Abstract
The resistance of malaria parasites to current anti-malarial drugs is an issue of major concern globally. Recently we identified a Plasmodium falciparum cell membrane aspartyl protease, which binds to erythrocyte band 3, and is involved in merozoite invasion. Here we report the complete primary structure of P. falciparum signal peptide peptidase (PfSPP), and demonstrate that it is essential for parasite invasion and growth in human erythrocytes. Gene silencing suggests that PfSPP may be essential for parasite survival in human erythrocytes. Remarkably, mammalian signal peptide peptidase inhibitors (Z-LL)2-ketone and L-685,458 effectively inhibited malaria parasite invasion as well as growth in human erythrocytes. In contrast, DAPT, an inhibitor of a related γ-secretase/presenilin-1, was ineffective. Thus, SPP inhibitors specific for PfSPP may function as potent anti-malarial drugs against the blood stage malaria.
Keywords: Malaria, Plasmodium falciparum, Signal peptide peptidase, Intramembrane aspartyl protease, Erythrocyte, Band 3, Presenilins
Malaria is one of the most common infectious diseases with enormous public health significance [1]. Because of emerging drug resistance, there is an urgent need to identify new drug targets against malaria. Malaria parasite proteases have long been considered potential therapeutic targets. A variety of proteases, such as aspartic, cysteine, and serine proteases have been evaluated as drug targets [2–5]. Two Plasmodium falciparum rhomboid intramembrane serine proteases, termed PfROM1 and PfROM4, have been identified in the micronemes and merozoite surface, and both can cleave a number of transmembrane adhesins implicated in merozoite invasion [6,7]. Despite these extensive efforts, the drug development efforts targeting parasite proteases have not been successful mainly because no single protease unique to malaria parasite has been characterized as absolutely essential for parasite survival in erythrocytes. The signal peptide peptidase (SPP) belongs to a family of intramembrane cleaving proteases, which include the rhomboid-type serine proteases, site-2-protease (S2P) family of putative metalloproteases, and γ-secretase complex [8–11].
A single SPP gene exists in the malaria parasite genome. Originally, we identified this gene as a hypothetical protein clone in a yeast two hybrid screen of malaria proteins interacting with an exofacial loop of erythrocyte receptor, band 3 [12,13]. Another study chemically synthesized the malaria SPP gene and expressed it in mammalian cells [14]. In the present study, we provide evidence that PfSPP is a highly conserved gene and plays an essential role in parasite invasion as well as growth in human erythrocytes. Gene disruption attempts of PfSPP suggest that this enzyme may be lethal at the blood stage of parasite development, and selective SPP inhibitors completely block merozoite invasion and growth. These results suggest a critical role of PfSPP in malaria parasite life cycle, and identify the intramembrane aspartyl protease as a potential drug target against malaria across species.
Materials and methods
Materials
Plasmodium falciparum strains 3D7, 7G8, Dd2, HB3, K1, and FCR3 were obtained from MR4. The γ-secretase/presenilin- 1 inhibitors DAPT and L-685,458 were gifts from Dr. S. Sisodia of the University of Chicago. (Z-LL)2-ketone inhibitor was purchased from Calbiochem/EMD Biosciences. Plasma samples were obtained from 10 healthy female adults (mean age 38 years, range 28–51 years) living in the rural village of Kambila, Mali where transmission of P. falciparum is seasonal and intense [15]. Three of the 10 subjects were infected with low levels of P. falciparum (range 75–575 asexual parasites/μl of blood) at the time of plasma collection.
Total RNA preparation and PfSPP RT-PCR
Total RNA from 6 strains of P. falciparum (3D7, 7G8, Dd2, HB3, K1, and FCR3) was isolated, and primers corresponding to PfSPP sequence (Gene ID: PF14_0543) were designed: 5′-GCCGGATCCATGAATTTATTAAAATT AATT-3′ and 5′-GCCGTCGACTCATTTATTGGTAATTCTTT-3′. Exons encoding PfSPP-exofacial loop were amplified from genomic DNA extracted from 64 blood samples of malaria patients attending the Albert Schweitzer Hospital in Lambaréné, using the primers F:ACAGTCTGGTTTGTTTGTATATGA and R:CTGGTATAATAATAT CTCCTAAACCAAGC. The PCR products were sequenced with the primers ATACATATTAATTGTTCTTGTT and TTGAAGCTCCAGTAAA ATTG. The sequences were analysed for polymorphisms using the BioEdit alignment program (North Carolina State University).
