Abstract
Objective
To describe NP and AOM otopathogens during the time frame 2007-2009, six to eight years after the introduction of 7-valent pneumococcal conjugate (PCV7) in the US and to compare nasopharyngeal (NP) colonization and acute otitis media (AOM) microbiology in children 6 to 36 months of age having 1st and 2nd AOM episodes with children who are otitis prone.
Methods
Prospectively, the microbiology of NP colonization and AOM episodes was determined in 120 children with absent or infrequent AOM episodes. NP samples were collected at 7 routine visits between 6 and 30 months of age and at the time of AOM. For 1st and subsequent AOM episodes, middle ear fluid (MEF) was obtained by tympanocentesis. Eighty otitis prone children were comparatively studied. All 200 children received age-appropriate doses of PCV7.
Results
We found PCV7 serotypes were virtually absent: (0.9% isolated from both NP and MEF) in both study groups. However, non-PCV7 serotypes replaced PCV serotypes such that the frequency of isolation of S. pneumoniae (Spn) was nearly equal to that of non-typeable Haemophilus influenzae (NTHi). M. catarrhalis (Mcat) was less common and Staphylococcus aureus infrequent in the NP and MEF from the two groups. The proportion of Spn, NTHi and Mcat causing AOM was similar in children with 1st and 2nd AOM episodes compared to otitis prone children. However, oxacillin-resistant Spn isolated from the NP and MEF was 19% for the absent/infrequent and 58% for the otitis prone groups, p<0.0001. Beta-lactamase producing NTHi occurred more frequently in the otitis prone group, p=0.04.
Conclusions
Six to 8 years after widespread use of PCV7, Spn strains expressing vaccine-type serotypes have virtually disappeared from the NP and MEF of vaccinated children. NP colonization and AOM has changed to non-PCV7 strains of Spn. NTHi continues to be a major AOM pathogen. The otopathogens in 1st and 2nd AOM and in otitis prone children are very similar although Spn and NTHi are more often antibiotic resistant in the otitis prone.
Keywords: Nasopharyngeal, AOM, S. pneumoniae, H. influenzae, M. catarrhalis
INTRODUCTION
Nasopharyngeal (NP) colonization is a necessary first step in the pathogenesis of acute otitis media (AOM). The major pathogens of AOM are Streptococcus pneumoniae (Spn), non-typeable Haemophilus influenzae (NTHi), and Moraxella catarrhalis (Mcat). In early studies after introduction of the 7-valent pneumococcal conjugate vaccine (PCV7) in the U.S., strains of Spn expressing capsular types included in PCV7 were detected less often from the NP [1-4], as causes of AOM [5], and invasive pneumococcal disease [6]. The success of PCV7 in reducing invasive pneumococcal disease and pneumococcal AOM has become clear and indisputable [7-9]. Furthermore, herd immunity has become established in the U.S. and the impact on unvaccinated children and adults evident [10].
NTHi emerged as the most common AOM isolate in 2001-2003 [11,12] and replacement by non-PCV7 serotypes (especially serotype 19A) has been occurring, leading to an increase in invasive pneumococcal disease and AOM by serotype 19A [13-17]. There have been conflicting reports on an increase in NP colonization by Staphylococcus aureus [18,19]. An increase in S. aureus colonization is a concerning possibility in light of the increasing prevalence of serious community-acquired methicillin resistant Staphylococcus aureus infections [19].
The objectives of this study were to describe changes in NP colonization and AOM otopathogens during 2007-2009, 6 to 8 years after the introduction of PCV7 in the US and to compare the microbiology of NP colonization and AOM episodes in children 6 to 36 months of age having absent/infrequent AOM with that of children who are otitis prone.
MATERIALS AND METHODS
Study populations
Children in group 1, (absent/infrequent AOM group) were enrolled at 6 months of age and followed to 30 months of age; the children had no prior AOM at the time of enrollment. NP and oropharyngeal samples were obtained at 7 routine visits when the children were 6, 9, 12, 15, 18, 24, and 30 months of age. With the first and any subsequent episodes of AOM NP and oropharyngeal cultures and middle ear fluid (MEF) by tympanocentesis were obtained. A follow up visit occurred 3 weeks later after each AOM and NP and oropharyngeal samples were again collected. Two children met the definition of otitis prone (3 AOM episodes in 6 months, n=1; or 4 AOM episodes in 12 months, n=1. Data for their 3rd / 4th AOM episodes meeting the otitis prone definition was included in group 2 (see below).
A second cohort (Group 2) were children less than 36 months of age (otitis prone group) enrolled when they had a 3rd AOM episode within 6 months of time or a 4th episode within 12 months of time. NP and oropharyngeal cultures and MEF by tympanocentesis were obtained at the time of AOM. NP and oropharyngeal samples were collected again at a 3-week follow up visit.
Demographic data collected included family history of AOM, daycare attendance, antibiotic exposure in the prior month, presence of upper respiratory infection, number of AOM episodes before enrollment, age of first AOM episode, and PCV-7 vaccine history. The study was approved by the University of Rochester and Rochester General Hospital IRB and written informed consent was obtained from parents before enrollment in the study.
Definition of AOM
AOM was diagnosed by pneumatic otoscopy by two of the authors (JC, MP), who are both validated otoscopists, when children with acute onset of otalgia had tympanic membranes (TMs) that were: (1) bulging or full; and (2) a cloudy or purulent effusion was observed, or the TM was completely opacified; and (3) TM mobility was reduced or absent.
