Abstract
Objective
To investigate hip shape by active shape modeling (ASM) as a potential predictor of incident radiographic and symptomatic hip OA (rHOA and srHOA).
Methods
All hips developing rHOA from baseline (Kellgren-Lawrence [KL] grade 0/1) to mean 6 year follow up (KL ≥2, 190 hips), and 1:1 control hips (KLG 0/1 at both times, 192 hips) were included. Proximal femur shape was defined on baseline AP pelvis radiographs and submitted to ASM, producing a mean shape and continuous variables representing independent modes of shape variation. Mode scores (n=14, explaining 95% of shape variance) were simultaneously included in logistic regression models, with incident rHOA and srHOA as dependent variables, adjusted for intra-person correlations, sex, race, body mass index (BMI), baseline KL and/or symptoms.
Results
We evaluated 382 hips from 342 individuals: 61% women, 83% white, with mean age 62 years and BMI 29 kg/m2. Several modes differed by sex and race, but no modes were associated with incident rHOA overall. Among men only, modes 1 and 2 were significantly associated (for a 1-SD decrease in mode 1 score, OR 1.7 [95% CI 1.1, 2.5], and for a 1-SD increase in mode 2 score, OR 1.5 [95% CI 1.0, 2.2]) with incident rHOA. A 1-SD decrease in mode 2 or 3 score increased the odds of srHOA by 50%.
Conclusion
This study confirms other reports that variations in proximal femur shape have a modest association with incident hip OA. The observation of proximal femur shape associations with hip symptoms requires further investigation.
Hip osteoarthritis leads to the majority of total hip arthroplasty (THA) procedures in the United States and the rate of hospitalization for THA is rising, increasing 33% from 1997 to 2009 [1]. Estimates of the prevalence of radiographic hip OA (rHOA) range widely, from <1% to 27% of adults [2]. Symptomatic radiographic hip OA (srHOA) is less well-studied but clearly less frequent than rHOA, and estimates of both are dependent on the definitions used [3]. At the baseline visit (1991–1997) of the Johnston County Osteoarthritis Project, 27% of participants had rHOA (Kellgren-Lawrence [KL] grade of 2 or more) and nearly 10% had srHOA (KL≥2 with symptoms present) [4]. Alterations in hip morphology, such as acetabular dysplasia and femoroacetabular impingement, have gained attention recently as potential risk factors for the development of rHOA [5–9]. Early recognition of individuals at risk for hip osteoarthritis based on such morphologic characteristics could allow early preventative interventions, encourage enrollment or improve stratification in randomized clinical trials of therapeutic modalities.
For the purpose of epidemiologic research in large cohorts, such morphologic alterations are typically assessed using visual and simple geometric measures on anteroposterior hip radiographs, which only include one aspect of hip shape at a time. Active shape modeling (ASM) is a method to model shape variation from a set of images, providing a way to model the shape of the proximal femoral head as a whole, and to compare mean shapes and variations in shape between groups using mode scores. Gregory, et al, the first to apply ASM in rHOA, used radiographs from the Rotterdam study [10]. They found differences in mode scores for the femoral head at baseline between those hips that developed rHOA after 6 years of follow up and those that did not. They also identified changes in hip morphology over time in hips developing rHOA, but not in control hips. Lynch, et al, used a similar but more comprehensive model on radiographs from the Study of Osteoporotic Fractures and identified several modes that were associated with incident rHOA after 8 years of follow up [11]. A more extensive ASM, including points along the proximal femur, acetabulum, and pelvis, has been applied to hip radiographs from the familial Genetics, Osteoarthritis, and Progression (GARP) study and the prospective Cohort Hip and Cohort Knee (CHECK) study [12, 13]. In the GARP study, 4 shape modes were associated with prevalent rHOA; mode scores were generally more highly correlated within-persons (right and left hip) than between sibling pairs [12]. Agricola, et al, using data from CHECK, found that 5 shape modes were associated with progression to THA in 5 years [13].
Studies of ASM to date have used white populations, either primarily or exclusively among women, and have focused on rHOA or THA. In the Johnston County Osteoarthritis Project (JoCo OA), African Americans compared with whites had a similar but slightly higher prevalence of rHOA (32 vs. 27%) and srHOA (12 vs. 9%) [4], were found to have individual radiographic features of rHOA that may predict progression [14], but were less likely to develop rHOA [15]. Racial differences in dysplasia and impingement have recently been reported between white and Chinese women [16]. The present analysis uses data from the JoCo OA, a longitudinal, community-based cohort that includes African American and white men and women. Using a nested case control study design, our aim was to use ASM (after the methods of Lynch, et al [11]) to describe baseline proximal femur shape by sex and race, and to determine the associations between femur shape and incident rHOA and srHOA.
