SUMMARY
Introduction:
The effects of dietary fatty acid supplementation on lipoprotein fatty acid composition have rarely been described.
Patients and Methods:
Sixty-one overweight and obese adults with dyslipidemia and insulin resistance were randomized to placebo, 2 g/day extended-release nicotinic acid (ERN), 4 g/day prescription omega-3 fatty acid ethyl ester (P-OM3), or combination therapy for sixteen weeks. Lipoprotein fatty acid composition was analyzed by gas chromatography pre- and post-treatment.
Results:
Treatment with P-OM3 or combination, but not ERN, increased proportions of eicosapentaenoic acid, docosahexaenoic acid, and docosapentaenoic acid, and reduced those for arachidonic acid in all lipoprotein fractions, with greatest impact in the high-density lipoprotein fraction. P-OM3-induced changes in eicosapentaenoic acid within low-density lipoproteins and very low-density lipoproteins were associated with beneficial effects on mean arterial pressure and pulse pressure.
Conclusions:
P-OM3 supplementation, with or without ERN, was associated with differentially altered lipoprotein fatty acid composition and improved blood pressure parameters.
Keywords: LDL, HDL, VLDL, fish oil, nicotinic acid, DHA, EPA, LA, AA
INTRODUCTION
Changes in lipoprotein concentration and composition play a role in the development of atherosclerosis and cardiovascular disease events [1]. Increased circulating levels of very low-density lipoproteins (VLDL; particularly VLDL remnants) and low-density lipoproteins (LDL) contributes to atherosclerosis and cardiovascular disease by promoting ectopic lipid accumulation, particularly of cholesterol [2]. Decreased levels of high-density lipoproteins (HDL) can in theory slow reverse cholesterol transport, and result in loss of their anti-inflammatory, anti-oxidative and anti-atherogenic effects [3-7].
In addition to lipoproteins, omega-6 (n6) and omega-3 (n3) fatty acids play important mechanistic roles in cardiovascular health [8, 9]. Linoleic acid (LA), found in vegetable oils, accounts for 85-90% of dietary n6 fatty acids and can be converted (sparingly) into arachidonic acid (AA), which is the precursor for many proinflammatory and anti-inflammatory eicosanoids and promotes platelet aggregation [8]. Eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and docosapentaenoic acid (DPA) are n3 fatty acids derived almost exclusively from seafood ingestion and have demonstrated protective and therapeutic effects against cardiovascular disease [10]. Supplementation of long-chain n3 fatty acids, primarily EPA and DHA, in fish oil has demonstrated the following effects: improved arterial endothelial function, reduced platelet aggregation, decreased circulating triacylglycerols, improved blood pressure, and reduced risk of cardiac death after an acute myocardial infarction [11-17]. Additionally, the fatty acid composition of lipoproteins has long been associated with lipoprotein oxidizability [18] and more recently with the activity of associated proteins such as paraoxonase [19], suggesting indirect means whereby fatty acids might impact lipoprotein metabolism. Although these are promising biochemical effects, the potential clinical benefit of dietary fatty acid supplementation is clouded by more recent studies, which did not support a cardioprotective effect of fish oil supplementation [20-23]. Amidst these controversies, there is a lack of literature about the effects of fish oils on the fatty acid compositions of the various lipoprotein fractions (HDL, VLDL, and LDL) and plasma.
Similar to dietary fatty acid supplementation, nicotinic acid favorably modulates lipoprotein concentrations, but there is much debate regarding its use in the prevention and treatment of cardiovascular disease [20-22, 24-26]. Nicotinic acid is thought to reduce cardiovascular disease events by increasing HDL-C while decreasing LDL-C and triacylglycerols [27, 28], but recent data indicate that increasing HDL-C may not confer cardioprotective benefits [25, 26]. At present, there is no reason to believe that nicotinic acid would alter fatty acid profiles, but no data on that question currently exist.
Here, we describe the biochemical effects of n3 fatty acid supplementation (Lovaza®) alone or in combination with extended-release nicotinic acid (Niaspan®) on the fatty acid content of lipoproteins. We also explored whether these changes could be associated with changes in blood pressure or vascular function.
METHODS
Study Design
This was a randomized, double-blind, placebo-controlled clinical trial of 4g/day prescription n3 fatty acid ethyl ester formulation (Lovaza®, GSK; P-OM3), and 2g/day of extended-release nicotinic acid (Niaspan, Abbott; ERN), for the treatment of cardiovascular disease risk in overweight and obese adults with dyslipidemia. The effects on the primary endpoints have been published [29]. A 6-week, single-blind run-in period to control for lifestyle changes and non-compliance preceded a baseline visit, at which time subjects were randomized to a 16- week treatment arm (placebo, ERN alone, P-OM3 alone, or combination). Lipid and vascular outcomes were measured at baseline (pre-intervention) and week 16 (post-intervention). A cohort of healthy subjects was included at baseline as a comparator group. The study was carried out by Sanford Research/USD in conjunction with the Sanford Clinic Clinical Research Services in Sioux Falls, SD. The study was approved by the local IRB and written informed consent was obtained from each subject prior to participating in the trial. The trial was registered with clinicaltrials.gov (#NCT00286234).
