Abstract
Heparanase, the sole mammalian endoglycosidase degrading heparan sulfate, is causally involved in cancer metastasis, angiogenesis, inflammation and kidney dysfunction. Despite the wide occurrence and impact of heparan sulfate proteoglycans in vascular biology, the significance of heparanase in vessel wall disorders is underestimated. Blood vessels are highly active structures whose morphology rapidly adapts to maintain vascular function under altered systemic and local conditions. In some pathologies (restenosis, thrombosis, atherosclerosis) this normally beneficial adaptation may be detrimental to overall function. Enzymatic dependent and independent effects of heparanase on arterial structure mechanics and repair closely regulate arterial compliance and neointimal proliferation following endovascular stenting. Additionally, heparanase promotes thrombosis after vascular injury and contributes to a pro-coagulant state in human carotid atherosclerosis. Importantly, heparanase is closely associated with development and progression of atherosclerotic plaques, including stable to unstable plaque transition. Consequently, heparanase levels are markedly increased in the plasma of patients with acute myocardial infarction. Noteworthy, heparanase activates macrophages, resulting in marked induction of cytokine expression associated with plaque progression towards vulnerability. Together, heparanase emerges as a regulator of vulnerable lesion development and potential target for therapeutic intervention in atherosclerosis and related vessel wall complications.
Keywords: Extracellular matrix, Heparan sulfate, Heparanase, Atherosclerosis, Vulnerable plaque, Thrombosis, Stenosis, Inflammation, Macrophages, Colitis, Diabetic nephropathy
1. Introduction
1.1 Heparan sulfate proteoglycans (HSPGs)
HSPGs exert their multiple functional repertoires via several distinct mechanisms that combine structural, biochemical and regulatory aspects. By interacting with other macromolecules such as laminin, fibronectin, and collagens I and IV, HSPGs contribute to the structural integrity, self-assembly and insolubility of the extracellular matrix (ECM) and basement membrane, thus intimately modulating cell-ECM interactions (Bernfield et al., 1999; Timpl and Brown, 1996; Udo Hacker, 2005). HSPGs also directly transfer information from the extracellular space to intracellular kinases and cytoskeletal elements and thus afffect cell signaling, adhesion and motility (Aalkjaer and Boedtkjer, 2009; Couchman, 2010). The sulfated saccharide domains of heparan sulfate (HS) provide numerous docking sites for a multitude of protein ligands, ensuring that a wide variety of bioactive molecules (i.e., cytokines, chemokines, growth factors, enzymes, protease inhibitors, ECM proteins) bind to the cell surface and ECM (Bernfield et al., 1999; Lindahl and Li, 2009) and thereby function in the control of normal and pathological processes, among which are morphogenesis, tissue repair, cancer metastasis, inflammation, vascularization, atherosclerosis, thrombosis and diabetes (Iozzo and Sanderson, 2011; Lindahl and Li, 2009). Cleavage of HSPGs would ultimately release these proteins and convert them into bioactive mediators, ensuring rapid tissue response to local or systemic cues. This function of HS provides the cell with a rapidly accessible reservoir, precluding the need for de novo synthesis when the requirement for a particular protein is increased (Vlodavsky et al., 1991; Vlodavsky et al., 2011).
The biosynthesis of HS takes place in the Golgi system and has been studied in great detail. Briefly, the polysaccharide chains are modified at various positions by sulfation, epimerization and N-acetylation, yielding clusters of sulfated disaccharides separated by low or non-sulfated regions (Iozzo and Sanderson, 2011; Lindahl and Li, 2009). Unlike the well resolved biosynthetic pathway, the mode of HS breakdown is less characterized. While synthesis and modification of HS chains require the activity of an array of enzymes, degradation of mammalian HS is primarily carried out by one enzyme, heparanase (HPSE), which cleaves the HS side chains of HSPGs into fragments of 10–20 sugar units (Vlodavsky et al., 1999). Enzymatic activity capable of cleaving glucuronidic linkages and converting macromolecular heparin to physiologically active fragments was first identified by Ogren and Lindahl (Ogren and Lindahl, 1975). Subsequent studies revealed that the same enzyme (heparanase) is critically involved in various pathologies such as cancer (Arvatz et al., 2011; Ilan et al., 2006; Parish et al., 2001; Vlodavsky et al., 2011; Vlodavsky and Friedmann, 2001), chronic inflammation (Lerner et al., 2011; Li and Vlodavsky, 2009), thrombosis (Baker et al., 2012; Nadir et al., 2010), atherosclerosis (Blich et al., 2012; Osterholm et al., 2012; Planer et al., 2011) and kidney dysfunction (Gil et al., 2012; van den Hoven et al., 2006). As a direct result of these studies heparanase was advanced from being an obscure enzyme with a poorly understood function to a highly promising drug target, offering new treatment strategies for various cancers and other diseases. Several up-to-date reviews nicely summarize basic and translational aspects related to the involvement of heparanase in cancer progression and inflammation (Hermano et al., 2012; Li and Vlodavsky, 2009; McKenzie, 2007; Vreys and David, 2007). The present review focuses on the emerging role of heparanase in vessel wall pathologies such as atherosclerosis (Baker et al., 2010; Blich et al., 2012; Osterholm et al., 2012; Planer et al., 2011), restenosis (Baker et al., 2009) and thrombosis (Baker et al., 2012; Nadir et al., 2010).
1.2 Mammalian heparanase
Heparanase is an endo-β-glucuronidase that cleaves HS side chains presumably at sites of low sulfation (Peterson and Liu, this series; Peterson and Liu, 2012; Peterson and Liu, 2010), releasing saccharide products with appreciable size (4–7 kDa) that can still associate with protein ligands and facilitate their biological potency. Mammalian cells express a single dominant functional heparanase enzyme (heparanase-1) (Barash et al., 2010; Ilan et al., 2006). A second heparanase (heparanase-2) has been cloned but has not been shown to have HS degrading activity (Levy-Adam et al., 2010; McKenzie et al., 2000). For simplification, we refer to heparanase-1 as heparanase. The heparanase mRNA encodes a 65 kDa pro-enzyme that is post translationally cleaved into 8 and 50 kDa subunits that non-covalently associate to form the active heparanase (Levy-Adam et al., 2003; McKenzie et al., 2003). The heparanase structure delineates a TIM-barrel fold harboring the enzyme’ active site and substrate binding domains, and a C-terminus domain that is critical for heparanase secretion and signaling function (Fux et al., 2009b). Similar to other glycosyl hydrolases, heparanase has a common catalytic mechanism that involves two conserved acidic residues, a putative proton donor at Glu225 and a nucleophile at Glu343 (Hulett et al., 2000). Cellular processing of the secreted latent enzyme involves uptake and delivery into late endosomes and lysosomes followed by removal of a 6 kDa linker segment brought about by cathepsin L (Abboud-Jarrous et al., 2008; Arvatz et al., 2011).
1.3. Heparanase in cancer progression
Heparanase is up-regulated in essentially all human tumors examined (Vlodavsky et al., 2011). A direct role of heparanase in tumor metastasis was demonstrated by the increased lung, liver and bone colonization of cancer cells following over-expression of the heparanase gene, and by a marked decrease in the metastatic potential of cells subjected to heparanase gene silencing (Ilan et al., 2006; Vlodavsky et al., 2011). A significant role of heparanase in tumor angiogenesis and lymphangiogenesis was demonstrated applying a similar experimental approach (Cohen-Kaplan et al., 2008; Ilan et al., 2006). Notably, heparanase expression levels correlate with tumor vascularity in cancer patients, further indicating a significant role in tumor angiogenesis (Vlodavsky et al., 2011). Cancer patients exhibiting high levels of heparanase had a significantly shorter postoperative survival time than patients whose tumors contained low levels of heparanase (Vlodavsky et al., 2011). Collectively, these results indicate that heparanase is causally involved in cancer progression and hence is a valid target for anti-cancer drug development and a promising tumor marker. This statement was reinforced by in vivo studies indicating a marked inhibition of tumor progression in mice treated with heparanase-inhibiting compounds (Casu et al., 2008; Dredge et al., 2011; Ritchie et al., 2011; Shafat et al., 2011a; Zhou et al., 2011; Yang et al, 2007). Of increasing significance are observations that heparanase promotes gene expression (i.e., VEGF, tissue factor, HGF, RANKL, TNFα) (Parish et al., this series; He et al., 2011; Nadir et al., 2006; Sanderson et al., 2004; Yang et al., 2010; Zetser et al., 2006; Ramani et al, 2011) and signaling pathways (i.e., phosphorylation of Akt, Src, Erk, EGF-receptor, insulin receptor) (Fux et al., 2009b; Ilan et al., 2006; Sanderson et al., 2004; Purushothaman et al, 2012) of which some are mediated by its C-terminus domain, devoid of heparanase enzymatic activity (Fux et al., 2009a).
2. Heparanase in atherosclerosis, stenosis and thrombosis
2.1. Heparanase & atherosclerosis (introduction)
Atherosclerosis represents the major cause of death and disability in the adult population. Atherosclerotic lesions are asymmetric focal thickenings of the intima, consisting of inflammatory and immune cells, connective tissue elements, lipids, debris, and vascular endothelial and smooth muscle cells. Proteoglycans were one of the earliest class of molecules identified to be associated with atherosclerotic lesions and lipid deposition in the vascular wall (Camejo et al., 1998). Proteoglycans with predominantly chondroitin or dermatan sulfate chains can retain low density lipoprotein (LDL) and are considered atherogenic (Wight and Merrilees, 2004). However the role of HSPGs in atherogenesis is less clear. Proteoglycans bearing HS chains have often been considered anti-atherogenic due to their ability to inhibit monocyte adhesion and vascular smooth muscle cell (SMC) growth. However, studies in mice with HS deficient perlecan have indicated that HS can have pro-atherogenic effects in mouse models of atherosclerosis (Vikramadithyan et al., 2004), suggesting that atherogenesis is stimulated by HS degradation. Heparin and HSPGs are important regulators of cellular and molecular processes in atherogenesis. They mediate lipoprotein clearance, recruitment of inflammatory cells and are essential modulators of SMC function (Aalkjaer and Boedtkjer, 2009; Osterholm et al., 2012). In addition, HSPGs bind lipoprotein lipase (LPL) to the endothelial cell surface where it promotes cellular uptake of chylomicron remnants, cholesterol-rich lipoproteins, and free fatty acids (Aalkjaer and Boedtkjer, 2009).