Gene disruption of PfSPP
To disrupt the PfSPP gene in 3D7 strain, 5′ and 3′ segments of PfSPP gene were cloned into the P. falciparum transfection plasmid pCC-1. The 5′ segment (616 bp) was PCR amplified from the genomic DNA (3D7) using primers 5′-GGCTTCCGCGGATGAATTTATTAAAATTAAT-3′ and 5′-TACAGCTTAAGAGTAAGCAAAGCTGCAGATC, and it was cloned into the SacII and AflII sites of pCC-1 upstream of the hDHFR cassette. The 3′ segment (711 bp) of PfSPP was amplified using the primers 5′-GCCGAATTCTCTGGTTTGTTTGTATATG-3′ and 5′-GCCGAATTCTCATTTA TTGGTAATTCTTT-3′, and cloned downstream of the hDHFR cassette. Ring-stage parasites were transfected with 100 μg of pCC-1ΔPfSPP plasmid in a 0.2-cm cuvette using a Gene Pulser (Bio-Rad) at 0.31 kV, 950 μF, with a maximum resistance. WR99210 (5 nM) was added 48 h after the electroporation, and maintained thereafter.
Results
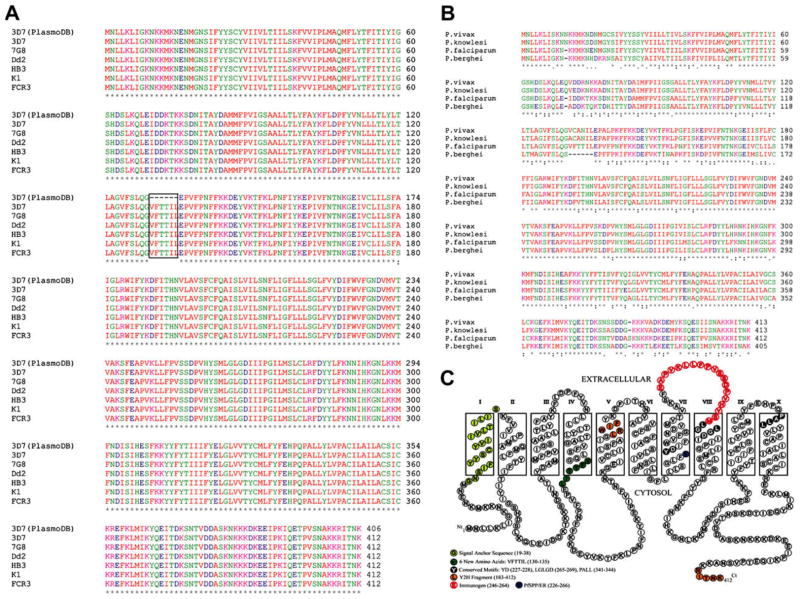
To investigate the sequence conservation of PfSPP in various parasite strains, we sequenced cDNAs amplified from 6 strains of P. falciparum (3D7, 7G8, Dd2, HB3, K1, and FCR3). No size variation was observed in the RT-PCR fragments of 6 parasite strains. PCR products were either sequenced directly or cloned in the pMAL-p2X vector for subsequent sequencing and expression. The complete cDNA sequence of PfSPP comprises 1239 bp encoding 412 amino acids with a predicted molecular mass of 47.6 kDa and an isoelectric point of 8.83. The total AT content of the coding region is 72.5%. The deduced amino acid sequence of PfSPP is highly conserved among 6 strains with only a single amino acid change from alanine to serine at position 180 in the FCR3 strain (Fig. 1A). Importantly, we found an additional 6 amino acid residues (VFTTIL) after glycine-129 of PfSPP in all 6 parasite strains (Fig. 1A), as compared to the published PfSPP amino acid sequence in the PlasmoDB (3D7 strain, PF14_0543). The PfSPP gene consists of 9 exons and 8 introns. The first 18 nucleotides of the 4th intron were incorrectly predicted and not included in the 4th exon of PfSPP gene leading to the publication of incorrect primary structure of PfSPP in the PlasmoDB. It is noteworthy that a previous study has chemically synthesized the PfSPP gene based on the published sequence in the PlasmoDB, and expressed the synthetic gene in mammalian cells to measure its protease activity [14]. Therefore, in future studies, it would be essential to measure the enzyme activity of full length PfSPP protein to gain further functional insights into the protease activity of the native PfSPP protein.
