Abstract
Dlg (Discs Large) is a multidomain protein that interacts with glutamate receptors and potassium channels at Drosophila neuromuscular junctions (NMJs) and at mammalian central nervous system synapses. Dlg also localizes postsynaptically at cholinergic mammalian NMJs. We show here that α-amino-3-hydroxy-5-methylisoxazole-4-proprionate (AMPA) receptor subunits, together with glutamate, are present at the mammalian NMJ. Both AMPA and NMDA (N-methyl-D-aspartate) glutamate receptor subunits display overlapping postsynaptic localization patterns with Dlg at all NMJs examined in normal mice. Kir2 potassium channels also localize with Dlg and glutamate receptors at this synapse. Localization of the components of a glutamatergic system suggests novel mechanisms at mammalian neuromuscular synapses.
Keywords: Dlg/SAP97, AMPA, NMDA, Kir2, neuromuscular junction
Dlg (Discs Large) and its mammalian homologs are members of the membrane-associated guanylate kinase (MAGUK) family of scaffolding proteins that contain multiple protein interaction domains. Drosophila dlg mutant NMJs have mislocalized inward-rectifying potassium channels and functional deficits resulting from a specific decrease of glutamate receptors.5,8 The mammalian ortholog of Dlg (SAP97 in rat; hDlg in human; mDlg in mouse; hereafter referred to as Dlg) interacts with and is capable of regulating the trafficking and synaptic activity of AMPA and NMDA glutamate receptors in experiments conducted in neuronal cultures and hippocampal slices.1,6,12,22,24,27,28,31,33,36,37 Dlg PDZ domains also interact with inward-rectifying potassium channels of the Kir2 family at postsynaptic densities and axons in the mammalian central nervous system (CNS).11,14,21,39 We have recently shown that Dlg is present at the postsynaptic membrane of the NMJ, raising the question of whether any of its interactors at other synapses are present postsynaptically at the mammalian NMJ.35
Mammalian cholinergic neuromuscular junctions (NMJs) have been contrasted with CNS synapses where a diversity of glutamatergic receptors are present.34 However, glutamatergic NMDA receptor subunit 1 (NR1) has been shown to localize to the mammalian NMJ, although its specific subcellular localization was not investigated.2,13,23
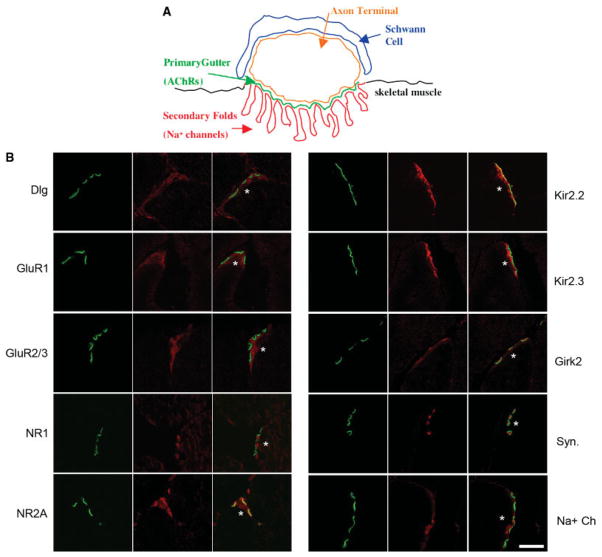
Mature postsynaptic skeletal muscle membranes are topologically complex structures where proteins differentially localize (Fig. 1A) (reviewed in Refs. 16,18). For instance, acetylcholine receptors (AChRs) are concentrated in the primary gutter and Na+ channels are concentrated at the depths of secondary folds.10
FIGURE 1.