While the vast majority of atherosclerotic lesions remain stable, some undergo alterations that make them vulnerable to rupture. Inflammatory process creates a thin cap of fibrous tissue over a lipid rich and metabolically active core which is the hallmark feature of vulnerable, high risk plaques (Fig. 1), associated with acute coronary syndrome and sudden cardiac death. The mechanism(s) underlying the progression from asymptomatic fibroatheromatous plaque to a lesion at high risk for rupture (“vulnerable plaque”) (VP) is largely unclear. In the continuum of atheroma morphology, vulnerable plaques are thought to be those with lowered collagen content, indicating that regulation of the ECM is a key aspect to stabilizing these plaques by preventing plaque cap thinning and eventual rupture. While the essential role of matrix metalloproteases (MMPs) in the atherosclerotic process is well recognized, little is known about the enzymatic regulation of HSPGs during the formation and progression of atherosclerotic plaques. Recent studies on the involvement of heparanase in the pathogenesis of athresclerosis are presented below.
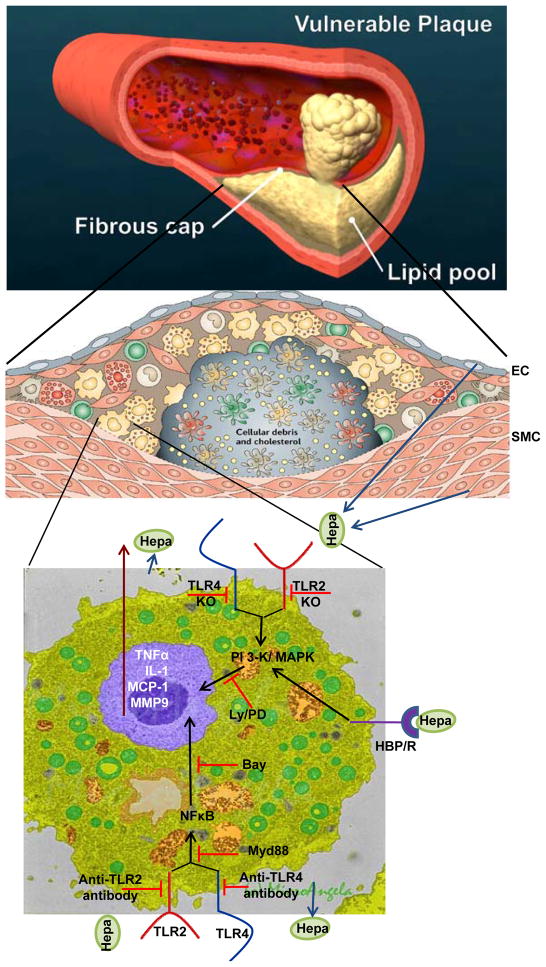
Figure 1.
Summary and proposed model for heparanase function in plaque vulnerability. A schematic illustration of blood vessel occluded with a vulnerable plaque is shown in the upper panel. A more detailed structure of the plaque is shown in the middle panel. A fibrous cap composed of endothelial cells (EC), smooth muscle cells (SMC) and collagen surrounds and stabilizes the plaque which is populated by macrophages, foam cells, monocytes, T cells and dendritic cells. Heparanase, secreted by EC, SMC or macrophages is abundantly present in the plaque and contributes to plaque rupture and vulnerability directly and indirectly. Heparanase activity, together with proteolytic activity exerted by MMP9 and other proteases weaken the fibrous cap making it more susceptible to rupture. Inactive heparanase activates macrophages and induces the expression of MMP9 and pro-inflammatory cytokines (i.e., TNF-α, MCP-1, IL-1), leading to the recruitment of more immune cells and plaque progression. Cytokine induction by heparanase involves the PI-3K and MAPK signaling pathways, NFκB, and TLR-2 and -4. The exact order of the complex sequence of events that lead to TLR activation and signaling cascade is not clear but is thought to be mediated by presently unidentified heparanase binding protein/receptor (HBP/R; lower panel).
Adopted from Hansson and Libby, Nature Immunology 6:508–519, 2006; and Microangela (http://www5.pbrc.hawaii.edu/microangela).
2.2. Heparanase expression in coronary artery disease
Baker et al (Baker et al., 2008) examined the enzymatic regulation of HS by heparanase during the natural history of atherosclerotic plaque development in the coronary arteries of hyperlipidemic, diabetic swine. They reported that heparanase protein levels and activity increased with progression of atherosclerotic lesions from minimal lesions to thin cap fibroatheromas (TCFAs). A critical comparison is that of the intermediate and TCFA lesions as this represents the transition from a stable to potentially unstable lesion. A greater than 3-fold increase in heparanase between these two groups suggests that heparanase may be involved not only in initial lesion formation but in the progression of stable lesions to vulnerable plaques. Immunohistochemical analysis revealed a pattern of heparanase accumulation that was similar to lipid deposition and inflammatory cell distribution suggesting that the major source of heparanase was the inflammatory cells. Notably, lipids have been shown to regulate heparanase expression in some cells (Chen et al., 2004) and heparanase immunostaining in human coronary arteries is increased in arteries with atherosclerosis versus non-diseased arteries (Baker et al., 2008; Osterholm et al., 2012) (see 2.3). In vitro studies on human macrophages demonstrated that heparanase activity can be increased by oxidized LDL and angiotensin II (Baker et al., 2008). Thus, oxidized lipid- or angiotensin-induced expression of heparanase in macrophages may be a primary mechanism increasing heparanase in these plaques.
Both collagen and elastin play an integral role in the atherosclerotic plaque development process. Destruction of these ECM molecules is therefore key to facilitating plaque thinning and rupture. In this context, heparanase could act as a facilitator of MMP and cathepsin-mediated destruction of ECM molecules (Baker et al., 2008). Notably, removal of HS from the cell surface has been shown to increase activation of MMP-2 (Munesue et al., 2007) and MMP mediated shedding of syndecan 1 from the cell membrane (Purushothaman et al., 2008; Purushothaman et al., 2010; Ramani et al, 2012). Heparanase and MMP-9 can also work synergistically to enhance the differentiation of vascular smooth muscle cells (Fitzgerald et al., 1999). More information on the cross-talk between heparanase and MMPs is presented in 2.6.
van den Berg et al demonstrated that the dimension and composition of the glycocalyx was altered in high risk atherogenic regions of the mouse carotid artery (van den Berg et al., 2009). Their results imply that loss of the glycocalyx layer, by disturbed flow and/or hyperlipidemia, may lead to enhanced arterial accumulation of lipids. In this context, heparanase activity, increased in arterial regions with low endothelial shear stress (ESS), would lead to loss of HS from the glycocalyx and enhanced permeability to lipoproteins. Thus, heparanase may have an important role in the well-known atherogenic effects of low or disturbed flow within the artery through altering the glycocalyx (Baker et al., 2008). Notably, loss of the glycocalyx in response to elevated levels of TNFα and the associated up-regulation of heparanase was recently reported to enhance white cell infiltration, acute inflammation and sepsis (Schmidt et al., 2012).
2.3. Heparanase in symptomatic carotid atherosclerosis
Osterholm et al (Osterholm et al., 2012) investigated expression of the HPSE gene and protein in a biobank of human carotid endarterecomy samples obtained from patients undergoing surgery for symptomatic and asymptomatic carotid disease. Briefly, microarray analysis of RNA extracted from 127 human carotid plaques showed a 6.6-fold increase in levels of HPSE mRNA in comparison to control tissue from non-atherosclerotic iliac arteries. In addition, HPSE mRNA expression correlated with increased serum creatinine levels and a reduced glomerular filtration rate (GFR), in accordance with our studies on the causal involvement of heparanase in the development of diabetic nephropathy (Gil et al., 2012). Blinded semiquantitative grading of 170 endarterectomy tissue core sections revealed that HPSE staining intensity was increased in plaque tissue sections from symptomatic patients, when compared to tissue sections from asymptomatic patients. Previously, polymorphism within the HPSE gene has been associated with differential transcription levels (Ostrovsky et al., 2009). Notably, equivalent association between the same gene polymorphisms and HPSE expression was found in carotid plaque tissues (Osterholm et al., 2012). Furthermore, positive correlations were found between HPSE and markers of inflammation and apoptosis whereas a clear negative correlation was observed between HPSE and SMC differentiation markers (Osterholm et al., 2012). Tissue microarray analyses revealed a widespread immunoreactivity for HPSE, especially in areas with extensive infiltration of inflammatory cells. The majority of CD163+ macrophages and a proportion of CD3+ lymphocytes expressed the HPSE protein. It was also found that HPSE co-localizes to a high degree with fibrin deposition in human carotid plaques, suggesting a connection between HPSE and coagulation (Nadir et al., 2010) within carotid plaque tissue, regardless of the source of HPSE. A recent report by Baker et al (Baker et al., 2012) (see 2.10) emphasizes the role of HPSE in thrombosis after vascular injury and endovascular stenting, and supports the notion that HPSE contributes to a pro-coagulant state in human carotid atherosclerosis. Altogether, analysis of the expression of HPSE in human carotid endarterectomies revealed increased expression of HPSE in human atherosclerosis associated with inflammation, coagulation and plaque instability. Collectively, these results indicate a role for heparanase as a regulator of the progression and development of atherosclerotic plaques, including stable to unstable plaque transition (Fig. 1). In this context, heparanase was associated with proatherogenic risk factors and severe plaque remodeling. Further, co-localization of heparanase with macrophages (Blich et al., 2012; Osterholm et al., 2012) (Fig. 2) and MMP activity (Baker et al. 2008) suggests that heparanase may work in concert with proteases (see 2.6) to enhance TCFA formation. In a recent study (Blich et al., 2012) combining basic research and clinical data (see 2.4) we have demonstrated that heparanase activates macrophages, resulting in marked induction of cytokine expression associated with plaque progression towards vulnerability (Figs. 1 &2).