Fig. 1.
Parasite SPP sequences and topology model. (A) Alignment of 6 strains of PfSPP was performed using ClustalW2. The 3D7 sequence was taken from the PlasmoDB (Gene ID: PF14_0543). Only one amino acid difference was found in the FCR3 strain (180, A→S). (B) SPP alignment. (C) Topology model of PfSPP by ConPred II. Signal-anchor sequence (19–38); two active site motifs YD (227–228) and LGLGD (265–269); and the PALL (341–344) motif are indicated.
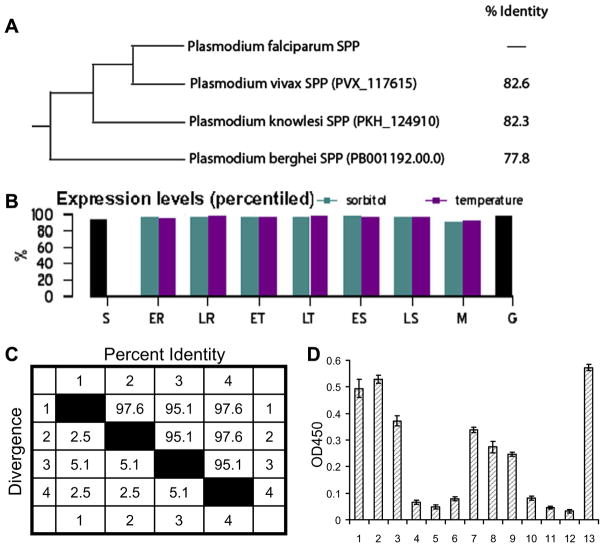
To examine whether the PfSPP sequence is conserved across malaria parasite species, the PfSPP sequence of 3D7 strain (FJ644538) was compared with its counterparts in P. vivax strain SaI-1 (PVX_117615), P. knowlesi strain H (PKH_124910), and P. berghei strain ANKA (PB001192.00.0) in the database (Fig. 1B). Sequence alignment of the PfSPP revealed 82.6% homology with human P. vivax, 82.3% homology with monkey P. knowlesi, and 77.8% homology with mouse P. berghei (Fig. 2B). Notably, one additional Ser-384 residue was found only in the P. falciparum SPP sequence whereas Asn-11 and Gln-71 residues were found only in the P. vivax and P. knowlesi SPPs but not in the P. falciparum and P. berghei SPPs (Fig. 1B). It is noteworthy that more recent software correctly predicted the 6 amino acid sequence between Ser-129 and Glu-130 in the SPPs of P. vivax and P. knowlesi but not in the P. berghei.
Fig. 2.
PfSPP antibodies in malaria patients. (A) Phylogenetic tree of malaria SPPs. (B) PfSPP-3D7 expression in the PlasmoDB. ER, early rings; LR, late rings; ET, early trophozoites; LT, late trophozoites; ES, early schizonts; LS, late schizonts; M, merozoites; S, sporozoites; G, gametozoites. (C) PfSPP-exofacial loop (AA: 226–266) sequence distances for P. falciparum (1), P. vivax (2), P. berghei (3), and P. knowlesi (4). (D) Antibodies against PfSPP-exofacial loop. Samples 1–10 indicate malaria patients’ plasma; samples 11–12 indicate two donors never exposed to malaria; and sample 13 indicates the rabbit anti-PfSPP/ER serum.