Sample Population
Subject inclusion/exclusion criteria have been published in detail [29]. Briefly, otherwise healthy men and women meeting the following criteria were eligible for randomization to the experimental groups: age 40 to 69 years, BMI 25 - 40 kg/m2, fasting triacylglycerols (TG) 150 - 750 mg/dL, HDL >10 mg/dL, and the ratio of TG/HDL >3.5. A reference cohort of fourteen healthy adults was also enrolled for baseline comparison purposes. This reference cohort was not randomized to treatment or evaluated beyond baseline.
Lipid,fatty acids and other measurements
A fasting (≥8hr) blood sample was obtained at baseline and after 16 weeks of treatment. Lipoproteins were purified by sequential flotation and fatty acid composition was determined by gas chromatography as described [30, 31]. The following fatty acids in plasma and within each lipoprotein fraction are reported here as a proportion of the total fatty acid composition: linoleic acid (LA, C18:2n6), arachidonic acid (AA, C20:4n6), eicosapentaenoic acid (EPA, C20:5n3), docosapentaenoic acid (DPA, C22:5n3), and docosahexaenoic acid (DHA, C22:6n3). The analytic error for these measurements can be found in the study reported by Shearer et al, in which the same methodology was used [32]. Blood pressure was measured per standard clinical procedures.
Statistical Analysis
Statistical analyses were performed using Stata12® software. Analysis of variance and chisquare tests were used to compare baseline characteristics among the treatment groups. Differences between baseline and follow-up lipoprotein fatty acid proportions within groups were determined using paired t-tests. The changes in proportions were compared among treatments and among lipoprotein fractions using two-sample t-tests with unequal variances. A Bonferroni correction (alpha = 0.0125) was included to account for multiple comparisons across the four groups. Two-sample t-tests were conducted to compare the mean change of cardiovascular parameters with each treatment by fraction. Pair-wise correlations between the changes cardiovascular parameters and the changes in lipoprotein fatty acid proportions by treatment group were performed.
RESULTS
Baseline characteristics (Table 1)
Table 1.
Baseline Data by Group [mean (SD) unless otherwise noted]
| Healthy Controls | Insulin Resistant Subjects | |
|---|---|---|
|
|
||
| Variable | n=14 | n=61 |
| Age (years) | 44.9 (12.3) | 47.7 (10.3) |
| Sex male, n (%) | 9 (64.3) | 36 (59.0) |
| BMI (kg/m2) | 22.9 (1.2) | 32.3 (4.0)c |
| Systolic BP (mmHg) | 112.4 (8.8) | 132.2 (11.5)c |
| Diastolic BP (mmHg) | 68.7 (6.6) | 82.7 (7.4)c |
| Triacylglycerols (mg/dL) | 76.4 (22.4) | 213.0 (81.6)c |
| Total Cholesterol (mg/dL) | 181.2 (30.0) | 203.1 (43.2) |
| HDL-C (mg/dL) | 55.9 (8.6) | 41.7 (8.3)c |
| Non-HDL-C (mg/dL) | 125.3 (28.1) | 161.6 (39.7)b |
| Glucose (mg/dL) | 88.2 (9) | 100.8 (10.8)c |
| Insulin (uU/mL) | 3.2 (1.5) | 14.0 (8.4)c |
| HOMA-IR | 0.7 (0.3) | 3.5 (2.2)c |
| Current Smoker, n (%) | 0 (0) | 5 (8.2) |
| Current statin use, n (%) | 0 (0) | 8 (13.1) |
| LA (% total) | ||
| HDL fraction | 25.4 (3.8) | 24.1 (4.2) |
| LDL fraction | 29.8 (5.4) | 29.3 (4.6) |
| VLDL fraction | 15.4 (3.5) | 20.1 (3.4)b |
| Plasma fraction | 29.6 (0.2) | 27.5 (4.3) |
| AA (% total) | ||
| HDL fraction | 10.3 (1.7) | 11.5 (2.7)a |
| LDL fraction | 7.3 (1.5) | 8.5 (1.9)a |
| VLDL fraction | 3.7 (0.8) | 5.6 (1.6)b |
| Plasma fraction | 8.0 (1.4) | 7.4 (1.8) |
| EPA (% total) | ||
| HDL fraction | 0.8 (0.4) | 0.6 (0.3) |
| LDL fraction | 0.7 (0.4) | 0.5 (0.3) |
| VLDL fraction | 0.3 (0.2) | 0.3 (0.2) |
| Plasma fraction | 0.8 (0.6) | 0.4 (0.2)a |
| DHA (% total) | ||
| HDL fraction | 2.8 (1.0) | 2.3 (0.6) |
| LDL fraction | 1.8 (0.7) | 1.5 (0.4) |
| VLDL fraction | 1.1 (0.7) | 1.3 (0.5) |
| Plasma fraction | 2.1 (0.9) | 1.4 (0.4)a |
| DPA (% total) | ||
| HDL fraction | 0.8 (0.2) | 0.8 (0.2) |
| LDL fraction | 0.5 (0.2) | 0.5 (0.1) |
| VLDL fraction | 0.4 (0.1) | 0.5 (0.1)a |
| Plasma fraction | 0.6 (0.2) | 0.5 (0.1) |
p<0.05,
p<0.01,
p<0.001 when control was compared to healthy controls HOMA-IR, homeostatic model assessment of insulin resistance; LA, linoleic acid; AA, arachidonic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid
The final trial cohort consisted of 61 insulin resistant subjects, with an additional reference cohort of 14 healthy subjects for baseline comparisons. At baseline, the insulin resistant subjects had higher BMI, blood pressure, and homeostatic model assessment of insulin resistance (HOMA-IR), as well as increased serum concentrations of triacylglycerols, non-HDL-C, glucose, and insulin compared to the healthy reference population. Additionally, insulin resistant subjects had lower HDL-C concentrations.
