira et al., 2002; Hampson and Deadwyler, 1999; Lichtman et al., 1995; Suenaga and Ichitani, 2008; Varvel et al., 2001; Yim et al., 2008). Although the cellular mechanisms are not clear, one of the major side effects of marijuana intoxication is the impairment of working memory in humans (Ranganathan and D'Souza, 2006) and animals (Puighermanal et al., 2012).
Until recently, cannabinoid abuse and dependence in humans had been restricted to plant-derived cannabinoids such as Δ9-THC. However, within the last decade, synthetic cannabinoids have been sprayed onto plant material, which is subsequently packaged and sold under generic names such as “Spice” or “K2” to mimic the effects of marijuana (Vardakou et al., 2010). Although labeled “not for human consumption,” these products are smoked, resulting in a marijuana-like high as well as other physiological effects, some of which may differ from those of marijuana (e.g., elevated blood pressure, vomiting) (Young et al., 2012). While it is widely known that most Spice drugs are potent CB1 agonists, exact molecular mechanisms underlying their toxic effects remain to be determined. These compounds and their metabolites have been found to possess higher binding affinity for cannabinoid receptors than marijuana, which implies greater potency, greater adverse effects, and perhaps a longer duration of action (Aung et al., 2000; Hermanns-Clausen et al., 2013; Hoffman et al., 2005). Spice products contain several chemicals, including JWH-081, JWH-018 and JWH-073, which are assumed to be similar to Δ9-THC in their mechanism of action but appear to be associated with additional symptoms (Hermanns-Clausen et al., 2013). JWH-081 binds to CB1 receptors with high affinity (1.2 nM) (Aung et al., 2000; Huffman et al., 2005) and causes acute toxicity as experienced by emergency patients possibly through strong CB1 receptor stimulation (Hermanns-Clausen et al., 2013). Further, another derivative of JWH (JWH-018) inhibits forskolin-stimulated cAMP production (Chin et al., 1999), exhibits agonistic (9 nM) activity at CB1 receptors (Seely et al., 2012) and also produces the tetrad of behaviors classically associated with cannabinoids in the rodent model (analgesia, catalepsy, hypomotility and hypothermia) (Brents et al., 2011; Wiebelhaus et al., 2012) but less potent compared to JWH-081. The emerging abuse problem and listing JWH-081 and its analogs as Schedule I controlled substances (Government, 2012) has placed an emphasis on the need for further characterization of JWH-081 with respect to brain function. However, there have been no studies of JWH-081 on synaptic plasticity, learning and memory. In the present study, we examined the effects of this compound on synaptic plasticity, learning and memory and signaling in CB1 receptor wild type (WT) and knock out (KO) mice.
MATERIALS AND METHODS
Animals and treatment
CB1 receptor WT and KO mice (Subbanna et al., 2013) on C57BL/6J background were generated from heterozygous breeding. C57BL/6J and CB1 receptor WT and KO mice were housed in groups under standard laboratory conditions (12 hrs light / 12 hrs dark cycle) with food and water available ad libitum. Animal care and handling procedures followed Institutional (NKI IACUC) and National Institutes of Health guidelines. The genotype of CB1 receptor WT and KO mice was determined by polymerase chain reaction (PCR) of genomic DNA obtained from mouse tails as described before (Basavarajappa et al., 2003). For the JWH-081 [(4-methoxy-1-naphthalenyl)(1-pentyl-1H-indol-3-yl)-methanone] (Cayman, Ann Arbor, MI, USA) experiments, JWH-081 was dissolved in DMSO (final concentration of DMSO was less than 2%) followed by a few drops of Tween 80 and then volume was made up with sterile saline solution. The JWH-081 solution was administered (0–1.25 mg/kg) by IP injection at a volume of 10 ml/kg body weight 30 min before behavioral test. The JWH-081 vehicle solution was injected as a control. The male animals were subjected to behavioral studies after 30 min. For the SR141716A (SR) experiments, SR (gift from RBI, Natick, MA) was dissolved in 10µl of ethanol followed by a few drops of Tween 80 and then volume was made up with sterile saline solution. The SR solution was administered (3 mg/kg) by IP injection at a volume of 10ml/kg body weight 30 min (Avdesh et al., 2013; Lichtman and Martin, 1996; Varvel et al., 2005) before JWH-081 administration. The SR vehicle solution was injected as a control. In some experiments, brains were processed for biochemical analyses, as described below. Five to 15 animals were used for each data point.
Electrophoresis and immunoblotting
For Western blot analysis, 0, 30 and 60 min after the vehicle or JWH-081 (0–1.25 mg/kg) injection, male mice were sacrificed by decapitation, hippocampus was dissected, flash frozen and stored at −80°C. In some experiments, SR was administered 30 min before vehicle or JWH-081 (1.25 mg/kg) injection and hippocampus was collected and stored as described above. Hippocampus was homogenized using homogenization buffer (0.01 M Tris, 250 mM Sucrose, 25 mM KCl, 0.5 mM of PMSF, 0.01 M sodium fluoride, 0.01 M beta-glycerol phosphate, 25 mM Na3VO4, pH 7.5, 1 mM EDTA) containing freshly added 1% protease inhibitor mixture (Roche, Indianapolis, IN, USA). Homogenates from the hippocampus was processed as described previously (Lubin and Sweatt, 2007; Subbanna et al., 2013). Tissue homogenates were centrifuged at 7700 g for 1 min, and the supernatant (total extract) was aspirated and stored at −80°C until use. The nuclear pellet was then resuspended in a nuclear extraction reagent (NER) (# 78833, Thermo Fisher Scientific, Suwanee, GA) (Grabowski, 2005). Nuclear fraction was prepared [according to the manufacturer instruction (Thermo Fisher Scientific, Suwanee, GA)] by dissolving nuclear pellet in ice cold NER and the samples were vortexed for 15 s. Then samples were placed on ice and continued vertexing for 15 s every 10 min, for a total of 40 min. The samples were sonicated for 30 s followed by centrifugation at 16000 g for 10 min at 4° C. The supernatant was transferred to prechilled tubes and were stored at −80°C until use. The samples were prepared in a sample buffer as previously described by our laboratory (Basavarajappa et al., 2008; Subbanna et al., 2013). The blots were incubated in primary antibody; anti-mouse CaMKIV (Sc-55501, 1: 1000), anti-rabbit pCaMKIV (Sc-28443-R, 1: 1000) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-rabbit p44/42 MAPK (ERK1/2) (# 9102, 1:2000), anti-rabbit-phospho-p44/42 MAPK (# 9101, 1:1000), anti-mouse-β-actin (#3700, 1:5000, Cell Signaling Technology, Danvers, MA, USA), anti-rabbit-pCREB (ser133) (# 05–807, 1:1000) and anti-mouse-CREB (# 04–218, 1:1000) (Millipore, Billerica, MA, USA) for 3 hrs at room temperature or overnight at 4°C and processed as previously described by our laboratory (Basavarajappa et al., 2008; Subbanna et al., 2013). Incubation of blots with a secondary antibody (goat anti-mouse peroxidase conjugate, #AP 124P, 1:5000; goat anti-rabbit, #AP132P, 1:5000, Millipore, Billerica, MA, USA) alone did not produce any bands.
Long-term potentiation (LTP)
Three month old male CB1 receptor WT and KO mice (n=5/group) were sacrificed by cervical dislocation followed by decapitation. Hippocampi were quickly removed. Transverse hippocampal slices (400 µm) were cut and recorded according to standard procedures (Sadrian et al., 2012; Subbanna et al., 2013; Vitolo et al., 2002). Following cutting, hippocampal slices were transferred to a recording chamber where they were maintained at 29° C and perfused with artificial cerebrospinal fluid (ACSF) continuously bubbled with 95% O2 and 5% CO2. The ACSF composition in mM was: 124.0 NaCl, 4.4 KCl, 1.0 Na2HPO4, 25.0 NaHCO3, 2.0 CaCl2, 2.0 MgSO4, 10.0 glucose, osmolarity 290–300. CA1 fEPSPs were recorded by placing both the stimulating and the recording electrodes in CA1 stratum radiatum. Responses were recorded for 2 hrs after and measured as fEPSP slope expressed as percentage of baseline as described in detail before (Subbanna et al., 2013). In some experiments, after 10 min baseline recording, hippocampal slices were perfused with JWH-081 (1.0 µM in DMSO) or vehicle (0.001% DMSO), for 30 min before inducing LTP with tetanic stimulation of the Schaeffer collateral pathway. The selection of JWH-081 concentration is based on the previous electrophysiological studies (Atwood et al., 2010) of the other JWH compounds. The data were expressed as mean ± Standard Error Mean (SEM).