Recently, a new program termed ConPred II was developed based on a consensus approach of methods including KKD, TMpred, TopPred II, DAS, TMAP, MEMSAT 1.8, SOSUI, TMHMM 2.0, and HMMTOP 2.0. The prediction accuracy of ConPred II is relatively high (~99%), thus improving the TM topology accuracy by ~11% over other methods [16]. Using ConPred II, the complete PfSPP amino acid is predicted to have 10 TM domains with preference for a cytosolic orientation of both the N- and the C-termini (Fig. 1C). Two intramembrane active site motifs YD and LGLGD are located in the center of TM7 and TM8, respectively, an orientation that is consistent with the active site motifs of human SPP. The conserved PALL motif near the C-terminus of PfSPP is located at the boundary of TM10 region. Thus, the PfSPP model suggests that the malaria enzyme has characteristics similar to signal peptide peptidase but not of presenilins. No signal peptide is predicted in the PfSPP, but an uncleaved signal-anchor sequence (Gly19–Ser38) is predicted by SignalP3.0. The newly identified 6 amino acid insert of PfSPP is predicted to be located at the membrane-cytosol interface of TM4 (Fig. 1C). Because of the high sequence identity between SPPs of various malaria species (Fig. 2A), it is likely that all parasite SPPs will conform to the same topology model as predicted for PfSPP.
In the PlasmoDB, the absolute transcription expression level of the intraerythrocytic PfSPP is relatively high at the trophozoite and schizont stage of development. In comparison, the percentile expression of PfSPP is high (>90%) at all intraerythrocytic stages including the rings, trophozoites, and schizonts (Fig. 2B). The PfSPP gene is also expressed in the gametocytes and sporozoites (Fig. 2B). Evidence from mass spectrometry has confirmed the expression of PfSPP in the merozoites. We have previously localized PfSPP in the micronemes and on the surface of merozoites with gold particles occasionally visible in the interior of the merozoite [13]. Together, these observations suggest a dynamic nature of the PfSPP polypeptide trafficking between various cellular compartments in infected erythrocytes.
We have shown that a major 41 amino acid ectoplasmic loop of PfSPP, termed PfSPP/ER (226–266), binds to human erythrocyte band 3 [13]. The putative surface exposed PfSPP/ER region is highly conserved with the P. falciparum sequence showing 97.6% identity with P. vivax and P. knowlesi, and 95.1% identity with P. berghei (Fig. 2C). To investigate the conservation of PfSPP/ER in malaria patients, parasite genomic DNA was isolated from 64 blood samples of field isolates, and exons encoding the PfSPP/ER region were amplified. Direct sequencing of the PCR products revealed only a single synonymous mutation in the codon of serine-256 changing the codon from TCG to TCC in seven field isolates. These results indicate that PfSPP/ER exofacial loop is highly conserved, and non-synonymous mutations are not tolerated within this part of the gene. To determine if the antibodies against PfSPP/ER region exist in malaria patients, recombinant PfSPP/ER region was expressed as a fusion to the maltose binding protein [13]. The immobilized fusion protein was used to detect serum antibodies against PfSPP in 10 malaria patients living in the malaria endemic areas. An ELISA screen revealed high plasma reactivity in 6 malaria patients and low plasma reactivity in 3 patients; and the response was specific as little plasma reactivity was observed in individuals who had never been exposed to malaria (Fig. 2D). These results suggest that the PfSPP/ER region is highly conserved, and is exposed to the immune system at some stage of malaria infection.