Localization of glutamate receptors and potassium channels at NMJs. A: Schematic of the mammalian NMJ shows a Schwann cell (blue), the axon terminal (orange), and the skeletal muscle postsynaptic membrane composed of the primary gutter where AChRs localize (green) and the secondary folds (red) where Na+ channels localize to the bottom of the folds. B: Confocal analysis of immunolocalization at the NMJ, corresponding to the schematic in A, of Dlg and candidate interactors in 5-week-old normal mice. Transverse sections of quadriceps muscles were costained with each antibody (red) and Alexafluor 488 anti-bungarotoxin (green) to show localization of the AChRs located in the primary gutter of the postsynaptic membrane. Merged images are shown in the right panel. An asterisk (*) denotes the postsynaptic side of the NMJ. Antibodies specific for GluR1, GluR2/3, NR1, NR2A, Kir2.2, and Kir2.3 all show specific localization to the postsynaptic side of the NMJ in the skeletal muscle fiber. Girk2 specifically localizes to the presynaptic motor neuron axon terminal. The presynaptic marker synaptophysin (Syn.) and Na+ channels, known to localize to secondary folds of the postsynaptic membrane, are shown as controls. Scale bar = 3.7 μm.
Here we show that NR1 and NR2A NMDA receptor subunits, along with AMPA receptor subunits GluR1 and GluR2/3, specifically localize postsynaptically at the NMJ. Both these glutamate receptors and potassiumchannels of the Kir2 family are not coincident with AChRs, but instead show overlapping localization with Dlg. The localization of glutamate and its receptors at the NMJ suggests the presence of mechanisms that have not yet been elucidated at this synapse.
MATERIALS AND METHODS
Confocal Analysis of Immunolocalization
Quadriceps muscles were removed from C57BL/10 mice and were fixed in 1% paraformaldehyde in potassium phosphate-buffered saline (KPBS) for 1 h, soaked in 20% sucrose in KPBS overnight, embedded in OCT, and frozen on solid-phase liquid-nitrogen-cooled isopentane. Then 8 μm cross-sections were cut, affixed to positively charged slides, and stored at −80°C. Sections were equilibrated in KPBS, washed in 0.1 M glycine in KPBS for 1 h at room temperature, extracted with 0.5% Triton in KPBS for 15 min on ice, washed again in KPBS, and blocked in 1% gelatin in KPBS for 1 h. The sections were then incubated overnight in the following rabbit polyclonal primary antibodies: hdlg at 1:10,000, GluRI total (Chemicon, Temecula, California) at 1:10, GluR 2/3 (Chemicon) at 1:50, NR1 (Chemicon) at 1:50, NR2A (Upstate Biotechnology, Lake Placid, New York) at 1:100, Kir 2.2 (Chemicon) at 1:200, and Kir 2.3 (Chemicon) at 1:200, Girk2 (Chemicon) at 1:50, synaptophysin (Novacastra, Newcastle, UK) at 1:100, Na+Ch (Upstate) at 1:100, and glutamate (Sigma, St. Louis, Missouri) at 1:1,000. The slides were washed three times for 15 min in KPBSG + 0.1% Tween-20 and incubated for 2–3 h in Alexafluor 594 goat antirabbit at 1:1,000+ Alexafluor 488 alpha-bugarotoxin at 1:1,000 (Molecular Probes, Eugene, Oregon) in KPBSG + 1% normal goat serum (Vector Laboratories, Burlingame, California). The slides were washed again in KPBSG +.1% Tween-20 for 3 × 15 min, mounted in Vectashield, and coverslipped. Images were taken using a Zeiss 510 Meta laser scanning microscope through a ×63 objective and ×3 zoom. The confocal analyses were carried out on mice at 5 weeks of age. The data for each antibody are representative of repeated experiments on at least six animals. The specific localization of glutamate receptors at the postsynaptic membrane was confirmed in animals at 12 weeks and 1 year of age.
Colocalization experiments were performed on rat quadriceps sections as above with Alexafluor 488-alpha-bungarotoxin, the rabbit polyclonal antibodies against NR1, GluR2/3, or Na+Ch together with monoclonal dlg antibody (BD-Biosciences-Transduction, Mississauga, Canada). CY3 goat antimouse (preabsorbed to rat) (Jackson Immunoresearch, West Grove, Pennsylvania) and Alexafluor 633 goat antirabbit (Molecular Probes) were used to detect monoclonal and polyclonal primary antibodies, respectively.