Novel object recognition task
The object recognition task (ORT) is based on ‘spontaneous novelty preference’, i.e. the natural predisposition of rodents to explore novel objects (Ennaceur and Delacour, 1988). ORT was evaluated as previously described (Subbanna et al., 2013). In brief, three to four month old male mice (n=8/group) were submitted to a habituation session where they were allowed to freely explore the open field for 5 min × 2 for two days. No objects were placed in the box during the habituation trial. Twenty-four hours after habituation, mice were treated with and without JWH-081. After 30 min, training (T1) was conducted by placing individual mice for 3 min in the open field, in which two identical objects (objects a1 and a2) were positioned in two adjacent corners at 10 cm from the walls. In a short-term recognition memory test given at 1 and 4 hrs (retention) after the training (T2), the mice explored the open field for 3 min in the presence of one familiar (a1) and one novel (b1, 1 hr; b2, 4 hrs) object. In a long-term recognition memory test given at 24 hrs (retention) after training (T2), the mice explored the open field for 3 min in the presence of one familiar (a1) and one novel object (b3; different from b1 and b2). All combinations and locations (left and right) of the objects were used in a balanced manner in order to reduce potential biases due to preferences for particular locations or objects. All objects had similar textures and sizes but had distinctive shapes and colors (Stoelting, Wood Dale, IL USA). Between trials, the objects were washed with 10% ethanol solution. Exploration was defined as directing the nose to the object at a distance of no more than 2 cm and/or touching the object with the nose. Sitting on the object was not considered as exploratory behavior. e1 and e2 are measures of the total exploration time of both objects during T1 and T2 (1, 4 and 24 hrs), respectively. d2 was considered as index measures of discrimination between the new and the familiar objects. d2 is a relative measure of discrimination which corrects the difference between exploring the familiar and the novel object for exploration activity (e2) and appears to be independent of the total exploration times (Sik et al., 2003). The times spent exploring each object during T1 and T2 were recorded manually with a personal computer.
Spontaneous alternation on Y maze
Spontaneous alternation was tested as described previously (Holcomb et al., 1998) using Y maze. The symmetrical Y maze is made of acrylic and consists of three identical arms (5 cm lane width, 35 cm arm length, 10 cm arm height) converging at the center of a triangular area so that they formed a symmetrical Y shape (120° of angular deviation from each other) (Stoelting, Wood Dale, IL USA). Male mice were treated with and without JWH-081. After 30 min, each mouse was placed in the center of the Y maze and was allowed to explore freely through the maze during an 8 min session. The sequence, time spent in each arm and total number of arms entered was recorded. Arm entry was considered to be completed when the hind paws of the mouse had been completely placed in the arm. Percentage alternation is the number of triads containing entries into all three arms divided by the maximum possible alternations (the total number of arms entered minus 2) × 100.
Spatial recognition memory using the Y maze
Spatial recognition memory was tested as described previously (Sarnyai et al., 2000). This ethologically relevant test is based on the rodents’ natural curiosity to explore novel areas. Male mice were treated with and without JWH-081. After 30 min, mice were placed into one of the arms of the Y maze (Stoelting, Wood Dale, IL USA) (start arm) and allowed to explore the maze with one of the arms closed for 10 min (training trial). After a 1 hr intertrial interval, mice were returned to the Y maze by placing them in the start arm. Then, the mice were allowed to explore freely all three arms of the maze for 3 min (test trial). The number of entries into, the time spent (dwell time) in each arm and the first choice of entry were registered manually from video recordings by an observer blind to the treatment or genotype of the mice. Discrimination ratio [Preference for the Novel arm over the familiar Other arm (Novel/Novel + Other)] for arm entries and dwell time of WT and KO mice treated with or without JWH-081 were calculated.
Statistical analysis
Unless indicated otherwise, the experiments were performed using equal number of animals per treatment. All of the data are presented as the mean ± SEM. A statistical comparison of the data was performed by either a one-way analysis of variance ANOVA or a two-way ANOVA with Bonferroni’s post hoc test. In all of the comparisons, p < 0.05 was considered to indicate statistical significance. The statistical analyses were performed using the Prism software (GraphPad, San Diego, CA).
RESULTS
JWH-081 inhibits CaMKIV and CREB phosphorylation in CB1 receptor WT but not in KO mice and does not alter ERK phosphorylation
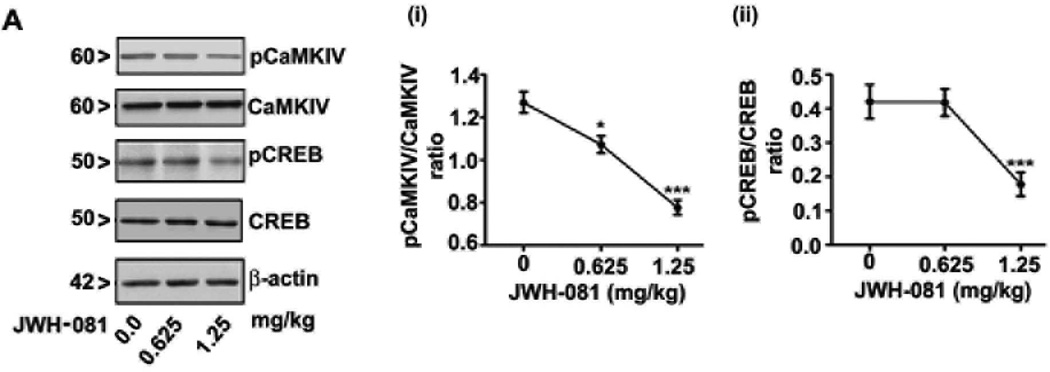
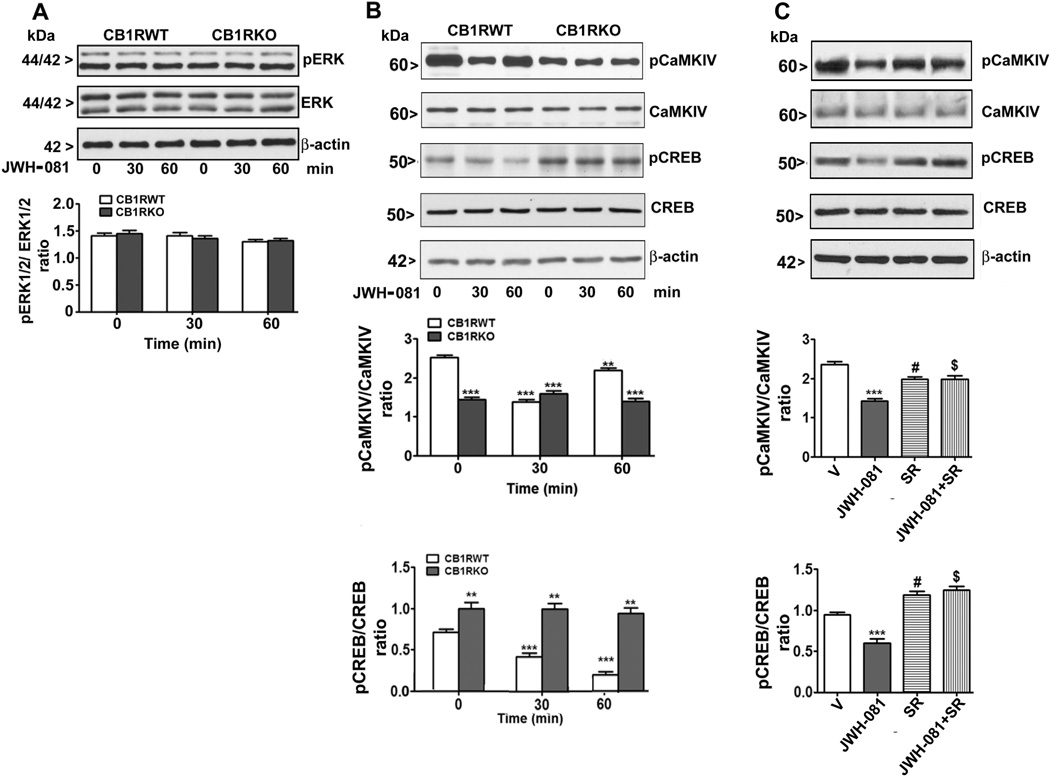
To elucidate the CB1 receptor mediated downstream intracellular pathways involved in the effects of JWH-081, we studied the involvement of pCaMKIV, pCREB and pERK1/2, key regulators of synaptic plasticity and learning and memory (Martin et al., 2000). Although a previous study had shown that ERK phosphorylation was reduced after treatment with another JWH derivative (JWH-018) in cultured hippocampal neurons (Atwood et al., 2010), it is not clear whether CaMKIV, CREB and ERK phosphorylation are changed by JWH-081 treatments. First, we determined the effect of various doses of acute administration of JWH-081 (30 min) on CaMKIV and CREB phosphorylation in the hippocampus of CB1 receptor WT mice. CaMKIV [Fig. 1A (i)] phosphorylation was reduced by JWH-081 in a dose-dependent manner whereas inhibition of CREB [Fig. 1A (ii)] phosphorylation was observed only at higher dose of JWH-081 (1.25 mg/kg, 30 min). We used higher dose (1.25 mg/kg) in all our subsequent experiments. We assessed pERK