PARTICIPANTS & METHODS
Participants
Data were from the JoCo OA, an ongoing community-based study of OA and its risk factors in Johnston County, NC. The recruitment of participants and overall project design have been detailed elsewhere [17]. In brief, the JoCo OA is a prospective, longitudinal cohort study in African American and White men and women aged 45 years and older, who were residents of one of six Johnston County townships for at least one year and capable of completing the study protocol. All participants completed informed consent followed by 2 home interviews and a clinic visit where radiographic and physical examinations were performed. The current analysis is a case-control study nested in the parent cohort, using baseline data collected from 1991–1997, and first follow up data from 1999–2004. The JoCo OA has been continuously approved by the Institutional Review Boards of the University of North Carolina at Chapel Hill and the Centers for Disease Control and Prevention.
Radiographs
All men and women at least 50 years of age had standardized supine anteroposterior pelvis radiographs, taken with the feet in 15 degrees of internal rotation, first at baseline and then at follow up after a mean of 6 ± 1.4 years. Women under 50 years of age did not have pelvic radiography to avoid pelvic area radiation and are not included in these analyses. Hip radiographs were read paired for Kellgren-Lawrence (KL) grades without knowledge of participant clinical status or chronological order by a single musculoskeletal radiologist (JBR) with high inter- and intra-rater reliability (κ=0.859 and 0.886, respectively, [18]) using the KL radiographic atlas for overall hip radiographic grades [19], such that hips with a small osteophyte of doubtful significance were graded 1, those with a definite osteophyte but no joint space narrowing received a KL grade of 2, those with definite joint space narrowing received a KL grade of 3, or a grade of 4 if sclerosis was also present [14].
Symptoms
Symptoms were determined based upon the answer to the question “on MOST days do you have pain/aching/stiffness in your right|left hip?” followed by “is the pain in your right|left hip mild, moderate, or severe?”
Definitions of outcomes
Radiographic hip OA (rHOA) was defined as KL grade ≥2. Incident rHOA was defined in hips with a baseline KL grade of 0 or 1 and a follow up KL grade of 2 or more. As only two cases of incident joint replacement were observed, these were not included in the analysis. Symptomatic radiographic hip OA (srHOA) was defined when both radiographic hip OA AND symptoms were present in the same hip. Incident srHOA was defined in hips that developed incident rHOA and had reported symptoms at follow up.
Case and control selection
Of the 1,726 individuals with paired radiographic data from both baseline and follow up time points, and after exclusion of hips with prevalent rHOA at baseline, 193 hips developed incident rHOA. All case hips were selected, along with 1:1 control hips (those without rHOA at either baseline or follow up) in approximately equal numbers from the 4 race-by-gender strata. Four hips (3 case hips and 1 control hip) were unsuitable for ASM analysis as the required anatomic landmarks were not captured in the image (usually greater or lesser trochanters) and were excluded, resulting in a total analysis sample of 190 case hips and 192 control hips in 342 individuals. Of the incident rHOA cases, there were 55 cases of incident srHOA, and all other hips (n=326, one hip was missing symptoms data) were included as controls for srHOA. The case and control definitions were hip-specific, such that both hips could be included from one individual in any combination (n=40 people with both hips included).
Covariates
Self-reported sex and race were obtained during home interviews. Height (cm) and weight (kg) measured during the baseline clinic visit were used to calculate body mass index. Baseline KL grade and hip symptoms at baseline were also used as covariates.
Analysis
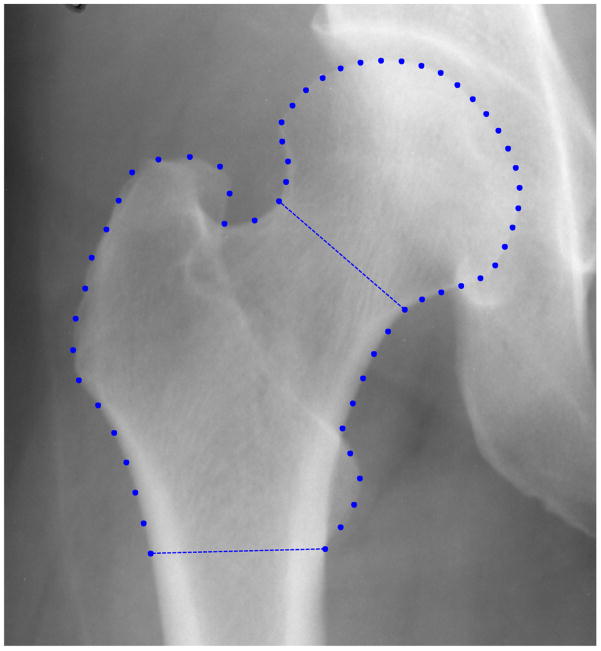
All 680 hips suitable for ASM from these 342 individuals were used to build the ASM model using the method of Cootes, et al [20] as adapted by Lynch, et al [11]. The shape of the proximal femur was defined on baseline pelvis radiographs for all hips by a trained reader (AEN), who placed landmark points to mark the femoral shaft at the level of the lesser trochanter, the femoral neck, a circle fitting the femoral head, and 2 points at the most superior and lateral points of the greater trochanter. The software then displayed the best matching femoral contour from a library of shape templates; this contour was manually adjusted by the reader to match the actual hip shape. Sixty equidistant landmark points (automatically created from the final fitted contour) were input into the ASM (10 between the lesser trochanter and femoral neck, 30 around the femoral head, and 20 around the greater trochanter and femoral shaft, Figure 1). The ASM produced a mean shape, and a set of continuous variables representing independent modes of variation in that shape, using principal components analysis. Reliability of the measurements were assessed by determining the number of points that were within 2–3mm by a single reader placing the points twice (AEN, n=30 hips, 1800 total points) and between two independent, uncalibrated readers (n=33 hips, 1980 total points). We also determined intra-class correlation coefficients (ICCs) for intra- and inter-reader reliability of mode scores, and within-person between hips for each mode using the method of Shrout [21] via icc23, a user-defined program in Stata 11 (College Station, TX).