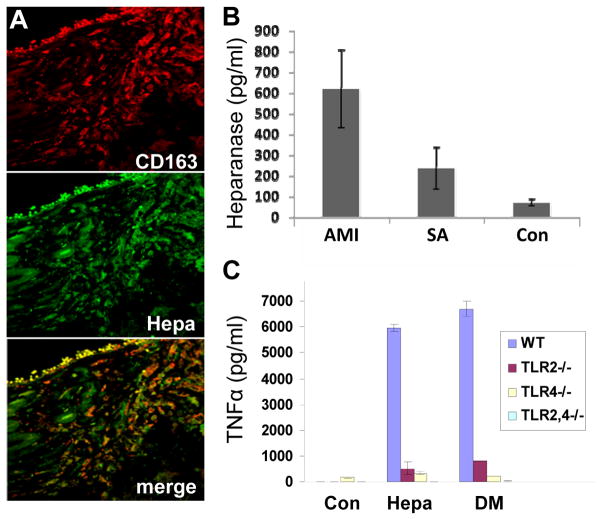
Figure 2.
A. Specimens of vulnerable plaques (VP) lesion were stained for CD163 (upper panel, red) and heparanase (middle panel, green). Merge image stained for CD 163 and heparanase is shown in the lower panel. Note that heparanase staining localizes, in part, to CD163-positive cells (appears yellow-orange in lower panel). Original magnification: x63.
B. Elevation of heparanase levels in the plasma of atherosclerotic patients. Plasma samples of control healthy donors (Con) and patients exhibiting stable angina (SA) or acute myocardial infarction (AMI) were collected on admission and heparanase levels were quantified by ELISA. Note marked increase of heparanase levels in patients exhibiting SA and even more so in patients exhibiting AMI (p=0.0006). C. TLR-deficient macrophages. Macrophages were harvested from control wild type mice (WT, blue bars), and mice deficient for TLR2 (TLR2−/−, red bars), TLR4 (TLR4−, yellow bars), or TLR2 and 4 (TLR2,4−/−, green bars). Macrophages were left untreated (Con) or incubated with native (Hepa) or mutated heparanase (DM; 5 μg/ml). Medium was collected after 20 h and TNFα levels were quantified by ELISA.
2.4. Heparanase associates with plaque instability
Blich et al (Blich et al., 2012) provided evidence for the clinical significance of heparanase in atherosclerosis. We examined by immunostaining heparanase expression in specimens of stable (SP) and vulnerable (VP) plaques compared with control arteries. Weak staining of heparanase was observed in the media of control and SP lesions, likely decorating SMC. In striking contrast, intense staining of heparanase was seen in the intima of VP lesions. Morphometric analysis revealed a significant increase in the staining percent for heparanase in specimens of VP as compared to specimens of SP. In order to identify heparanase-positive cells in the intima of VP, specimens were double stained for CD163, a macrophage cell surface marker, and heparanase. Results indicated that most of the heparanase positive cells within plaques were macrophages. We next evaluated the levels of heparanase in the plasma of patients with acute myocardial infarction (MI), stable angina and healthy subjects by ELISA. Heparanase levels were increased nearly 9-fold in patients with acute MI, and three fold in patients with stable angina (SA) as compared to healthy individuals (Blich et al., 2012). Subsequently, on day 3–5 after admission with acute MI, mean heparanase levels were reduced significantly. We compared the clinical manifestation of patients exhibiting low vs. high levels of plasma heparanase and found that high levels of heparanase were associated with acute MI and elevated white blood cell count (Fig. 2).
Noteworthy, presence of high levels of heparanase systemically may further damage the vasculature. For example, accumulating evidence suggests that heparanase functions also as a pro-coagulation mediator, enhancing expression of tissue factor and generation of factor Xa, two critical components in blood coagulation (Baker et al., 2012; Nadir et al., 2010; Nadir et al., 2006) thus providing another mode by which heparanase affects the vasculature in general and plaque development and progression in particular. It is evident that activation of the atherosclerotic plaque rather than stenosis causes ischemia and infarction. Major advance in prevention of the disease will thus require early detection of rupture-prone, vulnerable plaques. Our results (Blich et al., 2012) imply that heparanase levels are associated with plaque vulnerability and progression and may thus be considered as diagnostic marker and potentially therapeutic target in acute heart diseases.
2.5. Heparanase activates macrophages via TLR
As discussed above, extensive research has shown that inflammation plays a key role in coronary artery disease. Activated immune cells are abundant at sites of rupture; produce numerous inflammatory molecules and proteolytic enzymes that transform the stable plaque into vulnerable, unstable structures (Hansson, 2005). The following experiments were undertaken to elucidate the role and mode of heparanase action in these processes. Addition of recombinant latent heparanase to the culture medium of J774 mouse macrophage-like cells, resulted in marked increase in MCP-1 levels, in agreement with increased MCP-1 expression in injured neointima of transgenic mice over expressing heparanase (Baker et al., 2012). Heparanase addition elicited even higher increase in TNFα and IL-1 levels, while MMP-9 was elevated to a lesser degree. In order to substantiate these results, heparanase was similarly added to primary macrophages isolated from the peritoneum of thioglycolate-treated mice, resulting in a comparable increase in the levels of TNFα, MCP-1, MMP-9, and IL-1 (Blich et al., 2012). Marked increase of TNFα and IL-1 was similarly obtained following addition of heparanase to monocytes isolated from human peripheral blood, further demonstrating the ability of heparanase to activate macrophages. An increase in TNFα and IL-1 was also observed following the addition of mutated (glutamic acid residues 225 and 343) enzymatically inactive heparanase to J774 cells, thus strongly implying that HS-degrading activity is not required for elevation of cytokines by heparanase. RT-PCR analyses revealed that the observed increase in cytokine levels is due to enhanced gene transcription (Blich et al., 2012). TNFα transcription was similarly induced by the heparanase C-terminus protein domain shown previously to mediate signaling properties of heparanase (Fux et al., 2009a,b), further supporting this mode of action. These results clearly imply that over expression or exogenous addition of heparanase activates macrophages and stimulates the transcription of selected genes. Induction of MMP-9 expression by macrophages following heparanase over expression or exogenous addition suggests an intimate cooperation between the two distinct enzymatic activities in cancer, inflammation, and atherosclerosis (see 2.6.). Notably, macrophages exhibit heparanase activity that can be detected inside and outside the cells, resulting in macrophage activation in an autocrine manner. Macrophages can also be activated by heparanase originating from other cell types residing in the atherosclerotic plaque. For example, treatment of human microvascular endothelial cells with the pro-inflammatory cytokines TNFα and IL-1β resulted in a marked increase of heparanase secretion (Chen et al., 2004). This suggests co-operation between cellular compartments of the atherosclerotic plaque, in which cytokines (i.e., TNFα) secreted by activated macrophages stimulate the secretion of heparanase from endothelial cells, leading to further augmentation of macrophage activation. Subsequently, latent and active heparanase secreted by macrophages, together with enzymatic activity responsible for proteolytic cleavage of protein constituents of the ECM (i.e., MMPs) likely cooperate in remodeling the ECM, leading to plaque rupture.
The molecular mechanism underlying cytokine induction by heparanase is not entirely clear but involves toll like receptors (TLRs). TLRs are a family of type I transmembrane proteins which bind a range of bacterial products, leading to downstream signaling via NFκB which activates the transcription of pro-inflammatory mediators (Kawai and Akira, 2010). TLR-2 and TLR-4 are expressed by macrophages and are well characterized in terms of their contribution to atherosclerotic lesion development. Cytokine induction by heparanase appears to involve TLR-2, TLR-4, and NFκB. The involvement of TLRs is concluded since TNFα elevation was markedly attenuated in cells treated with MyD88 inhibitor, an adaptor molecule critical for TLRs signaling, or anti-TLR-2 and anti-TLR-4 neutralizing antibodies prior to heparanase addition (Blich et al., 2012) (Fig. 2). Even more striking were the results utilizing mouse peritoneal macrophages (MPM) derived from TLR-null mice. Clearly, induction of TNFα, MMP-9, and MCP-1 by heparanase was not seen in MPM deficient for TLR-2 or TLR-2 and -4 (Blich et al., 2012).
A growing body of evidence suggests that TLR signaling is elicited in the absence of infection through endogenous ligands generated at sites of tissue remodeling and inflammation. Of note, ECM components and their degradation products generated during tissue injury or remodeling have been found to function as TLR ligands (Brunn et al., 2005). Examples are hyaluronic acid, decorin, and soluble biglycan recognized as ligands for TLR-2 and TLR-4 (Schaefer and Iozzo, 2012), and versican which activates tumor-infiltrating myeloid cells through TLR-2 and its co-receptors TLR-6 and CD14. Thus, although activation of TLRs does not require binding or cleavage of HSPG, heparanase may activate TLRs by introducing conformational changes in cell membrane HS proteoglycans (i.e., syndecans, glypicans) following their clustering and activation, or by HS cleavage products (Brunn et al., 2005) in addition to its HS-independent function (Blich et al., 2012) (Fig. 1).
Utilizing a colitis model, we have reported that recruitment and infiltration of macrophages to the colon of transgenic mice over-expressing heparanase is substantially increased and sustained during the chronic phase of experimental colitis compared with control mice (Lerner et al., 2011; Goldberg et al, this series). Thus, it appears that in both the colitis and atheroscloris models, heparanase not only functions to recruit but also activates macrophages. Our data indicate that up-regulation of heparanase enables enhanced activation of macrophages, reprogramming their response from resolution of inflammation to unresolved chronic inflammation. In addition, we found that activated macrophages are capable of inducing heparanase expression in cells, most likely through TNFα-mediated stimulation of the Egr1 transcription factor, a powerful inducer of heparanase transcription (Lerner et al., 2011). Moreover, due to their unique ability to secrete mature cathepsin L, macrophages appear to be responsible for proteolytic activation of secreted latent proheparanase. Thus, macrophages not only represent a cellular target for heparanase action (i.e., macrophage activation), but can also upregulate heparanase, both at the transcriptional and posttranslational levels (Lerner et al., 2011; (Lerner et al., 2011; Goldberg et al, this series).