and the total amount of ERK protein. ERK phosphorylation and total ERK protein were not altered by JWH-081 treatment (p > 0.05) in the hippocampus of WT or KO mice. ERK phosphorylation and total ERK levels did not differ between WT and KO mice (p>0.05) (Fig. 2A). Then, we assessed CaMKIV phosphorylation and the total amount of CaMKIV protein levels at various time points. We found that in WT mice, CaMKIV phosphorylation was significantly (p<0.001) inhibited at 30 min after JWH-081 treatment and this inhibition was lost at 60 min (Fig. 2B). However, total CaMKIV protein levels were not significantly (p > 0.05) different in CB1 receptor KO compared to WT mice. JWH-081 failed to alter total CaMKIV levels either in WT or in KO mice (p > 0.05). We found lowered pCaMKIV/CaMKIV ratio in KO mice compared to WT mice. Next, we determined CREB phosphorylation and CREB protein levels at various time points. We found that in WT mice, CREB phosphorylation was significantly (p<0.001) inhibited at 30 and 60 min after JWH-081 treatment (Fig. 2B). We also found that total CREB protein levels were not significantly (p < 0.05) different between KO and WT mice. JWH-081 failed to alter total CREB levels either in WT or in KO mice (p > 0.05). We found increased pCREB/CREB ratio in KO mice compared to WT mice. In C57BL/6J mice, SR administration 30 min before JWH-081 treatment (30 min) rescued both CaMKIV and CREB phosphorylation (p > 0.05). We also found decreased pCaMKIV/CaMKIV ratio (p > 0.05) and increased pCREB/CREB ratio in mice treated with SR alone (p > 0.01) (Fig. 2C). Collectively, our data suggest that JWH-081 impairs CaMKIV and CREB phosphorylation via downstream of CB1 receptor signaling mechanism.
Figure 1.
Effect of various doses of acute administration of JWH-081 on CaMKIV and CREB phosphorylation levels in the hippocampus. (A) Hippocampal nuclear extracts from WT mice treated with or without various doses of JWH-081 (0–1.25 mg/kg) for 30 min were processed for Western blot to analyze the levels of pCaMKIV, pCREB, CaMKIV and CREB. β-actin was used as a loading control. Representative blots are shown for the hippocampal nuclear extracts (n = 6 mice/group). One-way ANOVA with Bonferroni’s post hoc test was used for statistical analysis. The error bars represent SEM. *p < 0.05 and ***p < 0.001 vs. Vehicle (0 dose).
Figure 2.
Effect of acute administration of JWH-081 on ERK, CaMKIV and CREB phosphorylation levels in the hippocampus. (A) Western blot analyses were performed to determine the levels of pERK1/2 and ERK1/2 in hippocampal total extracts. β-actin was used as a loading control. Representative blots are shown for the hippocampal total extracts (n = 6 mice/group). (B) Hippocampal nuclear extracts from WT and KO mice treated with or without JWH-081 (1.25 mg/kg) for 30 or 60 min were processed for Western blot to analyze the levels of pCaMKIV, pCREB, CaMKIV and CREB. β-actin was used as a loading control. ***p < 0.001; **p < 0.01. (C) Hippocampal nuclear extracts from vehicle and SR pretreated C57BL/6J mice treated with or without JWH-081 (1.25 mg/kg) for 30 were processed for Western blot to analyze the levels of pCaMKIV (# and $ p< 0.05), pCREB (# and $ p< 0.01), CaMKIV and CREB. β-actin was used as a loading control. Representative blots are shown for the hippocampal nuclear extracts (n = 6 mice/group). One-way ANOVA with Bonferroni’s post hoc test was used for statistical analysis. The error bars represent SEM. ***p < 0.001 vs Vehicle (V); # vs Vehicle; $ vs JWH-081.
JWH-081 impairs LTP in CB1 receptor WT but not in KO hippocampal slices
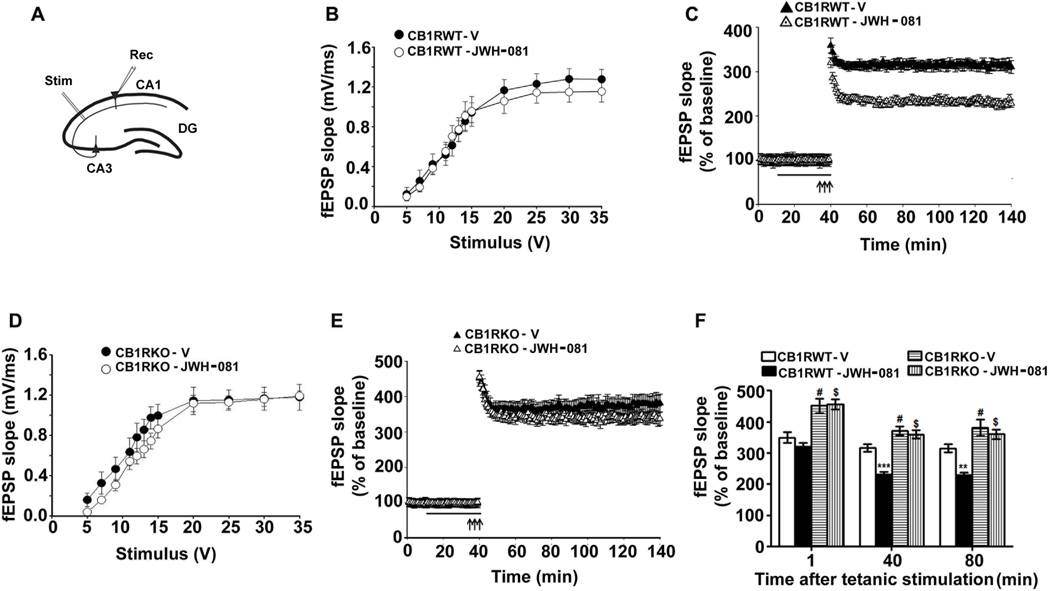
In our input/output (I/O) responses of fEPSPs in the Schaffer collateral pathway (Fig. 3A), increasing stimulus intensity evoked robust I/O responses of fEPSP in hippocampal slices prepared from adult CB1 receptor WT and KO animals treated with vehicle or JWH-081 (1µM). The I/O curve of fEPSP was not altered by vehicle or JWH-081 treatment (p > 0.05) (Fig. 3B) in WT slices. These findings suggest that neither vehicle nor JWH-081 significantly affects the fEPSP slope in pyramidal cells over the entire range of stimulation intensities. Prior to tetanic stimulations, the baseline fEPSP (10 min) was recorded in 60-s intervals with stimulation at an intensity equivalent to ~35% of the maximum evoked response. Tetanic stimulation evoked a typical LTP of fEPSP (Sadrian et al., 2012; Subbanna et al., 2013; Vitolo et al., 2002) in CB1 receptor WT slices treated with vehicle (p < 0.001). These responses were stable over 120 min. However, tetanic stimulation evoked a significantly reduced LTP in slices (n = 10 slices/5 mice/group) treated with JWH-081 (1µM for 30 min) (p < 0.001) (Fig. 3C). We then examined whether CB1 receptor KO mice are resistant to JWH-081-induced inhibition of LTP. Our results suggest that KO mice exhibited robust I/O responses of fEPSP evoked by increasing stimulus intensity, similar to WT mice. The I/O curve of fEPSP was not altered by JWH-081 in the KO mice (p > 0.05) (Fig. 3D). These findings suggest that neither JWH-081 nor genetic deletion of CB1 receptors significantly affects the fEPSP slope in pyramidal cells over the entire range of stimulation intensities. Tetanic stimulation evoked an enhanced LTP in CB1 receptor KO mice (1 min = 451 ± 23, 40 min = 371 ± 14 and 80 min = 381 ± 26) compared with WT mice (1 min: F3, 36 = 44, p < 0.001; 40 min: F3, 36 = 34, p < 0.001; 80 min: F3, 36 = 24, p < 0.05; one-way ANOVA) (Fig. 3E). These findings are consistent with previous studies that demonstrate enhanced hippocampal LTP in CB1 receptor KO mice (Bohme et al., 2000; Slanina et al., 2005; Subbanna et al., 2013). LTP magnitude of fEPSP was reduced by JWH-081 treatment in CB1 receptor WT slices (1 min = 320 ± 11, 40 min = 316 ± 12 and 80 min = 315 ± 15) compared with vehicle (1 min: F3, 36 = 2, p > 0.05; 40 min: F3, 36 = 54, p < 0.001; 80 min: F3, 36 = 60, p < 0.05; one-way ANOVA). JWH-081 treatment in CB1 receptor KO slices failed to inhibit LTP (p > 0.05) (Fig. 3E). Taken together, these findings suggest that JWH-081 impairs LTP (Fig. 3F) through the activation of CB1 receptors.