NP sampling
At each sampling visit a cotton-tipped wire swab was inserted into both nares and a culture obtained, an oropharyngeal swab was obtained by rubbing both tonsils and the posterior pharynx. Then 1 ml of sterile phosphate buffered saline was instilled and aspirated from both nares as a third sample for culture.
Tympanocentesis
MEF for culture was obtained by puncture of the inferior portion of an intact TM with a 20-gauge spinal needle attached to a 3-mL syringe using a hand-held operating otoscope. If a small sample of MEF was obtained on aspiration, 0.5 mL of trypticase soy broth was aspirated through the spinal needle and then aliquoted and inoculated onto agar plates and into broth, as described below.
Microbiology
NP and oropharyngeal swabs, the secretions from nasal wash and MEF were inoculated onto sheep blood and chocolate agar plates immediately after collection. All plates and broths were incubated at 37°C with 5% carbon dioxide. Bacterial isolates were identified by standard methods. All NTHi and M. cat isolated were tested for beta-lactamase production with the chromogenic cephalosporin disk method. Serotypes of Spn were determined by latex agglutination (Pneumotest-Latex, Statens Serum Institute, Copenhagen, Denmark) according to the manufacturer’s instructions. Quellung reactions were used to identify the serotype subgroup. Oxacillin sensitivity was determined for Spn and S. aureus by disc diffusion test.
PCR of 6A Spn serotypes
To distinguish between 6A and 6C serotypes PCR was performed with oligonucleotide primers 5106 and 3101 to amplify wciN as previously described [20].
Statistics
In the analysis of NP colonization over time if a child had the same Spn isolate on sequential visits then the isolate was counted only once. Differences were analyzed with the Fishers exact test and p < 0.05 (2-tailed) was considered significant.
RESULTS
The demographics of the study population are shown in Table 1 (on line only). The mean age at the time of the first episode of AOM in the absent/infrequent AOM group (10 months SD=4.4 months) was younger than the age of children in the otitis prone group (13 months SD= 6.5 months) (p=0.01). Daycare attendance differed significantly between the two groups with 27% of the absent/infrequent AOM group and 53% of the otitis prone group attending daycare (p=0.0006). Ninety-two percent of all patients had a concurrent URI at the time of an AOM; whereas 19% of the children had a URI at the time of the routine visits. In the 2 weeks prior to an AOM visit, 18% of absent/infrequent AOM children and 50% of otitis prone children had taken or were taking antibiotics (p<0.0001).
Table 1.
Demographics of the population of children.
| Group 1 Absent/Infrequent AOM |
Group 2 Otitis Prone |
|
|---|---|---|
| Number studied | 120 | 80 |
| Mean Age at initial AOM or rAOM # |
10 months (SD=4.4 mos.) | 13 months (SD=6.5 mos) |
| Male | 63 (53%) | 43 (49%) |
| Female | 57 (47%) | 37 (46%) |
| Daycare Attendance (N, %)* | 33 (27%) | 42 (53%) |
| Upper Respiratory Infection during routine visit (%) |
19 | N/A |
| No. of AOM prior 12 mos (% ≥ 4) |
N/A | 18 (23%) |
| No. of AOM prior 6 mos (% ≥ 3) |
N/A | 62 (77%) |
| Family History of AOM (N,%) |
61 (51%) 45 | (56%) |
p=0.01
p=0.0006
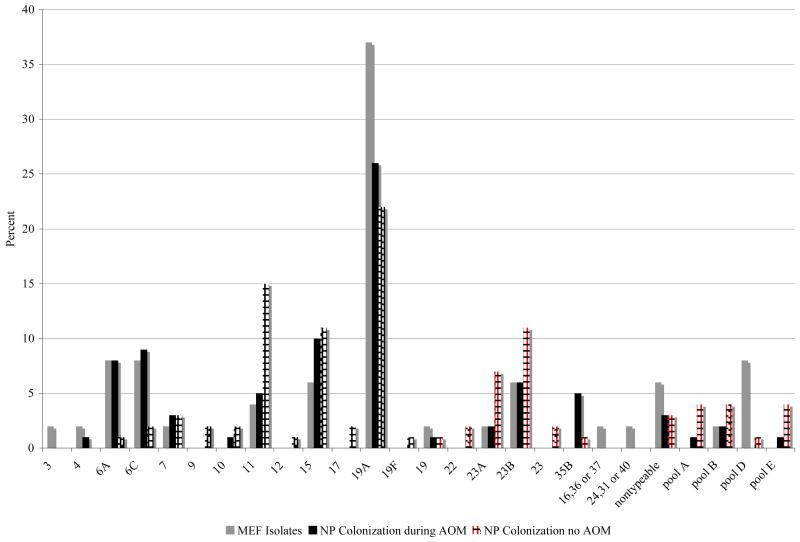
Figure 1 (on-line only) shows the Spn serotypes in our study. There were a total of 110 Spn isolates from the NP during routine visits when the children did not have AOM and 87 Spn isolates from the NP during AOM. There was a total of 49 Spn MEF isolates: 21 in the infrequent AOM group and 28 in the otitis prone group. The number of individual Spn serotypes was too small to detect a difference between the 2 groups during AOM. Overall, 19A was the most common serotype isolated from the NP when the children were well and when they had an AOM and isolated from the MEF as a cause of AOM: 22/110 of the NP isolates during routine visits, 23/87 of the NP during AOM and 18/49 of the MEF isolates. At the time of routine visits without AOM, of the 110 Spn NP isolates there were 22 different serotypes with 19A, 11, 15 and 23B the most common isolates. Of the 87 Spn NP isolates during AOM there were 16 different serotypes, with 19A, 15 and 6C the most common. Of the 49 Spn MEF isolates there were 16 different serotypes with 19A, 6A and 6C the most common.
