Abstract
This study confirms the presence of iron, co-localized with Aβ plaques, in PS/APP mouse brain, using Perls’ stain for Fe3+ supplemented by 3,3′-diaminobenzidine (DAB) and Aβ immunohistochemistry in histological brains sections fixed with formalin or methacarn. In this study, the fixation process and the slice thickness did not interfere with the Perls’ technique. The presence of iron in β-amyloid plaques in PS/APP transgenic mice, a model of Alzheimer’s disease (AD) pathology, may explain previous reports of reductions of transverse relaxation time (T2) in MRI studies and represent the source of the intrinsic Aβ plaque MR contrast in this model.
Keywords: β-amyloid, Alzheimer’s disease, Brain, Iron, MRI, Transgenic mice
INTRODUCTION
Iron is important for normal brain development and for most of the critical metabolic functions, including energy metabolism and synthesis of neurotransmitters. Brain iron increases with age and different cerebral regions accumulate iron at different rates and concentrations (1–3). The highest concentrations of iron in human adult brains are found in the basal ganglia, more specifically in the globus pallidus, red nucleus and substantia nigra. Iron is also present in white matter, especially during brain development, where it is found in high levels in oligodendrocytes and it is required for myelin production (1).
The blood–brain barrier (BBB) and blood-cerebrospinal fluid (CSF) barrier control the iron uptake into the brain by regulating the expression of transport proteins’ receptors, such as transferrin receptor expression in endothelial and choroids plexus cells (2,4,5). In normal brain, iron is not toxic despite its high levels, probably because of efficient homeostatic mechanism. Brain iron is contained in enzymes, in structural proteins, in transport proteins and in storage proteins such as ferritin. If there is an excess of iron or disruption of the homeostasis, iron-induced oxidative damage occurs (6–8).
Brain iron is abnormally elevated in several neurodegenerative disorders, including Alzheimer’s disease (AD) (9,10,11), with two times more iron in the neuropil of AD patients than in non-demented patients (12). It is known that Aβ protein has metal-ion-binding sites and that the interaction between Aβ and iron is one of the factors responsible for the aggregation and deposition of Aβ (11,13). The role of iron in the pathogenesis of AD is not completely clear. There are evidence that in binding with iron, Aβ is acting as a chelator to diminish the oxidative damage of high concentrations of iron (7,8), but in the end, the imbalance of the iron metabolism and its accumulation appears to contribute to enhance the oxidative stress implicated as one of the potential mechanisms for the neurodegenerative changes seen in AD.
The presence of iron in and around amyloid plaques in post mortem human brain tissue has been demonstrated using Perls’ technique despite the fact that the brain tissue was in general submitted to different concentrations of formaldehyde-based fixation (14,15). Indeed, iron histochemistry using Perls’ technique has been the method of choice for non-heme iron detection in tissue sections. Although this is a universally accepted method, its sensitivity and poor penetration on fixed and paraffin embedded tissue has limited the iron detection in certain studies, resulting in controversy about the accuracy of this staining technique. Even with modifications like DAB enhancement (3,3′-diaminobenzidine) introduced to enhances the signal and to improve the sensitivity of the standard Perls’ stain (16), there have been conflicting reports about Perls’ method not working on formalin-fixed tissue, but only when using tissue fixed in methacarn or fresh unfixed tissue (6). This controversy is also present on studies of the iron distribution in animal brain tissue (1,17,18). Although iron has been demonstrated in formalin fixed mouse (19,20) and rat (21) brain, Smith et al. (18) studying one model of AD transgenic mouse, were able to demonstrate the presence of iron only in brain tissue fixed in methacarn. Despite the vast literature about transgenic mouse models of AD, to our knowledge only the Smith et al. (18) study has attempted to demonstrate, by histochemical methods, the presence of iron in the brain of these transgenic mice. Other studies using biochemical and analytical methods have demonstrated an increase in the level of metals, including iron, in the brain of different mouse models of AD (22,23).