Figure 3.
JWH-081 inhibits LTP in hippocampal slices from adult CB1 receptor WT but not KO mice. (A) A schematic diagram showing the stimulating and recording electrode positions in the CA1 region of the hippocampus. (B) A summary graph showing the field input/output relationships in slices after treating WT slices with vehicle or JWH-081. (C) A time course of the averages of the fEPSP slopes in slices after treating WT slices with vehicle or JWH-081. (D) A summary graph showing the field input/output relationships in slices after treating KO slices with vehicle or JWH-081. (E) A time course of the averages of the fEPSP slopes in slices after treating KO slices with vehicle or JWH-081.The fEPSP slopes were normalized to the average value 10 min before stimulation in each experiment. Arrows denote the time of tetanic stimulation (4 pulses at 100 Hz, with bursts repeated at 5 Hz, each tetanus included three 10-burst trains separated by 15 s). For JWH-081 treatment experiments, after 10 min of baseline recording, hippocampal slices were perfused with JWH-081 (1.0 µM in DMSO) or vehicle (0.001% DMSO) for 30 min before inducing LTP with tetanic stimulation of the Schaeffer collateral pathway. (F) A combined plot of the averages of the fEPSP slopes for WT and KO slices with and without JWH-081 at several time points. Each point is presented as the mean ± SEM (n= 5 mice/group; 10 slices/group). One-way ANOVA with Bonferroni’s post hoc tests; ***p < 0.001.
JWH-081 impairs learning and memory in CB1 receptor WT mice but not in KO mice
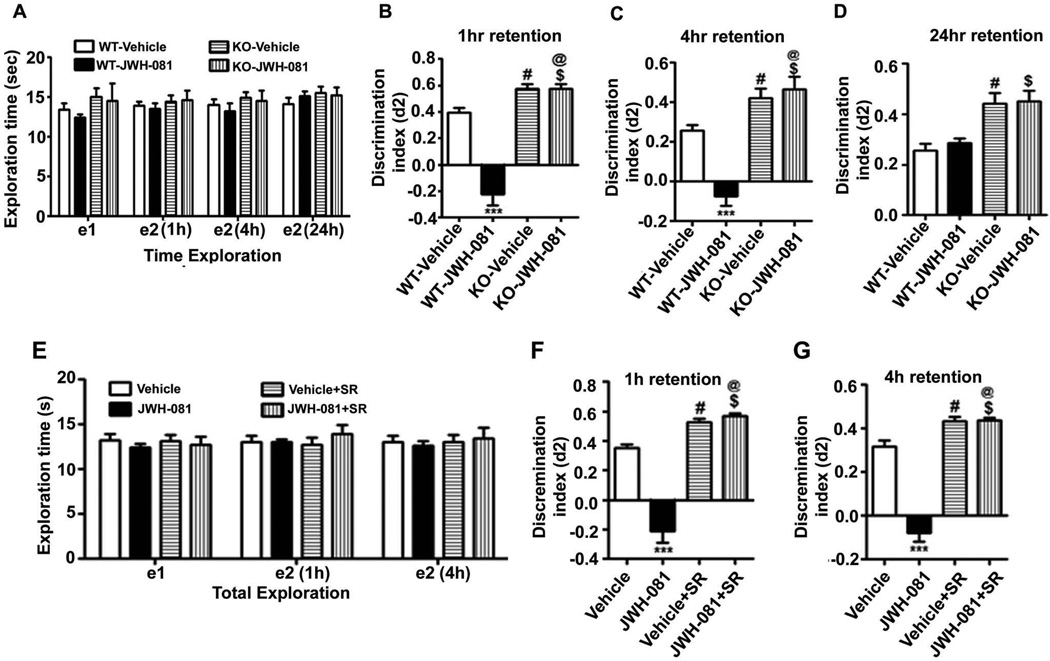
We used three memory tasks, object recognition, spontaneous alternation and spatial recognition, to examine whether JWH-081 impairs these memory tasks. The effects of JWH-081 treatment on ORT performance are shown in Figure 4A–G. The results showed no significant effects of JWH-081 treatment in CB1 receptor WT and KO mice on total exploration times at e1 or e2 (1 hr or 4 hrs or 24 hrs retention) in the ORT task (Fig. 4A). JWH-081 treatment significantly impaired ORT performance both at 1 h [F3,28 = 51; p<0.001] and 4 hrs retention [F3,28 = 26; p<0.001] (T2) in WT mice but not in KO mice (p>0.05) (Fig. 4B and C). In addition, KO mice exhibited enhanced ORT performance both at 1 hr, 4 hrs and 24 hrs retention (T2) compared to WT mice (p<0.01) (Fig. 4B–D). In addition, at 24 h retention, JWH-081 failed to impair ORT performance [F3,28 = 1.5; p>0.05] in WT and KO mice (Fig. 4D). Although we found no significant effects of SR pre-administration on total exploration times at e1 or e2 (1 hr or 4 hrs retention) in the ORT task (Fig. 4E), SR was able to prevent JWH-081-impaired ORT performance both at 1 h [F3,28 = 41; p<0.001] and 4 hrs retention [F3,28 = 32; p<0.001] (T2) in mice (p < 0.001) (Fig. 4F and G). In addition, SR treated mice exhibited enhanced ORT performance both at 1 hr and 4 hrs retention (T2) compared to vehicle treated mice (p<0.01) (Fig. 4F and G). These data together suggest that acute administration of JWH-081 impairs short-term memory but not long-term memory in the ORT task in a CB1 receptor-dependent manner in mice.
Figure 4.
Acute administration of JWH-081 impairs novel object recognition memory in adult mice. (A) The level of exploration was measured at el and e2 (1 or 4 or 24 hrs), and the time spent exploring the two objects was measured at T1 and T2 (at 1, 4 and 24 hrs) in WT or KO mice treated with vehicle or JWH-081. (B–D) Discrimination indices (d2) obtained from the WT or KO mice treated with vehicle or JWH-081 at 1 (b), 4 (c) and 24 hrs (d) retention intervals. ***p < 0.001 vs. CB1WT + Vehicle; # p < 0.05 vs. CB1WT + Vehicle; $ p < 0.05 vs. CB1WT + JWH-081; @ p < 0.001 vs. CB1WT + Vehicle. (E) The level of exploration was measured at el and e2 (1 or 4 hrs), and the time spent exploring the two objects was measured at T1 and T2 (at 1 and 4 hrs) in C57BL/6J mice pretreated with vehicle or SR and treated with vehicle or JWH-081. (F and G) Discrimination indices (d2) obtained from the SR pretreated mice treated with vehicle or JWH-081 at 1 (F), 4 hrs (G) retention intervals. One-way ANOVA with Bonferroni’s post hoc test; ***p < 0.001 vs. Vehicle; # p < 0.05 vs. Vehicle; $ p < 0.05 vs. or JWH-081; @ p < 0.001 vs. Vehicle.