Figure 1. Sixty landmark points used by ASM.
Scores for modes of variation which together explained 95% of the total shape variance were simultaneously entered in logistic regression models as independent predictors of the outcomes; models were used to identify baseline associations between modes with race and gender, and longitudinal associations with incident rHOA and incident srHOA. As all modes were examined in a single model, no correction for multiple comparisons was applied. The ASM scales each shape prior to applying the statistical model, so any effect due to the overall size of the femur is removed. The association of modes with incident hip outcomes was tested with OA case or control status as the outcome, adjusting for intra-person correlations (using the cluster option in Stata), sex, race, baseline BMI, KL grade and/or baseline symptoms. Pre-specified analyses stratified by sex, race, and baseline symptoms were also performed.
RESULTS
The case-control sample (n=342 persons, n=382 hips, Table 1) consisted of 39% men, 18% African Americans, with a mean age of 62 years and mean baseline BMI of 29 kg/m2. Participants had an average of 6 years (range 4–11 years) of follow up time between visits. Hips included as rHOA controls were from younger participants and more often had baseline symptoms and a baseline KL grade of 0 compared with incident rHOA case hips. Symptomatic radiographic HOA case hips were infrequently from African American participants, had more frequent baseline symptoms, and were less likely to have a baseline KL grade of 0 compared with control hips for srHOA.
Table 1.
Baseline descriptors for included individuals and hips, by case control status for incident rHOA and srHOA
| INDIVIDUALS | HIPS (n=382) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Overall (n=342) n (%) | rHOA Controls (n=192) n (%) | rHOA cases (n=190) n (%) | p value* | srHOA controls (n=326) n (%) | srHOA cases (n=55) n (%) | p value | |
| Men | 132 (38.6) | 74 (38.5) | 74 (38.9) | -- | 127 (39.0) | 20 (36.4) | 0.724 |
| African American | 60 (17.5) | 33 (17.2) | 32 (16.8) | -- | 60 (18.4) | 4 (7.3) | 0.048 |
| Present hip symptoms | 119 (35.1) | 79 (41.1) | 53 (27.9) | 0.007 | 101 (31.0) | 30 (54.5) | 0.002 |
| KL grade=0 | 69 (18.1) | 47 (24.4) | 22 (11.6) | 0.003 | 64 (19.6) | 5 (9.1) | 0.064 |
|
| |||||||
| mean (SD) | mean (SD) | mean (SD) | mean (SD) | mean (SD) | |||
|
| |||||||
| Age (years) | 61.7 (9.0) | 60.4 (8.6) | 63.0 (9.2) | 0.006 | 61.5 (8.9) | 62.9 (9.4) | 0.330 |
| BMI (kg/m2) | 29.1 (5.9) | 29.1 (5.9) | 29.2 (6.0) | 0.962 | 29.0 (5.9) | 30.3 (5.6) | 0.089 |
| Follow up (years) | 6.0 (1.4) | 5.9 (1.3) | 6.0 (1.5) | 0.863 | 6.0 (1.4) | 5.9 (1.5) | 0.935 |
No comparisons performed for the matching variables (sex and race) for rHOA. P values assessed using univariable models with cluster option to account for intra-person correlations.
Fourteen modes were needed to account for 95% of the total variance in shape among all 680 hips available for ASM (Table 2). Intra-reader agreement on point placement within 2mm was 97%; inter-reader agreement was 84% within 2mm and 91% within 3mm. The range of intra- and inter-reader ICCs for the 14 mode scores was 0.57 to 0.96 and 0.70 to 0.99, respectively; for comparisons between hips within a person, ICCs ranged from 0.45 to 0.79. A 2-SD change in mode shape, for most modes, represented a change of more than 2mm; the actual mm difference was predictably largest for the first modes, which explain the greatest proportion of variation (10mm for mode 1, 5–6mm for modes 2–4, 2–3mm for modes 5–14).
Table 2.
