Abstract
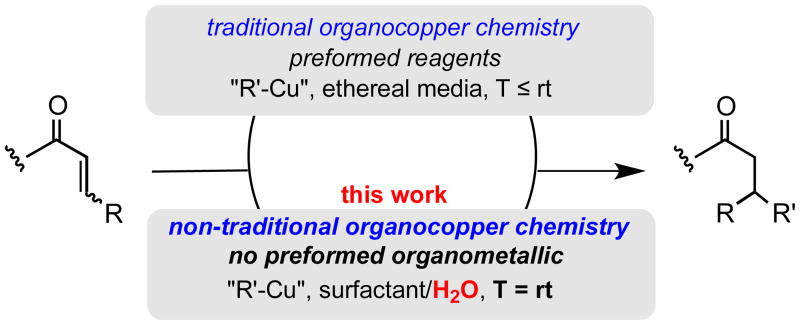
Conjugate addition reactions to enones can now be done in water at room temperature with in situ-generated organocopper reagents. Mixing an enone, zinc powder, TMEDA, and an alkyl halide in a micellar environemnt containing catalytic amounts of Cu(I), Ag(I), and Au(III), leads to 1,4-adducts in good isolated yields: no organometallic precursor is involved.
The origins of organocopper chemistry in synthesis date back to the late 1940's and early 1950's when Kharasch1 and Gilman2 first disclosed the preparation of Grignard- and organolithium-derived organocuprates, respectively. These fundamental reagents are generally scribed as “R2CuMgX” and “R2CuLi”, the hallmark of each being their ability to deliver both Csp3 and Csp2 residues from copper to carbon,3 most notably in a conjugate addition sense.4 Indeed, such species and their reactions with Michael acceptors are standard entries in textbooks on basic organic chemistry. But along with such instruction comes an appreciation for the incompatibility of organocopper reagents of almost all varieties with water; hence, practitioners must go to considerable lengths to ensure use of dry copper salt precursors, dry organic, aprotic solvents, as well as proper handling of organometallic precursors, whether obtained as items of commerce or freshly prepared prior to use.5 And while alternative inroads to organocopper reagents have been devised over time (e.g., transmetalations, etc.6), the overall level of tolerance to moisture, in general, is essentially nil. In this report, as counter-intuitive as it may seem, new technology for achieving organocopper-mediated 1,4-additions is reported that not only is tolerant of moisture, but in fact, is performed entirely in water as the gross reaction medium (Scheme 1). Moreover, unlike most conjugate additions (e.g., to enones) that occur best at lower temperatures,3 these proceed smoothly at ambient temperature. Hence, no investment of energy beyond that provided at room temperature need be made.
Scheme 1. Comparison approaches: traditional vs. micellar catalysis.
There is no true precedent for Cu-catalyzed conjugate additions of alkyl (or alkenyl, or aryl) groups to enones in aqueous media, with the exception of alkynyl conjugate additions by Carreira.7a Early work by Luche demonstrated promising results using a Zn/Cu couple in aqueous media, using copper or NH4Cl to activate zinc under ultrasonication leading to a radical pathway.7b-d
Related methodology developed by Fleming performed preferentially on silica applies solely to unsaturated nitriles,8 partners that while excellent radical accepters,9 are typically not ready participants toward copper-mediated 1,4-additions.3b As such, excessive amounts of alkyl halide (8 equiv) and Zn (6 equiv) are needed. More recently, Loh and co-workers described the use of indium/copper leading to conjugate additions of unactivated (mainly secondary) alkyl iodides (and not bromides) to α,β-unsaturated compounds in water, also via a proposed radical mechanism.10
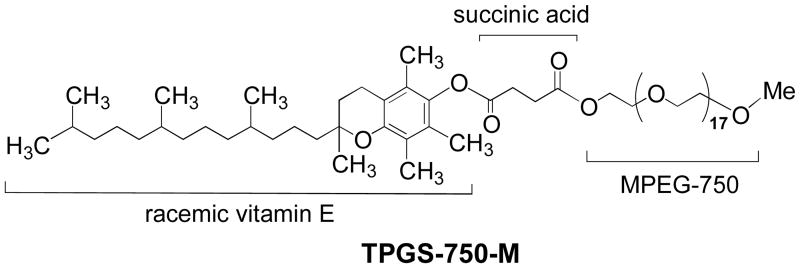
We recently disclosed highly moisture-sensitive Negishi-like cross-couplings that could be carried out in water using either alkyliodides11a or bromides.11b This methodology is enabled by small amounts of commercially available amphiphiles (e.g., 2 wt. % TPGS-750-M; Figure 1)12 that form nanomicelles in water within which the coupling occurs, where the precursor halides are used directly in the presence of Zn metal; i.e., no prior formation of RZnX is required.11a, 13
Figure 1.