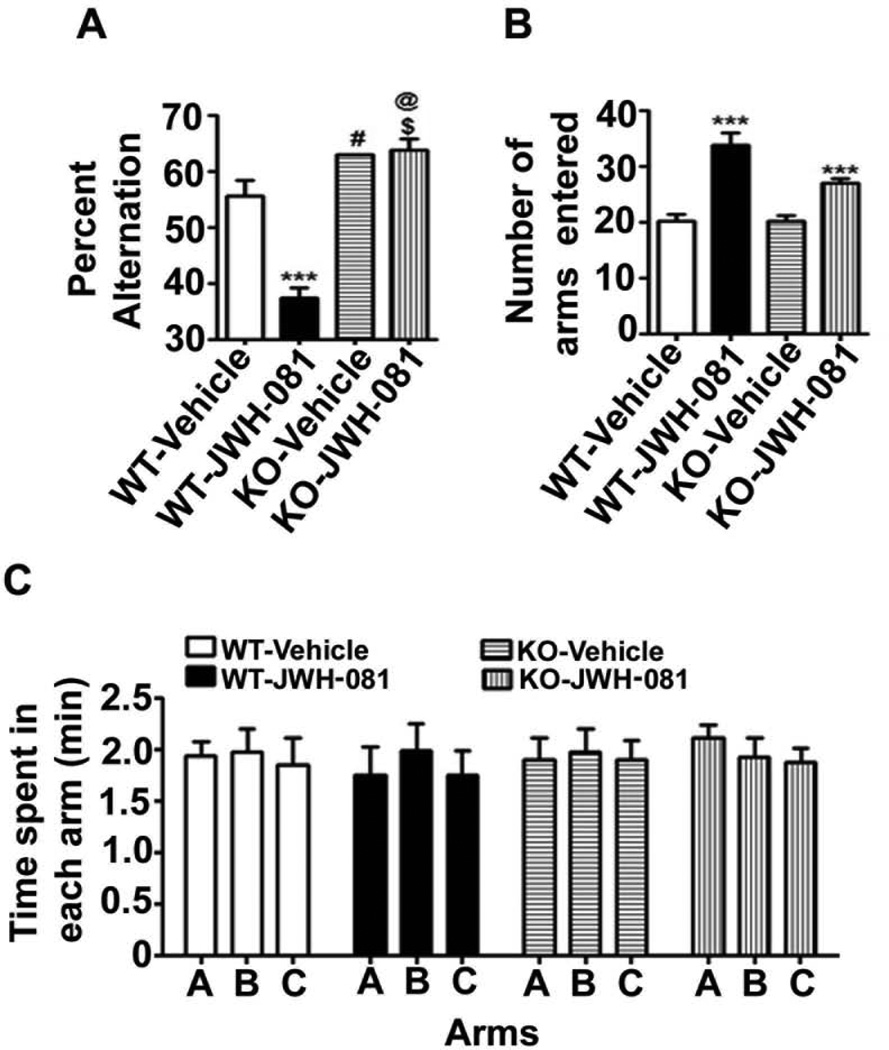
In our second behavioral test, animals treated with JWH-081 were tested for spontaneous alternation in the Y maze (Lalonde, 2002). CB1 receptor WT and KO mice were treated with JWH-081 30 min before the test. Consistent with the ORT performance, KO mice exhibited significantly enhanced spontaneous alternation behavior (p < 0.05) compared to WT mice. One-way ANOVA analysis revealed that JWH-081 treatment dramatically reduced spontaneous alternation performance in the Y-maze compared with WT mice [F3,28 = 40, p < 0.001] (Fig. 5A). Importantly, JWH-081 treatment failed to induce a spatial working memory deficit in the Y maze test in KO mice (p > 0.05). Furthermore, JWH-081 significantly enhanced (p < 0.01) exploratory activity, as assessed by the number of arm entries during Y-maze testing in both WT and KO mice (Fig. 5B). However, the amount of time spent in each arm was not altered by JWH-081 or vehicle in WT and KO mice (Fig. 5C). These findings together suggest that acute administration of JWH-081 impairs spontaneous alteration in a CB1 receptor-dependent manner in mice.
Figure 5.
Acute administration of JWH-081 impairs spontaneous alternation performance in adult mice. (A) Spatial working memory of CB1 receptor WT and KO mice treated with or without JWH-081 was tested using spontaneous alternation performance in the Y-maze. Note that JWH-081 treated WT mice perform poorly (well below 50% chance levels) compared to WT mice treated with vehicle (*** p < 0.001). Overall, KO mice treated with either vehicle or JWH-081 show significantly enhanced levels of alternation performance (# p < 0.05 versus CB1WT + V; @ p < 0.05 versus CB1WT + V; $ p < 0.05 versus CB1WT + JWH-081) when compared to WT mice with or without JWH-081 treatment. (B) The number of arm entries was not different between WT and KO mice (p > 0.05) but JWH-081 treatment enhanced the number of arm entries in both WT (*** p < 0.001 versus CB1WT + V) and KO mice (*** p < 0.001 versus CB1KO + V). (C) The time spent in each arm was not different between WT and KO mice (p > 0.05) with or without JWH-081 treatment (p > 0.05 versus CB1WT + V). Each point is the mean ± SEM (n= 8 mice/group). One-way ANOVA with Bonferroni’s post hoc test.
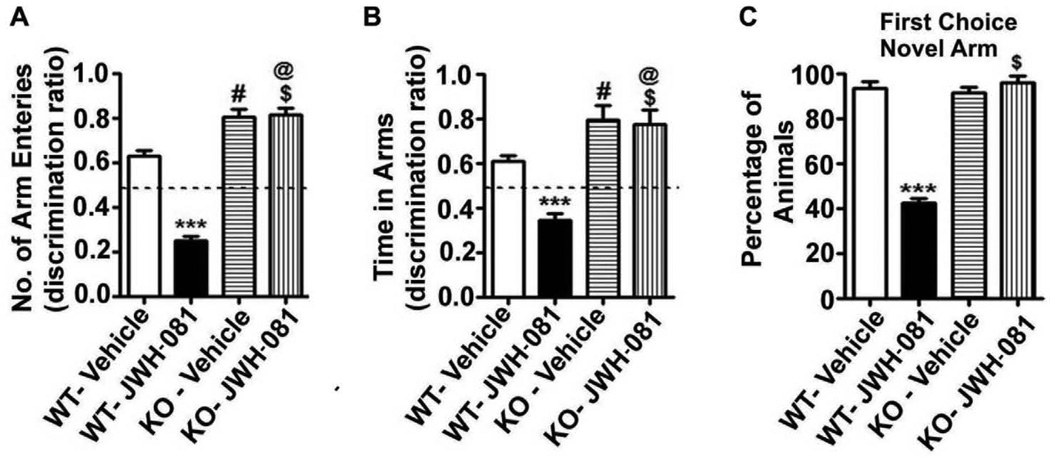
In our third behavioral test, we used another spatial memory test, spatial recognition memory using Y-maze. WT mice entered more frequently into (Arm Entry) and spent more time in the novel, previously unvisited arm of the maze. KO mice showed an enhanced preference toward the novel arm (Arm Entry, p < 0.001) and spent more time in the novel arm (Dwell Time, p < 0.001) compared to WT mice (Fig. 6A and B). Although all WT and KO mice selected the novel arm as the first choice, JWH-081 treated WT animals showed impaired preference for the novel arm as the first choice (Fig. 6C). JWH-081 treatment impaired the ability of WT mice to enter more frequently into [Arm Entry: F3,28 = 90, p < 0.001] and spend more time in [Dwell Time: F3,28 = 18, p < 0.001] the novel, previously unvisited arm of the maze. In contrast, JWH-081 treatment failed to alter KO mice preference toward the novel arm or the time spent in the novel arm (p > 0.05). These findings together suggest that acute administration of JWH-081 impairs spatial recognition memory in a CB1 receptor-dependent manner in mice.
Figure 6.
