Abstract
Free fatty acid receptor 4 (FFA4), previously known as GPR120, is a G protein-coupled receptor that promotes numerous anti-inflammatory and antidiabetic effects upon its agonism by long chained unsaturated fatty acids. We have previously demonstrated that agonism of FFA4 with docosahexaenoic acid (DHA) and alpha-linoleic acid (ALA) facilitates rapid and transient phosphorylation of FFA4 expressed ectopically on the surface of HEK293 cells. However, the precise mechanisms that promote FFA4 phosphorylation remain elusive. In the current study, we examined the mechanisms behind both heterologous and homologous phosphorylation of FFA4 and set out to identify the foci of FFA4 phosphorylation. Our results demonstrate that basal and heterologous phosphorylation of FFA4 are mediated by protein kinase C (PKC), while G protein-coupled receptor kinase 6 (GRK6) plays the predominant role in DHA-mediated phosphorylation of FFA4. Furthermore, we identify Thr347, Ser350, and Ser357 in the C-terminal tail as major sites of FFA4 phosphorylation. Concurrent mutation of these three sites leads to a FFA4 receptor that seemingly affects Gαq/11 signaling in a positive manner as demonstrated by heightened intracellular Ca+2 responses following agonism with DHA. Importantly, this phosphodefective FFA4 mutant lacked the ability to promote β -arrestin-2 recruitment to the cell membrane. Since many of the functionally beneficial physiological effects of FFA4 are noted to be β -arrestin mediated, these findings could provide insight into the structural requirements for FFA4 function.
1. INTRODUCTION
G protein-coupled receptors (GPCR) represent the largest and most diverse family of cell surface receptors, regulating a myriad of physiological processes (1). Agonism of GPCRs by their cognate endogenous ligands or synthetic agonists leads to activation of heterotrimeric guanine-nucleotide binding proteins (G-proteins) that, in turn, activate second-messenger-generating effectors such as adenylyl cyclase or phospholipase enzymes (1). Agonist-occupied GPCRs are quickly phosphorylated on serine/threonine residues by G protein-coupled receptor kinases (GRKs), a process termed homologous phosphorylation (2). GRK-mediated phosphorylation leads to high-affinity recruitment of β -arrestin partner proteins to the receptor, which physically uncouples the GPCR from further G-protein interactions and effectively desensitizes G-protein signaling (2). Importantly, β -arrestins behave as important scaffolding proteins and in doing so, link the GPCR with other cytosolic proteins thereby initiating G-protein-independent intracellular signals, as well as receptor endocytosis and trafficking (3). In addition to homologous, agonist-mediated phosphorylation, GPCRs can be phosphorylated by downstream mediators of other GPCRs that employ the same G-protein effector pathways. This process, commonly termed heterologous phosphorylation, typically involves the second messenger-activated serine/threonine kinases protein kinase A (PKA) and protein kinase C (PKC), which are linked to adenylyl cyclase and phospholipase cascades, respectively. As a consequence, GPCR phosphorylation is a critical regulator of G-protein signaling, initiation of β -arrestin signaling, and cell-surface expression, internalization, and recycling (3).
Recently, a subfamily of free-fatty acid receptors (FFA receptors) belonging to the GPCR superfamily have been discovered (4). This FFA receptor family includes FFA1 (originally described as GPR40), FFA2 (GPR43) and FFA3 (GPR41). Additionally, FFA4, also referred to as GPR120, has been shown to be densely expressed in human lungs and colon, as well as in adipocytes and macrophages, where it recognizes long-chained FFAs including palmitic acid, oleic acid, myristic acid, and importantly, the family of polyunsaturated omega-3 fatty acids, including α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) (5-6). Agonism of FFA4 has been shown to mediate endocrine processes including secretion of glucagon-like peptide-1 (GLP-1) and cholecystokinin from intestinal enteroendocrine cells (6-7), inhibition of ghrelin secretion from gastric ghrelin cells (8), and regulation of adipocyte differentiation (9). These results suggest that FFA4 may play an important role in regulation of endocrine function and indeed, FFA4 agonism has recently been shown to modulate numerous anti-inflammatory and insulin-sensitizing effects including suppression of TNF-α and IL-6 secretion from macrophages and enhancement of GLUT4 translocation and glucose uptake in adipocytes (5). FFA4−/− mice fed a high fat diet become obese, demonstrate decreased adipocyte differentiation and glucose metabolism, enhanced hepatic lipogenesis, and develop glucose intolerance (10). In humans, FFA4 expression in the adipose of obese subjects is elevated and a dysfunctional R270H receptor polymorphism is linked to increased risk of obesity (10). More recently, fatty acid agonism of FFA4 in mice has been shown to counteract diet-induced hypothalamic inflammation, leading to reduced adiposity and body weight (11). Taken together, these results make FFA4 a highly attractive target for treatment of type 2 diabetes and obesity.
While FFA4 has been shown to be linked to Gαq/11/IP3/Ca+2/PKC signaling pathway (12), the antidiabetic effects produced by suppression of macrophage-induced tissue inflammation and enhancement of insulin-sensitivity are fully dependent on FFA4-β -arrestin signaling (5). Furthermore, secretion of GLP-1 in response to FFA4 agonism was independent of the phospholipase C modulator U73122 (6), suggesting that as a whole, the beneficial antidiabetic effects of FFA4 agonism may be mediated by biased-signaling linked to the β -arrestin pathway.
Using a transient expressing HEK293 cell system, we have previously demonstrated that FFA4 is phosphorylated in its basal state and that agonism with ALA or DHA leads to an approximately 2-fold increase in phosphorylation, an effect that was rapid, occurring within one minute, and transient (13). Since β -arrestin, and hence, the putative beneficial signaling outcomes of FFA4 agonism are dependent on receptor phosphorylation, the goal of the present study was to characterize the intracellular mechanisms involved in homologous and heterologous phosphorylation of FFA4 and to begin to localize the site(s) of phosphorylation. Our results demonstrate that heterologous phosphorylation occurs via PKC while both GRK6 and PKC contribute to homologous phosphorylation. Using a site-directed mutagenesis based-approach, we also identify Thr347, Ser350, and Ser357 in the C-terminal tail as primary sites of phosphorylation by both kinases. Creation of a phosphodefective FFA4 mutant demonstrates a marked inability to recruit and interact with β -arrestin-2, while it shows enhanced ability to mobilize Ca+2 signals.
2. MATERIALS AND METHODS
2.1 Reagents and Chemicals
Flag-tagged FFA4 (short isoform) was created and inserted into the pcDNA3.1 plasmid as described previously (13). The pcDNA3-β2-adrenergic receptor plasmid was a kind gift from Dr. Robert J. Lefkowitz (Duke University Medical Center) and the pcDNA3.1-histamine H1 receptor plasmid was purchased from the Missouri S&T cDNA Resource Center (www.cdna.org). Purified docosahexaenoic acid oil was purchased from Nu-Chek Prep (Elysian, MN). Phorbol 12-myristate 13-acetate (PMA), H-89, forskolin, histamine, bisindolylmaleimide II, and isoproterenol hydrochloride were obtained from Sigma-Aldrich (St. Louis, MO). All other chemicals used were obtained at the highest available purity from Thermo Fisher Scientific (Waltham, MA) or Sigma-Aldrich.
2.2 Site-directed mutagenesis of FFA4
For generation of FFA4 mutants, the appropriate residue of the wild-type receptor was mutated to alanine via overlapping polymerase chain reactions using the specific mutation-containing primers and the QuikChange II XL site-directed mutagenesis kit (Agilent Technologies, Cedar Creek, TX), as directed by the manufacturer’s directions. All mutants were sequence verified by DNA sequencing (MCLAB, S. San Francisco, CA).