Structure for polyoxyethanyl-α-tocopheryl succinate (TPGS-750-M).
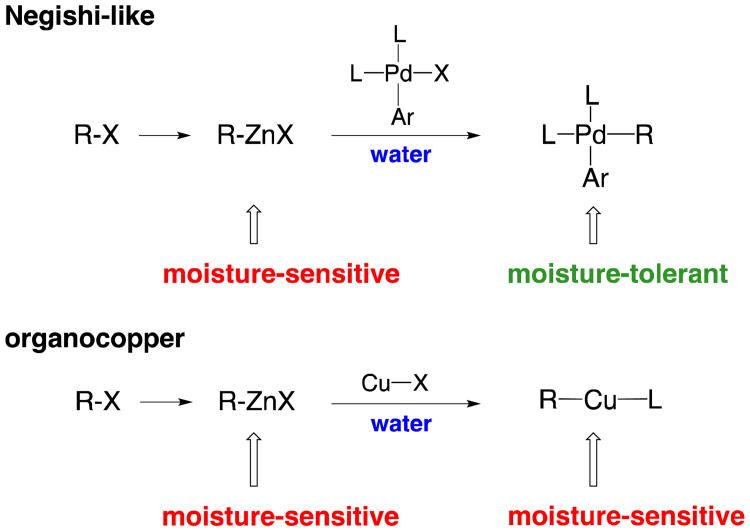
If conditions could be found such that transmetalation to copper, rather than palladium, takes place, in this case, in the presence of a Michael acceptor, 1,4-addition might ensue. However, there is a key distinguishing feature between these two processes; one that suggests that a process involving organocopper species is potentially far more demanding. That is, transmetalation from Zn to Pd would provide a moisture-insensitive intermediate, while in situ-generated organozinc and organocopper reagents are both highly intolerant of water (Figure 2).
Figure 2.
Distinctions between intermediates in Pd- vs. Cu-catalyzed reactions.
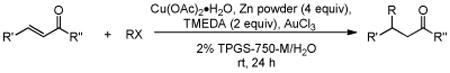
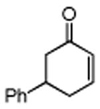
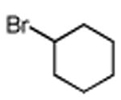
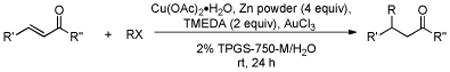
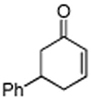
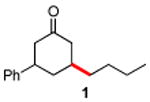
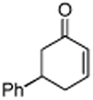
Initially, the combination of 5-phenylcyclohexenone and 1-iodobutane was studied in aqueous TPGS-750-M as a representative substrate, in the presence of Cu(OAc)2•H2O (5 mol %) and excess zinc powder and TMEDA (Table 1).