The histological demonstration of iron in Aβ plaques is important not only because of its possible role in the pathogenesis of the disease, but also because Aβ-iron complex could be used as a mechanism of detection and progression of the disease by MRI – histology correlation studies. Our laboratory and others have investigated iron in association with reduced T2 in MRI studies of normal and diseased human brain (24–26). Recently, using high resolution MRI, we have reported reduced mean T2 values in the hippocampus and cortex of 18 months old PS/APP mice in vivo (27) and detected Aβ plaques in vitro without the use of any contrast reagents (28). The PS/APP transgenic mouse is a model of Alzheimer pathology overexpressing both mutante human APP and PS1, and showing an early and extensive amyloid deposition. We hypothesize that the presence of iron in Aβ plaques, in this model, is the significant factor responsible for reducing T2 and the source of the plaque intrinsic MRI contrast seen in these animals. The objective of the present study is to investigate the presence of iron in Aβ plaques of the transgenic mouse model of Alzheimer pathology (PS/APP), in which we have previously demonstrated changes in MRI parameters. Additionally, this study also addresses the question of the role of formalin fixation on the accuracy of the Perls’ staining.
EXPERIMENTAL PROCEDURE
Three 18-month-old PS/APP transgenic mice and one age-matched non-transgenic (NTg) control mouse were used. The PS/APP transgenic mouse is a model of Alzheimer pathology over-expressing both mutante human APP and PS1, and showing an early and extensive amyloid deposition (29).
For histochemical analysis, two PS/APP and one NTg were perfused with phosphate-buffered saline (PBS) (pH 7.4) through the left cardiac ventricle, followed by 10% buffered formalin. After perfusion fixation, the brains were removed and stored in 10% buffered formalin at room temperature. A third 18-month-old PS/APP mouse, after PBS perfusion, had the brain fixed in methacarn as described by Smith et al. (6). After fixation, one PS/APP mouse brain fixed in formalin, one fixed in methacarn and the NTg mouse brain, were then cut serially in 40 µm-thick coronal sections using a vibrotome (series 1000). The other PS/APP mouse brain fixed in formalin was cut in 20 µm-thick coronal sections. To show the presence of iron in Aβ plaques, neighboring brain sections were staining with either Aβ antibody or DAB-enhanced Perls’ reaction and counterstained with nuclear fast red. The Aβ immunohistochemistry was performed using antibody 6E10 at 1:1000 (Sigma, St. Louis, MO, USA). Sections were immunostained by a standard avidin–biotin complex method using diaminobenzidine as chromogen. The Perls’ reaction following 3,3-diaminobenzidine (DAB) enhancement (16) staining is based on the formation of ferric ferrocyanide (Prussian blue) when ferric ion (Fe3+), released from iron-containing compounds by hydrochloric acid (HCl), reacts with potassium ferrocyanide. The ferric ferrocyanide then catalyzes the oxidation of DAB with formation of a brown precipitate.
RESULTS
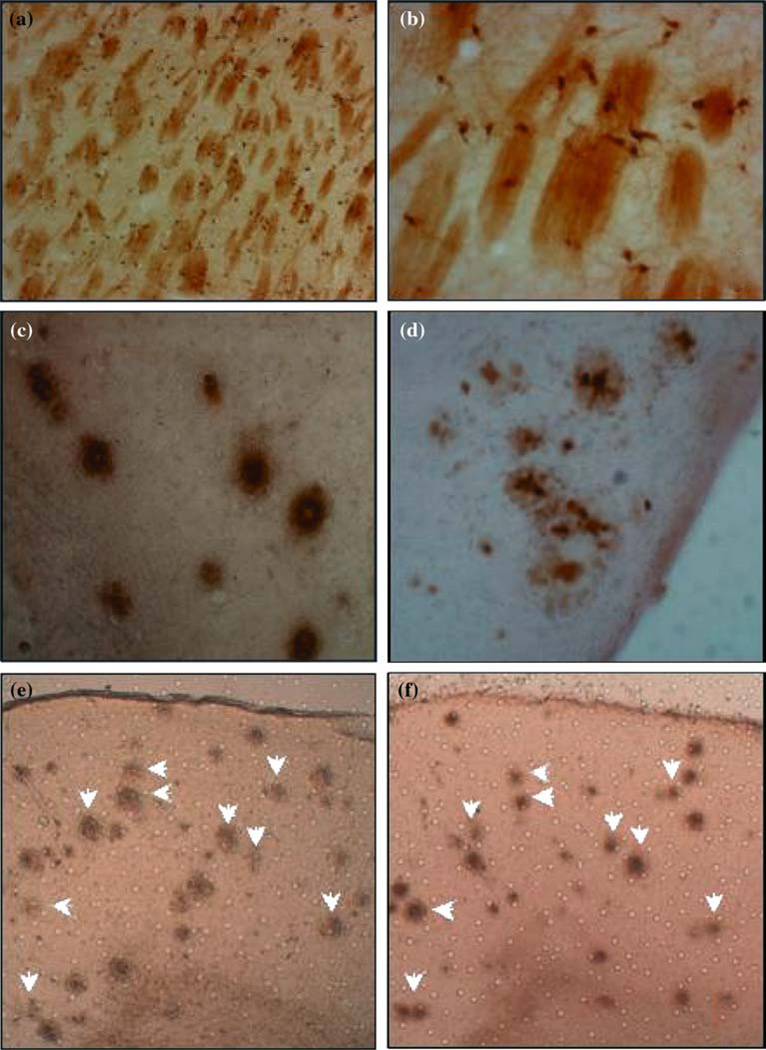
The presence of iron in brain sections of all mice was observed, stained with similar intensity in both transgenic and NTg mice cells in and around white matter tracks at the level of the Caudate-Putamen (Fig. 1a, b). Additionally, histological sections fixed with formalin (Fig. 1c) or methacarn (Fig.1d) from PS/APP mice brains showed the presence of iron in the majority of Aβ plaques. A similar pattern of distribution of Aβ plaques and iron staining could be seen in these mice brains (Fig. 1e, f), which serves to support the co-localization of iron in Aβ plaques. There was no positive staining for Aβ plaques in the negative control sections or sections stained only with DAB, indicating the specificity of the staining method.