2.3 Cell culture, transfection, and treatment
Human embryonic kidney (HEK293) cells were obtained from ATCC (Manassas, VA) and were cultured in 100 mm plates containing Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (Life Technologies, Grand Island, NY). Transfections were performed using LipoD293 reagent (Signagen Laboratories, Gaithersburg, MD), according to the manufacturer’s directions, exactly as we have previously reported (13). For experiments with siRNA, HEK293 cells were cotransfected with plasmid encoding for FFA4 along with siRNA specific to each GRK, or scrambled control, exactly as described previously (14).
2. 4 Immunoblotting
Immunoblotting was performed as we have described previously (15). Briefly, cells were lysed in RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 5 mM EDTA, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 10 mM NaF, 10 mM Na2HPO4, pH 7.4) plus protease inhibitor cocktail for 30 min and cleared for insoluble debris by centrifugation at 14,000 x g for 15 minutes at 4°C. Protein concentrations were standardized using DC Protein Assay (Bio-Rad, Hercules, CA) and an aliquot of the lysate was denatured in 2X SDS-sample buffer. For GRK immunoblots, samples were boiled for 5 minutes while FLAG-FFA4 samples were denatured at room temperature for 20 minutes. Equivalent concentrations of lysates were resolved by SDS–PAGE, followed by transfer to PVDF membranes, and immunoblotted with the appropriate antibody. Blots were visualized with HRP-conjugated secondary antibody followed by ECL. Where blots were stripped for reprobing, they were done so by incubation in glycine IgG stripping buffer for 30-45 minutes with agitation (25 mM Glycine, 1% SDS, 50°C, pH 2.0). Subtype-specific GRK antibodies against GRK2 (sc-562), 3 (sc-563), 5 (sc-565), and 6 (sc-566) and actin-HRP antibody were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
2.5 Whole cell phosphorylation assay
Assays were performed as we have previously described (13). Briefly, 48 hours following transfection, cells were starved in phosphate-free DMEM for 75 minutes and incubated with 0.2 mCi H332PO4 (Perkin Elmer, Waltham, MA) for 45 minutes, and then stimulated as described. Unstimulated control conditions replicated the vehicle for each given agent. Following treatment, cells were lysed at 4°C in RIPA/protease inhibitor cocktail for 60 min and then diluted with detergent-free RIPA buffer prior to centrifugation at 14,000 x g for 15 minutes at 4°C. Lysate protein content was assessed using the DC Protein Assay (Bio-Rad, Hercules, CA) and equivalent protein concentrations (1.0 - 1.5 mg/ml) were immunoprecipitated overnight at 4°C with anti-FLAG M2 antibody (Sigma Aldrich, St. Louis, MO) and protein G-agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA). Beads were washed three times with RIPA buffer and proteins eluted by addition of 1X SDS-sample buffer with 2.5% β-mercaptoethanol followed by resolution by SDS-PAGE. Gels were then dried and subjected to autoradiography at −80°C to detect 32P incorporation.
2.6 Cell surface expression assay
Cell surface expression of wild-type and mutant FLAG-FFA4 receptors was assessed by cell surface biotinylation followed by immunoblotting. Briefly, cell surface proteins were biotinylated using the cell-impermeable biotinylating agent Sulfo-NHS-SS-Biotin, based on the manufacturer’s directions (Thermo Scientific Pierce, Rockford, IL). Cells were washed three times in ice-cold PBS (pH 8), followed by tumbling for 2 hours in the presence of 2 mM sulfo-NHS-SS-Biotin at 4°C. Excess biotin was subsequently quenched by triplicate washes in ice-cold PBS/0.1 M glycine (pH 8) and cells were solubilized in RIPA buffer as described for phosphorylation assays. Lysates were standardized, an aliquot reserved, and equivalent protein concentrations tumbled overnight at 4°C with 30 μl of streptavidin-agarose (Thermo Scientific Pierce) to pull-down biotinylated proteins. Beads were washed three times in wash buffer (50 mM Tris-HCl, 150 NaCl, 5 mM EDTA, 0.5% Triton-X-100) and proteins eluted in 2X SDS-sample buffer with 2.5% 2-mercaptoethanol/0.1 M DTT to uncouple the biotinylated proteins from the beads. Samples, as well as the corresponding reserved inputs were subjected to SDS-PAGE, transferred to PVDF membranes and immunoblotted with anti-FLAG antibody to detect biotinylated, cell surface-expressed FFA4 receptors.
2.7 Intracellular Ca+2 Assay
HEK293 cells were co-transfected with pcDNA3-Gα15 and either wild-type or T347A/S350A/S357A FFA4 plasmids, as reported by others (5). Cells were seeded at 400,000 cells/well and the assays were performed 48 hr post-transfection using the Fluo-4 NW assay (Life Technologies, Grand Island, NY) as per the manufacturer’s instructions, with minor modifications. Briefly, cells were serum starved for 4 hr, washed twice with PBS, and incubated under light exclusion at 37 °C with Fluo-4 NW in 1x HBSS/20 mM HEPES/2.5 mM probenecid for 45 min. Following determination of basal fluorescence at 485 ± 20 nm excitation/528 ± 20 nm emission, equal volumes of 2X DHA were added to treatment wells and fluorescence measured for 10 intervals at 4 seconds each using a Synergy HT fluorescence plate reader.
2.8 Visualization of β-arrestin recruitment
HEK293 cells were transiently transfected with the appropriate FFA4 plasmid as in the phosphorylation assays, but also in the presence of pGFP(N1)-topaz-β-arrestin-2-YFP (2 μg) (a kind gift from from Dr. Michel Bouvier, University of Montreal). Cells were seeded onto glass coverslips 24 hr post-transfection. On the day of the experiment, cells were treated exactly as in the phosphorylation assays with a two hour serum-starvation period, followed by addition of 100 μM DHA for 5 min. Following agonism, cells were washed in ice-cold PBS and fixed in 4% paraformaldehyde for 15 min at 4°C. Once dried, cover slips were mounted onto glass slides and images were obtained using an Olympus CX41 microscope equipped with an argon ion laser emitting at 488 ± 20 nm.
2.9 Coimmunoprecipitation
Cells were transfected and stimulated as in recruitment assays above. Following agonism, cells were washed three times in iced-PBS and lysed in coimmunoprecipitation lysis buffer (25mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 2% glycerol) for 30 min on ice. Lysates were cleared of insoluble debris as above and protein content normalized. An aliquot of the input lysate was reserved and the remaining lysate was immunoprecipitated with anti-FLAG M2 antibody and protein G-agarose beads, as in whole cell phosphorylation assays. Following resolution of proteins by SDS-PAGE and transfer to PVDF, membranes were immunoblotted with anti-arrestin-1/2 antibody (Santa Cruz Biotechnology).
2.10 Data analysis
Autoradiographic data were quantified by densitometric analysis within the calibrated linear range using NIH Image J (Bethesda, MD) and graphed using Graphpad Prism 3.0 (San Diego, CA). Data are expressed as mean ± S.E.M for representative experiments repeated at least three independent times. Where not visible, error bars fall within the symbol size. Statistical analysis was performed, as appropriate, either by one-way analysis of variance and post-hoc Tukey’s test or by Student’s t-test using Graphpad Instat (San Diego, CA). Statistical significance is represented as a single symbol for p < 0.05, a double symbol for p < 0.01, and a triple symbol for p < 0.001, as noted in the figure legends.