Table 1. Optimization of Reaction Conditionsa.
| ||
|---|---|---|
| entry | conditions | yield of 1 (%)b |
| 1 | Cu(OAc)2•H2O (5 mol %) Zn powder (4 equiv), TMEDA (5 equiv) |
54 |
| 2 | Cu(OAc)2•H2O (5 mol %) Zn powder (4 equiv), TMEDA (2 equiv) |
67 |
| 3 | Cu(OAc)2•H2O (5 mol %), LiCIO4 (5 mol %) Zn powder (4 equiv), TMEDA (2 equiv) |
78 |
| 4 | Cu(OAc)2•H2O (5 mol %), AuCI3 (5 mol %) Zn powder (4 equiv), TMEDA (2 equiv) |
87 |
| 5c |
Cu(OAc)2•H2O (5 mol %), AuCI3(5 mol %) Zn powder (4 equiv), TMEDA (2 equiv) |
93 |
| 6d | Cu(OAc)2•H2O (5 mol %), AuCI3 (5 mol %) Zn powder (4 equiv), TMEDA (2 equiv) |
50 |
| 7 | AuCI3 (5 mol %) Zn powder (4 equiv), TMEDA (2 equiv) |
2 |
For details, see Supporting Information.
Determined by GC on crude material.
Iodobutane was added in two portions: t = 0 h, 1.5 equiv; t = 6 h, 1.5 equiv.
Water only was used as the medium (no surfactant).
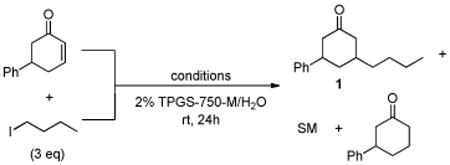
Only moderate yields of the desired product 1 (as a mixture of cis/trans isomoers) were obtained due to a significant amount of unreacted starting material (>40%), along with lesser quantities (ca. 5%) of reduced enone (entry 1). A drop in the level of TMEDA led to a rise in conversion, with two equivalents being optimal (entry 2).
Addition of a catalytic amount of a Lewis acid (e.g., LiClO4; entry 3) further increased the level of conversion, presumably by complexation of lithium with the enone.14 Other salts (e.g., Li halides, NiCl2, Sc(OTf)3) were screened, but ultimately AuCl3 was found to be the most effective (entry 4). By adding the alkyl halide and zinc in two portions, the conversion could be further increased to 93% (entry 5). A control reaction carried out “on water”15 (i.e., in the absence of TPGS-750-M) confirmed the importance of micellar catalysis in facilitating conjugate additions in aqueous media (entry 6). Attempts to vary the surfactant, including trials with Brij 30 and 35, Triton X-100, cremophor EL, and solutol HS (see Supporting Information), led in all cases to significantly lower yields of 1,4-adduct. The absence of a copper salt results in almost no formation of the product, suggesting that both Cu(OAc)2•H2O and AuCl3, along with the proper choice of amphiphile, are essential for realization of a synthetically useful process based on organocopper chemistry (entry 7).
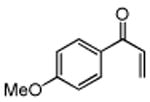
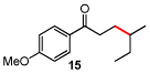
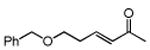
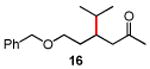
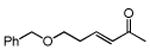
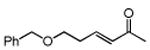
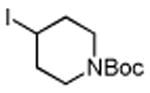
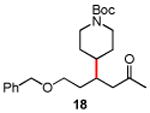
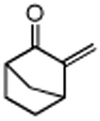
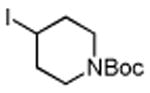
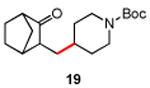
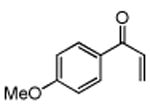
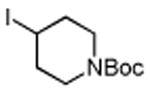
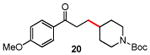
Several enones and alkyl halides bearing functional groups can be utilized, given the mildness of the reaction conditions and the functional group tolerance for which organozinc reagents are well known (Table 2).16
Table 2. Conjugate Additions of Alkyl Halides to Enone-sa.