Figure 1.
S. pneumoniae serotype distribution from the MEF, the NP and during an AOM episode and the NP with no AOM for both the infrequent and otitis prone groups. Pool A contains serotypes 13, 28, Pool B contains serotypes 21, 39, Pool D contains serotypes 25, 38, 43, 44, 45, 46, 48, and Pool E contains serotypes 29, 34, 35, 42, 47.
There were 2 children who had PCV-7 vaccine type serotypes isolated (2 of 220 (0.9%) of all Spn isolates). Spn serotype 4 was isolated from the NP and MEF of an 18 month-old child at the time of an AOM and a serotype 19F was isolated from the NP of an 18 month-old child during a routine visit without AOM.
There were 25 Spn identified as serotype 6A by Quellung reaction. All 25 strains were PCR amplified to differentiate 6A from 6C. Sixteen (63%) of the 6A isolates were found to be 6C. Three were isolated from the NP without AOM, 8 from the NP during AOM and 4 from the MEF. Non-typeable serotypes occurred infrequently, 4/110 (4%), 3/87 (3%) and 3/49 (6%) for NP without AOM, NP during AOM and MEF, respectively.
In 89% of the AOM episodes, the Spn that colonized the NP and was isolated from the MEF were concordant. Eleven percent of the time 2 Spn serotypes were isolated from the NP during AOM and only one of the colonizing serotypes was present in the MEF.
Table 2 shows the percentage of NTHi, Spn, Mcat, and S. aureus isolated from NP cultures at age 6, 9, 12, 15, 18, 24, and 30 months from the absent/infrequent AOM group when they did not have AOM. Spn was isolated most commonly, followed by Mcat then NTHi. The rates of NP colonization at the different ages did not differ significantly for the 3 otopathogens. Of the 434 visits when children did not have AOM, one or more otopathogens were present in 272 visits (63% of the visits). Colonization with more than one otopathogen occurred in 16% (71 of 434) of the visits when children did not have AOM. Among the 71 visits when subjects had more than 1 otopathogen in the NP, the most frequent combination was Spn and Mcat (51% of the multiple otopathogen visits). S. aureus was isolated most often during the first year of life but accounted for only 9% of the total isolates.
Table 2.
Nasopharyngeal culture results at the time of healthy visits for the absent/infrequent AOM group.
| Age |
H.
influenzae |
S.
pneumoniae |
M.
catarrhalis |
S. aureus | Non- pathogens |
Number of Visits |
|---|---|---|---|---|---|---|
| 6 | 15 (19%) | 29 (36%) | 30 (37%) | 7 (9%) | 44 (40%) | 111 |
| 9 | 16(30%) | 14(26%) | 15 (28%) | 9 (17%) | 35 (36%) | 96 |
| 12 | 11 (21%) | 23 (44%) | 15 (29%) | 3 (6%) | 35 (42%) | 83 |
| 15 | 9 (24%) | 20 (54%) | 7 (19%) | 1 (3%) | 22 (36%) | 61 |
| 18 | 13 (29%) | 14 (31%) | 14 (31%) | 4 (9%) | 16 (30%) | 53 |
| 24 | 8 (30%) | 9 (33%) | 8 (30%) | 2 (7%) | 4 (17%) | 24 |
| 30 | 1 (50%) | 1 (50%) | 0 (0%) | 0 (0%) | 4 (67%) | 6 |
| All Visits |
73 (24%) | 110 (37%) | 89 (30%) | 26 (9%) | 160 (37%) | 434 |
The percentage was based on the number of newly acquired specific otopathogen at a given age over the total number of newly acquired otopathogens isolated at a given age. The total number of otopathogens and non-pathogens is greater than the number of visits because of isolation of more than one organism at some visits. Age is denoted in months. The non-pathogen column denotes the visits where only normal flora was observed in the NP.