JWH-081 administration impairs spatial memory performance as measured by Y maze. (A–B) Discrimination ratio [Preference for the Novel arm over the familiar Other arm (Novel/Novel + Other)] for arm entries (A) and dwell time (B) of WT and KO mice treated with or without JWH-081 in the Y maze, 1 hr after the first encounter with the partially opened maze. The dashed line indicates chance performance (0.5). (C) The percentage of animals selecting the novel arm as the first choice is shown for WT and KO mice treated with or without JWH-081, 1 hr after the first encounter with the partially opened maze. Each point is the mean ± SEM (n= 8 mice/group). One-way ANOVA with Bonferroni’s post hoc test; ***p < 0.001 vs. CB1WT + Vehicle; # p < 0.05 vs. CB1WT + Vehicle; $ p < 0.05 vs. CB1WT + JWH-081; @ p < 0.001 vs. CB1WT + Vehicle.
DISCUSSION
This study demonstrates for the first time that the synthetic cannabinoid JWH-081 found in “Spice” and “K2” (Auwarter et al., 2009; Hermanns-Clausen et al., 2013) and recently classified as a schedule 1 controlled substances inhibits CaMKIV and CREB phosphorylation, LTP in hippocampal slices and impairs memory in a CB1 receptor-dependent manner in mice. Furthermore, significant deficits in object recognition, spontaneous alternation and spatial recognition memory were induced by acute administration of JWH-081 in CB1 receptor WT mice. The lack of JWH-081-induced LTP and behavioral impairments in CB1 receptor KO or in some cases SR treated mice indicates the specificity of this emerging drug of abuse in vivo. All Δ9-THC metabolites except one are inactivated by oxidative metabolism (Maurer et al., 2006), which prevents further CB1 receptor activation. However, JWH-081 (Razdan et al., 2009), metabolism leads to other active metabolites (Wohlfarth et al., 2013), which may continue to activate CB1 receptors. Although further research is required, it is conceivable to suggest that both the acute and chronic effects of JWH-081 including other constituent of spice on hippocampal function might be greater than a similar level of exposure to Δ9-THC.
Identification of specific signaling pathways mediating the effect of JWH-081 on the hippocampus may provide clues for candidate cellular mechanisms involved in synaptic plasticity and learning and memory deficits. Our findings of impaired CaMKIV and CREB phosphorylation by JWH-081 treatment are consistent with previous data in which Δ9-THC significantly reduced CREB phosphorylation (Rubino and Parolaro, 2008) and another calmodulin kinase related molecule such as CaMKII phosphorylation in a CB1 receptor-dependent manner (Rubino et al., 2007). Interestingly, our data show that JWH-081 inhibition of CaMKIV and CREB phosphorylation was absent in CB1 receptor KO mice or rescued by pre-administration of SR. These findings suggest that JWH-081 affect cellular events and hippocampal function through CB1 receptor-mediated downstream signaling mechanisms. Activation of CB1 receptor signaling is associated with activation of mitogen-activated protein kinase (MAPK) activity in cultured cells (Bouaboula et al., 1995; Daigle et al., 2008), hippocampal slices (Derkinderen et al., 2003) and embryonic rat cortices (Berghuis et al., 2007). Although in vivo studies of the intracellular signaling events coupling MAPK activation to the binding of CB1 receptors by Δ9-THC or other cannabinoids are limited (Rubino et al., 2007), several studies using cell lines suggest both up- and down regulation of MAPKs by Δ9-THC (De Petrocellis et al., 1998; Galve-Roperh et al., 2000). In a recent study, another JWH derivative such as JWH-018 enhanced ERK1/2 phosphorylation in HEK293 cells stably expressing CB1 receptors. In our in vivo studies, JWH-081 failed to alter total ERK1/2 and ERK1/2 phosphorylation in the hippocampus of CB1 receptor WT and KO mice. In general, the CaMKIV mediated phosphorylation of CREB at Ser133 is essential to the transcriptional activation of CREB/CRE-mediated - signaling pathway (Bito et al., 1996) and have been thought to play a central role in memory consolidation and LTP (Martin et al., 2000). To our knowledge, this is the first study suggesting the association between CaMKIV and CREB phosphorylation deficits with that of LTP and memory impairing effects of JWH-081 and it indicates that CB1 receptor-mediated inhibition of CaMKIV and CREB phosphorylation are important players. Future studies addressing the involvement of other specific signaling pathways in the effects of JWH-081 could provide additional mechanistic leads on the deleterious consequences of synthetic cannabinoids on hippocampus function.
The present findings on LTP deficits in mice hippocampal slices by JWH-081 are in accordance with previous studies describing impairment of hippocampal LTP formation by Δ9-THC and the psychoactive cannabinoid agonist HU-210, but not the non-psychoactive HU-211 (Collins et al., 1994). Other CB1 receptor ligands such as WIN55212-2 and anandamide were also shown to prevent the induction of LTP in rat hippocampal slices (Terranova et al., 1995). The selectivity ratio of JWH-081 (1.2 nM/ 9 nM) (Aung et al., 2000; Huffman et al., 1994) for CB1/CB2 receptors is bit higher than for most designer cannabimimetic examined to date (e.g., ACEA, arachidonyl-2'-chloroethylamide (1.4 nM/ >2000 nM); ACPA, arachidonylcyclopropylamide (2.2 nM/715 nM) (Hillard et al., 1999). In spite of these limitations the effects we observed are clearly due to CB1 receptor activation, the potential role of CB2 receptors in the effects of “Spice” and “K2” requires further study. The present study focused on JWH-081; however, various preparations of “Spice” and “K2” in fact contain different uncharacterized synthetic additives (Auwarter et al., 2009; Huffman et al., 2008), which may also act as agonists of CB1 receptors and may influence synaptic function in the brain. Investigation into these additional synthetic additives requires further attention.
The use of various memory tests, including object recognition, spontaneous alternation and spatial recognition tests, allowed for assessment of cognitive performance after acute administration of JWH-081 in CB1 receptor WT and KO mice. The object recognition task offers several advantages in the assessment of object recognition, particularly the ability to measure recognition of novelty based on novel object attributes (Kesner and Rogers, 2004). We found a selective deficit in novel object recognition performance after acute administration of JWH-081 in CB1 receptor WT but not in KO or SR treated mice, suggesting that the effects of JWH-081 are CB1 receptor-mediated. The present findings are congruent with previous reports of impairments in novel object recognition resulting from acute injections of various cannabinoids (Kosiorek et al., 2003). In addition, we also found that CB1 receptor KO or SR treated mice exhibited enhanced novel object recognition memory compared to vehicle treated C57BL/6J or WT littermates, suggesting that genetic deletion or pharmacological blockade of CB1 receptor enhances memory. Our results are consistent with the enhanced memory observed in CB1 receptor KO mice (Subbanna et al., 2013) and with CB1 receptor blockade in rats (Reibaud et al., 1999; Terranova et al., 1996). We also used the Y-maze spontaneous alternation task, which is commonly viewed as a spatial working memory task and the performance of this task is dependent on the integrity of the hippocampus (Lalonde, 2002). Acute administration of JWH-081 significantly impaired the spontaneous alternation performance of CB1 receptor WT but not KO mice. Moreover, KO mice exhibited enhanced spontaneous alternation performance compared to WT mice. The Y maze test is based on the natural drive of rodents to explore novel environments. It reliably investigates spatial memory. Acute administration of JWH-081 impaired spatial learning on the Y-maze in CB1 receptor WT but not KO mice. In addition, KO mice performed better compared to WT littermates. Similarly, Δ9-THC has been shown to produce impairments in hippocampal-dependent spatial learning in rodents (Puighermanal et al., 2012). Thus, in agreement with earlier reports with other cannabinoids (Puighermanal et al., 2012), our results show that JWH-081 impairs hippocampus-dependent learning and memory performance through CB1 receptors. Taken together, our findings suggest that an acute single dose of JWH-081 can cause cognitive deficits in mice.
In conclusion, the relevance of our findings in mice to the effect of Spice/K2 preparations on humans remains to be examined. It is likely that the additional compounds identified in Spice/K2 preparations might also contribute to the behavioral effects produced by smoking “Spice/K2”, and their different pharmacologies might cause different preparations of “Spice/K2” to vary in their cognitive effects. Investigation into these additional synthetic additives requires further attention. Despite these limitations, we have shown that acute JWH-081 administration has profound CB1 receptor-mediated effects on synaptic plasticity and learning and memory, which are likely to have a significant impact on cognitive function. “Spice/K2” is marketed as a “natural” herbal blend but contains at least one very potent synthetic CB1 receptor agonist, which likely accounts for the cognitive deficits/psychoactive effects produced when “Spice/K2” is smoked.