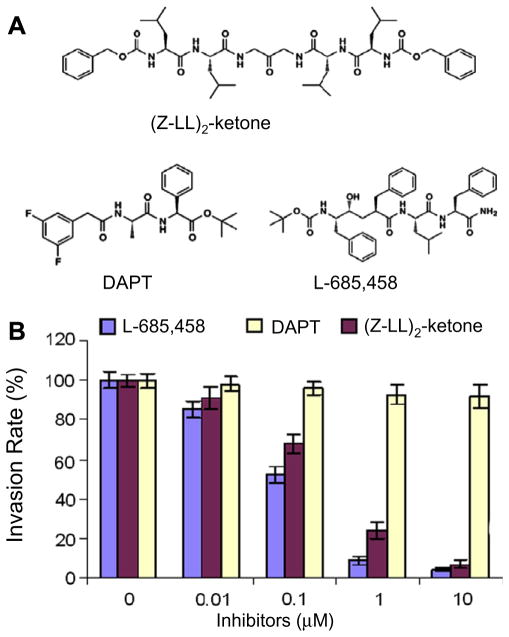
To investigate the role of PfSPP in parasite invasion, we used a chemical approach by employing three synthetic inhibitors against mammalian membrane aspartyl proteases. The (Z-LL)2-ketone is a specific inhibitor of mammalian SPP, whereas L-685,458 and DAPT inhibitors are relatively more specific for the γ-secretase/Presenilin-1 complex (Fig. 3A). The (Z-LL)2-ketone does not inhibit the γ-secretase/Presenilin-1 activity in live mammalian cells up to a concentration of 100 μM[17]. In contrast, L-685,458 inhibited both SPP and γ-secretase/Presenilin-1, whereas the DAPT had no effect on SPP activity at 100 μM [17]. A previous study demonstrated in vitro enzyme activity of a mutant version of PfSPP lacking the 6 amino acids was inhibited by the SPP inhibitor, (Z-LL)2-ketone, and the γ-secretase/PS1 inhibitors [14]. We incubated human erythrocytes with highly synchronized P. falciparum schizonts in the presence of increasing concentrations of (Z-LL)2-ketone, L-685,458, and DAPT inhibitors. After 20 h of incubation, the rings were counted from the inhibitor and DMSO-treated samples. The DAPT inhibitor had no effect on parasite invasion, whereas (Z-LL)2-ketone and L-685,458 caused a significant decrease in the number of new rings in a dose-dependent manner (Fig. 3B). Parasite cultures incubated with 10 μM of (Z-LL)2-ketone and L-685,458 resulted in >95% inhibition of new ring formation with no accumulation of the schizonts (Fig. 3C-Supplementary). These results suggest that the enzyme activity of PfSPP is essential for efficient merozoite invasion of human erythrocytes.
Fig. 3.
Effect of SPP inhibitors on parasite invasion. (A) Structures of inhibitors. (B) P. falciparum at the schizont stage treated with inhibitors for 20 h. (C) The Giemsastained smears were made after 20 h of inhibitor treatment at 10 μM.
Next we examined the effect of 3 inhibitors on malaria parasite growth in human erythrocytes. Synchronized ring stage parasites were incubated with inhibitors in the presence of 0.2% DMSO. Both (Z-LL)2-ketone and L-685,458 caused a significant inhibition of parasite growth in erythrocytes, whereas DAPT had no effect (Fig. 4A-Supplementary). A complete inhibition of parasite replication was observed at 10 μM of (Z-LL)2-ketone and L-685,458 with no detection of the ring stage parasites after one cycle of asexual multiplication (Fig. 4B). The precipitous decline in parasitemia presumably originated by the inhibition of late rings or early trophozoites, as pyknotic parasites were observed after 24–48 h of culture in the presence of (Z-LL)2-ketone and L-685,458 (Fig. 4). The destruction of the inhibitor-treated parasites was irreversible as no progression to the second asexual cycle was observed. In contrast, DAPT had little or no effect on parasite growth as compared to the DMSO-treated control (Fig. 4A-Supplementary). Both (Z-LL)2-ketone and L-685,458 inhibited 3D7 parasite growth in a dose-dependent manner with IC50 values of 0.985 and 0.174 μM, respectively (Fig. 4B). Since the primary structure of PfSPP is highly conserved, we tested the effect of these inhibitors on the growth of other parasite strains. The parasite strains included two chloroquine-resistant P. falciparum strains (7G8, Dd2), one chloroquine-sensitive strain (HB3), and one mildly chloroquine-resistant strain (FCR3). The parasite growth was evaluated in 4 strains by measuring the [3H]-hypoxanthine incorporation starting at 24 h post-invasion. Both (Z-LL)2-ketone and L-685,458 inhibited parasite growth in all 4 parasite strains with the IC50 values similar to 3D7 strain, while the DAPT had no effect on any strain (Table 1).