Baseline associations between mode scores and race (left) or gender (right)
| Mode | % Variance Explained | Mode score1 ↑ or ↓ |
African American (n=60 persons) OR2 (95% CI) | Mode score1 ↑ or ↓ |
Men (n=132 persons) OR3 (95% CI) |
|---|---|---|---|---|---|
| 1 | 37.4 | ↑ | 1.04 (0.74, 1.45) | ↓ | 3.03 (2.00, 4.55) |
| 2 | 16.0 | ↓ | 1.35 (0.93, 1.92) | ↑ | 1.29 (0.94, 1.77) |
| 3 | 12.5 | ↑ | 1.73 (1.18, 2.53) | ↓ | 1.45 (1.03, 2.04) |
| 4 | 9.9 | ↑ | 1.89 (1.30, 2.77) | ↓ | 2.22 (1.47, 3.33) |
| 5 | 5.1 | ↓ | 1.16 (0.83, 1.61) | ↓ | 1.75 (1.23, 2.44) |
| 6 | 3.4 | ↑ | 1.20 (0.87, 1.63) | ↓ | 1.54 (0.99, 2.38) |
| 7 | 2.6 | ↓ | 1.20 (0.90, 1.61) | ↓ | 1.27 (0.88, 1.82) |
| 8 | 2.3 | ↑ | 1.13 (0.83, 1.54) | ↑ | 1.77 (1.34, 2.35) |
| 9 | 1.7 | ↑ | 1.33 (0.97, 1.83) | ↓ | 1.54 (1.15, 2.04) |
| 10 | 1.3 | ↑ | 1.90 (1.33, 2.72) | ↓ | 1.67 (1.16, 2.38) |
| 11 | 1.1 | ↓ | 1.30 (0.91, 1.89) | ↓ | 1.20 (0.85, 1.72) |
| 12 | 0.9 | ↓ | 1.11 (0.81, 1.52) | ↑ | 1.23 (0.88, 1.71) |
| 13 | 0.7 | ↓ | 1.37 (1.00, 1.85) | ↑ | 1.29 (0.99, 1.69) |
| 14 | 0.6 | ↑ | 1.28 (0.93, 1.76) | ↓ | 1.12 (0.83, 1.52) |
Association with increase (↑) or decrease (↓) in mode score (e.g. for mode 3, the odds of being African American (compared with white) are 73% higher for every one SD increase in mode 3 score; the odds of being male are 45% higher for every one SD decrease in mode 3 score).
Adjusted for sex, age, BMI, baseline KL grade, and case or control status at follow up, referent category=white
Adjusted for race, age, BMI, baseline KL grade, and case or control status at follow up, referent category=women
Associations between baseline characteristics and ASM modes
At baseline (when all hips had KL grades 0 or 1), several modes were significantly different by race and by gender, both before and after adjustment for other covariates. Modes 3, 4, and 10 were significantly associated with being African American (Table 2). For every 1-SD increase in modes 3, 4, or 10, the odds of being African American compared with white were 70 to 90% higher. Modes 1, 3–5, and 8–10 were associated with being male, such that for every 1-SD decrease in each of modes 1, 3–5, 9, or 10, the odds of being male compared with female increased by 1.5 to 3 times. For every 1-SD increase in mode 8, the odds of being male compared with female were 77% higher (Table 2). Additionally, a KL grade of 1 (compared with 0) was associated with 1-SD increases in mode 6 and 9 scores (adjusted odds ratio [aOR] 1.61 [95% CI 1.08, 2.39] and aOR 1.24 [95% CI 1.24, 2.13], respectively), and the presence of baseline symptoms was associated with 1-SD increases in mode 11 score only (aOR 1.50 [95% CI 1.17, 1.94]).
Associations between baseline ASM modes and incident radiographic hip osteoarthritis
None of the modes was significantly associated with rHOA, either before or after adjustment for sex, race, age, BMI, or baseline KL grade (Table 3). In these models, a baseline KL grade of 1 doubled the odds of incident rHOA compared with a baseline KL grade of 0 (aOR 2.17 [95% CI 1.16, 4.04]), and every year of increasing age increased the odds of developing incident rHOA by 3% (aOR 1.03 [95% CI 1.01, 1.06]); BMI was not associated with the outcome.
Table 3.