Taken together, our results emphasize the clinical relevance of heparanase in plaque progression and rupture, and identify TLR family members as mediators of this function (Blich et al., 2012) (Figs. 1 & 2). The signal transduction initiated by heparanase is transmitted to the cell nucleus, actively inducing the transcription of pro-inflammatory genes that further fuels the inflammatory reaction. Heparanase inhibitors (see 2.11.) are thus expected to attenuate plaque progression.
2.6. Heparanase-protease cooperation
Cross-talk between heparanase and MMPs has been demonstrated. Enhanced expression of heparanase stimulates sustained ERK phosphorylation that in turn drives MMP-9 expression, while heparanase gene silencing resulted in reduced MMP-9 activity (Purushothaman et al., 2008). Moreover, not only MMP-9 but also urokinase-type plasminogen activator (uPA) and its receptor (uPAR), molecular determinants responsible for MMP-9 activation and plaque rupture, are up-regulated by heparanase (Purushothaman et al., 2008). Interestingly, heparanase appears to have a duel effect in stimulating syndecan-1 shedding; it increases expression of MMP-9 (a sheddase of syndecan-1) and it trims the heparan sulfate chains of syndecan-1 which enhances susceptibility of the core protein to MMP cleavage (Ramani et al, 2012; Purushothaman et al, 2008). These findings provide evidence for cooperation between heparanase and MMPs in regulating HSPGs on the cell surface and likely in the ECM, and are supported by our generation and characterization of heparanase knockout mice. Despite the complete lack of heparanase gene expression and enzymatic activity, heparanase null mice develop normally, are fertile, and exhibit no apparent anatomical or functional abnormalities (Zcharia et al., 2009). Notably, heparanase deficiency was accompanied by a marked elevation of MMP family members such as MMP-2 and MMP-14, in an organ-dependent manner, suggesting that MMPs provide tissue-specific compensation for heparanase deficiency (Zcharia et al., 2009). Co-regulation of heparanase and MMPs was also noted by a marked decrease in MMP (primarily MMP-2,-9 and -14) expression following over-expression of the heparanase gene in cultured human mammary carcinoma cells (Zcharia et al., 2009). These and other results suggest that heparanase acts as a regulator of protease expression (Arvatz et al., 2011).
2.7. Involvement of nuclear heparanase in cardiovascular complications
Patients with diabetes are predisposed to developing atherosclerosis, and, as discussed above, inflammation is considered an important regulatory process in early development of the atheromatous lesion. Hyperglycemia and hyperlipidemia are characteristic features associated with diabetes. A novel regulation of endothelial heparanase by these substrates was reported (Wang et al., 2009). Briefly, high glucose was identified as a potent stimulator of endothelial heparanase production (via Egr1) and secretion, which helps in the transfer of lipoprotein lipase (LPL) from the cardiomyocyte to the vascular lumen, permitting augmented fatty acid provision to cardiomyocytes (Wang et al., 2009). Unlike high glucose, augmented concentrations of fatty acid increased the nuclear content of heparanase (Wang et al., 2012). Heparanase is first synthesized as a latent 65 kDa enzyme that undergoes secretion followed by reuptake into the cells (Ilan et al., 2006). After proteolytic cleavage (primarily by cathepsin-L in lysosomes), a highly active heterodimer composed of 8 and 50 kDa polypeptides is formed. Within the acidic compartment of the lysosome, active heparanase is stored in a stable form and can assist in the turnover of HS side chains. Mobilization by demand can occur, where the enzyme is either secreted to degrade cell surface HS and release HS-bound proteins, or translocated to the nucleus to affect gene transcription (Ilan et al., 2006; Parish et al., this series). Wang et al (Wang et al., 2012) investigated the mechanisms by which fatty acid induces nuclear translocation of heparanase. It was found that palmitic acid (PA) triggers lysosome permeabilization, nuclear shuttling of released heparanase by Hsp90, and induction of genes that affect glucose metabolism and inflammation. Notably, nuclear localization of heparanase is not uncommon and several groups have detected the enzyme in the nucleus of cancer cells that were stably transfected with heparanase and in biopsy specimens of human tumors (Doweck et al., 2006; Kobayashi et al., 2006). Interestingly, nuclear heparanase was associated with good prognosis in head & neck cancer patients (Doweck et al., 2006).
The presence of HS in the nucleus has been reported in various cell types including endothelial cells. In response to palmitic acid (PA), staining for intact nuclear HS was lost, coinciding with appearance of cleaved HS exclusively in the nucleus, suggesting that PA-induced nuclear entry of heparanase facilitates HS cleavage (Wang et al., 2012). HS has been reported to be a potent repressor of gene transcription through its inhibitory effect on HAT, the enzyme that acetylates the lysine side chain of histone, allowing access of transcription factors to bind DNA and initiate transcription. Indeed, augmented HAT activity and histone acetylation were observed when nuclear HS was cleaved by heparanase (Purushothaman et al., 2011). It was also reported that nuclear heparanase is associated with euchromatin in both resting and activated human T cells (He et al., 2011). At the chromatin level, heparanase regulates histone H3K4 and H3K9 methylation by binding to target gene control regions in association with the demethylase LSD1 and thereby plays a central role in mediating the on/off transcription switch of inducible immune response genes (He et al., 2011). Thus, heparanase belongs to an emerging class of proteins that play an important role in regulating transcription in addition to their well-recognized extra-nuclear functions (Parish et al., this series).
It should be noted that in addition to genes controlling glucose metabolism (i.e., glucose oxidation - PDK2; and lactate formation - LDHA), nuclear entry of heparanase was also associated with an increase in genes related to inflammation, such as VCAM1, SERPINE1 and VEGFA, effects that were prevented by heparanase siRNA (Wang et al., 2012). The critical role of VCAM1, plasminogen activator inhibitor type 1 (SERPINE1), and VEGFA in the development of atherosclerosis has been reported. Altogether, it appears that fatty acid can provoke lysosomal release of heparanase, its nuclear translocation, and activation of genes controlling glucose metabolism and inflammation. The ensuing uncoupling between glucose oxidation and glycolysis leads to accumulation of lactate (Wang et al., 2012). Given that lactate and its lowering of pH has been implicated in the progression of atherosclerosis through its promotion of lipoprotein binding to proteoglycans, angiogenesis, and plaque rupture, and the critical role of VCAM, plasminogen activator inhibitor type 1, and VEGF in the development of atherosclerosis, data detailing the mechanism by which fatty acids shuttles endothelial heparanase into the nucleus may serve to reduce the increased susceptibility of patients with diabetes to atherosclerosis (Wang et al., 2012).
2.8. Lipid metabolism and fatty streak formation
Both animal and human studies indicated that triglyceridemia is an independent risk factor for early atherosclerosis and subjects with coronary artery diseases exhibit delayed metabolism of triglyceride rich lipoproteins (TRPs) (Boquist et al., 1999). Part of the increased susceptibility to atherosclerosis of patients with type 2 diabetes mellitus and the metabolic syndrome has been attributed to the decreased clearance of TRPs (Tabas et al., 2007). TRPs are extremely atherogenic and there is direct evidence showing high levels of TRPs in human atherosclerotic plaques. Appying heparanase transgenic mice (Hpa-Tg) we have demonstrated that over-expression of heparanase is associated with delayed clearance of TRPs and reduced uptake by the liver resulting in elevated plasma levels of these atherogenic particles. This could, at least in part, be due to the effect of heparanase on enhancing shedding of syndecan-1 (Yang et al, 2007; Deng et al, 2012). Indeed, in Hpa-Tg mice the increased triglyceride (TG) levels and decreased clearance of post-prandial lipoprotein remnants by the liver are associated with increased cross sectional area of fatty streaks (Planer et al., 2011). Clearly, shortening of the HS chains appears to have a significant impact on remnant clearance as there are fewer sites available for the binding of LPL and lipoproteins resulting in less sequestration and internalization. Over-expression of the heparanase gene indicates the importance of HSPGs for the uptake of TRPs and its protective effect on fatty streak formation and potentially atherosclerosis initiation. From a clinical perspective, changes in HS structure and/or expression may contribute to atherosclerotic manifestations in patients predisposed to mild but clinically relevant hypertriglyceridemia. Potential pharmacologic modulation of heparanase expression or activity may thus retard the development of atheromas (Planer et al., 2011).
2.9. Heparanase alters arterial structure and repair following endovascular stenting
Enzymatic dependent and independent effects of heparanase on arterial structure and function are likely to play a role in arterial compliance and neointimal proliferation following endovascular stenting. Baker et al (Baker et al., 2009) examined the role of heparanase in controlling arterial structure, mechanics, and remodeling. In vitro studies revealed that heparanase expression in endothelial cells serves as a negative regulator of endothelial inhibition of vascular smooth muscle cell (vSMC) proliferation. Transgenic mice overexpressing heparanase had increased arterial thickness, cellular density, and mechanical compliance. Aortic stiffness and ultimate strength were both decreased, accompanied by increased incidence of spontaneous aneurysm, implying that excessive heparanase expression can compromise the mechanical integrity of the aorta (Baker et al., 2009). Endovascular stenting is a common clinical treatment for arteries with occlusive disease due to atherosclerosis. While this treatment immediately increases blood flow to the ischemic tissue, the vascular injury and indwelling stent material can lead to restenosis of the artery through the development of neointimal hyperplasia. Endovascular stenting studies in rats demonstrated increased heparanase expression in the neointima of obese, hyperlipidemic rats in comparison to lean rats. The extent of heparanase expression within the neointima strongly correlated with the neointimal thickness following injury. To test the effects of heparanase overexpression on arterial repair, Baker et al (Baker et al., 2009) developed a novel murine model of stent injury using small diameter self-expanding stents. Using this model, they found that increased neointimal formation and macrophage recruitment occurs in transgenic mice overexpressing heparanase. Consistent with these findings, the concentration of MCP-1 was increased in the transgenic heparanase mice following acute vascular injury (Baker et al., 2009). Taken together, Baker et al demonstrated that heparanase is a potent regulator of vascular remodeling, both on the level of paracrine regulation of vascular homeostasis and as an effector molecule in vascular response to injury (Baker et al., 2009). It appears that heparanase can act through multiple mechanisms to alter vascular remodeling and response to injury. Heparanase can serve as a control point allowing endothelial cells to modulate between inhibition and stimulation of vascular SMC. Further, overexpression of heparanase can alter elastin fiber integrity as well as increase response to vascular injury through enhancing macrophage recruitment. A common unifying feature of these processes is the involvement of HS and, thus, vulnerability to disruption by heparanase. Consequently, aberrant heparanase may serve as a common pathophysiological mechanism governing vascular remodeling under different pathological disease states.