With a few exceptions, baseline fatty acid proportional compositions of lipoprotein fractions were similar between healthy controls and the experimental groups (Table 1). Compared to healthy controls, insulin resistant subjects had higher proportions of LA, AA, and DPA in the VLDL fraction and higher proportions of AA in the HDL and LDL fractions. Additionally, EPA and DHA were lower in whole plasma in the insulin resistant subjects compared to healthy controls.
As previously published [29], randomization of insulin resistant subjects to one of four treatment arms resulted in similar distribution of these baseline characteristics across treatment groups.
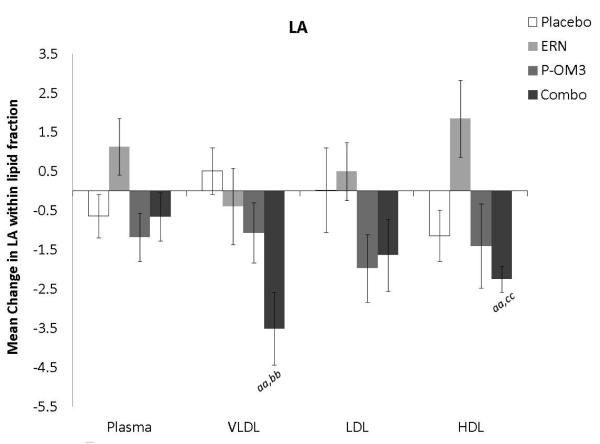
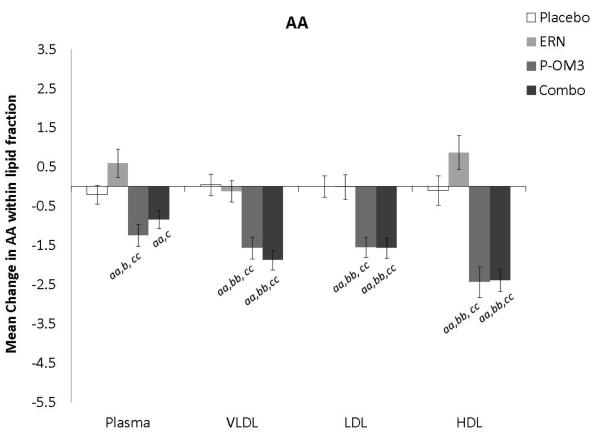
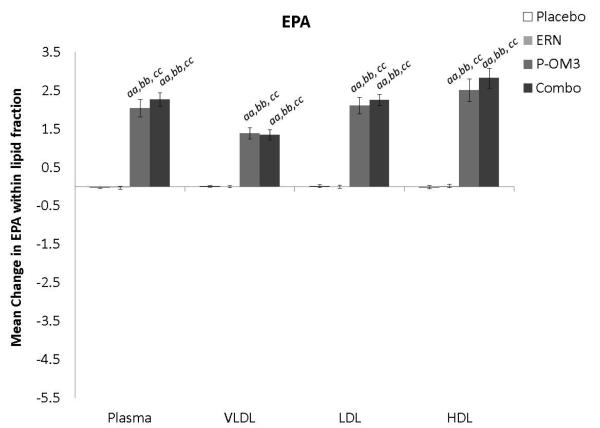
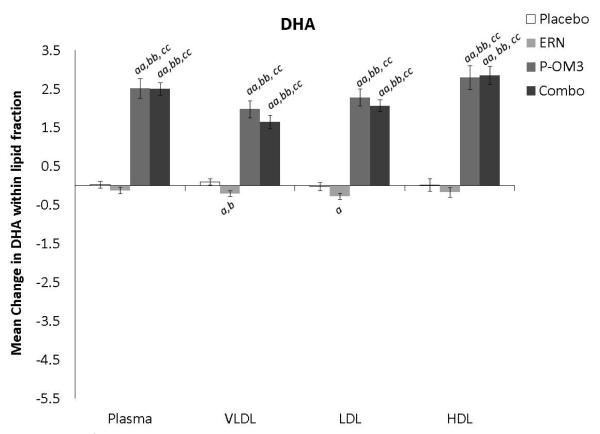
Changes in fatty acid proportional composition of lipoprotein fractions in response to treatment (Figure 1)
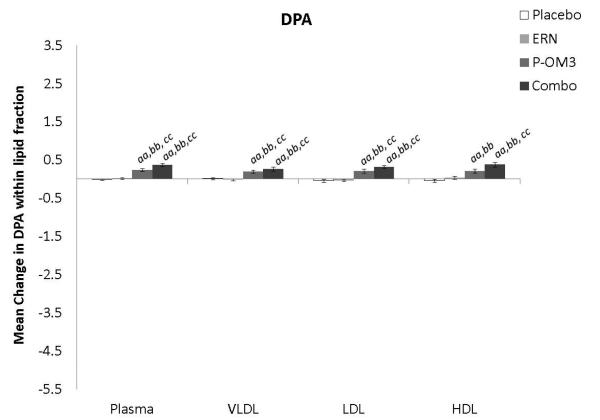
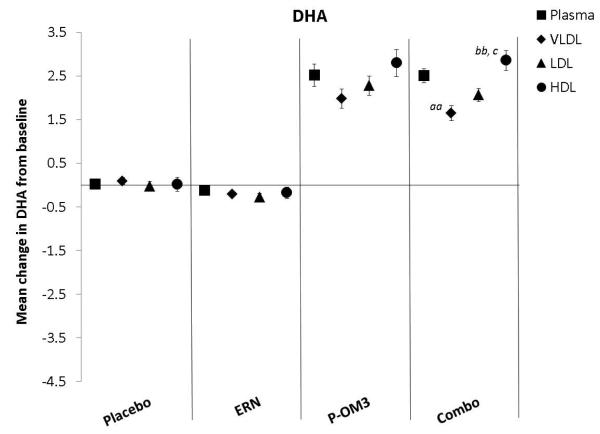
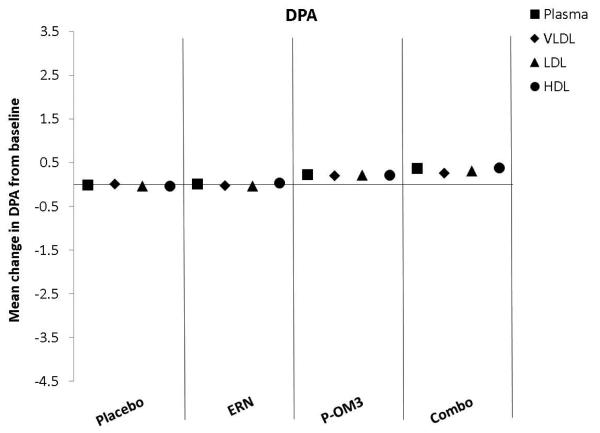
Figure 1. Mean change in fatty acid proportion within individual lipid fractions from baseline to follow-up in response to treatment.
ap<0.05; aap<0.01, treatment effect from baseline to follow-up visit; bp<0.05; bbp<0.01, treatment effect compared to change in placebo group; ccp<0.01, treatment effect compared to change in ERN group; LA, linoleic acid; AA, arachidonic acid; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid; DHA, docosahexaenoic acid.
Figure 1 displays the treatment effects (change from baseline) on fatty acid proportion within each lipoprotein fraction. Assignment to placebo did not affect the fatty acid composition within any lipoprotein fraction compared to baseline. The only effect of ERN alone was a significant reduction in the proportion of DHA within the VLDL fraction compared to baseline. Treatment with P-OM3 or in combination with ERN significantly decreased AA and significantly increased EPA, DPA, and DHA in all fractions compared to baseline. These effects of P-OM3 or Combo were also significantly different than those of placebo or ERN alone. Additionally, Combo reduced LA in the HDL and VLDL fractions compared to baseline. This effect was greater than placebo in the VLDL fraction and greater than ERN alone in the HDL fraction.