3. RESULTS
3.1 Homologous phosphorylation of FFA4 is mediated by GRK6
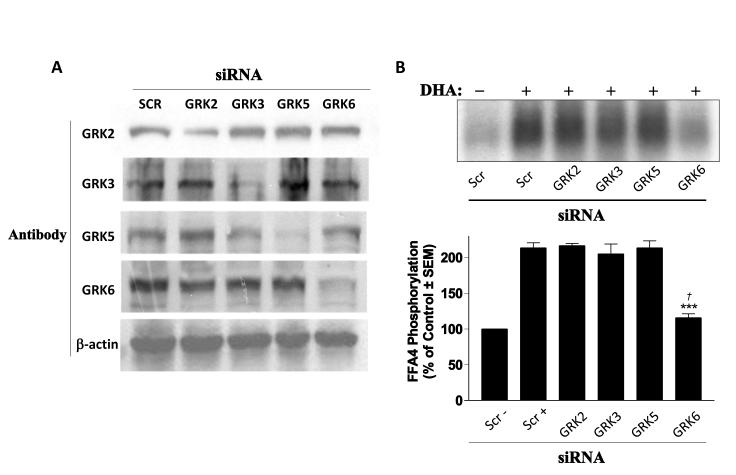
To date, seven distinct GRK isoforms have been identified (16). While GRK1 and 7 are expressed almost exclusively in the retina and GRK4 is limited to the testes, GRKs 2, 3, 5, and 6 are expressed ubiquitously (17). Here, we utilized the HEK293 cell model, which expresses the four ubiquitous GRK isoforms (14) and, in which, we have previously demonstrated rapid and transient agonist-mediated FFA4 phosphorylation (13). Transfection of isoform-specific GRK siRNA into HEK293 cells significantly reduced (≥70%) expression of the intended GRK compared to scrambled siRNA used as a control (Fig. 1A) and support the use of these siRNA in reducing GRK expression to assess FFA4 phosphorylation. Next, we performed whole cell phosphorylation assays using cells that express scrambled siRNA, or the GRK2, 3, 5, and 6-targeting siRNA, along with FLAG-FFA4. In cells expressing only scrambled siRNA, agonism with DHA for 5 min substantially increased FFA4 phosphorylation compared to the vehicle-treated control (Fig. 1B), similar to our previous report (13). DHA agonism increased FFA4 phosphorylation to the same level as the scrambled control condition (213 ± 7% of control) in cells treated with GRK2, GRK3, and GRK5-targeting siRNA (Fig. 1B). However, in cells treated with GRK6-targeting siRNA, the DHA-mediated effect was significantly reduced (115 ± 5% of control; p < 0.001 versus scrambled stimulated control) (Fig. 1B), demonstrating that of the ubiquitously-expressed GRK isoforms, GRK6 is responsible for significant homologous phosphorylation of FFA4. Despite significant reduction of the DHA-mediated signal, GRK6 knockdown was not sufficient to fully inhibit DHA-mediated FFA4 phosphorylation (p < 0.05 versus scrambled unstimulated control).
FIGURE 1. GRK-mediated phosphorylation of FFA4.
(A) siRNA-mediated knockdown of endogenous GRK isoforms in HEK293 cells. Specific GRK-targeting siRNA used is indicated at the top with Scr indicating scrambled siRNA control. The GRK-specific antibody used for immunoblot is indicated at the left with β-actin utilized as a loading control for each set. (B) DHA-mediated phosphorylation (213 ± 7% of scrambled control) is not affected by siRNA knockdown of GRK2 (217 ± 3%), GRK3 (205 ± 13%), or GRK5 (213 ± 9%), but is significantly attenuated upon GRK6 knockdown (11 5 ± 5% of control ). A representative autoradiograph is shown while the graph depicts pooled data from four independent experiments. *** denotes p < 0.001 versus scrambled, DHA-stimulated control. † denotes p < 0.05 versus scrambled unstimulated control.
3.2 Heterologous phosphorylation of FFA4 is mediated by PKC
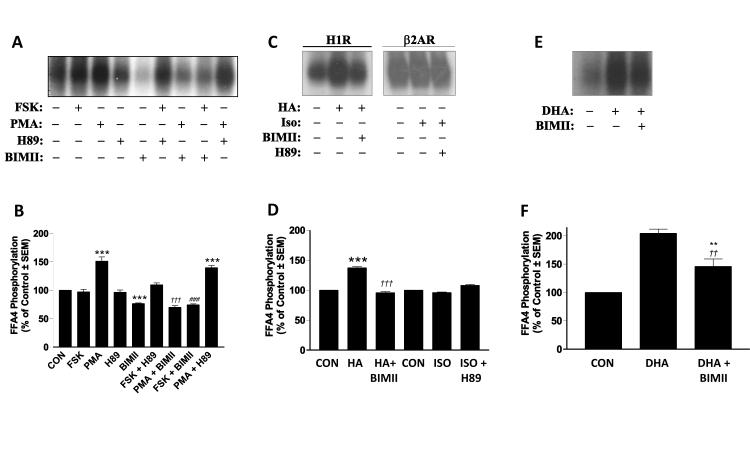
Next, we wished to determine which of the two primary second messenger-activated serine/threonine kinases, PKA and PKC, are responsible for heterologous phosphorylation of FFA4. Since HEK293 cells are known to express PKA in addition to the gamut of conventional (α, β), novel (δ, ε, η, θ), and atypical (ξ) PKC isozymes (18-21), which would make targeted siRNA-based approaches tedious, we utilized selective PKA and PKC activators and inhibitors to assess the role of the respective kinases in heterologous phosphorylation of FFA4. Stimulation of HEK293 cells expressing FLAG-FFA4 with the adenylyl cyclase activator forskolin (1μM, 20 min) had no significant effect on FFA4 phosphorylation (Fig. 2A-B). On the contrary, stimulation with the phorbol ester phorbol-12-myristate-13-acetate (PMA) (1μM, 20 min) resulted in a significant increase in FFA4 phosphorylation (151 ± 7.6% of control; p < 0.001 versus unstimulated control), demonstrating that PKC activation can facilitate FFA4 phosphorylation (Fig. 2A-B). Moreover, the selective PKA inhibitor H-89 (10μM, 20 min) had no effect on FFA4 phosphorylation while treatment with the PKC-selective inhibitor bisindolylmaleimide II (BIMII) reduced basal phosphorylation of FFA4 (77 ± 0.9% of control; p < 0.001 versus unstimulated control), again demonstrating that PKC is involved in second messenger-mediated phosphorylation of FFA4 (Fig. 2A-B). Importantly, FFA4 phosphorylation upon treatment with PMA was fully blocked by BIMII (70 ± 2.9% of control; p < 0.001 versus PMA alone), but not by H-89 (140 ± 3.8% of control; p < 0.001 versus unstimulated control) (Fig. 2A-B).
FIGURE 2. Heterologous phosphorylation of FFA4. (A-B).