Modes of variation with % variance explained and associations with incident rHOA in men and women
| OVERALL (n=382 hips) | MEN ONLY (n=148 hips) | WOMEN ONLY (n=234 hips) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mode | % Variance Explained | Mode score1 ↑ or ↓ |
RHOA cases (n=190) OR2 (95% CI) | Mode score1 ↑ or ↓ |
Male RHOA cases OR2 (95% CI) | Mode score1 ↑ or ↓ |
Female RHOA cases OR2 (95% CI) |
| 1 | 37.4 | ↓ | 1.15 (0.90, 1.47) | ↓ | 1.66 (1.11, 2.48) | ↓ | 1.03 (0.67, 1.57) |
| 2 | 16.0 | ↑ | 1.02 (0.82, 1.28) | ↑ | 1.49 (1.01, 2.19) | ↓ | 1.21 (0.90, 1.63) |
| 3 | 12.5 | ↓ | 1.03 (0.83, 1.28) | ↑ | 1.30 (0.83, 2.05) | ↓ | 1.21 (0.91, 1.60) |
| 4 | 9.9 | ↓ | 1.12 (0.88, 1.43) | ↑ | 1.00 (0.68, 1.47) | ↓ | 1.11 (0.71, 1.71) |
| 5 | 5.1 | ↑ | 1.03 (0.82, 1.29) | ↓ | 1.10 (0.74, 1.64) | ↑ | 1.07 (0.78, 1.46) |
| 6 | 3.4 | ↓ | 1.18 (0.93, 1.49) | ↓ | 1.22 (0.82, 1.82) | ↓ | 1.07 (0.73, 1.55) |
| 7 | 2.6 | ↓ | 1.04 (0.83, 1.30) | ↑ | 1.07 (0.74, 1.55) | ↓ | 1.13 (0.82, 1.57) |
| 8 | 2.3 | ↑ | 1.00 (0.81, 1.24) | ↑ | 1.00 (0.68, 1.49) | ↑ | 1.18 (0.88, 1.57) |
| 9 | 1.7 | ↑ | 1.10 (0.88, 1.38) | ↓ | 1.13 (0.79, 1.61) | ↑ | 1.28 (0.94, 1.75) |
| 10 | 1.3 | ↑ | 1.01 (0.82, 1.25) | ↓ | 1.25 (0.89, 1.74) | ↑ | 1.15 (0.83, 1.61) |
| 11 | 1.1 | ↑ | 1.17 (0.93, 1.48) | ↑ | 1.17 (0.80, 1.72) | ↓ | 1.10 (0.78, 1.54) |
| 12 | 0.9 | ↓ | 1.12 (0.91, 1.39) | ↑ | 1.06 (0.76, 1.49) | ↓ | 1.30 (0.95, 1.78) |
| 13 | 0.7 | ↑ | 1.01 (0.82, 1.24) | ↓ | 1.05 (0.69, 1.58) | ↑ | 1.08 (0.82, 1.42) |
| 14 | 0.6 | ↑ | 1.08 (0.87, 1.34) | ↑ | 1.00 (0.70, 1.43) | ↑ | 1.21 (0.87, 1.67) |
Association with increase (↑) or decrease (↓) in mode score (e.g. for mode 1, the odds of rHOA are 15% higher for every one SD decrease in mode 1 score overall; among men, the odds of rHOA are 66% higher for every one SD decrease in mode 1 score).
Adjusted for sex, race, age, BMI, baseline KL grade
In analyses stratified by sex, no modes were associated with rHOA among women. However, among men, modes 1 and 2 (37% and 16% of total variance, respectively) were associated with incident rHOA (for a 1-SD decrease in mode 1 score, OR 1.66 [95% CI 1.11, 2.48], and for a 1-SD increase in mode 2 score, OR 1.49 [95% CI 1.01, 2.19]). Analyses stratified by race did not show any significant associations.
Associations between baseline ASM modes and incident symptomatic radiographic hip osteoarthritis
In unadjusted models of srHOA, only a 1-SD decrease in mode 3 was associated with incident srHOA (OR 1.54 [95% CI 1.13, 2.10]). After adjustment for sex, race, age, BMI, baseline KL grade and baseline hip symptoms, 1-SD decreases in modes 2, 3, and 11 (16, 13, and 1% of the total variance, respectively) were significantly associated with srHOA (Figure 2, Table 3). In these models, baseline hip symptoms increased the odds of incident srHOA (OR 3.16 [95% CI 1.71, 5.85]) and African Americans compared with whites had lower odds of srHOA (aOR 0.27 [95% CI 0.09, 0.84]); no other covariates were significantly associated with the outcome.
Figure 2. Examples of modes associated with hip outcomes.
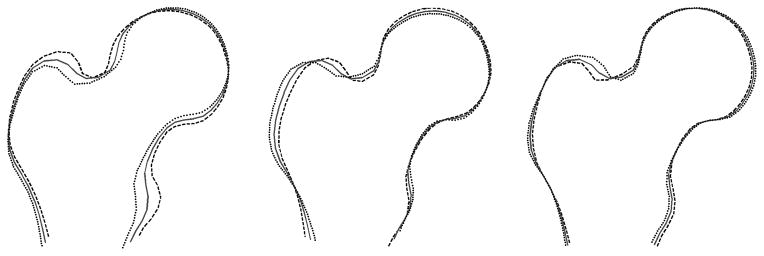
Proximal femur shape variation in modes 2, 3, and 6 (from left). Each figure shows the mean (solid gray), +2SD (black dashed line) and −2SD (black dotted line) shapes in each mode.
Sex and race stratification was not feasible due to small numbers for srHOA. Analyses stratified by the presence of baseline symptoms showed a consistent association between mode 3 and incident srHOA. Among those without baseline symptoms, a 1-SD increase in mode 6 (representing 3% of the shape variance) was associated with incident srHOA (OR 1.94 [95% CI 1.20, 3.11], while among those with baseline symptoms, the association was with a 1-SD decrease in mode 6 (OR 2.11 [95% CI 1.28, 3.50], Figure 2). The association with mode 11 was seen only in the group without baseline symptoms, and in those with baseline symptoms, mode 14 (0.6% of total variance) was also associated with srHOA (1-SD increase in mode 14 OR 1.80 [95% CI 1.06, 3.07].