Immunolocalization of glutamate for epifluorescence analysis was performed on unfixed frozen sections of mouse quadriceps muscles with the above protocol except for a primary incubation time of 2 h instead of overnight. Images were taken through a ×20 objective on a Nikon Eclipse 800 epifluorescent microscope (Nikon, Tokyo, Japan) using a SPOT-RT slider camera and software (Diagnostic Instruments, Sterling Heights, Michigan).
RESULTS
We used confocal analysis of immunolocalization to investigate the subcellular localization of glutamate receptor subunits at mouse NMJs. We and others have previously established that this technique differentiates between pre- and postsynaptic components at the NMJ.35,40 Immunolocalization with antibodies specific for the GluR1 subunit and the GluR23 subunits of AMPA receptors showed all subunits concentrated specifically at the skeletal muscle postsynaptic membrane of the NMJ (Fig 1). Fluorescently conjugated alpha-bungarotoxin was used to costain each section to detect AChRs in the primary gutter of the postsynaptic membrane (Fig. 1A,B). Na+ channels were used as a marker of postsynaptic secondary folds (Fig. 1A,B)10 and synaptophysin was used as a marker of presynaptic axon terminals (Fig. 1A,B). As previously shown, Dlg is excluded from AChR-containing regions and is localized on the postsynaptic side of AChRs. Antibodies against both GluR1 and GluR2/3 showed a similar postsynaptic localization pattern with respect to AChRs, as did Dlg (Fig. 1B). Antibodies specific for a phosphorylated active form of GluR1 showed the same localization pattern (not shown). AMPA receptor subunits were present postsynaptically at all AChR-stained NMJs in at least six normal 5-week-old mice analyzed. Postsynaptic NMJ localization of AMPA receptors was confirmed in mice at 12 weeks and 1 year of age.
Immunolocalization of the NR1 subunit con-firmed initial reports of NMJ localization of this NMDA receptor subunit and demonstrated that it localizes specifically to the postsynaptic membrane on the skeletal muscle fiber. NR1 and Dlg also showed similar localization with respect to AChRs at all NMJs (Fig. 1B). The NMDA receptor NR2A subunit overlapped and extended below AChRs (Fig. 1B). NMDA receptor subunits localized postsynaptically at all NMJs in all normal mice analyzed at 5 weeks, 3 months, and 1 year of age.
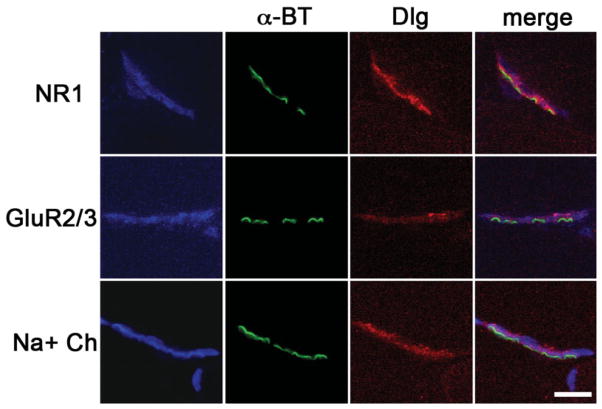
To address the localization of NMDA and AMPA receptors with respect to Dlg, rat skeletal muscle sections were triple-stained with a mouse monoclonal antibody to Dlg, alpha-bungarotoxin, and rabbit polyclonal antibodies to individual glutamate receptor subunits. Dlg showed overlapping localization patterns with NR1 and GluR2/3 (Fig. 2). Additionally, Dlg and Na+ channels showed overlapping, but not identical localization patterns (Fig. 2).
FIGURE 2.
Confocal coimmunofluorescence of AChRs (α-BT) (green) with Dlg (red), and NR1 or GluR2/3 (blue) show overlapping localization of Dlg and glutamate receptors at postsynaptic NMJs in rat. Dlg localization is also shown to overlap with Na+ Channels (blue), known to localize to secondary folds of the postsynaptic membrane. Merged images from the green, red, and blue channels are shown in the last column for each NMJ. Purple represents overlap of Dlg (red) with glutamate receptors (blue). Scale bar = 3.7 μm.