Acknowledgements
This work in part was supported by NIH/NIAAA grant AA019443 (BSB).
Footnotes
Disclosure
The authors declare no conflict of interest.
REFERENCES
- Atwood BK, Huffman J, Straiker A, Mackie K. JWH018, a common constituent of 'Spice' herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br J Pharmacol. 2010;160(3):585–593. doi: 10.1111/j.1476-5381.2009.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung MM, Griffin G, Huffman JW, Wu M, Keel C, Yang B, Showalter VM, Abood ME, Martin BR. Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB(1) and CB(2) receptor binding. Drug Alcohol Depend. 2000;60(2):133–140. doi: 10.1016/s0376-8716(99)00152-0. [DOI] [PubMed] [Google Scholar]
- Auwarter V, Dresen S, Weinmann W, Muller M, Putz M, Ferreiros N. 'Spice' and other herbal blends: harmless incense or cannabinoid designer drugs? J Mass Spectrom. 2009;44(5):832–837. doi: 10.1002/jms.1558. [DOI] [PubMed] [Google Scholar]
- Avdesh A, Hoe Y, Martins RN, Martin-Iverson MT. Pharmacological effects of cannabinoids on the reference and working memory functions in mice. Psychopharmacology (Berl) 2013;225(2):483–494. doi: 10.1007/s00213-012-2834-6. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Ninan I, Arancio O. Acute Ethanol Suppresses Glutamatergic Neurotransmission through Endocannabinoids in Hippocampal Neurons. J Neurochem. 2008;107(4):1001–1013. doi: 10.1111/j.1471-4159.2008.05685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Chronic ethanol inhibits the anandamide transport and increases extracellular anandamide levels in cerebellar granule neurons. Eur J Pharmacol. 2003;466:73–83. doi: 10.1016/s0014-2999(03)01557-7. [DOI] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A, et al. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316(5828):1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87(7):1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- Bohme GA, Laville M, Ledent C, Parmentier M, Imperato A. Enhanced long-term potentiation in mice lacking cannabinoid CB1 receptors. Neuroscience. 2000;95:5–7. doi: 10.1016/s0306-4522(99)00483-2. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Bourrie B, Canat X, Calandra B, Rinaldi-Carmona M, Le Fur G, Casellas P. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J. 1995;312(pt 2):637–641. doi: 10.1042/bj3120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brents LK, Reichard EE, Zimmerman SM, Moran JH, Fantegrossi WE, Prather PL. Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One. 2011;6(7):e21917. doi: 10.1371/journal.pone.0021917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin CN, Murphy JW, Huffman JW, Kendall DA. The third transmembrane helix of the cannabinoid receptor plays a role in the selectivity of aminoalkylindoles for CB2, peripheral cannabinoid receptor. J Pharmacol Exp Ther. 1999;291(2):837–844. [PubMed] [Google Scholar]
- Collins DR, Pertwee RG, Davies SN. The action of synthetic cannabinoids on the induction of long-term potentiation in the rat hippocampal slice. Eur J Pharmacol. 1994;259(3):R7–R8. doi: 10.1016/0014-2999(94)90666-1. [DOI] [PubMed] [Google Scholar]
- Daigle TL, Kearn CS, Mackie K. Rapid CB1 cannabinoid receptor desensitization defines the time course of ERK1/2 MAP kinase signaling. Neuropharmacology. 2008;54(1):36–44. doi: 10.1016/j.neuropharm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Melck D, Palmisano A, Bisogno T, Laezza C, Bifulco M, Di Marzo V. The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation. Proc Natl Acad Sci U S A. 1998;95(14):8375–8380. doi: 10.1073/pnas.95.14.8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadwyler SA, Hampson RE, Mu J, Whyte A, Childers S. Cannabinoids modulate voltage sensitive potassium A-current in hippocampal neurons via a cAMP-dependent process. J Pharmacol Exp Ther. 1995;273:734–743. [PubMed] [Google Scholar]
- Derkinderen P, Valjent E, Toutant M, Corvol JC, Enslen H, Ledent C, Trzaskos J, Caboche J, Girault JA. Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J Neurosci. 2003;23(6):2371–2382. doi: 10.1523/JNEUROSCI.23-06-02371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egashira N, Mishima K, Iwasaki K, Fujiwara M. Intracerebral microinjections of delta 9-tetrahydrocannabinol: search for the impairment of spatial memory in the eight-arm radial maze in rats. Brain Res. 2002;952(2):239–245. doi: 10.1016/s0006-8993(02)03247-x. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31(1):47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Galve-Roperh I, Sanchez C, Cortes ML, Gomez del Pulgar T, Izquierdo M, Guzman M. Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat Med. 2000;6(3):313–319. doi: 10.1038/73171. [DOI] [PubMed] [Google Scholar]
- Government US. 112th congress. Washington D.C: United State Government printing office; 2012. Synthetic Drug Abuse Prevention Act; pp. 138–139. [Google Scholar]
- Grabowski PJ. Splicing-active nuclear extracts from rat brain. Methods. 2005;37(4):323–330. doi: 10.1016/j.ymeth.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids, hippocampal function and memory. Life Sci. 1999;65(6–7):715–723. doi: 10.1016/s0024-3205(99)00294-5. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, de Costa BR, Richfield EK. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res. 1991;547:267–274. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- Hermanns-Clausen M, Kneisel S, Szabo B, Auwarter V. Acute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findings. Addiction. 2013;108(3):534–544. doi: 10.1111/j.1360-0443.2012.04078.x. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Manna S, Greenberg MJ, DiCamelli R, Ross RA, Stevenson LA, Murphy V, Pertwee RG, Campbell WB. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1) J Pharmacol Exp Ther. 1999;289(3):1427–1433. [PubMed] [Google Scholar]
- Hoffman AF, Macgill AM, Smith D, Oz M, Lupica CR. Species and strain differences in the expression of a novel glutamate-modulating cannabinoid receptor in the rodent hippocampus. Eur J Neurosci. 2005;22(9):2387–2391. doi: 10.1111/j.1460-9568.2005.04401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, et al. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4(1):97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Dong D, Martin BR, Compton DR. Design, synthesis and pharmacology of cannabimimetic indoles. Bioorg Med Chem Lett. 1994;4:563–566. [Google Scholar]
- Huffman JW, Thompson AL, Wiley JL, Martin BR. Synthesis and pharmacology of 1-deoxy analogs of CP-47,497 and CP-55,940. Bioorg Med Chem. 2008;16(1):322–335. doi: 10.1016/j.bmc.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman JW, Zengin G, Wu MJ, Lu J, Hynd G, Bushell K, Thompson AL, Bushell S, Tartal C, Hurst DP, et al. Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB(1) and CB(2) receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB(2) receptor agonists. Bioorg Med Chem. 2005;13(1):89–112. doi: 10.1016/j.bmc.2004.09.050. [DOI] [PubMed] [Google Scholar]
- Kellogg R, Mackie K, Straiker A. Cannabinoid CB1 receptor-dependent long-term depression in autaptic excitatory neurons. J Neurophysiol. 2009;102(2):1160–1171. doi: 10.1152/jn.00266.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Rogers J. An analysis of independence and interactions of brain substrates that subserve multiple attributes, memory systems, and underlying processes. Neurobiol Learn Mem. 2004;82(3):199–215. doi: 10.1016/j.nlm.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Kosiorek P, Hryniewicz A, Bialuk I, Zawadzka A, Winnicka MM. Cannabinoids alter recognition memory in rats. Pol J Pharmacol. 2003;55(5):903–910. [PubMed] [Google Scholar]
- Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev. 2002;26(1):91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Dimen KR, Martin BR. Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology (Berl) 1995;119(3):282–290. doi: 10.1007/BF02246292. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. Delta 9-tetrahydrocannabinol impairs spatial memory through a cannabinoid receptor mechanism. Psychopharmacology (Berl) 1996;126(2):125–131. doi: 10.1007/BF02246347. [DOI] [PubMed] [Google Scholar]