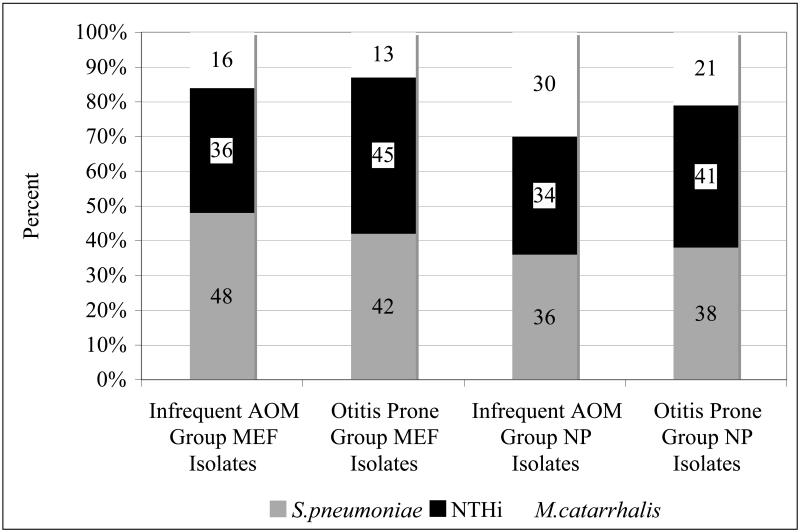
MEF and NP culture results at the time of an AOM for the infrequent AOM group and otitis prone group are shown in Figure 2 (on-line only). There were 52 1st or 2nd AOM episodes in the infrequent AOM group and 96 AOM episodes in the otitis prone group. NTHi was isolated from the MEF in 16 (36%) of the infrequent AOM group and 30 (45%) of the otitis prone group. There were 21 (48%) Spn isolates in the infrequent AOM group and 28 (42%) Spn isolates in the otitis prone group. Mcat was isolated from the MEF in 7 (16%) of children with infrequent AOM and 9 (13%) from the otitis prone group. The distribution of the MEF isolates did not differ between the 2 groups, p=0.43, 0.56, and 0.78 for NTHi, Spn and Mcat, respectively. More than one otopathogen isolated from the MEF occurred rarely in both the infrequent AOM and otitis prone groups, 2 (4%) (both Spn and Mcat) and 3 (3%) (2 Spn and Mcat and 1 NTHi and Spn) AOM episodes, respectively.
Figure 2.
MEF and NP culture results in the infrequent AOM group and in the otitis prone group during an AOM episode.
During AOM, the most common otopathogen NP isolate was Spn, 24/67 (36%), in the infrequent AOM group and NTHi, 48/117 (41%), in the otitis prone groups. There was no difference between the two groups in the percentage of the 3 otopathogens in the NP when an AOM occurred, p=0.43, p=0.87 and p=0.21 for NTHi, Spn, and Mcat, respectively. Colonization with multiple pathogens did not differ between the two groups. S. aureus was rarely isolated from the NP during an AOM episode: 3 of 52 and 3 of 96 AOM episodes for the infrequent AOM and otitis prone groups, respectively p=0.38.
NP colonization with multiple otopathogens at the time of an AOM episode occurred in both groups. There was a trend for a difference between the absent/infrequent group, 23 of 52 (44%) compared with the otitis prone group 27 of 96 (28%) p=0.07 (Table 3a and 3b). The most common otopathogen combination in the NP was NTHi + Spn in both of the groups.
Table 3a.
The combinations of multiple otopathogens colonizing the NP during an AOM episode in the infrequent AOM group and the organisms that caused the AOM episode.
| NTHi + Spn | NTHi + Mcat | Spn + Mcat | NTHi + Spn + Mcat |
|
|---|---|---|---|---|
| NP colonization during AOM |
9/23 (39%) | 3/23 (13%) | 9/23 (39%) | 2/23 (9%) |
| MEF culture | NTHi 6/9 (67%) NG 3/9 (33%) |
Spn 1/3 (33%) NG 1/3 (33%) NTHi 1 (33%) |
Spn 3/9(33%) Mcat 2/9 (22%) Spn + Mcat 2/9 (22%) NG 2/9 (22%) |
NTHi 1/2 (50%) NG 1/2 (50%) |
There were 52 AOM episodes in the infrequent AOM group: more than 1 otopathogen colonizing the NP in 23 of those episodes (44%). NG = no growth.
Table 3b.
The combinations of multiple otopathogens colonizing the NP at the time of an AOM in the otitis prone group and the organisms that caused the AOM episode.
| NTHi + Spn | NTHi + Mcat | Spn + Mcat | NTHi + Spn + Mcat |
|
|---|---|---|---|---|
| NP colonization during AOM |
11/27 (41%) | 5/27 (19%) | 10/27 (37%) | 1/27 (4%) |
| MEF culture | NTHi 5/11 (45%) Spn 2/11 (18%) NG 3/11 (27%) |
NTHi 3/5 (60%) Mcat 1/5 (20%) NG 1/5 (20%) |
Spn 5/10 (50%) Spn+Mcat 1/10 (10%) NG 4/10 (40%) |
Spn 100% |
There were 96 AOM episodes in the otitis prone group and 27 of those had more than 1 otopathogen colonizing the NP (28%). NG = no growth
All Spn and S. aureus isolates from the MEF and NP were tested for oxacillin resistance and NTHi and Mcat isolates were tested for beta lactamase production. Nineteen percent of all the Spn during an AOM episode were penicillin non-susceptible (PNSP) in the infrequent AOM group while 56% were PNSP in the otitis prone group by oxacillin disc testing, p<0.0001. (Table 4) There was no difference in oxacillin resistant Spn isolation from the MEF but NP colonization with oxacillin resistant Spn was more common in the otitis prone group, p=0.02. Thirty-two percent and 48% of all NTHi were beta-lactamase producing in both the infrequent AOM group and in the otitis prone group, respectively (p=0.04). All M. cat isolates were beta-lactamase producing in both the infrequent and otitis prone groups. All of the S. aureus isolated were methicillin susceptible.
Table 4.