Stimulation of HEK293 cells expressing FLAG-FFA4 with forskolin (FSK) (1μM, 20 min) had no significant effect on FFA4 phosphorylation while stimulation with phorbol-12-myristate-13-acetate (PMA) (1μM, 20 min) resulted in a significant increase in FFA4 phosphorylation (151 ± 7.6% of control). The selective PKA inhibitor H89 (10μM, 20 min) had no effect on basal or PMA-stimulated FFA4 phosphorylation while the selective PKC inhibitor BIMII (10μM, 20 min) reduced both basal (77 ± 0.9% of control) and PMA-stimulated (70 ± 2.9% of control) FFA4 phosphorylation. A representative autoradiograph is shown while the graph depicts pooled data from three independent experiments. *** denotes p < 0.001 versus unstimulated control; ††† denotes p < 0.001 versus PMA-stimulated; ### denotes p < 0.001 versus FSK alone. (C-D) Stimulation of cells expressing FLAG-FFA4/H1R (2μg) with histamine (10μM, 10 min) significantly increased FFA4 phosphorylation (139 ± 1.9% of control), an effect that was blocked by BIMII pretreatment (96 ± 1.5% of control). Stimulation of cells expressing FLAG-FFA4/β2AR (2μg) with ISO (1μM, 10 min) had no effect on FFA4 phosphorylation in the absence (96 ± 0.9% of control) or presence (108 ± 1.8% of control) of H89. Representative autoradiographs are shown while the graph depicts pooled data from two to three independent experiments. *** denotes p < 0.001 versus unstimulated control; ††† denotes p < 0.001 versus histamine-stimulated. (E-F) DHA-stimulated FFA4 phosphorylation (204 ± 7% of control) is partially, but significantly, decreased by BIMII pretreatment (146 ± 14% of control). A representative autoradiograph is shown while the graph depicts pooled data from four independent experiments ** denotes p < 0.01 versus unstimulated control; †† denotes p < 0.01 versus DHA-stimulated.
To confirm the effects of second messenger-associated kinase activity on FFA4 phosphorylation, we co-transfected HEK293 cells with FLAG-FFA4 and either the histamine H1 receptor (HR1) or the β2-adrenergic receptor (β2AR), agonism of which active the Gαq/11-PKC and GαS-PKA cascades, respectively. Stimulation of cells expressing FLAG-FFA4/H1R (2μg) with histamine (10μM, 10 min) caused a significant increase in phosphorylation of FFA4 (139 ± 1.9% of control; p < 0.001 versus unstimulated control) (Fig. 2C-D), and this effect was completely inhibited by BIMII pretreatment (96 ± 1.5% of control; p < 0.001 versus histamine-stimulated condition), confirming the role of PKC in heterologous phosphorylation of FFA4. Meanwhile, stimulation of cells expressing FLAG-FFA4/β2AR (2μg) with the β-receptor agonist isoproterenol (ISO; 1μM, 10 min) had no effect on FFA4 phosphorylation (Fig. 2C-D). Taken together, our results convincingly demonstrate that PKC, but not PKA, mediates basal and heterologous phosphorylation of FFA4. Since agonism of FFA4 with DHA would also be expected to activate the Gαq/11/IP3/Ca+2/PKC signaling cascade, we also assessed the contribution of PKC in this effect by pretreating DHA-stimulated cells with BIMII. These results show that DHA stimulated FFA4 phosphorylation (204 ± 7% of control; p < 0.001 versus unstimulated control) was slightly decreased upon BIMII pretreatment (150 ± 13 of control; p < 0.01 versus DHA-stimulated condition) (Fig. 2E-F). Together, our data demonstrate that DHA-stimulated phosphorylation of FFA4 is predominantly mediated by GRK, with a minor component that seems to be sensitive to the PKC cascade.
3.3 Identification of sites of FFA4 phosphorylation –
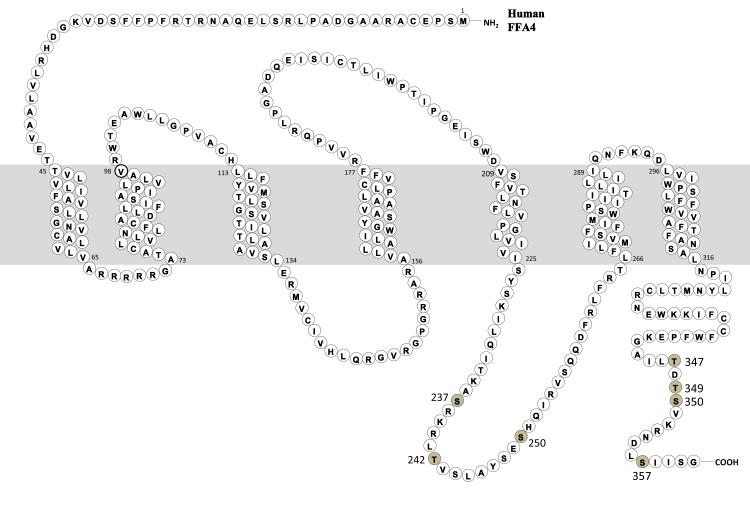
We utilized two distinct open-source computer algorithms to predict the site(s) of PKC and GRK mediated phosphorylation based on the protein sequence of FFA4 (data not shown). These results suggested that Ser237, Thr242 and Ser250 in the third intracellular loop (ICL) and Thr347, Ser350, and Ser357 in the C-terminus could be potential sites of FFA4 phosphorylation based on consensus flanking sequence (Fig. 3). To localize the specific foci of phosphorylation, we utilized site-directed mutagenesis to evaluate the role of each of these residues in FFA4 phosphorylation. Since DHA activates both the GRK and PKC phosphorylation axes, we screened the candidate Ser to Ala site-mutants for their ability to be phosphorylated following 5 min agonist stimulation. These results show that S237A, T242A, and S250A mutations to the 3rd ICL as well as T349A mutation in the C-terminus are phosphorylated to the same degree as wild-type (WT) FFA4, indicating that these are not significant sites of FFA4 phosphorylation (Fig. 4A).
FIGURE 3. Snake diagram of FFA4.
Phospholabile Serine/Threonine residues identified as potential sites of phosphorylation are depicted by shading and numbering.
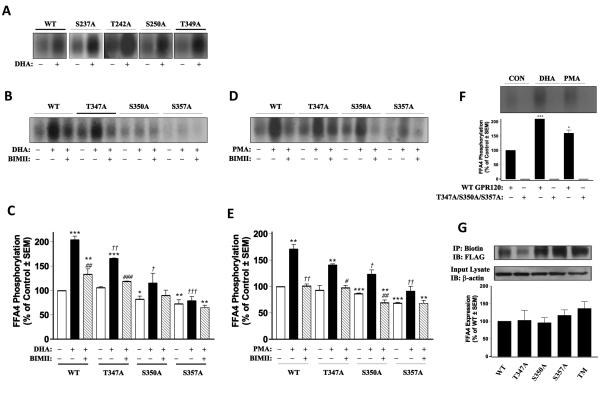
FIGURE 4. Identification of the foci of FFA4 phosphorylation.