To determine whether the presence of AMPA and NMDA receptors at the postsynaptic NMJ are part of a functional glutamatergic system, we investigated the presence of glutamate in skeletal muscle. We observed that glutamate localizes specifically to AChR-stained NMJs in quadriceps muscle sections (Fig. 3A). Higher-resolution confocal analyses of NMJs confirm the localization of glutamate (Fig. 3B), supporting the hypothesis that all of the components for a glutamatergic system are present at this synapse.
FIGURE 3.
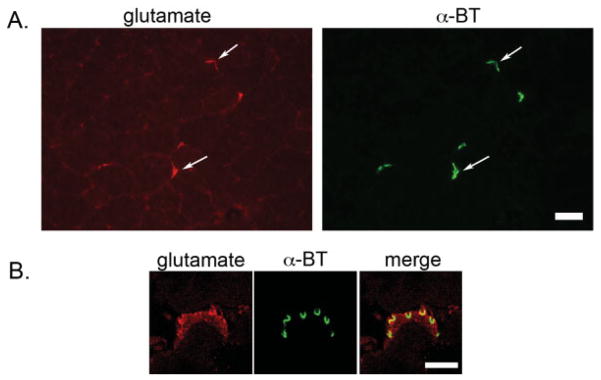
Localization of glutamate at mouse NMJs. A: Epifluorescence images of unfixed quadriceps sections shows localization of glutamate (red) specifically coincident with α-bungarotoxin-stained (α-BT) (green) AChRs at NMJs. Scale bar = 50 μm. B: Confocal images of fixed quadriceps sections show the subcellular localization of glutamate (red) with respect to AChRs (green) at the mammalian NMJ. Scale bar = 3.7 μm.
The Kir2 family of potassium channels has been shown to interact with the rat Dlg homolog, and Kir2.2 has been shown to grossly localize to the NMJ.19,20 We next determined the subcellular localization of Kir2 family members. Kir 2.3 displayed a very specific localization pattern on the postsynaptic side of AChRs coincident with Dlg (Fig. 1). Kir 2.2 displayed a broader postsynaptic localization and was present both coincident with AChRs in the primary gutter and also with Dlg (Fig. 1). Kir 2.1 also colocalized with Dlg (not shown).
To determine whether all Kir families show a similar pattern of localization, we analyzed the localization of the G-protein-coupled inward-rectifying potassium channel Girk2 (Kir3.2) of the Kir3 family, encoded by the Kcnj6 gene. Girk2 was present at the NMJ, but in contrast to the Kir2 family was concentrated at the presynaptic membrane of the motor neuron, coincident with the marker synaptophysin (Fig. 1).
DISCUSSION
Our results for the first time identify AMPA receptor localization at the adult mammalian NMJ. Furthermore, we show that AMPA and NMDA receptor subunits localize to the postsynaptic membrane with Dlg and that glutamate localizes specifically to this synapse in skeletal muscle. The Kir2 family of potassium channels also show a specific localization to the NMJ postsynaptic membrane.
Comparisons with CNS junctions or Drosophila NMJs do not make it clear what function AMPA and NMDA receptors may serve at the mammalian NMJ. In the CNS, AMPA receptors modulate fast synaptic transmission, while NMDA receptors are thought to play a crucial role in long-term potentiation and depression involved in learning and memory. Glutamate receptors are responsible for generating the action potential at Drosophila NMJs where glutamate instead of ACh is the primary neurotransmitter. Separate electrophysiological studies using NMDA to inhibit NMDA receptors at rodent NMJs have contrasting conclusions about the potential role of these receptors at this synapse. Recordings of rat diaphragm muscles bathed in NMDA receptor blockers have shown an inhibition of contractions elicited by indirect electrical stimulation, without altering the ACh part of the contraction cascade.15 A similarly designed study suggests that stimulation of NMDA receptors at the NMJ may modulate non-quantal release of ACh from nerve endings.25 Despite this support for a role of glutamate receptors at the mammalian NMJ, their physiological impact has not been further investigated.