Fig. 1.
Low (a) and High-power (b) view of histological sections from Caudate-Putamen showing the presence of Fe3+ in cells in and around white matter tracks; High-power view showing Fe3+ in amyloid plaques of transgenic PS/APP mice detected by Perl’s reaction followed by DAB enhancement in brains fixed with formalin (c) and methacarn (d). A similar pattern of distribution of Aβ plaques and iron staining was seen in neighboring brain sections (e and f, white arrows), which confirmed the co-localization of iron in Aβ plaques.
DISCUSSION
Despite the fact that the Perls’ technique is routinely used for histological detection of iron in tissue, a controversy about the accuracy and sensitivity of this staining technique exist. There are reports that this histochemical technique does not work or is greatly reduced by formalin fixation and will only work on frozen or tissue fixed in methacarn, mainly in studies aimed at detecting the presence of iron in amyloid deposits (6,18). Therefore, the histological confirmation of Aβ-iron complex in tissue fixed with different fixatives was important, particularly for many MRI – histology correlation studies.
We have demonstrated the presence of iron in PS/APP mouse brains using Perls’ stain for Fe3+ supplemented by 3,3′-diaminobenzidine (+DAB). Also, since formaldehyde-based fixation is supposedly considered to interfere with the iron localization by reducing the labeling, we tested two PS/APP mice brains, one fixed with methacarn and one fixed with formalin, and despite the fixation method, both brains showed the presence of Fe3+ in amyloid plaques. Thus, the fixation process and the slice thickness did not appear to interfere with the Perls’ technique, at least in our study.
The observation of iron accumulation in Aβ plaques in PS/APP mice is consistent with previous studies in humans (15,14), and helps to clarify the controversy about the accuracy of this staining technique in mice brain tissue. Despite the vast literature about transgenic mouse models of AD, this is the first study to demonstrate the co-localization of iron in Aβ plaques in PS/APP mouse brains using Perls’+DAB method.
The presence of iron in Aβ plaques in this transgenic mouse model of AD pathology confirms the source of the plaque intrinsic MRI contrast in these animals. In particular, this work supports our hypothesis that the presence of iron in the Aβ plaques is a major factor in the signal hypo-intensity seen in our in vitro images (28) and in the reduction of T2 measured in vivo (27) in PS/APP mouse brain. Additionally, the presence of iron could possibly be exploited as an endogenous contrast reagent utilizing new MRI techniques designed to be contrast sensitive to the presence of microscopic susceptibility effects (30), which will be important in future studies that aim to correlate different quantification indices applied to both MRI and histological images. We acknowledge that further work will be necessary to also investigate the presence of Fe2+ and ferritin in these transgenic mice, which would allow us to have a more complete view of the relationship between the effects of the imbalance of the iron metabolism and the MRI measurable parameters.
ACKNOWLEDGMENTS
This work was supported by grants from the NIH (RAN; P01 AG17617-02) and Wyeth (JAH).