(A) DHA-mediated phosphorylation of wild-type FLAG-FFA4 (WT) and individual S237A, T242A, S250A, and T349A FFA4 mutants reveals that each mutant is capable of being fully phosphorylated, similar to WT. Representative autoradiographs of at least two independent experiments are shown. (B-C) DHA-mediated phosphorylation of WT and individual T347A, S350A, and S357A mutants in the absence of presence of BIMII (10μM, 20 min). In WT-expressing cells, DHA-stimulated phosphorylation (204 ± 7.3% of unstimulated control) was partially inhibited by BIMII (133 ± 11% of control). In cells expressing T347A, DHA-stimulated phosphorylation was decreased (166 ± 0.8% of control) and BIMII further decreased (118 ± 0.6% of control) this effect. In cells expressing S350A, basal FFA4 phosphorylation was significantly reduced (84 ± 4% of control), as was the DHA-stimulated effect (115 ± 20% of control), which was further reduced by pretreatment with BIMII (90 ± 10.7% of control). In cells expressing S357A, basal FFA4 phosphorylation was significantly reduced (73 ± 5% of control), as was the DHA-stimulated effect (79 ± 8.5% of control), which was further reduced by pretreatment with BIMII (65 ± 5% of control). A representative autoradiograph is shown while the graph depicts pooled data from three to four independent experiments. A single symbol denotes p < 0.05, a double symbol denotes p < 0.01, and a triple symbol denotes p < 0.001. An * symbol denotes comparison versus unstimulated control, a # symbol denotes comparison versus the respective DHA-stimulated condition, a † symbol denotes comparison to the WT+DHA-stimulated condition. (D-E) PMA-mediated phosphorylation of WT and individual T347A, S350A, and S357A mutants in the absence of presence of BIMII (10μM, 20 min). In WT-expressing cells, PMA-stimulated phosphorylation (171 ± 8% of unstimulated control) was fully inhibited by BIMII (101 ± 3.5% of control). In cells expressing T347A, PMA-stimulated phosphorylation was decreased (141 ± 2% of control) versus WT+DHA, and BIMII inhibited this effect (98 ± 4.4% of control). In cells expressing S350A, basal FFA4 phosphorylation was significantly reduced (84 ± 4% of control), as was the PMA-stimulated effect (123 ± 8% of control), which was reduced to below levels by pretreatment with BIMII (70 ± 5% of control). In cells expressing S357A, basal FFA4 phosphorylation was basal significantly reduced (73 ± 5% of control), as was the PMA-stimulated effect (91 ± 9.2% of control), which was reduced below basal levels by pretreatment with BIMII (68 ± 5.5% of control). A representative autoradiograph is shown while the graph depicts pooled data from two to three independent experiments. A single symbol denotes p < 0.05, a double symbol denotes p < 0.01, and a triple symbol denotes p < 0.001. An * symbol denotes comparison versus unstimulated control, a # symbol denotes comparison versus the respective PMA-stimulated condition, a † symbol denotes comparison to the WT+PMA-stimulated condition. (F) DHA and PMA-mediated phosphorylation of WT and T347A/S350A/S357A triple mutant reveals an increase in phosphorylation of WT-FFA4 upon stimulation with DHA (222 ± 0.6% of control) and PMA (160 ± 11% of control), whereas stimulation of T347A/S350A/S357A-FFA4 was undetectable. The *** and * denote p < 0.001 and 0.05 compared to WT-unstimulated control, respectively. (G) Cell-surface biotinylation of WT and mutant FFA4 receptors reveals approximately equivalent receptor expression compared to WT. Cell surface receptors were biotinylated with NHS-S-S-biotin, immunoprecipitated with streptavidin-agarose and immunoblotted with anti-FLAG M2 antibody to determine expression (upper blot) which was quantified and compared to WT (graph). Blots were stripped and reprobed with β-actin to demonstrate equivalent protein loading (lower blot).
However, mutation of Thr347, Ser350, and Ser357 in the C-terminal tail caused significant alterations to the FFA4 phosphorylation profile. While basal phosphorylation of the T347A mutant was similar to WT, basal phosphorylation of both the S350A and S357A mutants were significantly decreased (84 ± 4 and 74 ± 5% of WT control, respectively; p < 0.01 and p < 0.01 versus WT control, respectively) (Fig. 4B-D). Agonism with DHA caused the standard approximately two-fold increase in phosphorylation of WT-FFA4 that we have previously shown, while DHA mediated-phosphorylation of T347A, S350A, and S357A was significantly reduced (166 ± 0.7%, 115 ± 20%, and 79 ± 4.9% of control, respectively; p < 0.01, p < 0.001, and p < 0.001, versus WT+DHA respectively) (Fig. 4B-C). Moreover, pretreatment with BIMII reduced the DHA-mediated response substantially for all three mutants (108 ± 7%, 90 ± 12%, and 65 ± 4.9% of control, respectively) (Fig. 4B-C). Notably, the effect of PKC inhibition by BIMII was complete for both S350A and S357A mutants but incomplete at the S347A mutant.
Additionally, we assessed the PKC mediated contribution by testing each mutant with PMA in the presence and absence of BIMII. These data show that while PMA is able to phosphorylate the T347A, S350A, and S357A mutants, the maximal level of PMA-induced phosphorylation is significantly reduced (Fig. 4D-E). Compared to wild-type FFA4 (171 ± 8% of unstimulated control; p < 0.01 versus unstimulated control), maximal PMA-induced phosphorylation at the T347A, S350A, and S357A mutants was 141 ± 2%, 123 ± 8%, and 91 ± 9%, respectively (p > 0.05, p < 0.05, p < 0.01 versus WT+DHA, respectively), demonstrating that PKC can also mediate phosphorylation at all three sites. Additional treatment with BIMII further reduced the PMA effect consistent with the PKC-mediated mechanism (Fig. 4D-E). Taken together, these data demonstrate that both GRK and PKC can phosphorylate FFA4 at Thr347, Ser350, and Ser357, with greater GRK influence on the former site and equal GRK/PKC contribution at the latter two sites.
To confirm these results, we created a T347A/S350A/S357A triple mutant and assessed its ability to be phosphorylated by both PMA and DHA. As demonstrated in Fig. 4F, contrary to WT-FFA4, the T347A/S350A/S357A triple mutant showed no appreciable phosphorylation upon treatment with DHA or PMA, validating that these sites are the three major foci of phosphorylation by both kinases.
To ensure that the phosphorylation results were not consequences of decreased cell surface expression of FFA4 mutants, we performed cell surface biotinylation experiments to visualize expression of WT-FFA4 compared to the T347A, S350A, S357A, and T347A/S350A/S357A triple mutant. Results of these experiments demonstrate all FFA mutants expressed appreciably to the cell surface and do not vary significantly from expression of WT-FFA4 (Fig. 4G).
3.4 Functional effect of phosphodefective FFA4
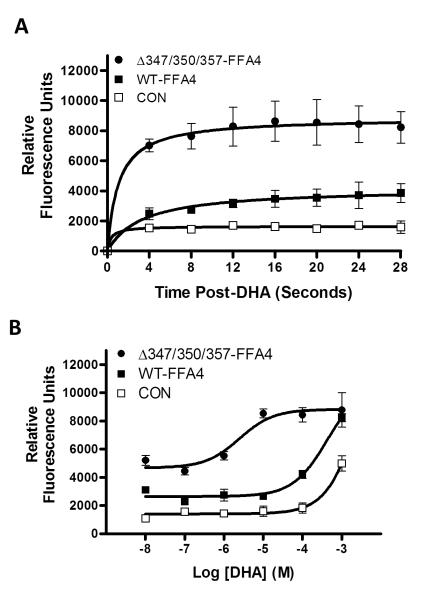
To assess if phosphorylation of FFA4 plays a role in receptor function, we compared the ability of WT-FFA4 and the T347A/S350A/S357A triple mutant to activate the Ca+2 and β-arrestin linked cascades. As shown in Fig. 5, agonism of cells expressing WT- FFA4 with DHA (100 μM) facilitated a rapid increase in intracellular Ca+2, and this effect was sustained for the first 30 seconds following agonism (12). Agonism of cells expressing the T347A/S350A/S357A FFA4 triple mutant lead to a significantly higher level of intracellular Ca+2 during this time course (Fig. 5A). Furthermore, DHA promoted a concentration-dependent increase in intracellular Ca+2 in WT-FFA4 expressing cells (pEC50 = −3.7 ± 0.2). In cells expressing the T347A/S350A/S357A triple mutant, the basal intracellular Ca+2 level was markedly higher compared to that seen in WT cells (5202 ± 352 RFU and 3099 ± 72 RFU, respectively) and the dose-response curve was significantly left-shifted (pEC50 = −5.6 ± 0.3) (Fig. 5B). These results imply that phosphodefective FFA4 is capable of signaling through the Gαq/11 pathway, where it is in fact, appreciably more efficacious compared to WT-FFA4.