Comparison of treatment response across lipoprotein fractions (Figure 2)
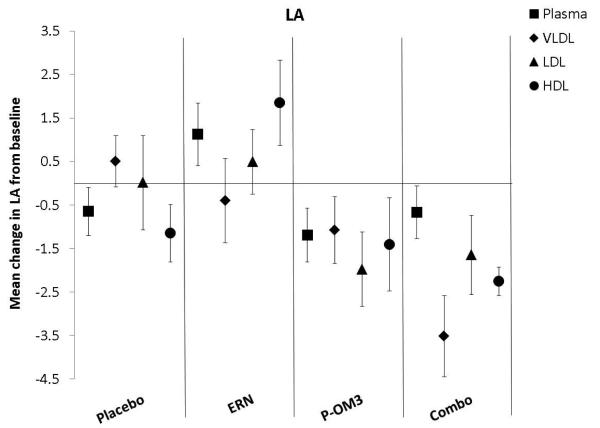
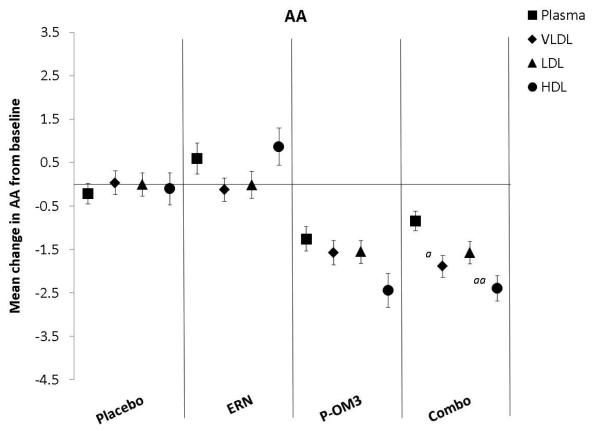
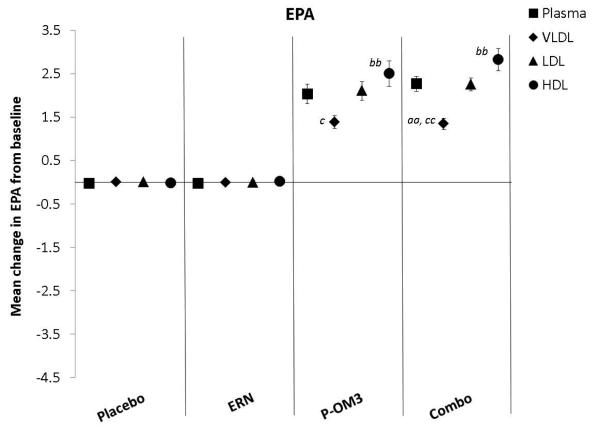
Figure 2. Mean changes in fatty acid proportion from baseline to follow-up in response to treatment between lipid fractions.
ap<0.05; aap<0.01, significantly different change compared to plasma; bp<0.05; bbp<0.01, significantly different change compared to the VLDL fraction; cp<0.05, ccp<0.01, significantly different change compared to the LDL fraction. LA, linoleic acid; AA, arachidonic acid; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid; DHA, docosahexaenoic acid.
Figure 2 compares the change in fatty acid proportion from baseline among lipoprotein fractions by treatment group. The placebo and ERN treatment (non)effects were similar across the lipoprotein fractions for all fatty acids examined. The proportions of LA or DPA were not significantly altered in response to any treatment. This was also true for treatment effect on AA. Treatment effects of P-OM3 or Combo on EPA appeared to be strongest in the HDL fraction and weakest in VLDL. This pattern was also observed for DHA in response to Combo therapy, but the effect was not significant in response to P-OM3.
Correlations between changes in fatty acid proportions and measures of endothelial function (Figure 3)
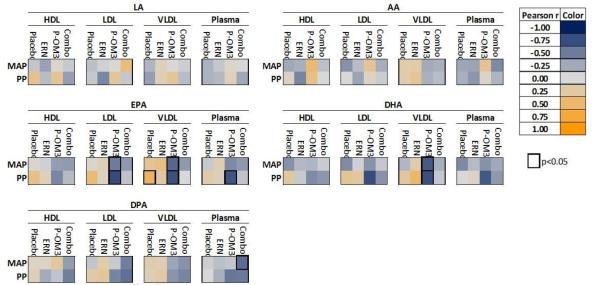
Figure 3. Heat map representation of correlation between change in percent fatty acid composition and vascular endpoints.
Each colored square represents the Pearson correlation between change in percent fatty acid within a specific fraction and change in vascular endpoint from baseline to follow-up. Significant associations (p<0.05) are highlighted by a thick black border. LA, linoleic acid; AA, arachidonic acid; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid; DHA, docosahexaenoic acid;MAP, mean arterial pressure; PP, pulse pressure.
Figure 3 is a heat map of the correlation (Pearson’s r) between change in fatty acid proportion in response to treatment and change in blood pressure parameters. Of the 61 subjects, 59 were eligible for vascular endpoint measurements and exploratory analyses were conducted. In general, changes in fatty acid composition of lipoprotein fractions were not associated with improvement or worsening of vascular endpoints using the EndoPAT outcome measure. Correlation analysis indicated significant beneficial changes in mean arterial pressure and pulse pressure (MAP and PP) in association with P-OM3-induced increases in EPA within LDL and VLDL, as well as DHA within VLDL.