Presumptive antibiotic resistance of the MEF and NP organisms.
| Infrequent AOM Group | Otitis Prone Group | ||||
|---|---|---|---|---|---|
| Organism | MEF | NP during AOM |
NP not during AOM |
MEF | NP during AOM |
|
S. pneumoniae oxacillin resistant (%) |
4/21 (19)*+ | 6/21 (29)+ | 18/106 (17) | 14/28 (50)*+ | 27/44 (61)+ |
|
H. influenzae beta lactamase + (%) |
6/16 (37)^ | 12/33 (36) | 21/73 (29) | 15/30 (50)^ | 18/39 (46) |
|
M. catarrhalis beta lactamase + N (%) |
7/7 (100) | 20/20 (100) | 89/89 (100) | 9/9 (100) | 25/25 (100) |
|
S. aureus methicillin resistant (%) |
0 | 0 | 0 | 0 | 0 |
P=0.04
MEF + NP during AOM p = <0.0001
p=0.54
DISCUSSION
This prospective study evaluated the MEF isolates and NP colonization patterns from PCV7 vaccinated children 6 to 8 years after the introduction of PCV7 vaccine in the U.S and compared the microbiology of AOM and NP colonization between a cohort of children without AOM, with children experiencing their 1st or 2nd AOM episode, and with children who are otitis prone. Our major findings were: (1) an increase in frequency of isolation of Spn since 2001-2003 due to non-PCV7 serotypes, most commonly 19A such that Spn and NTHi isolations from the MEF were nearly equal in children with infrequent AOM and otitis prone children; (2) the near complete elimination of vaccine-type Spn from the MEF and NP for all 200 children evaluated; (3) the rank order of NP colonization frequency among the 3 main otopathogens was Spn, Mcat and NTHi, the frequency of NP colonization did not differ across the age span of 6-30 months of age, and an otopathogen colonized the NP of children in 63% of all samplings; (4) the first US study to show no significant difference in the proportion of otopathogens from the MEF or NP in children with their 1st or 2nd AOM were compared to otitis prone children; (5) among Spn initially identified as serotype 6A, 64% were found to be serotype 6C by PCR; (6) more frequent isolation of PNSP and beta-lactamase producing NTHi from the MEF and NP of otitis prone children compared to children with their 1st or 2nd AOM; (7) no increase in S. aureus NP colonization.
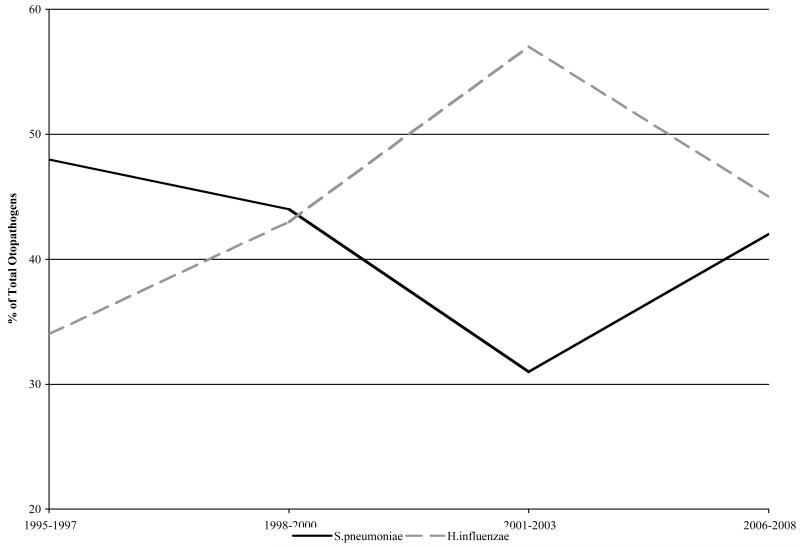
Prior to the introduction of PCV7, multiple studies identified Spn as the most common AOM pathogen, NTHi second and Mcat third. [21-24], including studies from our own center [25,26]. Initial studies of the PCV7 vaccine showed a significant decrease in AOM episodes in vaccine recipients. [5] In the first few years following PCV7 introduction, our group [11] and Block et al [12] described a shift in the predominant otopathogen among mostly otitis prone children from Spn to NTHi. Shortly thereafter, a shift to non-PCV 7 Spn isolates was described [27,28] and most recently a highly resistant serotype 19A Spn was identified. [29] In this current report, we described that the proportion of Spn causing AOM has surged back to equal that of NTHi in otitis prone children due to increasing AOM caused by non-PCV7 serotypes, (Figure 3). The consequences of the anticipated introduction of an expanded pneumococcal vaccine, PCV13, should have a favorable, immediate and positive impact in reducing the most common non-PCV7 serotypes, especially serotype 19A. . The PCV13 vaccine will include the PCV7 serotypes plus 1, 3, 5, 6A, 7F, and 19A. Our study shows that the 6 added serotypes in the PCV13 would include 23% of the Spn colonizing the NP during healthy visits. During an episode of AOM the added PCV13 serotypes were isolated from the NP 34% and MEF 47% of the time. Therefore, the PCV13 would add significant protection against additional serotypes of Spn that are currently colonizing the NP and causing AOM.
Figure 3.
Percentage of S. pneumoniae and NTHi causing rAOM in Rochester, NY 1995-2008.