The evidence for a functional glutamatergic system at vertebrate NMJs is growing. A recent report has demonstrated that NMJs in Xenopus embryonic muscles express functional NMDA and AMPA glutamate receptors, which are then replaced by AChRs during maturation of this synapse.3 Accumulation of postsynaptic glutamate receptor subunits and assembly of functional glutamatergic NMJs has been shown to occur after innervation of an adult rat muscle by a glutamate releasing central nonmotor axon.4 Recently, glutamate transporter 1 (GLT) has been shown to localize postsynaptically at NMJs in both rats and mice, while glutamate aspartate transporter (GLAST) has been shown to be restricted to rat NMJs.32 This localization overlaps with our observed localization of the glutamate receptors. The localization of glutamate receptors, glutamate, and glutamate transporters suggest that a functional glutamatergic system is present at the mammalian NMJ. Although glutamate is not the primary neurotransmitter at NMJs, co-release of glutamate could potentially function as part of the safety mechanism at this synapse or could be involved in plasticity such as maintenance of skeletal muscle fiber-type.
Interestingly, knockouts of both NMDA and AMPA receptor subunits have exhibited some phenotypes that are similar to those typically associated with neuromuscular disorders. Mice with a knockout of the AMPA subunit GluR2 are small and hunched, and die within 3 weeks of birth, similar to the utrophin/dystrophin-deficient mouse model of muscular dystrophy.9,30 GluR2/R3 double knockout mice show reduced locomotor activity, despite the normal appearance of synaptic structures in the brain.26 Mice with targeted point mutations in the gene for NR1 die from respiratory failure within an hour of birth, similar to many neuromuscular mutants.38 Transgenic expression of a GluR2 subunit with increased Ca2+ permeability causes adult-onset motor neuron disease.17 All analysis of all glutamate receptor mutant mice focused on the brain and no analysis of muscle or NMJs was conducted to investigate whether neuromuscular involvement contributed to the phenotypes.
It is also unclear what differential specific functions the multitude of inward-rectifying potassium channels may play at the postsynaptic membrane. A critical functional role of the Kir2 family in skeletal muscle is emphasized by Kir 2.1 dominant negative mutations (KCNJ2 gene) that lead to Andersen’s syndrome, characterized by periodic paralysis, a common feature of other skeletal muscle channelopathies.29 Possible mammalian in vivo interactions between Kir 2 family members and Dlg are suggested by a mouse knockout of Kir2.1 that displays the same features of cleft palate and neonatal lethality as the Dlg null mutant.7,41
The current study suggests an as-yet undefined contribution of glutamate receptors in mammalian muscle. Whether Dlg plays a crucial role in the localization or function of these receptors or channels remains unknown. Ultimately, the roles of glutamate receptors and Dlg at the mammalian NMJ can only be further delineated when comprehensive and time-consuming structural and functional analyses of skeletal muscle-specific conditional knockouts of these genes are carried out.
Acknowledgments
The authors thank the Campus Microscopy and Imaging Facility and Kathy Wolken for use of the confocal microscope, and Jonathan Edwards and Chad Groer for cutting sections. This work was supported by NIH grants R01AR047034 (to J.R.F.) and R01CA094414 (to A.H.C.).