Fig. 4.
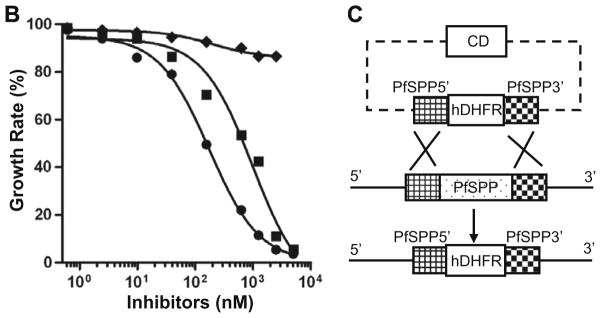
Effect of SPP inhibitors on parasite growth. (A) Blood smears of ring-stage parasites were made in the presence of inhibitors at 10 μM in 0.2% DMSO. (B) (Z-LL)2-ketone (■), L-685,458 (●), and DAPT (◆). The IC50 values of (Z-LL)2-ketone and L-685,458 for live parasites are 984.9 and 173.5 nM, respectively. (C) Disruption of the PfSPP gene in P. falciparum (3D7 strain). PfSPP gene disruption via double crossover mediated homologous recombination between the chromosomal PfSPP locus and the knockout plasmid pCC-1ΔPfSPP. Cytosine deaminase (CD) cassette was used for negative selection. PfSPP5′ and PfSPP3′ represent 5′ region (616 bp) and 3′ region (711 bp) of the PfSPP gene, respectively. Dashed lines represent bacterial vector sequences. Bold lines represent untranslated regions of the target PfSPP gene.
Table 1.
Inhibition of P. falciparum growth by PfSPP inhibitors as measured by [3H]- hypoxanthine incorporation assay.
| Parasite strain | IC50 ± SE (μM)
|
||
|---|---|---|---|
| (Z-LL)2-ketone | L-685,458 | DAPT | |
| Dd2 | 1.08 ± 0.07 | 0.18 ± 0.03 | >10 |
| 7G8 | 1.12 ± 0.05 | 0.21 ± 0.04 | >10 |
| FCR3 | 1.23 ± 0.04 | 0.23 ± 0.03 | >10 |
| HB3 | 1.06 ± 0.05 | 0.19 ± 0.02 | >10 |
Each assay was repeated at least two times. The IC50values are means ± standard errors (SE) of the mean for experiments run in triplicate.
To examine if the single PfSPP gene in the malaria genome is essential for parasite development, we attempted to genetically knockout the PfSPP gene in P. falciparum (Fig. 4C). To disrupt the PfSPP gene in 3D7 strain, the 5′ and 3′ segments of PfSPP gene were cloned into the P. falciparum transfection plasmid pCC-1 to generate the pCC-1ΔPfSPP vector. The 5′ segment (616 bp) of PfSPP was amplified from parasite genomic DNA (3D7) and cloned upstream of the hDHFR resistance cassette. The 3′ segment (711 bp) of PfSPP was cloned downstream of the hDHFR cassette. To disrupt the PfSPP gene, ring-stage parasites were transfected and viable parasites were monitored in the presence of 5 nM of WR99210. No live parasites were observed for up to 35 days in the presence of WR99210. Multiple attempts to disrupt the PfSPP gene were unsuccessful, suggesting an essential role of this protease in the erythrocytic life cycle of malaria parasite. Together with our chemical inhibition data, we conclude that PfSPP is indispensible to malaria parasite survival in human erythrocytes.