As far as we are aware, this is the first study from the US to comprehensively evaluate the proportion of otopathogens colonizing the NP of healthy children and children with AOM. In the early 1990s, nearly a decade before the introduction of PCV7, Faden et al [30] conducted a similar study exclusively involving NTHi colonization in healthy children. At 12 months of age, Faden et al found the cumulative acquisition rate of NTHi was 34%, which was similar to our rate of 36%. However, by 2 years of age, our cumulative NTHi acquisition rate of 84% was nearly two-fold higher than Faden et al (44%). Factors such as number of siblings, daycare attendance, frequency of upper respiratory tract infection, and diet (breast/bottle feeding) can affect NP colonization patterns [31-33]. The higher NTHi colonization rate in our group could be explained by an increase in NTHi due to a decrease in Spn NP colonization as a result of PCV7 vaccination or the higher daycare attendance rate in our study population. The epidemiology of pneumococcal colonization and infection during the first 24 months of age was studied in the pre-PCV7 era. [34] Shortly after the introduction of PCV7 several studies showed a reduction of NP colonization of PCV7 serotypes and then the beginning of serotype replacement with non-PCV7 serotypes. [13,35,36] Here, 6 to 8 years after introduction of PCV7, we describe the virtual elimination of PCV7 serotypes and a surging increase in pneumococcal colonization by non-PCV7 serotypes.
For many years, scientists in the field of AOM speculated on whether tympanocentesis findings predominantly obtained from otitis prone children were representative of children with their 1st or 2nd AOM episode. Studies from Finland suggested that the pathogen mix was similar [24]. Ours is the first study in the US to compare otopathogens from a cohort of children who are otitis prone with a cohort of children experiencing their 1st and 2nd AOM episodes. In agreement with the Finnish results, we found that the otopathogens and proportions are the same in these two populations. However, as might have been anticipated, due to antibiotic selection pressure more children in the otitis prone group, having previously received antibiotics, harbored organisms in their NP and became infected in their middle ear with PNSP and beta-lactamase producing NTHi.
The repeating capsular polysaccharide for serotype subtypes 6A (galactose-glucose-rhamnose-ribitol-phosphate) varies from 6C (glucose-glucose-rhamnose-ribitol-phosphate) by one carbohydrate [37] and current latex agglutination method for serotyping does not distinguish 6C from 6A. Previous work by Jacobs et al [38] and our current study demonstrated that at least half of isolated 6A serotypes identified by latex agglutination are actually 6C. Our study shows that both 6A and 6C are colonizing and causing AOM. Nahm et al [39] has demonstrated that the proportion of 6C isolates has increased since the introduction of PCV7. Therefore, future pneumococcal work needs to identify 6C from 6A and future vaccines may need to incorporate 6C.
The presence of 2 otopathogens in the NP was not uncommon in our study population. The possibility that combinations of potential otopathogens facilitate co-colonization (especially when a concurrent viral upper respiratory tract infection occurs) by modulating the innate and adaptive immune response of children and/or by forming biofilms is now under study by our group. Although 2 or 3 otopathogens colonized the NP typically only 1 pathogen caused AOM. This process of microbial competition and the virulence features of the organisms is also being evaluated by our group.
The possibility of increased colonization by S. aureus has been previously studied with differing conclusions. Two studies showed that S. aureus NP carriage occurred more frequently among children vaccinated with PCV7 [40,41]. However, in a French surveillance study [18], the proportion of children that carried S. aureus alone or with S. pneumoniae was similar among PCV7-vaccinated children (3.9% and 5.1%, respectively) and non-vaccinated children (3.5% and 5.1%, respectively). Among 1783 children in that study 60.8% carried Spn and 9% carried S. aureus. Among children < 2 years old with AOM, S. aureus carriage was found in 9% of PCV7 vaccinated and 8.7% of non-vaccinated children. Our colonization rate for S. aureus in a PCV7 immunized population was 9%, and all S. aureus were methicillin susceptible. This rate does not suggest that S. aureus is replacing S. pneumoniae as a frequent colonizer.
As far as we are aware we are the only center in the U.S. prospectively tracking the NP colonization patterns and performing tympanocentesis on young children with AOM. Limitations of our study include that it occurred in one center comprised mostly of children living in suburban, middle-income households in the US. The observations are made in a specific time frame (2007-2009), in a PCV7 vaccinated population, and in a community where widespread and early adoption of PCV7 vaccinations likely established herd immunity. Other study populations in the U.S. and in other countries where PCV7 has not been widely used or only recently adopted would likely have different results. Comprehensive antibiotic susceptibility testing of the organisms isolated in our study is underway and will be reported elsewhere by Hardy et al [42].
In conclusion Six to 8 years after widespread use of PCV7, Spn strains expressing vaccine-type serotypes have essentially disappeared from the NP and MEF of vaccinated children. NP colonization and AOM by Spn is returning to near pre-PCV7 rates due to non-PCV7 strains. The proportion of otopathogens in infrequent or absent AOM and in otitis prone children is very similar although otitis prone children are colonized and experience AOM more frequently from antibiotic resistant Spn and NTHi. NTHi continues to be a major AOM pathogen in children with their 1st or 2nd AOM and in otitis prone children.
Acknowledgements
Work supported by R01DC08671, Thrasher Research Fund and an investigator-initiated grant from Wyeth Vaccines (all to MEP). This study would not have been possible without the help and dedication of our study coordinator, Sally Thomas LPN, CCRC, our nurses and staff, collaborating pediatricians from Long Pond Pediatrics, Genesis Pediatrics, Rainbow Pediatrics and Lewis Pediatrics, and the parents who consented to this long and challenging study.