DISCUSSION
This sub-study was conducted to address a gap in the literature regarding the biochemical effects of dietary fatty acid supplementation on fatty acid composition of lipoprotein fractions, as well as dispel assumptions that dietary fatty acids distribute equally among the lipoprotein fractions. Data from the current study support the notion that long-chain n3 fatty acid supplementation (alone or in combination with nicotinic acid) can alter the fatty acid content of lipoproteins. As expected, this included significant increases in EPA, DHA, and DPA, accompanied by a decrease in AA across all lipoprotein fractions. More subtly, we demonstrated that these changes in response to P-OM3 supplementation (alone or in combination with nicotinic acid) were greater in HDL than in LDL or VLDL, indicating a stronger response in this fraction. Further, the changes in HDL fatty acids were related to changes in blood pressure. The concordance of these two parameters provides a first-level of evidence that fatty acid composition mediates some HDL function. Previously described data regarding the influence of ingested fatty acids on the composition of blood lipoproteins are limited in size and scope [33] in comparison to these data. One study noted that corn oil consumption in a single subject increased the ratio of linoleic acid to oleic acid in lipoprotein fractions of different density, but did not measure other fatty acids [34]. Another study evaluated the effect of a one-week, low-fat diet on fatty acids levels of cholesterol esters, phospholipids, and triacylglycerols in two subjects and found that linoleic acid predominated in the cholesterol esters, oleic acid in the triacylglycerols, and palmitic acid in phospholipids [35]. That study did not measure other lipoproteins, EPA, or DHA. A third study examined the effects of fish oil on plasma phospholipids, cholesterol ester, and triacylglycerols in five healthy individuals, but did not determine the lipoprotein fatty acid composition [36]. The current study provides evidence of changes in fatty acid composition of lipoproteins in response to dietary n3 fatty acid supplementation in a greater number of individuals. Additionally, these individuals are overweight and obese adults with dyslipidemia and insulin resistance, a population that is at greater risk for diabetes and CVD events [37].
The parent clinical trial for the current study established that the combination of n3 fatty acid supplementation and nicotinic acid could partially correct dyslipidemia [29]. It is hypothesized that fish oil may reduce triacylglycerol levels by lowering the hepatic availability of non-esterified fatty acids (mostly through increased β-oxidation in liver and target tissues), which subsequently decreases VLDL production [38]. It has remained unclear as to how the consumption of n3 fatty acids found in fish oil are incorporated into and distributed among the various lipoprotein fractions. Data from the current sub-study, however, demonstrate that the ingestion of fish oil alone (or in combination with ERN) leads to potentially beneficial changes in fatty acid composition of plasma lipoproteins, which can have numerous effects on lipoprotein function including the exchange of cholesterol esters [39] and so could impact the course of vascular disease.
Exploratory analyses revealed that P-OM3-induced changes in the EPA content of LDL and VLDL and the DHA content of VLDL were correlated with improved blood pressure measures, but the direct impact of the biochemical changes on the surrogate cardiovascular measures could not be expanded upon in this study. Beneficial changes in pulse pressure and mean arterial pressure have been associated with cardiovascular disease risk [40, 41]. It may be that the proportions of fatty acids within lipoproteins are more important than the concentration of lipoproteins in determining risk, but this remains to be tested.
The most dramatic treatment-induced increases in the proportions of EPA, DPA, and DHA were observed in the HDL fraction, but these effects did not appear to impart benefit on the surrogate cardiovascular endpoints (pulse pressure and mean arterial pressure) measured in this study. These data suggest that either this effect does not translate or that another clinical endpoint would be more appropriate or sensitive to these physiologic changes. This relationship would be a topic of future study as this data set did not contain the parameters necessary to examine this mechanism further.
In this study, proportional increases in EPA and DHA within VLDL were associated with beneficial effects on mean arterial pressure and pulse pressure. While VLDL is a source of remnant lipoproteins and other lipids that contribute to atherosclerosis, it is not included in a typical lipid profile and is an often underappreciated CVD risk factor, particularly for individuals with insulin resistance [42]. Additionally, VLDL serves as a major source of fatty acids for many tissues including heart, skeletal muscle and lungs. The results observed in this study suggest that P-OM3 supplementation could safely and inexpensively modulate the polyunsaturated fatty acid composition of VLDL, which could impact lipid-generated signaling in such tissues, resulting in changes in biochemical and downstream cardiovascular endpoints.
The relatively small sample size restricted the types of analyses and adjustments to evaluate treatment effect on blood pressure metrics. Although some subjects could not be classified at having overt metabolic syndrome as defined by the National Heart, Lung, and Blood Institute [43], all subjects were insulin resistant and overweight or obese. These risk factors are highly correlated with development of cardiovascular disease [43]. Strengths include the evaluation of two FDA-approved pharmaceutical agents that are used widely to treat dyslipidemia in clinical settings, the focus on subjects who were at relatively high cardiovascular disease risk, and the inclusion of a healthy group of control individuals.
In conclusion, n3 fatty acid supplementation (with or without niacin) in overweight and obese adults with dyslipidemia and insulin resistance altered fatty acid composition of lipoprotein fractions. Additionally, compositional changes in VLDL in response to n3 fatty acid supplementation were associated with changes in a measure of improved blood pressure, but future research is necessary to provide any evidence of causality. On the whole, these results contribute to a lack of literature regarding the biochemical effects of dietary fatty acid intake on the composition of circulating lipoproteins.