Abbreviations
- AChR
acetylcholine receptor
- AMPA
α-amino-3-hydroxy-5-methylisoxazole-4-propionate
- CASK
Ca+, calmodulin associated serine/threonine kinase
- Dlg
discs large
- MAGUK
membrane-associated guanylate kinase
- NMDA
N-methyl-D-aspartate
- NMJ
neuromuscular junction
- PDZ
PSD-95, Dlg, Zo-1
References
- 1.Bassand P, Bernard A, Rafiki A, Gayet D, Khrestchatisky M. Differential interaction of the tSXV motifs of the NR1 and NR2A NMDA receptor subunits with PSD-95 and SAP97. Eur J Neurosci. 1999;11:2031–2043. doi: 10.1046/j.1460-9568.1999.00611.x. [DOI] [PubMed] [Google Scholar]
- 2.Berger UV, Carter RE, Coyle JT. The immunocytochemical localization of N-acetylaspartyl glutamate, its hydrolysing enzyme NAALADase, and the NMDAR-1 receptor at a vertebrate neuromuscular junction. Neuroscience. 1995;64:847–850. doi: 10.1016/0306-4522(95)92578-8. [DOI] [PubMed] [Google Scholar]
- 3.Borodinsky LN, Spitzer NC. Activity-dependent neurotransmitter-receptor matching at the neuromuscular junction. Proc Natl Acad Sci U S A. 2007;104:335–340. doi: 10.1073/pnas.0607450104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunelli G, Spano P, Barlati S, Guarneri B, Barbon A, Bresciani R, et al. Glutamatergic reinnervation through peripheral nerve graft dictates assembly of glutamatergic synapses at rat skeletal muscle. Proc Natl Acad Sci U S A. 2005;102:8752–8757. doi: 10.1073/pnas.0500530102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budnik V, Koh Y, Guan B, Hartmann B, Hough C, Woods D, et al. Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron. 1996;17:627–640. doi: 10.1016/s0896-6273(00)80196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai C, Coleman SK, Niemi K, Keinanen K. Selective binding of synapse-associated protein 97 to GluR-A alpha-amino-5-hydroxy-3-methyl-4-isoxazole propionate receptor subunit is determined by a novel sequence motif. J Biol Chem. 2002;277:31484–31490. doi: 10.1074/jbc.M204354200. [DOI] [PubMed] [Google Scholar]
- 7.Caruana G, Bernstein A. Craniofacial dysmorphogenesis including cleft palate in mice with an insertional mutation in the discs large gene. Mol Cell Biol. 2001;21:1475–1483. doi: 10.1128/MCB.21.5.1475-1483.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen K, Featherstone DE. Discs-large (DLG) is clustered by presynaptic innervation and regulates postsynaptic glutamate receptor subunit composition in Drosophila. BMC Biol. 2005;3:1. doi: 10.1186/1741-7007-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldmeyer D, Kask K, Brusa R, Kornau HC, Kolhekar R, Rozov A, et al. Neurological dysfunctions in mice expressing different levels of the Q/R site-unedited AMPAR subunit GluR-B. Nat Neurosci. 1999;2:57– 64. doi: 10.1038/4561. [DOI] [PubMed] [Google Scholar]
- 10.Flucher BE, Daniels MP. Distribution of Na+ channels and ankyrin in neuromuscular junctions is complementary to that of acetylcholine receptors and the 43 kd protein. Neuron. 1989;3:163–175. doi: 10.1016/0896-6273(89)90029-9. [DOI] [PubMed] [Google Scholar]
- 11.Garcia RA, Vasudevan K, Buonanno A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proc Natl Acad Sci U S A. 2000;97:3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardoni F, Mauceri D, Fiorentini C, Bellone C, Missale C, Cattabeni F, et al. CaMKII-dependent phosphorylation regulates SAP97/NR2A interaction. J Biol Chem. 2003;278:44745–44752. doi: 10.1074/jbc.M303576200. [DOI] [PubMed] [Google Scholar]
- 13.Grozdanovic Z, Gossrau R. Co-localization of nitric oxide synthase I (NOS I) and NMDA receptor subunit 1 (NMDAR-1) at the neuromuscular junction in rat and mouse skeletal muscle. Cell Tissue Res. 1998;291:57–63. doi: 10.1007/s004410050979. [DOI] [PubMed] [Google Scholar]