FIGURE 5. Effects of phosphodefective FFA4 on intracellular Ca+2 signaling.
(A) Intracellular Ca+2 was measured by Fluo-4NW fluorescence for 4 seconds prior to addition of DHA (100 μM) and then for 28 seconds in control HEK293 cells as well as those expressing WT-FFA4 or the T347A/S350A/S357A triple mutant (ΔT347A/S350A/S357A). One-way ANOVA analysis revealed significant differences in the mean s of the three groups (p < 0.0001) with post-hoc Tukey’s comparison revealing p < 0.01 for the CON versus WT-FFA4 response, p < 0.001 for WT-FFA4 versus ΔT347A/S350A/S357A response, and p < 0.001 for CON versus ΔT347A/S350A/S357A response. Due to inter -experimental differences in control values in the FLUO-4NW assay, which can affect experimental RFUs, data from two independent experiments with similar control values were pooled. Experiments were conducted at least four times. (B) DHA-dose response reveals leftward shift in the dose-response of ΔT347A/S350A/S357A (pEC50 = −5.6 ± 0.3) compared to WT-FFA4 (−3.7 ± 0.2), while the Emax is reached at nearly two-log units sooner in ΔT347A/S350A/S357A (ca. 10 .M) compared to WT (ca. 1 mM). Results represent pooled data from three independent experiments with similar control values.
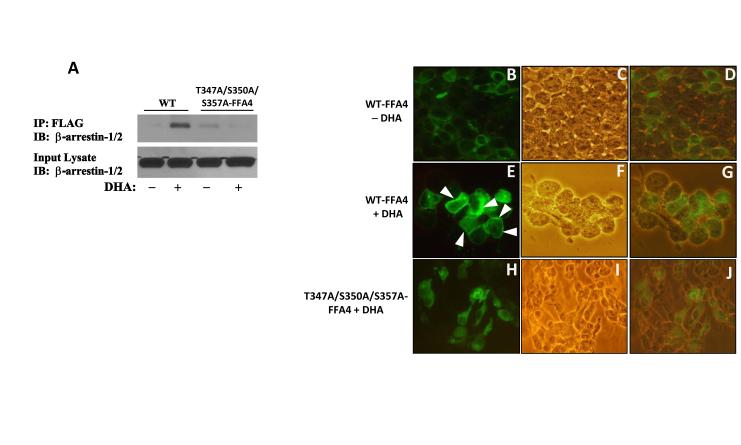
Since agonism of FFA4 has been shown to promote the association of the receptor with β-arrestin-2, but not β-arrestin-1, we next utilized coimmunoprecipitation to compare the ability of WT-FFA4 or the T347A/S350A/S357A FFA4 triple mutant to directly interact with β-arrestin-2. Cells were cotransfected with WT-FFA4 or the T347A/S350A/S357A FFA4 triple mutant, as well as β-arrestin-YFP and then stimulated for 5 min with DHA. Results of these experiments clearly demonstrated that agonism of WT-FFA4 with DHA (100 μM, 5 min) leads to pronounced interaction of the receptor with β-arrestin-2, while agonism of T347A/S350A/S357A-FFA4 with DHA (100 μM, 5 min) showed no increase in receptor-β-arrestin-2 association (Fig 6A). Next, we utilized fluorescent microscopy to visualize the effects that phosphodefective FFA4 may have on β-arrestin recruitment. In WT-FFA4 expressing cells (Fig. 6B-D), β-arrestin-YFP was found to be localized to the cytosol as expected (Fig. 6B). Upon agonism of WT-FFA4 with DHA (100 μM, 5 min) (Fig. 6E-G), the fluorescent signal representing β-arrestin-YFP was prominently associated with the cell-surface, indicative of agonist-directed recruitment of β-arrestin to FFA4 (Fig. 6E). On the contrary, in cells expressing the phosphodefective FFA4 triple mutant (Fig 6. H-J), there was no clear membrane association of β-arrestin-YFP following DHA stimulation (Fig. 6H). Furthermore, these cells displayed primarily cytosolic β-arrestin-YFP that mimicked unstimulated cells (Fig. 6A). Taken together, these results suggest that phosphodefective FFA4 (i.e., the T347A/S350A/S357A mutant) is incapable of recruiting and interacting with β-arrestin.
FIGURE 6. Interaction of FFA4 with β-arrestin and membrane recruitment of β-arrestin-2-YFP.
(A) Cells coexpressing either WT-FFA4 or T347A/S350A/S357A-FFA4 along with β-arrestin-YFP were left unstimulated or stimulated with DHA (100 μM) for 5 min, washed in PBS and lysed. Lysates were immunoprecipitated with FLAG antibody/protein G-agarose, resolved by SDS-PAGE and immunoblotted with anti-β-arrestin-1/2 antibodies. An aliquot of the input lysate was also probed to demonstrate equivalent arrestin expression. Experiments were repeated three times. For validation by microscopy, cells coexpressing either WT-FFA4 (B-G) or T347A/S350A/S357A-FFA4 (H-J) along with β-arrestin-YFP were left unstimulated (B-D) or stimulated with DHA (100 μM) for 5 min (E-J). Cells were washed in PBS, fixed in 4% paraformaldehyde and mounted on slides. The left columns repr esent fluorescence indicative of β-arrestin-YFP, the center columns represent phase-contrast images indicative of the cell population, and the right columns represent phase/fluorescent overlays. (B) In unstimulated cells expressing WT-FFA4, β-arrestin-YFP is localized to the cytosol. (E) In cells expressing WT -FFA4, agonism with DHA (100 μM, 5 min) causes marked recruitment of β-arrestin-YFP to the cell membrane. White arrows indicate dense membrane localization of β-arrestin-YFP. (H) In cells expressing T347A/S350A/S357A-FFA4, agonism with DHA (100 μM, 5 min) had no effect on recruitment of β-arrestin-YFP to the membrane but rather, β- arrestin-YFP remained localized in the cytosol as seen in unstimulated cells. Images, at 400X magnification, were obtained using an Olympus CX41 microscope equipped with an argon ion laser emitting at 488 ± 20 nm.
4. DISCUSSION
Previously, we have reported that FFA4 is phosphorylated in its basal state and that agonism with polyunsaturated (omega-3) fatty acids facilitates a rapid and transient increase in receptor phosphorylation (13). In the current study, we examined mechanisms of both heterologous and homologous phosphorylation of FFA4 in HEK293 cells. Importantly, our results demonstrate that FFA4 phosphorylation is localized to three primary sites, Thr347, Ser350, and Ser357. Our findings that the selective PKC inhibitor BIMII, but not GRK knockdown, decreased basal (i.e., in the absence of DHA) FFA4 phosphorylation (Fig. 2A-B) demonstrate that PKC plays a significant role in basal FFA4 phosphorylation. In support of this hypothesis, our results also reveal that mutation of the Ser350 and Ser357 residues leads to significant decreases in basal FFA4 phosphorylation (Fig. 4B-E). Taken together, these data suggest that heterologous activation of the Gαq/11/IP3/Ca+2/PKC signaling cascade can lead to constitutive phosphorylation of FFA4, at Ser350/Ser357, in its basal state. Meanwhile, activation of this signaling axis with PMA further increased FFA4 phosphorylation and this effect was also mimicked by agonism of exogenously expressed H1R, which is also linked to PKC signaling. These data unambiguously implicate PKC in heterologous phosphoregulation of FFA4.