DISCUSSION
Variations in hip shape, assessed using active shape modeling, were associated with sex and race at baseline and with incident rHOA in men and srHOA in all participants at follow up. We report the first comparison of proximal femur shape by ASM among African Americans and whites, finding several shape differences by race in the absence of rHOA and independent of age, BMI, or baseline KL grade. Gregory, et al identified differences between men and women only for modes 1 and 7 in their study, while several modes differed by gender in the JoCo OA [10]. Variations in proximal femur shape have been used in forensic medicine for sex identification, and racial differences have been recognized as well [22]. A recent study using 3-dimensional statistical shape analysis in normal knees also identified differences by sex and ethnicity [23]. Given known differences in rOA prevalence [4, 17] and in radiographic features of rOA at the hip [14] and knee [24] among African Americans and whites, these findings warrant further study to determine their clinical significance.
Although it is not possible to directly compare the modes found in this study to those identified in other studies using ASM, broad comparisons are possible. In the Study of Osteoporotic Fractures [11] and Rotterdam [10] populations, 10 modes were required to explain 95% of the total shape variance, compared with 14 modes in the JoCo OA, 23 modes in GARP [12], and 24 modes in CHECK (for 90% of variance) likely due primarily to differences in the number of hips analyzed, the model used and the number of points placed. However, our ASM methodology in the JoCo OA was identical to that of the SOF, suggesting that there may be true differences in the amount of variation in hip morphology in those two populations, possibly due to inclusion of African Americans and men in the JoCo OA. We found a similar within person correlation between the two hips as reported in the GARP study (our ICC range: 0.45–0.79, GARP: 0.41–0.82).
We found few associations with incident rHOA alone, in contrast to other studies using ASM. The Rotterdam and GARP studies included hips with both prevalent and incident rHOA in the ASM, such that some of the associations between rHOA and mode scores were due to changes from the osteoarthritis disease process [10, 12]. Gregory, et al, addressed this by separately analyzing a group of hips that developed incident rHOA and a group of hips that had KL grades of 0 at both baseline and 6 year follow up. A lower mode 6 score (reflecting a flattened transition at the femoral neck) was found in those hips developing rHOA compared to those that did not both at baseline and follow up [10]. Lynch, et al, used ASM methodology identical to the current report but for rHOA definition used a modified Croft score, which emphasizes joint space narrowing and femoral osteophytes, leading to a more stringent definition than used in the JoCo OA (KL grade of 2 or more) [11]. They found associations, in a population of older white women, between incident rHOA and increases in 3 modes of shape variation (modes 3, 5, and 9) [11]. Increased values of mode 3 in that study were related to a larger femoral head size and a longer femoral neck, while higher mode 5 scores were related to a larger greater trochanter and smaller femoral neck size. Higher values for mode 9 reflected a larger femoral head compared to femoral neck size along with a larger greater trochanter. In the current study, a decrease in mode 1 score was associated with rHOA in men and was related to a larger, flatter trochanter and a flattening of the transition between the femoral head and neck, features similar to mode 5 in the study by Lynch et al, and mode 6 in the study by Gregory et al. Flattening of the femoral head was also a feature of an increase in our mode 2 score, somewhat suggestive of a cam-type change of femoroacetabular impingement; in support of this, the modified triangular index height [7] was greater among those with positive mode 2 scores (2.83 ± 0.36 cm) compared to those with negative mode 2 scores (2.66 ± 0.33 cm, p<0.001).
Incident srHOA has not been assessed as an outcome in the prior ASM studies. Gregory, et al included information on hip pain as a covariate but found no association between self-reported hip pain at baseline or follow up and the 3 OA-associated modes of variation in their study [10]. Agricola, et al found no association between baseline shape modes and meeting clinical ACR criteria for hip OA after 5 years [13]. It is possible that the addition of symptoms to rHOA may have strengthened our case definition, which for rHOA was based solely on a KL grade of 2 or more and therefore likely represents an earlier stage of disease compared with the more stringent definition used in the SOF cohort. In the current study, 1-SD decreases in scores for each of modes 2, 3, and 11 were associated with incident srHOA. A decrease in mode 2 score reflects alterations in the transition between the greater trochanter and femoral neck, a slight reduction in femoral neck width (for those with negative mode 2 scores, femoral neck width was 3.86 ± 0.45 cm vs. 4.03 ± 0.44cm for those with positive scores, p<0.001), and a qualitative impression of a longer femoral neck, compared to the mean shape. The variation in shape of the transition from greater trochanter to femoral neck is a feature of reduced mode 3 and 11 scores as well, while a reduction in mode 3 score also suggests a somewhat flatter femoral head. After stratification by baseline symptoms, mode 6 was associated with srHOA but in opposite directions; an increase in mode 6 score was associated with srHOA only in those without baseline symptoms, while there was an association with decreased mode 6 score in those with baseline symptoms. Mode 6 reflects subtle differences in the size of the greater trochanter, the length of the femoral neck, and the transition between the two. The significance of this finding is unclear and will need to be verified in other populations.