- 14.Huang YZ, Wang Q, Won S, Luo ZG, Xiong WC, Mei L. Compartmentalized NRG signaling and PDZ domain-containing proteins in synapse structure and function. Int J Dev Neurosci. 2002;20:173–185. doi: 10.1016/s0736-5748(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 15.Koyuncuoglu H, Kara I, Gunel MA, Nurten A, Yamanturk P. N-methyl-D-aspartate antagonists, glutamate release inhibitors, 4-aminopyridine at neuromuscular transmission. Pharmacol Res. 1998;37:485–491. doi: 10.1006/phrs.1998.0318. [DOI] [PubMed] [Google Scholar]
- 16.Kummer TT, Misgeld T, Sanes JR. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr Opin Neurobiol. 2006;16:74–82. doi: 10.1016/j.conb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Kuner R, Groom AJ, Bresink I, Kornau HC, Stefovska V, Muller G, et al. Late-onset motoneuron disease caused by a functionally modified AMPA receptor subunit. Proc Natl Acad Sci U S A. 2005;102:5826–5831. doi: 10.1073/pnas.0501316102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai KO, Ip NY. Postsynaptic signaling of new players at the neuromuscular junction. J Neurocytol. 2003;32:727–741. doi: 10.1023/B:NEUR.0000020620.62318.01. [DOI] [PubMed] [Google Scholar]
- 19.Leonoudakis D, Conti LR, Anderson S, Radeke CM, McGuire LM, Adams ME, et al. Protein trafficking and anchoring complexes revealed by proteomic analysis of inward rectifier potassium channel (Kir2. x)-associated proteins. J Biol Chem. 2004;279:22331–22346. doi: 10.1074/jbc.M400285200. [DOI] [PubMed] [Google Scholar]
- 20.Leonoudakis D, Conti LR, Radeke CM, McGuire LM, Vandenberg CA. A multiprotein trafficking complex composed of SAP97, CASK, Veli, and Mint1 is associated with inward rectifier Kir2 potassium channels. J Biol Chem. 2004;279:19051–19063. doi: 10.1074/jbc.M400284200. [DOI] [PubMed] [Google Scholar]
- 21.Leonoudakis D, Mailliard W, Wingerd K, Clegg D, Vandenberg C. Inward rectifier potassium channel Kir2. 2 is associated with synapse-associated protein SAP97. J Cell Sci. 2001;114:987–998. doi: 10.1242/jcs.114.5.987. [DOI] [PubMed] [Google Scholar]
- 22.Lin L, Sahr K, Chishti A. Identification of the mouse homologue of human discs large and rat SAP97 genes. Biochim Biophys Acta. 1997;1362:1–5. doi: 10.1016/s0925-4439(97)00059-8. [DOI] [PubMed] [Google Scholar]
- 23.Luck G, Hoch W, Hopf C, Blottner D. Nitric oxide synthase (NOS-1) coclustered with agrin-induced AChR-specializations on cultured skeletal myotubes. Mol Cell Neurosci. 2000;16:269–281. doi: 10.1006/mcne.2000.0873. [DOI] [PubMed] [Google Scholar]
- 24.Lue R, Marfatia S, Branton D, Chishti A. Cloning and characterization of hdlg: the human homologue of the Drosophila discs large tumor suppressor binds to protein 4. 1. Proc Natl Acad Sci U S A. 1994;91:9818–9822. doi: 10.1073/pnas.91.21.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malomouzh AI, Nikolsky EE, Lieberman EM, Sherman JA, Lubischer JL, Grossfeld RM, et al. Effect of N-acetylaspartyl-glutamate (NAAG) on non-quantal and spontaneous quantal release of acetylcholine at the neuromuscular synapse of rat. J Neurochem. 2005;94:257–267. doi: 10.1111/j.1471-4159.2005.03194.x. [DOI] [PubMed] [Google Scholar]
- 26.Meng Y, Zhang Y, Jia Z. Synaptic transmission and plasticity in the absence of AMPA glutamate receptor GluR2 and GluR3. Neuron. 2003;39:163–176. doi: 10.1016/s0896-6273(03)00368-4. [DOI] [PubMed] [Google Scholar]