Homologous phosphorylation of GPCRs via GRK activity has been shown to be independent of agonist- induced second messenger signals for a variety of Gαq/11-coupled GPCRs including the α1B-adrenergic (22) muscarinic M1 and M3 (23-24), and the B2 bradykinin receptors (25). Our results demonstrate that DHA-mediated phosphorylation is predominantly facilitated by GRK6, however, a small component (ca. 15%) was insensitive to the GRK6 knockdown (Fig. 1B). This component could be the consequence of incomplete GRK6 knockdown (Fig. 1A), or alternatively, a sensitivity to PKC. Our results with DHA in the absence and presence BIMII demonstrate that a small component of agonist-mediated phosphorylation of FFA4 is indeed sensitive to PKC inhibition (Fig. 2E-F and Fig. 4B-C). However, our data do not exclude the possibility that the effects of PKC here may not be directly on the receptor, but may rather affect GRK6. In this regard, knockdown of GRK6 (Fig 1B) and pretreatment of DHA-agonism with BIMII (Fig. 2E) reveals a small fraction (ca. 15%) of agonist-mediated FFA4 phosphorylation, suggesting that there may be a corequirement of crosstalk between the two kinases. Such crosstalk between PKC and GRKs has been deliberated for phosphorylation of dopamine D2 receptors, where prior phosphorylation by the second-messenger linked kinase could presumably enhance the phosphorylation by GRK at neighboring serines or threonine residues (26). It could be conceivable then that basal PKC phosphorylation of FFA4 could affect further DHA-mediated phosphorylation. Nonetheless, the current study demonstrates that like the similarly Gαq/11-coupled angiotensin II-1A (27), platelet-activating factor (2894), and cholecystokinin receptors (29), agonist-induced phosphorylation of FFA4 is mediated predominantly by both G protein-coupled receptor kinases, with a minor contribution, either indirectly or directly, from PKC. In each of these instances, PKC inhibition completely inhibits PMA-mediated receptor phosphorylation and only inhibits agonist-mediated phosphorylation to a minor extent. Additionally, in each case, agonist-mediated phosphorylation was greater than that elicited by PMA, consistent with our observation that DHA causes a 1.75- to 2-fold increase in FFA4 phosphorylation, while the effect of PKC activation alone, either via PMA or H1R agonism, was comparably weaker (ca. 1.35- to 1.5- fold) (Fig. 2).
Our results here also identify Thr347, Ser350, and Ser357 as important foci for both PKC and GRK mediated phosphorylation. While mutation of the Thr347 site significantly decreased PMA-mediated phosphorylation compared to WT FFA4, there was still considerable phospho-incorporation with this residue mutated. PMA-mediated phosphorylation was more effectively reduced upon mutation of the Ser350 site, and mutation of the Ser357 site effectively abolished the PMA mediated effect, suggesting that Ser357 is a critical site of PKC-mediated phosphorylation, while the other two residues are minor foci. While the T347A mutant demonstrated retained ability to be phosphorylated upon agonism with DHA, mutation of the Ser350 or Ser357 sites lead to loss of DHA-mediated signal demonstrating that these residues are critical for the effect of agonist- induced phosphorylation of FFA4.
It has been well described that GRKs often, but not always, prefer to phosphorylate serine and threonine residues that are in close proximity to acidic residues located on the amino-terminal side of phosphorylated amino acid (16,30). Analysis of the C-terminus of FFA4 reveals the presence of two acidic aspartic acid residues near the three sites of phosphorylation we have identified: (T347-D348-T349-S350-V351-K352-R353-N354-D355-L356-S357) (Fig. 3). Asp348 is localized in between Thr347 and Thr349 and served as the basis for our initial hypothesis that these residues would be sites of GRK phosphorylation. However, as our data demonstrate, mutation of Thr349 had no effect on FFA4 phosphorylation (Fig. 4A), while mutation of Ser350, two residues downstream (towards the COOH-terminus) from Asp348, has a more prominent effect on overall receptor phosphorylation. Furthermore, Ser357, which our data implicate as a major site of both GRK and PKC phosphorylation (Fig. 4), is also located two residues downstream from an acidic residue, Asp355, suggesting that these two acidic residues may contribute to the GRK-induced phosphosensor. On the contrary, there are other reports that have demonstrated that GRK 5 and 6 may prefer substrate Ser/Thr residues that are flanked by nearby basic Lys or Arg residues (31). Interestingly, Lys342 located five residues upstream of Thr347 and the cluster of Lys352-Arg353, which flanks both the Thr347/Ser350 and Ser357 sites, represent potential basic GRK-induced phosphosensor sites. Further experiments are underway in our laboratory to characterize the role of PKC in potentiating GRK phosphorylation (e.g., kinase crosstalk) at the three sites of phosphorylation, and to further pinpoint the FFA4 residues that are critical for formation of the GRK-induced phosphosensor.
Agonism of FFA4 ectopically expressed in HEK293 cells with oleic acid has been shown to cause rapid increases in intracellular Ca+2, which occurs within seconds, as well as recruitment of β-arrestin to the cell membrane, which occurs at a slower rate, on the order of minutes (12). Furthermore, following agonism, FFA4 has been shown to be rapidly and robustly internalized and trafficked to intracellular lysosomal compartments, with minimal recycling back to the cell-surface (12). Our results demonstrate that while cell-surface expression of the four phosphodefective receptor mutants is approximately similar to that with WT-FFA4, removal of the T347/S350/S357 sites potentiated the effects of DHA on intracellular Ca+2 mobilization (Fig. 5), while it inhibited direct interaction with β-arrestin-2 and its recruitment to the cell membrane (Fig. 6). Expression of the T347A/S350A/S357A triple mutant caused significantly higher (ca. 2-fold) basal levels of intracellular Ca+2, effectively acting in a positive-cooperative manner and suggesting that phosphorylation of FFA4 may counteract constitutive FFA4-Gαq/11 signaling. Since phosphorylation would be expected to lead to β-arrestin recruitment and physical obstruction of G-protein/GPCR interactions, this effect would be lacking with T347A/S350A/ S357A-FFA4, suggesting that PKC may regulate constitutive FFA4 signaling to Gαq/11.
Furthermore, Oh and colleagues recently demonstrated that FFA4 agonism promotes profound anti-inflammatory and insulin-sensitizing effects. In mouse macrophages as well as in vivo, FFA4 agonism inhibits secretion and expression of pro-inflammatory mediators including, TNF-α, IL-6, IL-10, and MCP-1, amongst others, demonstrating that FFA4 can regulate anti-inflammatory effects (5). Additionally, FFA4 agonism abolishes activation of JNK and NF-κB, inhibits degradation of IκB, and enhances systemic insulin sensitivity in mice (5). Perhaps, most importantly, the anti-inflammatory and antidiabetic effects of FFA4 were independent of Gαq/11 but completely dependent on β-arrestin-2 (5). As a consequence, our results showing that DHA-mediated recruitment of β-arrestin-2 is effectively abolished in cells expressing T347A/S350A/S357A-FFA4 demonstrates an important functional significance of phosphodefective FFA4. Polymorphisms in alleles that encode for FFA4 which give rise to phosphodefective FFA4 would be expected to abrogate the anti-inflammatory and antidiabetic effects of the receptor. Furthermore, since FFA4 has become an attractive target for potential pharmacological treatment of diabetes and obesity, drug development processes must ensure that prospective preclinical FFA4 agonist candidates are properly screened to ensure that they give rise to significant phosphorylation responses that would be expected to lead to biased β-arrestin agonism. Development of phospho-specific FFA4 antibodies which arise as a result of the current study will aid in such efforts.
In summary, the current study demonstrates for the first time, that FFA4 is phosphorylated by PKC and GRK6 linked signals, and localizes the sites of FFA4 phosphorylation to Thr347, Ser350, and Ser357 within the C-terminal tail.