| ||||
|---|---|---|---|---|
| entry | enone | alkyl-X | product | yield (%)b |
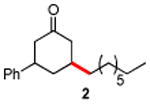
| 1 |
|
|
|
87 |
| 2 |
|
|
|
89 |
| 3 |
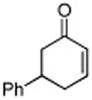
|
|
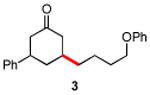
|
87 |
| 4 |
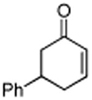
|
|
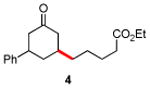
|
80 |
| 5 |
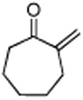
|
|
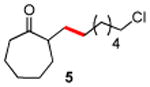
|
82 |
| 6c |
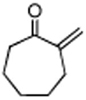
|
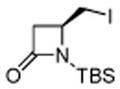
|
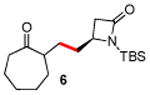
|
83 |
| 7 |
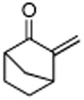
|
|
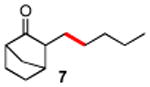
|
86 |
| 8 |
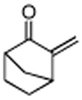
|
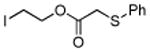
|
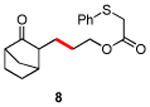
|
75 |
| 9 |
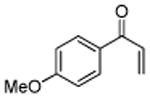
|
|
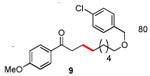
|
80 |
| 10 |
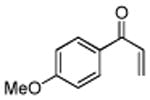
|
|
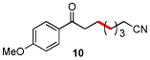
|
86 |
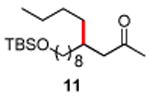
| 11 |
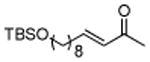
|
|
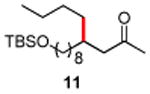
|
86 |
| 12 |
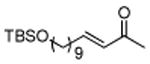
|
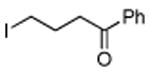
|
|
83 |
| 13 |
|
|
|
82 |
Conditions: alkyl-X (3 equiv), Zn powder (X = I) or Zn dust (X = Br) (4 equiv), TMEDA (2 equiv), 3-5 mol % [Cu], 5 mol % AuCl3, 0.5 mL 2 wt %-TPGS-750-M/H2O, rt, 24 h.
Isolated, chromatographically purified.
Zinc dust used.
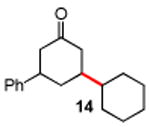
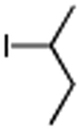
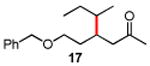
Secondary iodides and bromides also work well under these standard conditions (Table 3). Remarkably, no rearrangement of secondary centers was observed, unlike those known to occur in related reactions, presumably due to β-hydride elimination.17
Table 3. Conjugate Addition Reactions of Secondary Alkyl Halides to Enonesa.
Conditions: alkyl-X (3 equiv), Zn powder (X = I) or Zn dust (X = Br) (4 equiv), TMEDA (2 equiv), 3 mol % [Cu], 5 mol % AuCl3, 0.5 mL 2 wt %-TPGS-750-M/H2O, rt, 24h.
Isolated, chromatographically purified.
Tetraethyl derivative of TMEDA used.
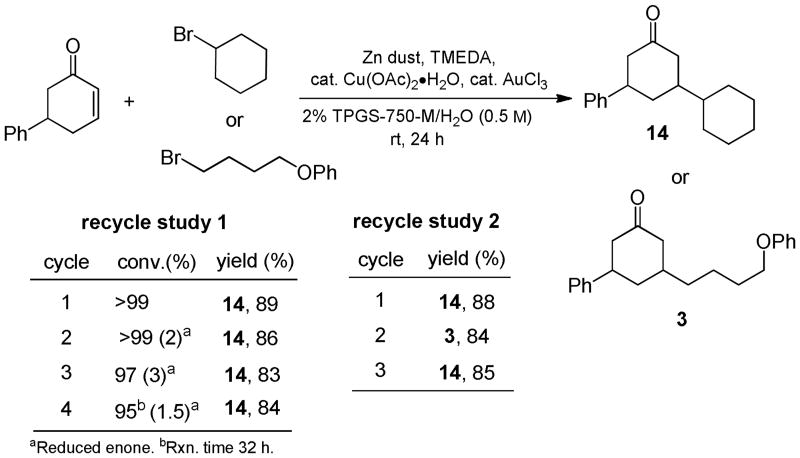
Studies on the potential for recycling of both the aqueous medium containing the surfactant as well as the gold catalyst show considerable potential. In-flask recycling was achieved using minimum amounts of hexanes as the extraction solvent (Scheme 2, study 1). Each recycle, without the addition of fresh surfactant or AuCl3, showed a minimal decrease in reaction rate, as well as minimal competitive enone reduction (see Supporting Information for details). Moreover, different substrate combinations can be used in each recycle, further broadening the utility of TPGS-750-M/H2O as a reaction medium (Scheme 2, study 2).