- Lubin FD, Sweatt JD. The IkappaB kinase regulates chromatin structure during reconsolidation of conditioned fear memories. Neuron. 2007;55(6):942–957. doi: 10.1016/j.neuron.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci U S A. 1992;89:3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci. 1995;15:6552. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Maurer HH, Sauer C, Theobald DS. Toxicokinetics of drugs of abuse: current knowledge of the isoenzymes involved in the human metabolism of tetrahydrocannabinol, cocaine, heroin, morphine, and codeine. Ther Drug Monit. 2006;28(3):447–453. doi: 10.1097/01.ftd.0000211812.27558.6e. [DOI] [PubMed] [Google Scholar]
- Monory K, Blaudzun H, Massa F, Kaiser N, Lemberger T, Schutz G, Wotjak CT, Lutz B, Marsicano G. Genetic dissection of behavioural and autonomic effects of Delta(9)-tetrahydrocannabinol in mice. PLoS Biol. 2007;5(10):e269. doi: 10.1371/journal.pbio.0050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminal. Neuron. 2001;29(3):729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58(3):389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacological actions of cannabinoids. Handb Exp Pharmacol. 2005;(168):1–51. doi: 10.1007/3-540-26573-2_1. [DOI] [PubMed] [Google Scholar]
- Puighermanal E, Busquets-Garcia A, Maldonado R, Ozaita A. Cellular and intracellular mechanisms involved in the cognitive impairment of cannabinoids. Philos Trans R Soc Lond B Biol Sci. 2012;367(1607):3254–3263. doi: 10.1098/rstb.2011.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan M, D'Souza DC. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology (Berl) 2006;188(4):425–444. doi: 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- Razdan RK, Vemuri VK, Makriyannis A, Huffman JW. Cannabimimetic indoles, pyrroles, and indenes: structure-activity relationships and receptor interactions. In: Reggio PH, editor. Cannabinoid Receptor Ligands and Structure–Activity Relationships. part I. Totowa: Humana Press; 2009. pp. 3–94. [Google Scholar]
- Reibaud M, Obinu MC, Ledent C, Parmentier M, Bohme GA, Imperato A. Enhancement of memory in cannabinoid CB1 receptor knock-out mice. Eur J Pharmacol. 1999;379:R1–R2. doi: 10.1016/s0014-2999(99)00496-3. [DOI] [PubMed] [Google Scholar]
- Robson P. Therapeutic aspects of cannabis and cannabinoids. Br J Psychiatry. 2001;178:107–115. doi: 10.1192/bjp.178.2.107. [DOI] [PubMed] [Google Scholar]
- Rubino T, Parolaro D. Long lasting consequences of cannabis exposure in adolescence. Mol Cell Endocrinol. 2008;286(1–2) Suppl 1:S108–S113. doi: 10.1016/j.mce.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Rubino T, Sala M, Vigano D, Braida D, Castiglioni C, Limonta V, Guidali C, Realini N, Parolaro D. Cellular mechanisms underlying the anxiolytic effect of low doses of peripheral Delta9-tetrahydrocannabinol in rats. Neuropsychopharmacology. 2007;32(9):2036–2045. doi: 10.1038/sj.npp.1301330. [DOI] [PubMed] [Google Scholar]
- Sadrian B, Subbanna S, Wilson DA, Basavarajappa BS, Saito M. Lithium prevents long-term neural and behavioral pathology induced by early alcohol exposure. Neuroscience. 2012;206:122–135. doi: 10.1016/j.neuroscience.2011.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z, Sibille EL, Pavlides C, Fenster RJ, McEwen BS, Toth M. Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin(1A) receptors. Proc Natl Acad Sci U S A. 2000;97(26):14731–14736. doi: 10.1073/pnas.97.26.14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely KA, Brents LK, Radominska-Pandya A, Endres GW, Keyes GS, Moran JH, Prather PL. A major glucuronidated metabolite of JWH-018 is a neutral antagonist at CB1 receptors. Chem Res Toxicol. 2012;25(4):825–827. doi: 10.1021/tx3000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Piser TM, Seybold VS, Thayer SA. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J Neurosci. 1996;16:4322–4334. doi: 10.1523/JNEUROSCI.16-14-04322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A, van Nieuwehuyzen P, Prickaerts J, Blokland A. Performance of different mouse strains in an object recognition task. Behav Brain Res. 2003;147(1–2):49–54. doi: 10.1016/s0166-4328(03)00117-7. [DOI] [PubMed] [Google Scholar]
- Slanina KA, Roberto M, Schweitzer P. Endocannabinoids restrict hippocampal long-term potentiation via CB1. Neuropharmacology. 2005;49(5):660–668. doi: 10.1016/j.neuropharm.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Subbanna S, Shivakumar M, Psychoyos D, Xie S, Basavarajappa BS. Anandamide-CB1 Receptor Signaling Contributes to Postnatal Ethanol-Induced Neonatal Neurodegeneration, Adult Synaptic and Memory Deficits. Journal of neuoscience. 2013;33(15):6350–6366. doi: 10.1523/JNEUROSCI.3786-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suenaga T, Ichitani Y. Effects of hippocampal administration of a cannabinoid receptor agonist WIN 55,212-2 on spontaneous object and place recognition in rats. Behav Brain Res. 2008;190(2):248–252. doi: 10.1016/j.bbr.2008.02.029. [DOI] [PubMed] [Google Scholar]
- Taura F, Sirikantaramas S, Shoyama Y, Morimoto S. Phytocannabinoids in Cannabis sativa: recent studies on biosynthetic enzymes. Chem Biodivers. 2007;4(8):1649–1663. doi: 10.1002/cbdv.200790145. [DOI] [PubMed] [Google Scholar]
- Terranova JP, Michaud JC, Le Fur G, Soubrie P. Inhibition of long-term potentiation in rat hippocampal slices by anandamide and WIN55212-2: reversal by SR141716 A, a selective antagonist of CB1 cannabinoid receptors. Naunyn Schmiedebergs Arch Pharmacol. 1995;352(5):576–579. doi: 10.1007/BF00169393. [DOI] [PubMed] [Google Scholar]
- Terranova JP, Storme JJ, Lafon N, Perio A, Rinaldi-Carmona M, Le Fur G, Soubrie P. Improvement of memory in rodents by the selective CB1 cannabinoid receptor antagonist, SR 141716. Psychopharmacology (Berl) 1996;126(2):165–172. doi: 10.1007/BF02246352. [DOI] [PubMed] [Google Scholar]
- Vardakou I, Pistos C, Spiliopoulou C. Spice drugs as a new trend: mode of action, identification and legislation. Toxicol Lett. 2010;197(3):157–162. doi: 10.1016/j.toxlet.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Anum EA, Lichtman AH. Disruption of CB(1) receptor signaling impairs extinction of spatial memory in mice. Psychopharmacology (Berl) 2005;179(4):863–872. doi: 10.1007/s00213-004-2121-2. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Hamm RJ, Martin BR, Lichtman AH. Differential effects of delta 9-THC on spatial reference and working memory in mice. Psychopharmacology (Berl) 2001;157(2):142–150. doi: 10.1007/s002130100780. [DOI] [PubMed] [Google Scholar]
- Vitolo OV, Sant'Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid beta -peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci U S A. 2002;99(20):13217–13221. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebelhaus JM, Poklis JL, Poklis A, Vann RE, Lichtman AH, Wise LE. Inhalation exposure to smoke from synthetic "marijuana" produces potent cannabimimetic effects in mice. Drug Alcohol Depend. 2012;126(3):316–323. doi: 10.1016/j.drugalcdep.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410(6828):588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Wohlfarth A, Scheidweiler KB, Chen X, Liu HF, Huestis MA. Qualitative Confirmation of 9 Synthetic Cannabinoids and 20 Metabolites in Human Urine Using LC-MS/MS and Library Search. Anal Chem. 2013;85(7):3730–3738. doi: 10.1021/ac3037365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim TT, Hong NS, Ejaredar M, McKenna JE, McDonald RJ. Post-training CB1 cannabinoid receptor agonist activation disrupts long-term consolidation of spatial memories in the hippocampus. Neuroscience. 2008;151(4):929–936. doi: 10.1016/j.neuroscience.2007.08.037. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, Watanabe M. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26(18):4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AC, Schwarz E, Medina G, Obafemi A, Feng SY, Kane C, Kleinschmidt K. Cardiotoxicity associated with the synthetic cannabinoid, K9, with laboratory confirmation. Am J Emerg Med. 2012;30(7):1320 e5–1320 e7. doi: 10.1016/j.ajem.2011.05.013. [DOI] [PubMed] [Google Scholar]