Previous studies have made use of potential heparanase inhibitors in the context of neointimal formation. Heparin (Edelman et al., 1990) and PI-88 (Khachigian and Parish, 2004) have been used in animal models to reduce neointimal formation. Both of these compounds inhibit heparanase activity but also have other activities including growth factor binding and direct activity on vSMCs. Local delivery of a neutralizing antibody to heparanase has also been used to reduce neointima formation following carotid balloon injury, resulting in a more specific outcome (Myler et al., 2006).
2.10. Heparanase in stent thrombosis
The placement of an endovascular stent presents several major stimuli for the formation of thrombosis including endothelial injury, arterial stretch with deep vascular injury and locally disturbed blood flow due to the indwelling stent. Drug eluting stent technology has allowed the delivery of therapeutic agents to inhibit the proliferation of vascular cells following stent implantation. While these therapeutics have reduced the incidence of restentosis, it became evident that these drugs also inhibit the normal healing of the artery leading to enhanced and prolonged risk of thrombosis and subsequent cardiac death (Hacker et al., 2005).
HS anticoagulatory properties arise from the interaction of HS with antithrombin III, an inhibitor of coagulation pathway enzymes including thrombin and factor Xa. As discussed above heparanase is increased during neointimal hyperplasia, a process that can be greatly increased in the presence of type II diabetes (Baker et al., 2009). Thus, heparanase overexpression has the potential for mediating disease induced changes in the thrombotic potential of endovascular stents. Baker et al (Baker et al., 2012) examined the role of heparanase in controlling thrombosis following vascular injury and stent thrombosis. In the absence of vascular injury wild type and heparanase overexpressing (Hpa-Tg) mice had similar time to thrombosis in a laser-induced arterial thrombosis model. However, in the presence of light or heavy vascular injury the time to thrombosis was dramatically reduced in Hpa-Tg mice (Baker et al., 2012). An ex-vivo flow system was used to flow blood from wild type and Hpa-Tg mice over stents and stented arterial segments. These studies revealed markedly increased thrombosis in stents with blood isolated from Hpa-Tg mice in comparison to blood from wild type animals (Baker et al., 2012). Stented arterial segments were also exposed to luminal flow in an ex-vivo flow system. It was found that blood from Hpa-Tg animals had markedly increased thrombosis when applied to stented arterial segments from either wild type or Hpa-Tg mice (Baker et al., 2012). Thus, blood from heparanase overexpressing mice had a near three fold increase in thrombin deposition in stents implanted in silicone tubing. This implies that in response to the altered hemodynamic environment provided by the stents, heparanase overexpression increases thrombus formation.
Previous studies have demonstrated that heparanase overexpression induces increased levels of tissue factor through a p38-dependent signaling pathway (Nadir et al., 2010; Nadir et al., 2006). In parallel, heparanase appears to also stimulate TF pathway inhibitor (TFPI) (Nadir et al., 2008). Additionally, exogenously applied heparanase has been shown to modify in-vitro coagulation on endothelial cells in culture (Nadir et al., 2010). Yet, in the absence of vascular injury there was little or no difference between the clotting times of wild type and Hpa-Tg mice, indicating that heparanase has only a moderate effect on the hypercoagulability of the blood (overexpression of TF with compensation by TFPI) (Baker et al., 2012). Loss of endogenous HS increases vWF/GPIb interactions thereby facilitating clot formation around in the flow surrounding stent struts. Thus, degradation of HS side chains and shedding of syndecan-1 (Yang et al., 2010) due to heparanase excess may contribute to increased thrombosis. Clinically, as discussed above, heparanase expression is increased in the plasma and urine of cancer patients (Shafat et al., 2007). In addition, heparanase is increased locally and systemically in patients with chronic inflammation (Li et al., 2008), renal disease (Shafat et al., 2012; Shafat et al., 2011b; van den Hoven et al., 2006) and atherosclerotic lesions (Blich et al., 2012). Heparanase is also increased in the neointima formed after stent implantation in diabetic animals and regulates the formation of neointimal hyperplasa following vascular injury (Baker et al., 2008; Baker et al., 2009). Patients with diabetes and end stage renal disease have increased risk of stent thrombosis (Hacker et al., 2005), implying that heparanase is a powerful mediator of thrombosis in the context of vascular injury and stent-induced flow disturbance. Consequently, inhibition of heparanase in addition to traditional anticoagulatory therapy may provide a reduced risk of stent thrombosis in patients with high levels of heparanase.
2.11. Heparanase inhibitors
Clearly, due to its critical role in many disease processes and to its perceived limited function in normal tissues, heparanase has emerged as an important therapeutic target. Several approaches hold potential for inhibition of heparanase including use of modified heparins, small molecule inhibitors and function-blocking monoclonal antibodies. Modified heparins or oligosaccharides that serve as heparin mimics have been developed and have taken on many forms with predictably wide-ranging results (Casu et al., 2008) (Coombe and Kett, 2012). Within this class of compounds PI-88, PG545, SST0001 and M402 are currently in various stages of clinical trials in cancer patients. PI-88 is a phosphosulfomannoligosaccharide having anti-heparanase and anti-angiogenic activity presumably due to its binding to heparanase and to heparin-binding factors such as VEGF (Cochran et al., 2003). It is reasonably well-tolerated by cancer patients and is efficacious against some cancers, most notably hepatocellular carcinoma (Liu et al., 2009). A second generation of anti-heparanase compounds similar to PI-88 yielded PG545, a tetrasaccharide that has shown anti-tumor activity in murine models of breast, prostate, liver, lung, colon, head and neck cancers and melanoma (Dredge et al., 2011). SST0001 (formerly designated as G4000) is a modified heparin that is 100% N-acetylated and 25% glycol split (Casu et al., 2008; Naggi et al., 2005). In pre-clinical cancer models, SST0001 has shown efficacy against Ewing’s sarcoma, multiple myeloma and pancreatic cancer (Meirovitz et al., 2011; Ritchie et al., 2011; Shafat et al., 2011; Yang et al., 2007). Pharmacodynamic studies in myeloma models demonstrated that SST0001 inhibits heparanase activity in vivo, regulates levels of growth factors (e.g., HGF, VEGF) and inhibits angiogenesis (Ritchie et al., 2011). Another glycol-split heparin compound similar to SST0001 yet smaller in molecular mass is M402. M402 showed efficacy in a melanoma model of experimental metastasis and in spontaneous metastasis (Zhou et al., 2011).
3. Concluding remarks, future perspectives and take-home message
Heparanase is a multifaceted protein endowed with enzymatic and non-enzymatic functions that appear to participate in major human pathologies. Since the cloning of the human heparanase gene in 1999, heparanase was advanced from being an obscure enzyme with a poorly understood function to a highly promising drug target. While most attention was addressed to heparanase function in tumor biology, emerging evidence indicate that heparanase is also engaged in other disease conditions often associated with degradation of HS, release of bioactive molecules anchored within the ECM network, disregulated signalling cascades and gene transcription, and activation of innate immune cells. Among these diseases are chronic inflammation (i.e., inflammatory bowel disease, rheumatoid arthritis) (Lerner et al., 2011; Li et al., 2008), autoimmunity (i.e., type 1 diabetes, psoriasis) (Ziolkowski et al., 2012 & our unpublished results), diabetic nephropathy (Gil et al., 2012), bone osteolysis (Yang et al., 2010) and, as emphasized in the present review, aterosclerosis (Baker et al., 2008; Blich et al., 2012; Osterholm et al., 2012). We have demonstrated that heparanase induces activation of macrophages (Lerner et al., 2011; Blich et al., 2012) which in turn may stimulate, via the effect of secreted cytokines (i.e., TNFα), transcription of the HPSE gene and further production of the enzyme by various cell types (Fig. 1). Similarly, the enzyme is up-regulated by glucose in endothelial, mesengial and kidney epithelial cells, thereby generating a vicious cycle and local environment that fuels disease (i.e., cancer, diabetic nephropathy, atherosclerosis) progression (Vlodavsky et al., 2011). Heparanase exerts a strong angiogenic response and was also noted to accelerate the proliferation of SMC, implying that the enzyme affects all major cellular components (i.e., endothelial cells, vSMC, macrophages) of the atherosclerotic lesion. The results emphasize the clinical relevance of heparanase in plaque progression and rupture, and identify TLR family members as mediators of this function (Osterholm et al., 2012; Blich et al., 2012) (Fig. 2). The molecular mechanism underlying heparanase mediated TLR activation and the resulting cytokine induction in macrophages within the plaque, has not been elucidated and likely involve heparanase-binding receptors on the cell surface. Likewise, the mode of heparanase action in promoting restenosis, coagulation and thrombosis, all associated with arterial plaque development and rupture, is the subject of intensive investigation. There is growing evidence that heparanase upregulates expression of genes that participate in glucose metabolism, immune response, inflammation, atherosclerosis, and plaque rupture, suggesting that heparanase belongs to an emerging class of proteins that play a significant role in regulating transcription in addition to their well-recognized extra-nuclear functions (Parish et al., this series). Heparanase-inhibiting compounds (heparin-mimicking compounds, sulfated oligosaccharides, neutralizing antibodies, small molecules) have been developed, of which some (i.e., PI-88, SST0001, M402, PG545, all saccharide based compounds) entered clinical trials in cancer patients (Sanderson and Iozzo, 2012). The clinical information gaind by these trials will certainly accelerate use of the same compounds for the treatment of vessel wall complications.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants CA106456 (IV) and CA138535, CA135075 and CA138340 (RDS), the United States-Israel Binational Science Foundation (BSF; to IV and RDS); the Ministry of Science & Technology of the State of Israel and the German Cancer Research Center (DKFZ), and by a research contract from Sigma-Tau Research Switzerland S.A. I. Vlodavsky is a Research Professor of the Israel Cancer Research Fund (ICRF). We gratefully acknowledge the contribution, motivation and assistance of the research teams in the Cancer and Vascular Biology Research Center of the Rappaport Faculty of Medicine (Technion, Haifa), the Hadassah-Hebrew University Medical Center (Jerusalem, Israel), the University of Alabama at Birmingham (Birmingham, Alabama, USA) and the Ronzoni Institute for Chemical and Biochemical Research (Milan, Italy). We thank Dr. Claudio Pisano and Dr. Allesandro Noseda (Sigma-Tau SpA, Pomezia, Italy) for their continuous support and active collaboration.