REFERENCES
- 1.Hill JA. The distribution of iron in the brain. Pages 1–24. In: MBH Youdim MBH, editor. Brain iron: Neurochemistry and behavioural aspects. London: Taylor & Francis; 1988. [Google Scholar]
- 2.Beard JL, Connor JR, Jones BC. Iron in the brain. Nutr. Rev. 1993;51:157–170. doi: 10.1111/j.1753-4887.1993.tb03096.x. [DOI] [PubMed] [Google Scholar]
- 3.Koeppen AH. A brief history of brain iron research. J. Neurol. Scis. 2003;207:95–97. doi: 10.1016/s0022-510x(02)00429-x. [DOI] [PubMed] [Google Scholar]
- 4.Takeda A. Significance of transferring in iron delivery to the brain. J. Health Sci. 2001;47:323–331. [Google Scholar]
- 5.Burdo JR, Antonetti DA, Wolpert EB, Connor JR. Mechanisms and regulation of transferrin and iron transport in a model blood-brain barrier system. Neuroscience. 2003;121:883–890. doi: 10.1016/s0306-4522(03)00590-6. [DOI] [PubMed] [Google Scholar]
- 6.Smith MA, Harris PL, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redoxgenerated free radicals. Proc. Natl. Acad. Sci. USA. 1997;94:9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bush AI. The metallobiology of Alzheimer’s disease. Trends Neurosci. 2003;26:207–214. doi: 10.1016/S0166-2236(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 8.Todorich BM, Connor JR. Redox metals in Alzheimer’s disease. Ann. NY Acad. Sci. 2004;1012:171–178. doi: 10.1196/annals.1306.014. [DOI] [PubMed] [Google Scholar]
- 9.Thompson KJ, Shoham S, Connor JR. Iron and neurodegenerative disorders. Brain Res. Bull. 2001;55:155–164. doi: 10.1016/s0361-9230(01)00510-x. [DOI] [PubMed] [Google Scholar]
- 10.Sipe JC, Lee P, Beutler E. Brain Iron Metabolism and Neurodegenerative Disorders. Dev. Neurosci. 2002;24:188–196. doi: 10.1159/000065701. [DOI] [PubMed] [Google Scholar]
- 11.Bishop GM, Robinson SR, Liu Q, Perry G, Atwood CS, Smith MA. Iron: a pathological mediator of Alzheimer disease? Dev. Neurosci. 2002;24:184–187. doi: 10.1159/000065696. [DOI] [PubMed] [Google Scholar]
- 12.Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998;158:47–52. doi: 10.1016/s0022-510x(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 13.Atwood CS, Martins RN, Smith MA, Perry G. Senile plaque composition and posttranslational modification of amyloid-beta peptide and associated proteins. Peptides. 2002;23:1343–1350. doi: 10.1016/s0196-9781(02)00070-0. [DOI] [PubMed] [Google Scholar]
- 14.LeVine SM. Iron deposits in multiple sclerosis and Alzheimer’s disease brains. Brain Res. 1997;760:298–303. doi: 10.1016/s0006-8993(97)00470-8. [DOI] [PubMed] [Google Scholar]
- 15.Connor JR, Menzies SL, St Martin SM, Mufson EJ. A histochemical study of iron, transferrin, and ferritin in Alzheimer’s diseased brains. J. Neurosci. Res. 1992;31:75–83. doi: 10.1002/jnr.490310111. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen-Legros J, Bizot J, Bolesse M, Pulicani JP. “Diaminobenzidine black” as a new histochemical demonstration of exogenous iron. Histochemistry. 1980;66:239–244. doi: 10.1007/BF00495737. [DOI] [PubMed] [Google Scholar]
- 17.Perl DP, Good PF. Comparative Techniques for Determining Cellular Iron Distribution in Brain Tissues. Ann. Neurol. 1992;32:S76–S81. doi: 10.1002/ana.410320713. [DOI] [PubMed] [Google Scholar]
- 18.Smith MA, Hirai K, Hsiao K, Pappolla MA, Harris PL, Siedlak SL, Tabaton M, Perry G. Amyloid-beta deposition in Alzheimer transgenic mice is associated with oxidative stress. J. Neurochem. 1998;70:2212–2215. doi: 10.1046/j.1471-4159.1998.70052212.x. [DOI] [PubMed] [Google Scholar]