Acknowledgments
The authors wish to thank Dr. Kathryn Momary for critical review of the manuscript, Mrs. Vivienne Brown for secretarial support, and Dr. Robert J. Lefkowitz (Duke University Medical Center) and Dr. Michel Bouvier (University of Montreal) for the noted reagents.
The abbreviations used are
- FFA4
Free-fatty acid receptor 4
- GRK
G-protein receptor kinase
- PKC
Protein Kinase C
- PKA
Protein Kinase A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FOOTNOTES
5. REFERENCES
- 1.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3(9):639–50. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 2.Kohout TA, Lefkowit RJ. Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol Pharmacol. 2003;63(1):9–18. doi: 10.1124/mol.63.1.9. [DOI] [PubMed] [Google Scholar]
- 3.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;280:24412–24419. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 4.Brown AJ, Jupe S, Briscoe CP. A family of fatty acid binding receptors. DNA Cell Biol. 2005;24(1):54–61. doi: 10.1089/dna.2005.24.54. [DOI] [PubMed] [Google Scholar]
- 5.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142(5):687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11(1):90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka T, Katsuma S, Adachi T, Koshimizu TA, Hirasawa A, Tsujimoto G. Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn Schmiedebergs Arch Pharmacol. 2008;377(4-6):523–527. doi: 10.1007/s00210-007-0200-8. [DOI] [PubMed] [Google Scholar]
- 8.Lu X, Zhao X, Feng J, Liou AP, Anthony S, Pechhold S, Sun Y, Lu H, Wank S. Postprandial inhibition of gastric ghrelin secretion by long-chain fatty acid through GPR120 in isolated gastric ghrelin cells and mice. Am J Physiol Gastrointest Liver Physiol. 2012;303(3):G367–376. doi: 10.1152/ajpgi.00541.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotoh C, Hong YH, Iga T, Hishikawa D, Suzuki Y, Song SH, Choi KC, Adachi T, Hirasawa A, Tsujimoto G, Sasaki S, Roh SG. The regulation of adipogenesis through GPR120. Biochem Biophys Res Commun. 2007;354(2):591–597. doi: 10.1016/j.bbrc.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 10.Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo, et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 2012;483(7389):350–354. doi: 10.1038/nature10798. [DOI] [PubMed] [Google Scholar]
- 11.Cintra DE, Ropelle ER, Moraes JC, Pauli JR, Morari J, Souza CT, Grimaldi R, Stahl M, Carvalheira JB, Saad MJ, Velloso LA. Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS One. 2012;7(1):e30571. doi: 10.1371/journal.pone.0030571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watson SJ, Brown AJ, Holliday ND. Differential signaling by splice variants of the human free fatty acid receptor GPR120. Mol Pharmacol. 2012;81(5):631–642. doi: 10.1124/mol.111.077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns RN, Moniri NH. Agonism with the omega-3 fatty acids alpha-linolenic acid and docosahexaenoic acid mediates phosphorylation of both the short and long isoforms of the human GPR120 receptor. Biochem Biophys Res Commun. 2010;396(4):1030–1035. doi: 10.1016/j.bbrc.2010.05.057. [DOI] [PubMed] [Google Scholar]
- 14.Ren XR, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci U S A. 2005;102(5):1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh M, Moniri NH. Reactive oxygen species are required for β2 adrenergic receptor-β-arrestin interactions and signaling to ERK1/2. Biochem Pharmacol. 2012;84(5):661–669. doi: 10.1016/j.bcp.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 17.Freedman NJ, Lefkowitz RJ. Desensitization of G protein-coupled receptors. Recent Prog Horm Res. 1996;51:319–351. [PubMed] [Google Scholar]
- 18.Brändlin I, Eiseler T, Salowsky R, Johannes FJ. Protein kinase C(mu) regulation of the JNK pathway is triggered via phosphoinositide-dependent kinase 1 and protein kinase C(epsilon) J Biol Chem. 2002;22(47):45451–45457. doi: 10.1074/jbc.M205299200. 277. [DOI] [PubMed] [Google Scholar]
- 19.Leaney JL, Dekker LV, Tinker A. Regulation of a G protein-gated inwardly rectifying K+ channel by a Ca2+-independent protein kinase C. J Physiol (Lond) 2001;534:367–379. doi: 10.1111/j.1469-7793.2001.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagey R, Hu J, Pelech SL, Raymond LA, Krieger C. Modulation of NMDA-mediated excitotoxicity by protein kinase C. J Neurochem. 2001;78:715–726. doi: 10.1046/j.1471-4159.2001.00459.x. [DOI] [PubMed] [Google Scholar]
- 21.Johnson EA, Oldfield S, Braksator E, Gonzalez-Cuello A, Couch D, Hall KJ, Mundell SJ, Bailey CP, Kelly E, Henderson G. Agonist-selective mechanisms of mu-opioid receptor desensitization in human embryonic kidney 293 cells. Mol Pharmacol. 2006;70(2):676–685. doi: 10.1124/mol.106.022376. [DOI] [PubMed] [Google Scholar]
- 22.Lattion AL, Diviani D, Cotecchia S. Truncation of the receptor carboxyl terminus impairs agonist-dependent phosphorylation and desensitization of the alpha 1B-adrenergic receptor. J Biol Chem. 1994;269(36):22887–22893. [PubMed] [Google Scholar]
- 23.Tobin AB, Nahorski SR. Rapid agonist-mediated phosphorylation of m3-muscarinic receptors revealed by immunoprecipitation. J Biol Chem. 1993;268(13):9817–9823. [PubMed] [Google Scholar]
- 24.Waugh MG, Challiss RA, Berstein G, Nahorski SR, Tobin AB. Agonist-induced desensitization and phosphorylation of m1-muscarinic receptors. Biochem J. 1999;338:175–183. [PMC free article] [PubMed] [Google Scholar]
- 25.Blaukat A, Alla SA, Lohse MJ, Müller-Esterl W. Ligand-induced phosphorylation/dephosphorylation of the endogenous bradykinin B2 receptor from human fibroblasts. J Biol Chem. 1996;271(50):32366–32374. doi: 10.1074/jbc.271.50.32366. [DOI] [PubMed] [Google Scholar]
- 26.Namkung Y, Sibley DR. Protein kinase C mediates phosphorylation, desensitization, and trafficking of the D2 dopamine receptor. J Biol Chem. 2004;279(47):49533–49541. doi: 10.1074/jbc.M408319200. [DOI] [PubMed] [Google Scholar]
- 27.Oppermann M, Freedman NJ, Alexander RW, Lefkowitz RJ. Phosphorylation of the type 1A angiotensin II receptor by G protein-coupled receptor kinases and protein kinase C. J Biol Chem. 1996;271(22):13266–13272. doi: 10.1074/jbc.271.22.13266. [DOI] [PubMed] [Google Scholar]
- 28.Ali H, Richardson RM, Tomhave ED, DuBose RA, Haribabu B, Snyderman R. Regulation of stably transfected platelet activating factor receptor in RBL-2H3 cells. Role of multiple G proteins and receptor phosphorylation. J Biol Chem. 1994;269(40):24557–24563. [PubMed] [Google Scholar]
- 29.Gates LK, Ulrich CD, Miller LJ. Multiple kinases phosphorylate the pancreatic cholecystokinin receptor in an agonist-dependent manner. Am J Physiol. 1993;264:G840–847. doi: 10.1152/ajpgi.1993.264.5.G840. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53(1):1–24. [PubMed] [Google Scholar]
- 31.Loudon RP, Benovic Expression, purification, and characterization of the G protein-coupled receptor kinase GRK6. J Biol Chem. 1994;269(36):22691–22697. [PubMed] [Google Scholar]