ACKNOWLEDGMENTS
AA and LL were supported by the National Heart Lung and Blood Institute Preventive Cardiology T32 granted to Thomas A. Pearson of the University of Rochester (5 T32 HL007937). Measurements of lipoprotein fatty acids were supported by a grant from GlaxoSmithKline (LVZ 122790). The original trial was supported by the National Institute of Health (5 R01 DK061486). Abbott (Niaspan®) and GlaxoSmithKline (Lovaza®) provided the drugs used in this study.
Sources of Support: AA and LL were supported by the National Heart Lung and Blood Institute Preventive Cardiology T32 granted to Thomas A. Pearson of the University of Rochester (5 T32 HL007937). Measurements of lipoprotein fatty acids were supported by a grant from GlaxoSmithKline (LVZ 122790). The original trial was supported by the National Institute of Health (5 R01 DK061486). Abbott (Niaspan®) and GlaxoSmithKline (Lovaza®) provided the drugs used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Shearer received research support and speakership honoraria from GlaxoSmithKline while the parent study was ongoing. Dr. Harris received speakership honoraria from GlaxoSmithKline while the parent study was ongoing.
REFERENCES
- 1.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr., Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 2.Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 3.Corti MC, Guralnik JM, Salive ME, Harris T, Field TS, Wallace RB, et al. HDL cholesterol predicts coronary heart disease mortality in older persons. JAMA. 1995;274:539–44. [PubMed] [Google Scholar]
- 4.Huxley RR, Barzi F, Lam TH, Czernichow S, Fang X, Welborn T, et al. Isolated low levels of high-density lipoprotein cholesterol are associated with an increased risk of coronary heart disease: an individual participant data meta-analysis of 23 studies in the Asia-Pacific region. Circulation. 2011;124:2056–64. doi: 10.1161/CIRCULATIONAHA.111.028373. [DOI] [PubMed] [Google Scholar]
- 5.Negi S, Ballantyne CM. Insights from recent meta-analysis: role of high-density lipoprotein cholesterol in reducing cardiovascular events and rates of atherosclerotic disease progression. J Clin Lipidol. 2010;4:365–70. doi: 10.1016/j.jacl.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 7.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 8.Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, et al. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009;119:902–7. doi: 10.1161/CIRCULATIONAHA.108.191627. [DOI] [PubMed] [Google Scholar]
- 9.Siddiqui RA, Harvey KA, Zaloga GP. Modulation of enzymatic activities by n-3 polyunsaturated fatty acids to support cardiovascular health. J Nutr Biochem. 2008;19:417–37. doi: 10.1016/j.jnutbio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Lavie CJ, Milani RV, Mehra MR, Ventura HO. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol. 2009;54:585–94. doi: 10.1016/j.jacc.2009.02.084. [DOI] [PubMed] [Google Scholar]
- 11.Iso H, Rexrode KM, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, et al. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA. 2001;285:304–12. doi: 10.1001/jama.285.3.304. [DOI] [PubMed] [Google Scholar]
- 12.Bucher HC, Hengstler P, Schindler C, Meier G. N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med. 2002;112:298–304. doi: 10.1016/s0002-9343(01)01114-7. [DOI] [PubMed] [Google Scholar]
- 13.Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS. n-3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the Cardiovascular Health Study. Am J Clin Nutr. 2003;77:319–25. doi: 10.1093/ajcn/77.2.319. [DOI] [PubMed] [Google Scholar]
- 14.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–55. [PubMed] [Google Scholar]
- 15.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 16.Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 17.Block RC, Harris WS, Reid KJ, Sands SA, Spertus JA. EPA and DHA in blood cell membranes from acute coronary syndrome patients and controls. Atherosclerosis. 2008;197:821–8. doi: 10.1016/j.atherosclerosis.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 18.Corso G, Trivellone E, Motta A, Postiglione A, Mancini FP, Carbone V, et al. Effect of low density lipoprotein fatty acid composition on copper-induced peroxidation: 1H-nuclear magnetic resonance analysis. Clin Chim Acta. 1997;258:193–200. doi: 10.1016/s0009-8981(96)06461-3. [DOI] [PubMed] [Google Scholar]
- 19.Boshtam M, Emami Razavi A, Pourfarzam M, Ani M, Naderi GA, Basati G, et al. Serum paraoxonase 1 activity is associated with Fatty Acid composition of high density lipoprotein. Dis Markers. 2013;35:273–80. doi: 10.1155/2013/612035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kowey PR, Reiffel JA, Ellenbogen KA, Naccarelli GV, Pratt CM. Efficacy and safety of prescription omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: a randomized controlled trial. JAMA. 2010;304:2363–72. doi: 10.1001/jama.2010.1735. [DOI] [PubMed] [Google Scholar]