- 27.Muller B, Kistner U, Veh R, Cases-Langhoff C, Becker B, Gundelfinger E, et al. Molecular characterization and spatial distribution of SAP97, a novel presynaptic protein homologous to SAP90 and the Drosophila discs-large tumor suppressor protein. J Neuroscience. 1995;15:2354–2366. doi: 10.1523/JNEUROSCI.15-03-02354.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakagawa T, Futai K, Lashuel HA, Lo I, Okamoto K, Walz T, et al. Quaternary structure, protein dynamics, and synaptic function of SAP97 controlled by L27 domain interactions. Neuron. 2004;44:453–467. doi: 10.1016/j.neuron.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Plaster NM, Tawil R, Tristani-Firouzi M, Canun S, Bendahhou S, Tsunoda A, et al. Mutations in Kir2. 1 cause the developmental and episodic electrical phenotypes of Andersen’s syndrome. Cell. 2001;105:511–519. doi: 10.1016/s0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]
- 30.Rafael JA, Brown SC. Dystrophin and utrophin: genetic analyses of their role in skeletal muscle. Microsc Res Tech. 2000;48:155–166. doi: 10.1002/(SICI)1097-0029(20000201/15)48:3/4<155::AID-JEMT4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Regalado MP, Terry-Lorenzo RT, Waites CL, Garner CC, Malenka RC. Transsynaptic signaling by postsynaptic synapse-associated protein 97. J Neurosci. 2006;26:2343–2357. doi: 10.1523/JNEUROSCI.5247-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rinholm JE, Slettalokken G, Marcaggi P, Skare O, Storm-Mathisen J, Bergersen LH. Subcellular localization of the glutamate transporters GLAST and GLT at the neuromuscular junction in rodents. Neuroscience. 2007;145:579–591. doi: 10.1016/j.neuroscience.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 33.Rumbaugh G, Sia GM, Garner CC, Huganir RL. Synapse-associated protein-97 isoform-specific regulation of surface AMPA receptors and synaptic function in cultured neurons. J Neurosci. 2003;23:4567–4576. doi: 10.1523/JNEUROSCI.23-11-04567.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 35.Sanford JL, Mays TA, Rafael-Fortney JA. CASK and Dlg form a PDZ protein complex at the mammalian neuromuscular junction. Muscle Nerve. 2004;30:164–171. doi: 10.1002/mus.20073. [DOI] [PubMed] [Google Scholar]
- 36.Sans N, Racca C, Petralia RS, Wang YX, McCallum J, Wenthold RJ. Synapse-associated protein 97 selectively associates with a subset of AMPA receptors early in their biosynthetic pathway. J Neurosci. 2001;21:7506–7516. doi: 10.1523/JNEUROSCI.21-19-07506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schluter OM, Xu W, Malenka RC. Alternative N-terminal domains of PSD-95 and SAP97 govern activity-dependent regulation of synaptic AMPA receptor function. Neuron. 2006;51:99–111. doi: 10.1016/j.neuron.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 38.Single FN, Rozov A, Burnashev N, Zimmermann F, Hanley DF, Forrest D, et al. Dysfunctions in mice by NMDA receptor point mutations NR1(N598Q) and NR1(N598R) J Neurosci. 2000;20:2558–2566. doi: 10.1523/JNEUROSCI.20-07-02558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tiffany AM, Manganas LN, Kim E, Hsueh YP, Sheng M, Trimmer JS. PSD-95 and SAP97 exhibit distinct mechanisms for regulating K(+) channel surface expression and clustering. J Cell Biol. 2000;148:147–158. doi: 10.1083/jcb.148.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trinidad JC, Fischbach GD, Cohen JB. The Agrin/MuSK signaling pathway is spatially segregated from the neuregulin/ErbB receptor signaling pathway at the neuromuscular junction. J Neurosci. 2000;20:8762–8770. doi: 10.1523/JNEUROSCI.20-23-08762.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaritsky JJ, Eckman DM, Wellman GC, Nelson MT, Schwarz TL. Targeted disruption of Kir2.1 and Kir2. 2 genes reveals the essential role of the inwardly rectifying K(+) current in K(+)-mediated vasodilation. Circ Res. 2000;87:160–166. doi: 10.1161/01.res.87.2.160. [DOI] [PubMed] [Google Scholar]