Scheme 2. In-Flask Recycling of TPGS-750-M and AuCl3.
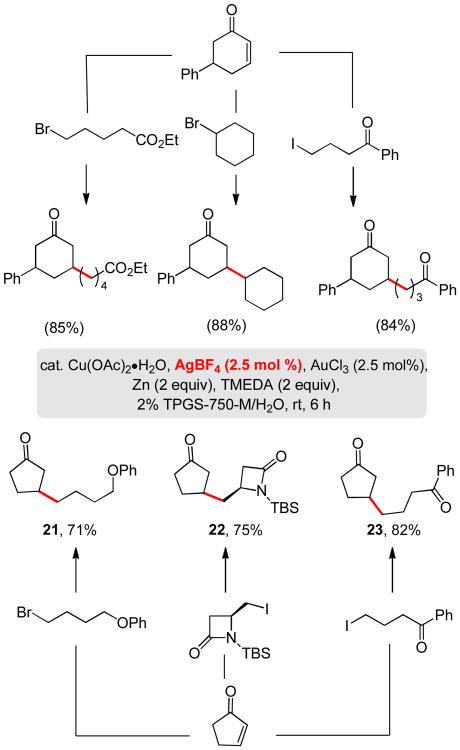
In an effort to further enhance the rate of these copper-catalyzed conjugate addition reactions, the Lewis acidity of AuCl3 was modified by introduction of an equimolar (catalytic) amount of AgBF4, a ploy oftentimes used in gold catalyzed reactions.18 Remarkably, in the presence of this additive, the amounts of alkyl halide, Zn, and AuCl3 needed were reduced dramatically, as were reaction times. As the examples in Scheme 3 illustrate, the overall efficiency remains high.
Scheme 3. The Coinage Metal Triad as Catalysts.
In summary, the first green methodology for effecting water-sensitive copper-catalyzed 1,4-additions in a totally aqueous environment has been developed. It takes advantage of micellar catalysis leveraged by use of a “designer” surfactant that forms nanoreactors of a favored size and within which organozinc reagents are formed in situ at the metal surface. The resulting organozinc species then undergo transmetalation to copper, and ultimately, conjugate addition to unsaturated ketones, giving good yields of the desired 1,4-adducts. A broad substrate scope has been demonstrated indicative of considerable generality, including tolerance to a wide range of functionality. Neither organic solvents nor energy in the form of applied heat or cooling need be invested. Further work on developing an enantioselective version of this process, as well as other coupling reactions involving organocopper complexes in water, are currently underway.
Supplementary Material
Acknowledgments
Financial support provided by the NIH (GM 86485) is greatly acknowledged.
Footnotes
Notes: The authors declare no competing financial interests.