REFERENCES
- 1.Dagan R, Melamed R, Muallem M, et al. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J Infect Dis. 1996;174:1271–1278. doi: 10.1093/infdis/174.6.1271. [DOI] [PubMed] [Google Scholar]
- 2.Dagan R, Muallem M, Melamed R, Leroy O, Yagupsky P. Reduction of pneumococcal nasopharyngeal carriage in early infancy after immunization with tetravalent pneumococcal vaccines conjugated to either tetanus toxoid or diphtheria toxoid. Pediatr Infect Dis J. 1997;16:1060–1064. doi: 10.1097/00006454-199711000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Dagan R, Givon-Lavi N, Zamir O, Fraser D. Effect of a nonavalent conjugate vaccine on carriage of antibiotic-resistant Streptococcus pneumoniae in day-care centers. Pediatr Infect Dis J. 2003;22:532–540. doi: 10.1097/01.inf.0000069761.11093.c3. [DOI] [PubMed] [Google Scholar]
- 4.Jones VF, Harrison C, Stout GG, Hopkins J. Nasopharyngeal colonization with heptavalent pneumococcal conjugate vaccine serotypes of Streptococcus pneumoniae with prolonged vaccine dosing intervals. Pediatr Infect Dis J. 2005;24:969–973. doi: 10.1097/01.inf.0000187030.83080.8a. [DOI] [PubMed] [Google Scholar]
- 5.Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344:403–409. doi: 10.1056/NEJM200102083440602. [DOI] [PubMed] [Google Scholar]
- 6.Poehling KA, Talbot TR, Griffin MR, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. Jama. 2006;295:1668–1674. doi: 10.1001/jama.295.14.1668. [DOI] [PubMed] [Google Scholar]
- 7.Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–1746. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 8.Black S, Shinefield H, Baxter R, et al. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal conjugate vaccine in Northern California Kaiser Permanente. Pediatr Infect Dis J. 2004;23:485–489. doi: 10.1097/01.inf.0000129685.04847.94. [DOI] [PubMed] [Google Scholar]
- 9.Whitney CG, Pilishvili T, Farley MM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368:1495–1502. doi: 10.1016/S0140-6736(06)69637-2. [DOI] [PubMed] [Google Scholar]
- 10.Millar EV, Watt JP, Bronsdon MA, et al. Indirect Effect of 7-Valent Pneumococcal Conjugate Vaccine on Pneumococcal Colonization among Unvaccinated Household Members. Clin Infect Dis. 2008;47:989–96. doi: 10.1086/591966. [DOI] [PubMed] [Google Scholar]
- 11.Casey JR, Pichichero ME. Changes in frequency and pathogens causing acute otitis media in 1995-2003. Pediatr Infect Dis J. 2004;23:824–828. doi: 10.1097/01.inf.0000136871.51792.19. [DOI] [PubMed] [Google Scholar]
- 12.Block SL, Hedrick J, Harrison CJ, et al. Community-wide vaccination with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr Infect Dis J. 2004;23:829–833. doi: 10.1097/01.inf.0000136868.91756.80. [DOI] [PubMed] [Google Scholar]
- 13.Pelton SI, Loughlin AM, Marchant CD. Seven valent pneumococcal conjugate vaccine immunization in two Boston communities: changes in serotypes and antimicrobial susceptibility among Streptococcus pneumoniae isolates. Pediatr Infect Dis J. 2004;23:1015–1022. doi: 10.1097/01.inf.0000143645.58215.f0. [DOI] [PubMed] [Google Scholar]
- 14.Huang SS, Hinrichsen VL, Stevenson AE, et al. Continued Impact of Pneumococcal Conjugate Vaccine on Carriage in Young Children. Pediatr. 2009;124:e1–e11. doi: 10.1542/peds.2008-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richter SS, Heilmann KP, Dohrn CL, et al. Changing Epidemiology of Antimicrobial-resistant Streptococcus Pneumoniae in the United States, 2004-2005. Clin Infect Dis. 2009;48:e23–e33. doi: 10.1086/595857. [DOI] [PubMed] [Google Scholar]
- 16.Byington CL, Samore MH, Stoddard GJ, et al. Temporal trends of invasive disease due to Streptococcus pneumoniae among children in the intermountain west: emergence of nonvaccine serogroups. Clin Infect Dis. 2005;41:21–29. doi: 10.1086/430604. [DOI] [PubMed] [Google Scholar]
- 17.Farrell DJ, Klugman KP, Pichichero M. Increased antimicrobial resistance among nonvaccine serotypes of Streptococcus pneumoniae in the pediatric population after the introduction of 7-valent pneumococcal vaccine in the United States. Pediatr Infect Dis J. 2007;26:123–128. doi: 10.1097/01.inf.0000253059.84602.c3. [DOI] [PubMed] [Google Scholar]
- 18.Cohen R, Levy C, Thollot F, et al. Pneumococcal conjugate vaccine does not influence Staphylococcus aureus carriage in young children with acute otitis media. Clin Infect Dis. 2007;45:1583–1587. doi: 10.1086/523734. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan SL. Community-acquired methicillin-resistant Staphylococcus aureus infections in children. Semin Pediatr Infect Dis. 2006;17:113–119. doi: 10.1053/j.spid.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Park IH, Park S, Hollingshead SK, Nahm MH. Genetic basis for the new pneumococcal serotype, 6C. Infect Immun. 2007;75:4482–4489. doi: 10.1128/IAI.00510-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long SS, Henretig FM, Teter MJ, McGowan KL. Nasopharyngeal Flora and Acute Otitis Media. Infect and Immun. 1983;41:987–991. doi: 10.1128/iai.41.3.987-991.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bluestone CD, Klein JO. Otitis Media in Infants and Children. 4th Edition BC Decker Inc; Hamilton ON: 2007. pp. 101–126. [Google Scholar]