- 19.LeVine SM. Oligodendrocytes and myelin sheaths in normal, quaking and shiverer brains are enriched in iron. J. Neurosci. Res. 1991;29:413–419. doi: 10.1002/jnr.490290317. [DOI] [PubMed] [Google Scholar]
- 20.Thompson K, Menzies S, Muckenthaler M, Torti FM, Wood T, Torti SV, Hentze MW, Beard J, Connor J. Mouse brains deficient in H-ferritin have normal iron concentration but a protein profile of iron deficiency and increased evidence of oxidative stress. J. Neurosci. Res. 2003;71:46–63. doi: 10.1002/jnr.10463. [DOI] [PubMed] [Google Scholar]
- 21.Hill JM, Switzer RC., 3rd The regional distribution and cellular localization of iron in the rat brain. Neuroscience. 1984;11:595–603. doi: 10.1016/0306-4522(84)90046-0. [DOI] [PubMed] [Google Scholar]
- 22.White AR, Reyes R, Mercer JF, Camakaris J, Zheng H, Bush AI, Multhaup G, Beyreuther K, Masters CL, Cappai R. Copper levels are increased in the cerebral cortex and liver of APP and APLP2 knockout mice. Brain Res. 1999;842:439–444. doi: 10.1016/s0006-8993(99)01861-2. [DOI] [PubMed] [Google Scholar]
- 23.Maynard CJ, Cappai R, Volitakis I, Cherny RA, White AR, Beyreuther K, Masters CL, Bush AI, Li QX. Overexpression of Alzheimer’s disease amyloid-beta opposes the age-dependent elevations of brain copper and iron. J. Biol. Chem. 2002;277:44670–44676. doi: 10.1074/jbc.M204379200. [DOI] [PubMed] [Google Scholar]
- 24.Helpern JA, Ordidge RJ, Gorell JM, Deniau JC, Welch KMA. Preliminary Observations of Transverse Relaxation Rates Obtained at 3 Tesla From the Substantia Nigra of Adult Normal Human Brain. NMR Biomed. 1995;8:25–27. doi: 10.1002/nbm.1940080106. [DOI] [PubMed] [Google Scholar]
- 25.Gorell JM, Ordidge RJ, Brown GG, Deniau JC, Buderer NM, Helpern JA. Increased Iron Related MRI Contrast in the Substantia Nigra in Parkinson’s Disease. Neurology. 1995;45:1138–1143. doi: 10.1212/wnl.45.6.1138. [DOI] [PubMed] [Google Scholar]
- 26.Bartzokis G, Sultzer D, Cummings J, Holt LE, Hance DB, Henderson VW, Mintz J. In vivo evaluation of brain iron in Alzheimer disease using magnetic resonance imaging. Arch. Gen. Psychiatry. 2000;57:47–53. doi: 10.1001/archpsyc.57.1.47. [DOI] [PubMed] [Google Scholar]
- 27.Helpern JA, Lee SP, Falangola MF, Dyakin VV, Bogart A, Ardekani B, Duff K, Branch CA, Wisniewski T, Leon MJ, de Wolf OT, O’Shea J, Nixon RA. In Vivo detection of neuropathology in an animal model of Alzheimer’s disease by magnetic resonance imaging. Magn. Reson. Med. 2004;51:794–798. doi: 10.1002/mrm.20038. [DOI] [PubMed] [Google Scholar]
- 28.Lee SP, Falangola MF, Nixon RA, Duff K, Helpern JA. Visualization of β-Amyloid Plaques in a Transgenic Mouse Model of Alzheimer’s Disease using MR Microscopy Without Contrast Reagents. Magn. Reson. Med. 2004;52:538–544. doi: 10.1002/mrm.20196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGowan E, Sanders S, Iwatsubo T, Takeuchi A, Saido T, Zehr C, Yu X, Uljon S, Wang R, Mann D, Dickson D, Duff K. Amyloid phenotype characterization of transgenic mice overexpressing both mutant amyloid precursor protein and mutant presenilin 1 transgenes. Neurobiol. Dis. 1999;6:231–244. doi: 10.1006/nbdi.1999.0243. [DOI] [PubMed] [Google Scholar]
- 30.Jensen JH, Chandra R. Method for measuring the magnetic field correlation function for water protons in biological tissues; Pages 2297, in Proceedings of the International Society for Magnetic Resonance in Medicine tenth scientific meeting; 2002. [Google Scholar]