The authors apologize for the inability, due to space limitations, to reference all studies relevant to this review.
Abbreviations
- ECM
extracellular matrix
- HS
heparan sulfate
- HSPGs
heparan sulfate proteoglycans
- GAG
glycosaminoglycan
- HAT
histone acetyltransferase
- MMP
matrix metalloproteinase
- VEGF
vascular endothelial growth factor
- SMC
smooth muscle cells
- TF
tissue factor
- TNFα
tumor necrosis factor α
- VP
vulnerable plaque
- TLR
toll like receptor
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
References
- Aalkjaer C, Boedtkjer DB. Getting neointimal: the emergence of heparanase into the vascular matrix. Circulation Res. 2009;104:277–279. doi: 10.1161/CIRCRESAHA.108.192625. [DOI] [PubMed] [Google Scholar]
- Abboud-Jarrous G, Atzmon R, Peretz T, Palermo C, Gadea BB, Joyce JA, Vlodavsky I. Cathepsin L is responsible for processing and activation of proheparanase through multiple cleavages of a linker segment. J Biol Chem. 2008;283:18167–18176. doi: 10.1074/jbc.M801327200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvatz G, Shafat I, Levy-Adam F, Ilan N, Vlodavsky I. The heparanase system and tumor metastasis: is heparanase the seed and soil? Cancer Metastasis Rev. 2011;30:253–268. doi: 10.1007/s10555-011-9288-x. [DOI] [PubMed] [Google Scholar]
- Baker AB, Chatzizisis YS, Beigel R, Jonas M, Stone BV, Coskun AU, Maynard C, Rogers C, Koskinas KC, Feldman CL, Stone PH, Edelman ER. Regulation of heparanase expression in coronary artery disease in diabetic, hyperlipidemic swine. Atherosclerosis. 2010;213:436–442. doi: 10.1016/j.atherosclerosis.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AB, Gibson WJ, Kolachalama VB, Golomb M, Indolfi L, Spruell C, Zcharia E, Vlodavsky I, Edelman ER. Heparanase regulates thrombosis in vascular injury and stent-induced flow disturbance. J Am Coll Cardiol. 2012;59:1551–1560. doi: 10.1016/j.jacc.2011.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AB, Groothuis A, Jonas M, Ettenson DS, Shazly T, Zcharia E, Vlodavsky I, Seifert P, Edelman ER. Heparanase alters arterial structure, mechanics, and repair following endovascular stenting in mice. Circulation Res. 2009;104:380–387. doi: 10.1161/CIRCRESAHA.108.180695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash U, Cohen-Kaplan V, Dowek I, Sanderson RD, Ilan N, Vlodavsky I. Proteoglycans in health and disease: new concepts for heparanase function in tumor progression and metastasis. The FEBS J. 2010;277:3890–3903. doi: 10.1111/j.1742-4658.2010.07799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- Blich M, Golan A, Arvatz G, Sebbag A, Shafat I, Sabo E, Cohen-Kaplan V, Petcherski S, Avniel-Polak S, Eitan A, Hammerman H, Aronson D, Axelman E, Ilan N, Nussbaum G, Vlodavsky I. Macrophage activation by heparanase is mediated by TLR-2 and TLR-4 and associates with plaque progression. Arterioscler Thromb Vasc Biol. 2013;33:e56–65. doi: 10.1161/ATVBAHA.112.254961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquist S, Ruotolo G, Tang R, Bjorkegren J, Bond MG, de Faire U, Karpe F, Hamsten A. Alimentary lipemia, postprandial triglyceride-rich lipoproteins, and common carotid intima-media thickness in healthy, middle-aged men. Circulation. 1999;100:723–728. doi: 10.1161/01.cir.100.7.723. [DOI] [PubMed] [Google Scholar]
- Brunn GJ, Bungum MK, Johnson GB, Platt JL. Conditional signaling by Toll-like receptor 4. Faseb J. 2005;19:872–874. doi: 10.1096/fj.04-3211fje. [DOI] [PubMed] [Google Scholar]
- Camejo G, Hurt-Camejo E, Wiklund O, Bondjers G. Association of apo B lipoproteins with arterial proteoglycans: pathological significance and molecular basis. Atherosclerosis. 1998;139:205–222. doi: 10.1016/s0021-9150(98)00107-5. [DOI] [PubMed] [Google Scholar]
- Casu B, Vlodavsky I, Sanderson RD. Non-anticoagulant heparins and inhibition of cancer. Pathophysiol Haemost Thromb. 2008;36:195–203. doi: 10.1159/000175157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Wang D, Vikramadithyan R, Yagyu H, Saxena U, Pillarisetti S, Goldberg IJ. Inflammatory cytokines and fatty acids regulate endothelial cell heparanase expression. Biochemistry. 2004;43:4971–4977. doi: 10.1021/bi0356552. [DOI] [PubMed] [Google Scholar]
- Cochran S, Li C, Fairweather JK, Kett WC, Coombe DR, Ferro V. Probing the interactions of phosphosulfomannans with angiogenic growth factors by surface plasmon resonance. J Med Chem. 2003;46:4601–4608. doi: 10.1021/jm030180y. [DOI] [PubMed] [Google Scholar]
- Coombe DR, Kett WC. Heparin mimetics. Handb Exp Pharmacol. 2012;207:361–383. doi: 10.1007/978-3-642-23056-1_16. [DOI] [PubMed] [Google Scholar]
- Couchman JR. Transmembrane signaling proteoglycans. Annu Rev Cell Dev Biol. 2010;26:89–114. doi: 10.1146/annurev-cellbio-100109-104126. [DOI] [PubMed] [Google Scholar]
- Cohen-Kaplan V, Naroditsky I, Zetser A, Ilan N, Vlodavsky I, Doweck I. Heparanase induces VEGF C and facilitates tumor lymphangiogenesis. Int J Cancer. 2008;123:2566–2573. doi: 10.1002/ijc.23898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Foley EM, Gonzales JC, Gordts PL, Li Y, Esko JD. Shedding of syndecan-1 from human hepatocytes alters very low density lipoprotein clearance. Hepatology. 2012;55:277–286. doi: 10.1002/hep.24626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doweck I, Kaplan-Cohen V, Naroditsky I, Sabo E, Ilan N, Vlodavsky I. Heparanase localization and expression by head and neck cancer: correlation with tumor progression and patient survival. Neoplasia. 2006;8:1055–1061. doi: 10.1593/neo.06577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dredge K, Hammond E, Handley P, Gonda TJ, Smith MT, Vincent C, Brandt R, Ferro V, Bytheway I. PG545, a dual heparanase and angiogenesis inhibitor, induces potent anti-tumour and anti-metastatic efficacy in preclinical models. Br J Cancer. 2011;104:635–642. doi: 10.1038/bjc.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman ER, Adams DH, Karnovsky MJ. Effect of controlled adventitial heparin delivery on smooth muscle cell proliferation following endothelial injury. Proc Natl Acad Sci USA. 1990;87:3773–3777. doi: 10.1073/pnas.87.10.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M, Hayward IP, Thomas AC, Campbell GR, Campbell JH. Matrix metalloproteinase can facilitate the heparanase-induced promotion of phenotype change in vascular smooth muscle cells. Atherosclerosis. 1999;145:97–106. doi: 10.1016/s0021-9150(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Fux L, Feibish N, Cohen-Kaplan V, Gingis-Velitski S, Feld S, Geffen C, Vlodavsky I, Ilan N. Structure-function approach identifies a COOH-terminal domain that mediates heparanase signaling. Cancer Res. 2009a;69:1758–1767. doi: 10.1158/0008-5472.CAN-08-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fux L, Ilan N, Sanderson RD, Vlodavsky I. Heparanase: busy at the cell surface. Trends Biochem Sci. 2009b;34:511–519. doi: 10.1016/j.tibs.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil N, Goldberg R, Neuman T, Garsen M, Zcharia E, Rubinstein A, van Kuppevelt T, Meirovitz A, Pisano C, Li JP, van der Vlag J, Vlodavsky I, Elkin M. Heparanase is essential for the development of diabetic nephropathy in mice. Diabetes. 2012;61:208–216. doi: 10.2337/db11-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: the sweet side of development. Nat Rev. 2005;6:530–541. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Eng J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- He YQ, Sutcliffe EL, Bunting KL, Li J, Goodall KJ, Poon IK, Hulett MD, Freeman C, Zafar A, McInnes RL, Taya T, Parish CR, Rao S. The endoglycosidase heparanase enters the nucleus of T lymphocytes and modulates H3 methylation at actively transcribed genes via the interplay with key chromatin modifying enzymes. Transcription. 2011;3:130–145. doi: 10.4161/trns.19998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermano E, Lerner I, Elkin M. Heparanase enzyme in chronic inflammatory bowel disease and colon cancer. Cell Mol Life Sci. 2012;69:2501–2513. doi: 10.1007/s00018-012-0930-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulett MD, Hornby JR, Ohms SJ, Zuegg J, Freeman C, Gready JE, Parish CR. Identification of active-site residues of the pro-metastatic endoglycosidase heparanase. Biochemistry. 2000;39:15659–15667. doi: 10.1021/bi002080p. [DOI] [PubMed] [Google Scholar]