Discussion
Our initial characterization of the PfSPP clone revealed a single ~47 kDa protein that was associated with membrane-vesicles in the infected erythrocytes [13]. Our original Y2H clone encoded the C-terminal half of PfSPP [13]. We assembled the full length amino acid sequence of PfSPP by combining our Y2H clone sequence with the N-terminal sequence of PfSPP available in the PlasmodDB [13]. While investigating the conservation of PfSPP, we noticed that the cDNAs from the corresponding mRNAs of 6 parasite strains encoded an additional 6-amino acid peptide (Fig. 1). This newly identified peptide, VFTTIL, is located within the N-terminal half of PfSPP and is present in all 6 strains (Fig. 1A). The VFTTIL peptide is predicted to be located at the membrane- cytoplasmic interface of PfSPP (Fig. 1C). To investigate if any part of PfSPP is exposed to the immune system during malaria infection, we first analyzed the exofacial loop of PfSPP because of the ease of its expression in bacteria and its high sequence conservation in various strains (Fig. 2C). Using PfSPP-exofacial loop, we developed an ELISA and optimized it for human plasma antibodies. An initial screen of plasma from 10 patients residing in the malaria endemic areas indicated >70% plasma-positivity against PfSPP (Fig. 2D). Although our results are based on a relatively small number of patient samples, this proof-of-principle finding suggests that the exofacial loop of PfSPP may serve as a target of immune response in malaria infection. At this stage, we have not examined whether the smaller loops that are predicted to be exposed in PfSPP are also target of the immune response in malaria patients.
Since mammalian presenilins and SPPs are closely related aspartyl proteases with opposite active site orientations, several inhibitors have been synthesized with specificity towards each class of these enzymes. For example, the DAPT preferentially inhibits presenilins, whereas the L-685,458 and (Z-LL)2-ketone are more specific for the SPPs. Consistent with this specificity, the L-685,458 and (Z-LL)2-ketone efficiently inhibited merozoite invasion in human erythrocytes whereas the DAPT inhibitor had no effect (Fig. 3). It is noteworthy that even at saturating concentrations of polyclonal antibodies against the exofacial loop of PfSPP, the merozoite invasion was blocked only by 40–50% [13], whereas the SPP inhibitors completely inhibited merozoite invasion. These results suggest that the enzyme activity of PfSPP may play an essential role in parasite invasion. Importantly, the same SPP inhibitors caused a complete arrest of the parasite development in erythrocytes suggesting that PfSPP also plays a critical role during parasite transition from ring to the trophozoite stage (Fig. 4).
In summary, our studies identify PfSPP as a potential target of anti-malarial drug development. The ubiquitous presence of PfSPP suggests that the newly developed drugs against PfSPP may be effective against other malaria parasites such as P. vivax. The presence of plasma antibodies against PfSPP in patients suggest that the 41 amino acid exofacial loop may also be considered for inclusion in the multi-subunit vaccine against malaria. Once the substrates of PfSPP have been identified, it would be important to determine whether direct binding of erythrocyte band 3 to PfSPP modulates its protease activity during invasion of the erythrocytes. The identification of PfSPP substrates may provide multiple points of intervention against malaria by combining the PfSPP inhibitors with the blockers of its substrates to mitigate the drug resistance that might arise against PfSPP. Given the availability of well characterized chemical libraries against the Alzheimer’s disease γ-secretase complex, it is likely that selective inhibitors of PfSPP will soon be identified as new anti-malarial drugs.