Supporting Information: Detailed experimental procedures, analytical and spectral data is available. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Kharasch MS, Tawney PO. J Am Chem Soc. 1941;63:2308. [Google Scholar]
- 2.Gilman H, Jones RG, Woods LA. J Org Chem. 1952;17:1630. [Google Scholar]
- 3.Erdik E. Tetrahedron. 1984;40:641.Lipshutz BH, Sengupta S. Org React. 1992;41:135.For a review on directed reactions of organocopper reagents, see: Breit B, Schmidt Y. Chem Rev. 2008;108:2928. doi: 10.1021/cr078352c.Krause N. In: Modern Organocopper Compounds. Krause N, editor. WILEY-VCH; Weinheim: 2005. Knochel P, Yang X, Gommerman N. In: Handbook of Functionalized Organometallics Applications in Synthesis. Knochel P, editor. Vol. 2. WILEY-VCH; Weinheim: 2005. pp. 379–398.
- 4.For early work on Grignard- and organolithium-based cuprates, see: Normant JF. Synthesis. 1972;2:63.Corey EJ, Beames DJ. J Am Chem Soc. 1972;94:7210.Corey EJ, Katzenellenbogen JA. J Am Chem Soc. 1969;91:1851.Corey EJ, Kim CU, Chen RHK, Takeda M. J Am Chem Soc. 1972;94:4395.For more recent examples, see: Müller D, Alexakis A. Org Lett. 2012;14:1842. doi: 10.1021/ol3004436.Tissot M, Hernández AP, Müller D, Mauduit M, Alexakis A. Org Lett. 2011;13:1524. doi: 10.1021/ol200219m.Gremaud L, Palais L, Alexakis A. Chimia. 2012;66:196. doi: 10.2533/chimia.2012.196.For focused reviews on Cu-catalyzed 1,4-addition and allylic substitution reactions, see: Alexakis A, Bäckvall JE, Krause N, Pàmies O, Diéquez M. Chem Rev. 2008;108:2796. doi: 10.1021/cr0683515.Harutyunyan SR, den Hartog T, Geurts K, Minnaard AJ, Feringa BL. Chem Rev. 2008;108:2824. doi: 10.1021/cr068424k.
- 5.Lipshutz BH. In: Organometallics in Synthesis A Manual. 2nd. Schlosser M, editor. John Wiley & Sons Ltd; Chichester: 2002. pp. 665–815. [Google Scholar]
- 6.For transmetalation from aluminum, see: May TL, Brown K, Hoveyda AH. Angew Chem Int Ed. 2008;47:7358. doi: 10.1002/anie.200802910.Brown MK, Hoveyda AH. J Am Chem Soc. 2008;130:12904. doi: 10.1021/ja8058414.For transmetalation with Zn, see: Endo K, Ogawa M, Shibata T. Angew Chem Int Ed. 2010;49:2410. doi: 10.1002/anie.200906839.Kacprzynski MA, May TL, Kazane SA, Hoveyda AH. 2007;47:4554. doi: 10.1002/anie.200700841.Lee K, Brown MK, Hird AW, Hoveyda AH. J Am Chem Soc. 2006;128:7182. doi: 10.1021/ja062061o.Hird AW, Hoveyda AH. J Am Chem Soc. 2005;127:14988. doi: 10.1021/ja0553811.For transmetalation with boron, see: Yoshida M, Ohmiya H, Sawamura M. J Am Chem Soc. 2012;134:11896. doi: 10.1021/ja304481a.Jung B, Hoveyda AH. J Am Chem Soc. 2012;134:1490. doi: 10.1021/ja211269w.Gao F, Carr JL, Hoveyda AH. Angew Chem Int Ed. 2012;51:6613. doi: 10.1002/anie.201202856.For a recent overview of transmetallation reactions leading to organocopper complexes, see: Rosenker CJ, Wipf P. In: The Chemistry of Organocopper Compounds Part 2. Rappoport Z, Marek I, editors. John Wiley & Sons Ltd; Chichester: 2009. pp. 443–525.