- Ilan N, Elkin M, Vlodavsky I. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int J Biochem Cell Biol. 2006;38:2018–2039. doi: 10.1016/j.biocel.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Sanderson RD. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med. 2011;15:1013–1031. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Khachigian LM, Parish CR. Phosphomannopentaose sulfate (PI-88): heparan sulfate mimetic with clinical potential in multiple vascular pathologies. Cardiovascular Drug Rev. 2004;22:1–6. doi: 10.1111/j.1527-3466.2004.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Liu CJ, Lee PH, Lin DY, Wu CC, Jeng LB, Lin PW, Mok KT, Lee WC, Yeh HZ, Ho MC, Yang SS, Lee CC, Yu MC, Hu RH, Peng CY, Lai KL, Chang SS, Chen PJ. Heparanase inhibitor PI-88 as adjuvant therapy for hepatocellular carcinoma after curative resection: a randomized phase II trial for safety and optimal dosage. J Hepatol. 2009;50:958–968. doi: 10.1016/j.jhep.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Naomoto Y, Nobuhisa T, Okawa T, Takaoka M, Shirakawa Y, Yamatsuji T, Matsuoka J, Mizushima T, Matsuura H, Nakajima M, Nakagawa H, Rustgi A, Tanaka N. Heparanase regulates esophageal keratinocyte differentiation through nuclear translocation and heparan sulfate cleavage. Differentiation. 2006;74:235–243. doi: 10.1111/j.1432-0436.2006.00072.x. [DOI] [PubMed] [Google Scholar]
- Lerner I, Hermano E, Zcharia E, Rodkin D, Bulvik R, Doviner V, Rubinstein AM, Ishai-Michaeli R, Atzmon R, Sherman Y, Meirovitz A, Peretz T, Vlodavsky I, Elkin M. Heparanase powers a chronic inflammatory circuit that promotes colitis-associated tumorigenesis in mice. J Clin Invest. 2011;121:1709–1721. doi: 10.1172/JCI43792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Adam F, Feld S, Cohen-Kaplan V, Shteingauz A, Gross M, Arvatz G, Naroditsky I, Ilan N, Doweck I, Vlodavsky I. Heparanase 2 interacts with heparan sulfate with high affinity and inhibits heparanase activity. J Biol Chem. 2010;285:28010–28019. doi: 10.1074/jbc.M110.116384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Adam F, Miao HQ, Heinrikson RL, Vlodavsky I, Ilan N. Heterodimer formation is essential for heparanase enzymatic activity. Biochem Biophys Res Commun. 2003;308:885–891. doi: 10.1016/s0006-291x(03)01478-5. [DOI] [PubMed] [Google Scholar]
- Li JP, Vlodavsky I. Heparin, heparan sulfate and heparanase in inflammatory reactions. Thromb Haemost. 2009;102:823–828. doi: 10.1160/TH09-02-0091. [DOI] [PubMed] [Google Scholar]
- Li RW, Freeman C, Yu D, Hindmarsh EJ, Tymms KE, Parish CR, Smith PN. Dramatic regulation of heparanase activity and angiogenesis gene expression in synovium from patients with rheumatoid arthritis. Arthritis Rheum. 2008;58:1590–1600. doi: 10.1002/art.23489. [DOI] [PubMed] [Google Scholar]
- Lindahl U, Li JP. Interactions between heparan sulfate and proteins-design and functional implications. Int Rev Cell Mol Biol. 2009;276:105–159. doi: 10.1016/S1937-6448(09)76003-4. [DOI] [PubMed] [Google Scholar]
- McKenzie E, Tyson K, Stamps A, Smith P, Turner P, Barry R, Hircock M, Patel S, Barry E, Stubberfield C, Terrett J, Page M. Cloning and expression profiling of Hpa2, a novel mammalian heparanase family member. Biochem Biophys Res Commun. 2000;276:1170–1177. doi: 10.1006/bbrc.2000.3586. [DOI] [PubMed] [Google Scholar]
- McKenzie E, Young K, Hircock M, Bennett J, Bhaman M, Felix R, Turner P, Stamps A, McMillan D, Saville G, Ng S, Mason S, Snell D, Schofield D, Gong H, Townsend R, Gallagher J, Page M, Parekh R, Stubberfield C. Biochemical characterization of the active heterodimer form of human heparanase (Hpa1) protein expressed in insect cells. Biochem J. 2003;373:423–435. doi: 10.1042/BJ20030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie EA. Heparanase: a target for drug discovery in cancer and inflammation. Br J Pharmacol. 2007;151:1–14. doi: 10.1038/sj.bjp.0707182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirovitz A, Hermano E, Lerner I, Zcharia E, Pisano C, Peretz T, Elkin M. Role of heparanase in radiation-enhanced invasiveness of pancreatic carcinoma. Cancer Res. 2011;71:2772–2780. doi: 10.1158/0008-5472.CAN-10-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munesue S, Yoshitomi Y, Kusano Y, Koyama Y, Nishiyama A, Nakanishi H, Miyazaki K, Ishimaru T, Miyaura S, Okayama M, Oguri K. A novel function of syndecan-2, suppression of matrix metalloproteinase-2 activation, which causes suppression of metastasis. J Biol Chem. 2007;282:28164–28174. doi: 10.1074/jbc.M609812200. [DOI] [PubMed] [Google Scholar]
- Myler HA, Lipke EA, Rice EE, West JL. Novel heparanase-inhibiting antibody reduces neointima formation. J Biochem (Tokyo) 2006;139:339–345. doi: 10.1093/jb/mvj061. [DOI] [PubMed] [Google Scholar]
- Nadir Y, Brenner B, Fux L, Shafat I, Attias J, Vlodavsky I. Heparanase enhances the generation of activated factor X in the presence of tissue factor and activated factor VII. Haematologica. 2010;95:1927–1934. doi: 10.3324/haematol.2010.023713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadir Y, Brenner B, Gingis-Velitski S, Levy-Adam F, Ilan N, Zcharia E, Nadir E, Vlodavsky I. Heparanase induces tissue factor pathway inhibitor expression and extracellular accumulation in endothelial and tumor cells. Thromb Haemost. 2008;99:133–141. [PubMed] [Google Scholar]
- Nadir Y, Brenner B, Zetser A, Ilan N, Shafat I, Zcharia E, Goldshmidt O, Vlodavsky I. Heparanase induces tissue factor expression in vascular endothelial and cancer cells. J Thromb Haemost. 2006;4:2443–2451. doi: 10.1111/j.1538-7836.2006.02212.x. [DOI] [PubMed] [Google Scholar]
- Naggi A, Casu B, Perez M, Torri G, Cassinelli G, Penco S, Pisano C, Giannini G, Ishai-Michaeli R, Vlodavsky I. Modulation of the heparanase-inhibiting activity of heparin through selective desulfation, graded N-acetylation, and glycol splitting. J Biol Chem. 2005;280:12103–12113. doi: 10.1074/jbc.M414217200. [DOI] [PubMed] [Google Scholar]
- Ogren S, Lindahl U. Cleavage of macromolecular heparin by an enzyme from mouse mastocytoma. J Biol Chem. 1975;250:2690–2697. [PubMed] [Google Scholar]
- Osterholm C, Folkersen L, Lengquist M, Ponten F, Renne T, Li J, Hedin U. Increased expression of heparanase in symptomatic carotid atherosclerosis. Atherosclerosis. 2013 Oct 23; doi: 10.1016/j.atherosclerosis.2012.09.030. [DOI] [PubMed] [Google Scholar]
- Ostrovsky O, Korostishevsky M, Shafat I, Mayorov M, Ilan N, Vlodavsky I, Nagler A. Inverse correlation between HPSE gene single nucleotide polymorphisms and heparanase expression: possibility of multiple levels of heparanase regulation. J Leuc Biol. 2009;86:445–455. doi: 10.1189/jlb.1208735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish CR, Freeman C, Hulett MD. Heparanase: a key enzyme involved in cell invasion. Biochim Biophys Acta. 2001;1471:M99–108. doi: 10.1016/s0304-419x(01)00017-8. [DOI] [PubMed] [Google Scholar]
- Peterson S, Liu J. Deciphering mode of action of heparanase using structurally defined oligosaccharides. J Biol Chem. 2012;287:34836–34843. doi: 10.1074/jbc.M112.390161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson SB, Liu J. Unraveling the specificity of heparanase utilizing synthetic substrates. J Biol Chem. 2010;285:14504–14513. doi: 10.1074/jbc.M110.104166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planer D, Metzger S, Zcharia E, Wexler ID, Vlodavsky I, Chajek-Shaul T. Role of heparanase on hepatic uptake of intestinal derived lipoprotein and fatty streak formation in mice. PloS one. 2011;6:e18370. doi: 10.1371/journal.pone.0018370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman A, Chen L, Yang Y, Sanderson RD. Heparanase stimulation of protease expression implicates it as a master regulator of the aggressive tumor phenotype in myeloma. J Biol Chem. 2008;283:32628–32636. doi: 10.1074/jbc.M806266200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman A, Hurst DR, Pisano C, Mizumoto S, Sugahara K, Sanderson RD. Heparanase-mediated loss of nuclear syndecan-1 enhances histone acetyltransferase (HAT) activity to promote expression of genes that drive an aggressive tumor phenotype. J Biol Chem. 2011;286:30377–30383. doi: 10.1074/jbc.M111.254789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman A, Uyama T, Kobayashi F, Yamada S, Sugahara K, Rapraeger AC, Sanderson RD. Heparanase-enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis. Blood. 