- 23.Del Beccaro MA, Mendelman PM, Inglis AF, et al. Bacteriology of acute otitis media: A new perspective. J Pediatr. 1992;120:81–84. doi: 10.1016/s0022-3476(05)80605-5. [DOI] [PubMed] [Google Scholar]
- 24.Kilpi T, Herva E, Kaijalainen T, et al. Bacteriology of acute otitis media in a cohort of Finnish children followed for the first two years of like. Pediatr Infect Dis J. 2001;20:654–662. doi: 10.1097/00006454-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Pichichero ME, Pichichero CL. Persistent acute otitis media:I. Causative pathogens. Pediatr Infect Dis J. 1995;14:178–183. doi: 10.1097/00006454-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Pichichero ME, McLinn S, Aronovitz G, et al. Cefprozil treatment of persistent and recurrent acute otitis media. Pediatr Infect Dis J. 1997;16:471–478. doi: 10.1097/00006454-199705000-00003. [DOI] [PubMed] [Google Scholar]
- 27.McEllistrem MC, Adams JM, Patel K, et al. Acute otitis media due to penicillin-nonsusceptible Streptococcus pneumoniae before and after the introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2005;40:1738–1744. doi: 10.1086/429908. [DOI] [PubMed] [Google Scholar]
- 28.McEllistrem MC, Adams J, Mason EO, Wald ER. Epidemiology of acute otitis media caused by Streptococcus pneumoniae before and after licensure of the 7-valent pneumococcal protein conjugate vaccine. J Infect Dis. 2003;188:1679–1684. doi: 10.1086/379665. [DOI] [PubMed] [Google Scholar]
- 29.Pichichero ME, Casey JR. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA. 2007;298:1772–1778. doi: 10.1001/jama.298.15.1772. [DOI] [PubMed] [Google Scholar]
- 30.Faden H, Duffy L, Wasielewski R, Wolf J, Krystofik D, Tung Y. Relationship between nasopharyngeal colonization and the development of otitis media in children. Tonawanda/Williamsville Pediatrics. J Infect Dis. 1997;175:1440–1445. doi: 10.1086/516477. [DOI] [PubMed] [Google Scholar]
- 31.Aniansson G, Alm B, Andersson B, et al. Nasopharyngeal colonization during the first year of life. J Infect Dis. 1992;165(Suppl 1):S38–S42. doi: 10.1093/infdis/165-supplement_1-s38. [DOI] [PubMed] [Google Scholar]
- 32.Howard AJ, Dunkin KT, Millar GW. Nasopharyngeal carriage and antibiotic resistance of Haemophilus influenzae in healthy children. Epidemiol Infect. 1988;100:193–203. doi: 10.1017/s0950268800067327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faden H, Waz MJ, Bernstein JM, Brodsky L, Stanievich J, Ogra PL. Nasopharyngeal flora in the first three years of life in normal and otitis-prone children. Ann Otol Rhinol Laryngol. 1991;100:612–615. doi: 10.1177/000348949110000802. [DOI] [PubMed] [Google Scholar]
- 34.Gray BM, Converse GM, Dillon HC. Epidemiologic Studies of Streptococcus pneumoniae in Infants: Acquisition, Carriage, and Infection during the First 24 months of Lile. J Infect Dis. 1980;142:923–933. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- 35.Moore MR, Hyde TB, Hennessy TW, et al. Impact of a conjugate vaccine on community-wide carriage of nonsusceptible Streptococcus pneumoniae in Alaska. J Infect Dis. 2004;190:2031–2038. doi: 10.1086/425422. [DOI] [PubMed] [Google Scholar]
- 36.Ghaffar F, Barton T, Lozano J, et al. Effect of the 7-valent pneumococcal conjugate vaccine on nasopharyngeal colonization by Streptococcus pneumoniae in the first 2 years of life. Clin Infect Dis. 2004;39:930–938. doi: 10.1086/423379. [DOI] [PubMed] [Google Scholar]
- 37.Park IH, Pritchard DG, Cartee R, Brandao A, Brandileone MC, Nahm MH. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J Clin Microbiol. 2007;45:1225–1233. doi: 10.1128/JCM.02199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobs MR, Bajaksouzian S, Bonomo RA, et al. Occurrence, distribution, and origins of Streptococcus pneumoniae Serotype 6C, a recently recognized serotype. J Clin Microbiol. 2009;47:64–72. doi: 10.1128/JCM.01524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nahm MH, Lin J, Finkelstein JA, Pelton SI. Increase in the prevalence of the newly discovered pneumococcal serotype 6C in the nasopharynx after introduction of pneumococcal conjugate vaccine. J Infect Dis. 2009;199:320–325. doi: 10.1086/596064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bogaert D, van Belkum A, Sluijter M, et al. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet. 2004;363:1871–1872. doi: 10.1016/S0140-6736(04)16357-5. [DOI] [PubMed] [Google Scholar]
- 41.Regev-Yochay G, Dagan R, Raz M. S. aureus (Sa) is inversely related to nasopharyngeal S. pneumonia (Sp) carriage in children and their parents; Program and Abstracts of the 5th International Symposium on Pneumococci and Pneumococcal Diseases; Alice Springs, Australia. 2006.p. 169. [Google Scholar]