Supplementary Material
Acknowledgments
This work was supported by the NIH Grant HL 60961 (AC). We are grateful to Dr. Sam Sisodia for sharing SPP inhibitors with us and Dr. Alan Cowman for providing the gene targeting vector. We gratefully acknowledge the gift of WR-99210 drug from Dr. Guy Schiehser of Jacobus Pharmaceutical Company, New Jersey. We are also grateful to Dr. Louis H. Miller for helping us to obtain plasma samples from the malaria patients. Critical comments and discussions with Drs. Ronald Dubreuil and John Quigley considerably improved this manuscript. We also thank Ms. Alex Chavez for help with the prediction and drawing of the PfSSP topology structure. Finally, we thank Dr. Anwar Khan, Farnaz Bakhshi, and Deanna Rybak for careful reading and editing of the manuscript.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bbrc.2009.01.083.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackman MJ. Proteases involved in erythrocyte invasion by the malaria parasite: function and potential as chemotherapeutic targets. Curr Drug Targets. 2000;1:59–83. doi: 10.2174/1389450003349461. [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal PJ. Proteases of malaria parasites: new targets for chemotherapy. Emerg Infect Dis. 1998;4:49–57. doi: 10.3201/eid0401.980107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenthal PJ. Hydrolysis of erythrocyte proteins by proteases of malaria parasites. Curr Opin Hematol. 2002;9:140–145. doi: 10.1097/00062752-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Hanspal M, Dua M, Takakuwa Y, Chishti AH, Mizuno A. Plasmodium falciparum cysteine protease falcipain-2 cleaves erythrocyte membrane skeletal proteins at late stages of parasite development. Blood. 2002;100:1048–1054. doi: 10.1182/blood-2002-01-0101. [DOI] [PubMed] [Google Scholar]
- 6.Baker RP, Wijetilaka R, Urban S. Two Plasmodium rhomboid proteases preferentially cleave different adhesins implicated in all invasive stages of malaria. PLoS Pathog. 2006;2:e113. doi: 10.1371/journal.ppat.0020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Donnell RA, Hackett F, Howell SA, Treeck M, Struck N, Krnajski Z, Withers-Martinez C, Gilberger TW, Blackman MJ. Intramembrane proteolysis mediates shedding of a key adhesin during erythrocyte invasion by the malaria parasite. J Cell Biol. 2006;174:1023–1033. doi: 10.1083/jcb.200604136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown MS, Ye J, Rawson RB, Goldstein JL. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 2000;100:391–398. doi: 10.1016/s0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- 9.Urban S, Lee JR, Freeman M. Drosophila rhomboid-1 defines a family of putative intramembrane serine proteases. Cell. 2001;107:173–182. doi: 10.1016/s0092-8674(01)00525-6. [DOI] [PubMed] [Google Scholar]
- 10.Weihofen A, Binns K, Lemberg MK, Ashman K, Martoglio B. Identification of signal peptide peptidase, a presenilin-type aspartic protease. Science. 2002;296:2215–2218. doi: 10.1126/science.1070925. [DOI] [PubMed] [Google Scholar]
- 11.Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Chen H, Oo TH, Daly TM, Bergman LW, Liu SC, Chishti AH, Oh SS. A co-ligand complex anchors Plasmodium falciparum merozoites to the erythrocyte invasion receptor band 3. J Biol Chem. 2004;279:5765–5771. doi: 10.1074/jbc.M308716200. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Chen H, Oh SS, Chishti AH. A Presenilin-like protease associated with Plasmodium falciparum micronemes is involved in erythrocyte invasion. Mol Biochem Parasitol. 2008;158:22–31. doi: 10.1016/j.molbiopara.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nyborg AC, Ladd TB, Jansen K, Kukar T, Golde TE. Intramembrane proteolytic cleavage by human signal peptide peptidase like 3 and malaria signal peptide peptidase. FASEB J. 2006;20:1671–1679. doi: 10.1096/fj.06-5762com. [DOI] [PubMed] [Google Scholar]
- 15.Crompton PD, Traore B, Kayentao K, Doumbo S, Ongoiba A, Diakite SA, Krause MA, Doumtabe D, Kone Y, Weiss G, Huang CY, Doumbia S, Guindo A, Fairhurst RM, Miller LH, Pierce SK, Doumbo OK. Sickle cell trait is associated with a delayed onset of malaria: implications for time-to-event analysis in clinical studies of malaria. J Infect Dis. 2008;198:1265–1275. doi: 10.1086/592224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arai M, Mitsuke H, Ikeda M, Xia JX, Kikuchi T, Satake M, Shimizu T. ConPred II: a consensus prediction method for obtaining transmembrane topology models with high reliability. Nucleic Acids Res. 2004;32:W390–W393. doi: 10.1093/nar/gkh380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weihofen A, Lemberg MK, Friedmann E, Rueeger H, Schmitz A, Paganetti P, Rovelli G, Martoglio B. Targeting presenilin-type aspartic protease signal peptide peptidase with gamma-secretase inhibitors. J Biol Chem. 2003;278:16528–16533. doi: 10.1074/jbc.M301372200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.