Our study has many strengths, such as the inclusion of African American and white men and women. We took advantage of the very well characterized JoCo OA cohort, allowing a nested case-control design for the current study while also making future studies of longer follow-up and functional outcomes possible. We had standardized AP pelvis radiographs on all participants, and ASM and KL grade assessments were reliable. However, this study also has a number of limitations, including the use of KL grades alone to define cases and controls. A more stringent definition such as that used in the SOF may provide sharper distinctions, although this is similar to defining an incident case using a KL grade of 3, which was infrequent in the JoCo OA. We had a relatively small number of cases even defining by a KL grade of 2 or more, and a very small number of incident srHOA cases, which did not allow further subgroup stratification for this outcome. Our ASM was limited to the proximal femoral head, and inclusion of the acetabulum [12] may provide further insights into OA risk at the hip. Currently, our results are specific to our population; in order to generalize such findings, films from different studies and populations would need to be simultaneously submitted to ASM.
In conclusion, morphologic variations at the proximal femur, assessed by active shape modeling, were associated with baseline characteristics such as sex and race, and with incident hip OA outcomes after 6 years of follow up. Such shape variations may contribute to hip OA risk and provide another avenue of exploration to identify those at risk for this potentially debilitating condition.
Table 4.
Modes of variation with % variance explained and associations with incident srHOA
| Mode | % Variance Explained | Mode score1 ↑ or ↓ |
SrHOA cases (n=55 hips) |
|---|---|---|---|
| OR2 (95% CI) | |||
| 1 | 37.4 | ↑ | 1.11 (0.79, 1.56) |
| 2 | 16.0 | ↓ | 1.47 (1.03, 2.08) |
| 3 | 12.5 | ↓ | 1.54 (1.09, 2.17) |
| 4 | 9.9 | ↑ | 1.10 (0.74, 1.65) |
| 5 | 5.1 | ↑ | 1.26 (0.92, 1.71) |
| 6 | 3.4 | ↑ | 1.07 (0.77, 1.48) |
| 7 | 2.6 | ↓ | 1.03 (0.76, 1.41) |
| 8 | 2.3 | ↓ | 1.08 (0.82, 1.39) |
| 9 | 1.7 | ↑ | 1.29 (0.91, 1.82) |
| 10 | 1.3 | ↓ | 1.12 (0.82, 1.56) |
| 11 | 1.1 | ↓ | 1.52 (1.05, 2.17) |
| 12 | 0.9 | ↑ | 1.00 (0.73, 1.37) |
| 13 | 0.7 | ↓ | 1.18 (0,86, 1.61) |
| 14 | 0.6 | ↑ | 1.17 (0.85, 1.63) |
Association with increase (↑) or decrease (↓) in mode score (e.g. for mode 1, the odds of srHOA are 11% higher for every one SD increase in mode 1 score).
Adjusted for sex, race, age, BMI, baseline KL grade, and baseline symptoms
SIGNIFICANCE AND INNOVATIONS.
Hip morphology likely contributes to hip osteoarthritis risk.
Active shape modeling allows a comprehensive comparison of shape characteristics on hip radiographs.
Variations in shape were found at baseline by sex and race, and in those who do or do not develop incident radiographic or symptomatic hip osteoarthritis at follow up.
Acknowledgments
The authors would like to thank the participants and staff of the Johnston County Osteoarthritis Project, without whom this work would not have been possible.
Funding was provided in part by: NIAMS K23 AR061406 (Nelson); NIH/NIAMS P60AR30701 (Jordan/Renner/Schwartz); CDC/ASPH S043 and S3486 (Jordan/Renner); K24-AR04884, P50-AR063043, and P50-AR060752 (Lane)
Footnotes
CONFLICT OF INTEREST
The authors have no financial conflicts in relation to this work.