- 7.(a) Knöpfel T, Carreira EK. J Am Chem Soc. 2003;125:6054. doi: 10.1021/ja035311z. [DOI] [PubMed] [Google Scholar]; (b) Petrier C, Dupuy C, Luche JL. Tetrahedron Lett. 1986;27:3149. [Google Scholar]; (c) Luche JL, Allavena C. Tetrahedron Lett. 1988;29:5369. [Google Scholar]; (d) Dupuy C, Petrier C, Sarandeses LA, Luche JL. Synth Commun. 1991;21:643. [Google Scholar]
- 8.Fleming FF, Gudipati S, Aitken JA. J Org Chem. 2007;72:6961. doi: 10.1021/jo0711539. [DOI] [PubMed] [Google Scholar]
- 9.(a) Ryu I, Kusano K, Yamazaki H, Sonoda N. J Org Chem. 1991;56:5003. [Google Scholar]; (b) Thoma G, Giese B. Tetrahedron Lett. 1989;30:2907. [Google Scholar]; (c) Pike P, Hershberger S, Hershberger J. Tetrahedron Lett. 1988;44:6295. [Google Scholar]
- 10.Shen ZL, Cheong HL, Loh TP. Tetrahedron Lett. 2009;50:1051. [Google Scholar]
- 11.(a) Krasovskiy A, Duplais C, Lipshutz BH. J Am Chem Soc. 2009;131:15592. doi: 10.1021/ja906803t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Duplais C, Krasovskiy A, Lipshutz BH. Organometallics. 2011;30:6090. doi: 10.1021/om200846h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipshutz BH, Ghorai S, Leong WY, Taft BR, Krogtad DVJ. Org Chem. 2011;76:5061. doi: 10.1021/jo200746y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritter S. Chemical and Engineering News “Cross-Coupling Made Easier”. 2009;87:6. [Google Scholar]
- 14.(a) Yamamoto Y, Maruyama K. J Am Chem Soc. 1978;100:3240. [Google Scholar]; (b) Yamamoto Y, Yamamoto S, Yatagai H, Ishihara Y, Maruyama KJ. J Org Chem. 1982;47:119. [Google Scholar]; (c) Yamamoto Y. Angew Chem Int Ed Engl. 1986;25:947. [Google Scholar]
- 15.Narayan S, Muldoon J, Finn MG, Fokin VV, Kolb HC, Sharpless KB. Angew Chem Int Ed. 2005;44:3275. doi: 10.1002/anie.200462883. [DOI] [PubMed] [Google Scholar]
- 16.Knochel P, Leuser H, Gong LZ, Perone S, Kneisel FF. In: Handbook of Functionalized Organometallics. Knochel P, editor. Vol. 1. WILEY-VCH; Weinheim: 2005. Chapter 7. [Google Scholar]
- 17.Isomerization in Zn-mediated, Pd-catalyzed cross-couplings, due to β-H elimination has been observed: Han C, Buchwald SL. J Am Chem Soc. 2009;131:7532. doi: 10.1021/ja902046m.Liu J, Deng Y, Wang H, Zhang H, Yu G, Wu B, Zhang H, Li Q, Marder TB, Yang Z, Lei A. Org Lett. 2008;10:2661. doi: 10.1021/ol8007342.Isomerization in Pd-catalyzed Suzuki-Miyaura cross-couplings with secondary alkyl Molander-ates, due to β-H elimination has been described: Dreher SD, Dormer PG, Sandrock DL, Molander GA. J Am Chem Soc. 2008;130:9257. doi: 10.1021/ja8031423.
- 18.For scattered reports, see: Arcadi A. Chem Rev. 2008;108:3266. doi: 10.1021/cr068435d.For use of silver additives in gold-catalyzed C–H functionalization reactions, see: Patrick SR, Boogaerts IIF, Gaillard S, Slawin AMZ, Nolan SP. Beilstein J Org Chem. 2011;7:892. doi: 10.3762/bjoc.7.102.For a study on the effects of silver in Au(I) catalysis, see: Wang D, Cai R, Sharma S, Jirak J, Thummanapelli SK, Akhmedov NG, Zhang H, Liu X, Petersen JL, Shi X. J Am Chem Soc. 2012;134:901. doi: 10.1021/ja303862z.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.