2010;115:2449–2457. doi: 10.1182/blood-2009-07-234757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman A, Babitz SK, Sanderson RD. Heparanase Enhances the Insulin Receptor Signaling Pathway to Activate Extracellular Signal-regulated Kinase in Multiple Myeloma. J Biol Chem. 2012;287:41288–41296. doi: 10.1074/jbc.M112.391417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani VC, Yang Y, Ren Y, Nan L, Sanderson RD. Heparanase plays a dual role in driving hepatocyte growth factor (HGF) signaling by enhancing HGF expression and activity. J Biol Chem. 2011;286:6490–6499. doi: 10.1074/jbc.M110.183277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani VC, Pruett PS, Thompson CA, Delucas LD, Sanderson RD. Heparan sulfate chains of syndecan-1 regulate ectodomain shedding. J Biol Chem. 2012;287:9952–9961. doi: 10.1074/jbc.M111.330803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie JP, Ramani VC, Ren Y, Naggi A, Torri G, Casu B, Penco S, Pisano C, Carminati P, Tortoreto M, Zunino F, Vlodavsky I, Sanderson RD, Yang Y. SST0001, a chemically modified heparin, inhibits myeloma growth and angiogenesis via disruption of the heparanase/syndecan-1 axis. Clin Cancer Res. 2011;17:1382–1393. doi: 10.1158/1078-0432.CCR-10-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson RD, Iozzo RV. Targeting heparanase for cancer therapy at the tumor-matrix interface. Matrix Biol. 2012;31:283–284. doi: 10.1016/j.matbio.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Sanderson RD, Yang Y, Suva LJ, Kelly T. Heparan sulfate proteoglycans and heparanase--partners in osteolytic tumor growth and metastasis. Matrix Biol. 2004;23:341–352. doi: 10.1016/j.matbio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Iozzo RV. Small leucine-rich proteoglycans, at the crossroad of cancer growth and inflammation. Curr Opin Gen Dev. 2012;22:56–57. doi: 10.1016/j.gde.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, Smith LP, Cheng SS, Overdier KH, Thompson KR, Geraci MW, Douglas IS, Pearse DB, Tuder RM. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med July. 2012;22:2012. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafat I, Agbaria A, Boaz M, Schwartz D, Baruch R, Nakash R, Ilan N, Vlodavsky I, Weinstein T. Elevated urine heparanase levels are associated with proteinuria and decreased renal allograft function. PloS one. 2012;7:e44076. doi: 10.1371/journal.pone.0044076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafat I, Ben-Arush MW, Issakov J, Meller I, Naroditsky I, Tortoreto M, Cassinelli G, Lanzi C, Pisano C, Ilan N, Vlodavsky I, Zunino F. Pre-clinical and clinical significance of heparanase in Ewing’s sarcoma. J Cell Mol Med. 2011a;15:1857–1864. doi: 10.1111/j.1582-4934.2010.01190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafat I, Ben-Barak A, Postovsky S, Elhasid R, Ilan N, Vlodavsky I, Ben Arush MW. Heparanase levels are elevated in the plasma of pediatric cancer patients and correlate with response to anticancer treatment. Neoplasia. 2007;9:909–916. doi: 10.1593/neo.07673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafat I, Ilan N, Zoabi S, Vlodavsky I, Nakhoul F. Heparanase levels are elevated in the urine and plasma of type 2 diabetes patients and associate with blood glucose levels. PloS one. 2011b;6:e17312. doi: 10.1371/journal.pone.0017312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- Timpl R, Brown JC. Supramolecular assembly of basement membranes. Bioessays. 1996;18:123–132. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- Udo Hacker KNaNP. Heparan sulphate proteoglycans: the sweet side of development. Nat Rev Mol Cell Biol. 2005;6:530–541. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]
- van den Berg BM, Spaan JA, Vink H. Impaired glycocalyx barrier properties contribute to enhanced intimal low-density lipoprotein accumulation at the carotid artery bifurcation in mice. Pflugers Arch. 2009;457:1199–1206. doi: 10.1007/s00424-008-0590-6. [DOI] [PubMed] [Google Scholar]
- van den Hoven MJ, Rops AL, Bakker MA, Aten J, Rutjes N, Roestenberg P, Goldschmeding R, Zcharia E, Vlodavsky I, van der Vlag J, Berden JH. Increased expression of heparanase in overt diabetic nephropathy. Kidney Int. 2006;70:2100–2108. doi: 10.1038/sj.ki.5001985. [DOI] [PubMed] [Google Scholar]
- Vikramadithyan RK, Kako Y, Chen G, Hu Y, Arikawa-Hirasawa E, Yamada Y, Goldberg IJ. Atherosclerosis in perlecan heterozygous mice. J Lipid Res. 2004;45:1806–1812. doi: 10.1194/jlr.M400019-JLR200. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I, Bar-Shavit R, Ishai-Michaeli R, Bashkin P, Fuks Z. Extracellular sequestration and release of fibroblast growth factor: a regulatory mechanism? Trends Biochem Sci. 1991;16:268–271. doi: 10.1016/0968-0004(91)90102-2. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I, Beckhove P, Lerner I, Pisano C, Meirovitz A, Ilan N, Elkin M. Significance of Heparanase in Cancer and Inflammation. Cancer Microenviron. 2012;5:115–132. doi: 10.1007/s12307-011-0082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I, Friedmann Y. Molecular properties and involvement of heparanase in cancer metastasis and angiogenesis. J Clin Invest. 2001;108:341–347. doi: 10.1172/JCI13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I, Friedmann Y, Elkin M, Aingorn H, Atzmon R, Ishai-Michaeli R, Bitan M, Pappo O, Peretz T, Michal I, Spector L, Pecker I. Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nat Med. 1999;5:793–802. doi: 10.1038/10518. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I, Ilan N, Naggi A, Casu B. Heparanase: Structure, Biological Functions, and Inhibition by Heparin-Derived Mimetics of Heparan Sulfate. Curr Pharm, Des. 2007;13:2057–2073. doi: 10.2174/138161207781039742. [DOI] [PubMed] [Google Scholar]
- Vreys V, David G. Mammalian heparanase: what is the message? J Cell Mol Med. 2007;11:427–452. doi: 10.1111/j.1582-4934.2007.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Kim MS, Puthanveetil P, Kewalramani G, Deppe S, Ghosh S, Abrahani A, Rodrigues B. Endothelial heparanase secretion after acute hypoinsulinemia is regulated by glucose and fatty acid. Am J Physiol. 2009;296:H1108–1116. doi: 10.1152/ajpheart.01312.2008. [DOI] [PubMed] [Google Scholar]
- Wang F, Wang Y, Zhang D, Puthanveetil P, Johnson JD, Rodrigues B. Fatty acid-induced nuclear translocation of heparanase uncouples glucose metabolism in endothelial cells. Arterioscler Thromb Vasc Biol. 2012;32:406–414. doi: 10.1161/ATVBAHA.111.240770. [DOI] [PubMed] [Google Scholar]
- Wight TN, Merrilees MJ. Proteoglycans in atherosclerosis and restenosis: key roles for versican. Circulation Res. 2004;94:1158–1167. doi: 10.1161/01.RES.0000126921.29919.51. [DOI] [PubMed] [Google Scholar]
- Yang Y, MacLeod V, Dai Y, Khotskaya-Sample Y, Shriver Z, Venkataraman G, Sasisekharan R, Naggi A, Torri G, Casu B, Vlodavsky I, Suva LJ, Epstein J, Yaccoby S, Shaughnessy JD, Jr, Barlogie B, Sanderson RD. The syndecan-1 heparan sulfate proteoglycan is a viable target for myeloma therapy. Blood. 2007;110:2041–2048. doi: 10.1182/blood-2007-04-082495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Macleod V, Miao HQ, Theus A, Zhan F, Shaughnessy JD, Jr, Sawyer J, Li JP, Zcharia E, Vlodavsky I, Sanderson RD. Heparanase enhances syndecan-1 shedding: A novel mechanism for stimulation of tumor growth and metastasis. J Biol Chem. 2007;282:13326–13333. doi: 10.1074/jbc.M611259200. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ren Y, Ramani VC, Nan L, Suva LJ, Sanderson RD. Heparanase enhances local and systemic osteolysis in multiple myeloma by upregulating the expression and secretion of RANKL. Cancer Res. 2010;70:8329–8338. doi: 10.1158/0008-5472.CAN-10-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zcharia E, Jia J, Zhang X, Baraz L, Lindahl U, Peretz T, Vlodavsky I, Li JP. Newly generated heparanase knock-out mice unravel co-regulation of heparanase and matrix metalloproteinases. PloS one. 2009;4:e5181. doi: 10.1371/journal.pone.0005181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetser A, Bashenko Y, Edovitsky E, Levy-Adam F, Vlodavsky I, Ilan N. Heparanase induces vascular endothelial growth factor expression: correlation with p38 phosphorylation levels and Src activation. Cancer Res. 2006;66:1455–1463. doi: 10.1158/0008-5472.CAN-05-1811. [DOI] [PubMed] [Google Scholar]
- Zhou H, Roy S, Cochran E, Zouaoui R, Chu CL, Duffner J, Zhao G, Smith S, Galcheva-Gargova Z, Karlgren J, Dussault N, Kwan RY, Moy E, Barnes M, Long A, Honan C, Qi YW, Shriver Z, Ganguly T, Schultes B, Venkataraman G, Kishimoto TK. M402, a novel heparan sulfate mimetic, targets multiple pathways implicated in tumor progression and metastasis. PloS one. 2011;6:e21106. doi: 10.1371/journal.pone.0021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziolkowski AF, Popp SK, Freeman C, Parish CR, Simeonovic CJ. Heparan sulfate and heparanase play key roles in mouse beta cell survival and autoimmune diabetes. J Clin Invest. 2012;122:132–141. doi: 10.1172/JCI46177. [DOI] [PMC free article] [PubMed] [Google Scholar]