- 21.Kromhout D, Giltay EJ, Geleijnse JM. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–26. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- 22.Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122:2152–9. doi: 10.1161/CIRCULATIONAHA.110.948562. [DOI] [PubMed] [Google Scholar]
- 23.Risk, Prevention Study Collaborative G. Roncaglioni MC, Tombesi M, Avanzini F, Barlera S, et al. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368:1800–8. doi: 10.1056/NEJMoa1205409. [DOI] [PubMed] [Google Scholar]
- 24.Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8:1245–55. doi: 10.1016/s0735-1097(86)80293-5. [DOI] [PubMed] [Google Scholar]
- 25.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–67. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 26.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–80. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Assmann G, Gotto AM., Jr. HDL cholesterol and protective factors in atherosclerosis. Circulation. 2004;109:III8–14. doi: 10.1161/01.CIR.0000131512.50667.46. [DOI] [PubMed] [Google Scholar]
- 28.Superko HR. Beyond LDL cholesterol reduction. Circulation. 1996;94:2351–4. doi: 10.1161/01.cir.94.10.2351. [DOI] [PubMed] [Google Scholar]
- 29.Shearer GC, Pottala JV, Hansen SN, Brandenburg V, Harris WS. Effects of prescription niacin and omega-3 fatty acids on lipids and vascular function in metabolic syndrome: a randomized controlled trial. J Lipid Res. 2012 doi: 10.1194/jlr.P022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–20. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 31.Harris WS, Pottala JV, Vasan RS, Larson MG, Robins SJ. Changes in erythrocyte membrane trans and marine fatty acids between 1999 and 2006 in older Americans. J Nutr. 2012;142:1297–303. doi: 10.3945/jn.112.158295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shearer GC, Pottala JV, Spertus JA, Harris WS. Red blood cell fatty acid patterns and acute coronary syndrome. PLoS One. 2009;4:e5444. doi: 10.1371/journal.pone.0005444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agheli N, Jacotot B, Ayrault-Jarrier M, Lemonnier D. Compositions of Serum Lipids, Lipoproteins, and Lipoprotein Fatty Acids in Type IV Primary Hypertriglyceridemia. Journal of Clinical and Biochemical Nutrition. 1992;12:131–40. [Google Scholar]
- 34.Bragdon J, Karmen A. Effect of ingested fat on fatty acid composition of serum lipoproteins. J Lipid Res. 1961;2:400–2. [Google Scholar]
- 35.Goodman DS, Shiratori T. Fatty acid composition of human plasma lipoprotein fractions. J Lipid Res. 1964;5:307–13. [PubMed] [Google Scholar]
- 36.Zuijdgeest-van Leeuwen SD, Dagnelie PC, Rietveld T, van den Berg JW, Wilson JH. Incorporation and washout of orally administered n-3 fatty acid ethyl esters in different plasma lipid fractions. Br J Nutr. 1999;82:481–8. doi: 10.1017/s0007114599001737. [DOI] [PubMed] [Google Scholar]
- 37.Lempiainen P, Mykkanen L, Pyorala K, Laakso M, Kuusisto J. Insulin resistance syndrome predicts coronary heart disease events in elderly nondiabetic men. Circulation. 1999;100:123–8. doi: 10.1161/01.cir.100.2.123. [DOI] [PubMed] [Google Scholar]
- 38.Shearer GC, Savinova OV, Harris WS. Fish oil - How does it reduce plasma triglycerides? Biochim Biophys Acta. 2012;1821:843–51. doi: 10.1016/j.bbalip.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cazita PM, Castilho LN, Carvalho MD, Sesso AC, Oliveira HC, Quintao EC. Reversible flow of cholesteryl ester between high-density lipoproteins and triacylglycerol-rich particles is modulated by the fatty acid composition and concentration of triacylglycerols. Braz J Med Biol Res. 2010;43:1135–42. doi: 10.1590/s0100-879x2010007500136. [DOI] [PubMed] [Google Scholar]
- 40.Domanski M, Mitchell G, Pfeffer M, Neaton JD, Norman J, Svendsen K, et al. Pulse pressure and cardiovascular disease-related mortality: follow-up study of the Multiple Risk Factor Intervention Trial (MRFIT) JAMA. 2002;287:2677–83. doi: 10.1001/jama.287.20.2677. [DOI] [PubMed] [Google Scholar]
- 41.Sesso HD, Stampfer MJ, Rosner B, Hennekens CH, Gaziano JM, Manson JE, et al. Systolic and diastolic blood pressure, pulse pressure, and mean arterial pressure as predictors of cardiovascular disease risk in Men. Hypertension. 2000;36:801–7. doi: 10.1161/01.hyp.36.5.801. [DOI] [PubMed] [Google Scholar]
- 42.Adiels M, Olofsson SO, Taskinen MR, Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:1225–36. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- 43.Grundy SM, Brewer HB, Jr., Cleeman JI, Smith SC, Jr., Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]