References
- 1.Wier LM, Pfuntner A, Maeda J, Stranges E, Ryan K, Collins Sharp B, et al. HCUP Facts and Figures: Statistics on Hospital-based Care in the United States, 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2011. [January 22, 2013]; Available from: http://www.hcup-us.ahrq.gov/reports.jsp. [PubMed] [Google Scholar]
- 2.Dagenais S, Garbedian S, Wai EK. Systematic review of the prevalence of radiographic primary hip osteoarthritis. Clin Orthop Relat Res. 2009;467(3):623–37. doi: 10.1007/s11999-008-0625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pereira D, Peleteiro B, Araujo J, Branco J, Santos RA, Ramos E. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage. 2011;19(11):1270–85. doi: 10.1016/j.joca.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of hip symptoms and radiographic and symptomatic hip osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2009;36(4):809–15. doi: 10.3899/jrheum.080677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McWilliams DF, Doherty SA, Jenkins WD, Maciewicz RA, Muir KR, Zhang W, et al. Mild acetabular dysplasia and risk of osteoarthritis of the hip: a case-control study. Ann Rheum Dis. 2010;69(10):1774–8. doi: 10.1136/ard.2009.127076. [DOI] [PubMed] [Google Scholar]
- 6.Gosvig KK, Jacobsen S, Sonne-Holm S, Palm H, Troelsen A. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population-based survey. J Bone Joint Surg Am. 2010;92(5):1162–9. doi: 10.2106/JBJS.H.01674. [DOI] [PubMed] [Google Scholar]
- 7.Nicholls AS, Kiran A, Pollard TC, Hart DJ, Arden CP, Spector T, et al. The association between hip morphology parameters and nineteen-year risk of end-stage osteoarthritis of the hip: a nested case-control study. Arthritis Rheum. 2011;63(11):3392–400. doi: 10.1002/art.30523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lane NE, Lin P, Christiansen L, Gore LR, Williams EN, Hochberg MC, et al. Association of mild acetabular dysplasia with an increased risk of incident hip osteoarthritis in elderly white women: the study of osteoporotic fractures. Arthritis Rheum. 2000;43(2):400–4. doi: 10.1002/1529-0131(200002)43:2<400::AID-ANR21>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 9.Doherty M, Courtney P, Doherty S, Jenkins W, Maciewicz RA, Muir K, et al. Nonspherical femoral head shape (pistol grip deformity), neck shaft angle, and risk of hip osteoarthritis: a case-control study. Arthritis Rheum. 2008;58(10):3172–82. doi: 10.1002/art.23939. [DOI] [PubMed] [Google Scholar]
- 10.Gregory JS, Waarsing JH, Day J, Pols HA, Reijman M, Weinans H, et al. Early identification of radiographic osteoarthritis of the hip using an active shape model to quantify changes in bone morphometric features: can hip shape tell us anything about the progression of osteoarthritis? Arthritis Rheum. 2007;56(11):3634–43. doi: 10.1002/art.22982. [DOI] [PubMed] [Google Scholar]
- 11.Lynch JA, Parimi N, Chaganti RK, Nevitt MC, Lane NE. The association of proximal femoral shape and incident radiographic hip OA in elderly women. Osteoarthritis Cartilage. 2009;17(10):1313–8. doi: 10.1016/j.joca.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waarsing JH, Kloppenburg M, Slagboom PE, Kroon HM, Houwing-Duistermaat JJ, Weinans H, et al. Osteoarthritis susceptibility genes influence the association between hip morphology and osteoarthritis. Arthritis Rheum. 2011;63(5):1349–54. doi: 10.1002/art.30288. [DOI] [PubMed] [Google Scholar]
- 13.Agricola R, Reijman M, Bierma-Zeinstra SM, Verhaar JA, Weinans H, Waarsing JH. Total Hip Replacement but not Clinical Osteoarthritis can be Predicted by the Shape of the Hip: A Prospective Cohort Study (Check) Osteoarthritis Cartilage. 2013 doi: 10.1016/j.joca.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Nelson AE, Braga L, Renner JB, Atashili J, Woodard J, Hochberg MC, et al. Characterization of individual radiographic features of hip osteoarthritis in African American and White women and men: the Johnston County Osteoarthritis Project. Arthritis Care Res (Hoboken) 2010;62(2):190–7. doi: 10.1002/acr.20067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopec JA, Sayre EC, Schwartz TA, Renner JB, Helmick CG, Badley EM, et al. Occurrence of radiographic osteoarthritis of the knee and hip among african americans and caucasians: The johnston county osteoarthritis project. Arthritis Care Res (Hoboken) 2012 doi: 10.1002/acr.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudda M, Kim YJ, Zhang Y, Nevitt MC, Xu L, Niu J, et al. Morphologic differences between the hips of Chinese women and white women: could they account for the ethnic difference in the prevalence of hip osteoarthritis? Arthritis Rheum. 2011;63(10):2992–9. doi: 10.1002/art.30472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34(1):172–80. [PubMed] [Google Scholar]
- 18.Jordan JM, Linder GF, Renner JB, Fryer JG. The impact of arthritis in rural populations. Arthritis Care Res. 1995;8(4):242–50. doi: 10.1002/art.1790080407. [DOI] [PubMed] [Google Scholar]
- 19.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cootes TF, Taylor CJ, Cooper DH, Graham J. Active Shape Models - Their Training and Application. Computer Vision and Image Understanding. 1995;61(1):38–59. [Google Scholar]
- 21.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 22.Gill GW. Racial variation in the proximal and distal femur: heritability and forensic utility. J Forensic Sci. 2001;46(4):791–9. [PubMed] [Google Scholar]
- 23.Mahfouz M, Abdel Fatah EE, Bowers LS, Scuderi G. Three-dimensional morphology of the knee reveals ethnic differences. Clin Orthop Relat Res. 2012;470(1):172–85. doi: 10.1007/s11999-011-2089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braga L, Renner JB, Schwartz TA, Woodard J, Helmick CG, Hochberg MC, et al. Differences in radiographic features of knee osteoarthritis in African-Americans and Caucasians: the Johnston county osteoarthritis project. Osteoarthritis Cartilage. 2009;17(12):1554–61. doi: 10.1016/j.joca.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]