Abstract
Asymmetry of the neonatal brain is not yet understood at the level of structural connectivity. We utilized DTI deterministic tractography and structural network analysis based on graph theory to determine the pattern of structural connectivity asymmetry in 124 normal neonates. We tracted white matter axonal pathways characterizing interregional connections among brain regions and inferred asymmetry in left and right anatomical network properties. Our findings revealed that in neonates, small-world characteristics were exhibited, but did not differ between the two hemispheres, suggesting that neighboring brain regions connect tightly with each other, and that one region is only a few paths away from any other region within each hemisphere. Moreover, the neonatal brain showed greater structural efficiency in the left hemisphere than that in the right. In neonates, brain regions involved in motor, language, and memory functions play crucial roles in efficient communication in the left hemisphere, while brain regions involved in emotional processes play crucial roles in efficient communication in the right hemisphere. These findings suggest that even at birth, the topology of each cerebral hemisphere is organized in an efficient and compact manner that maps onto asymmetric functional specializations seen in adults, implying lateralized brain functions in infancy.
Keywords: cerebral asymmetry, structural connectivity network, diffusion tensor imaging, neonates, tractography
Introduction
The human brain exhibits structural asymmetries to support its specific lateralized functions, such as language and motor control. Such asymmetry in structure is apparent even in early life. But these structural asymmetries at the neonatal period may or may not persist through life. Moreover it is unclear whether structural asymmetry is reflected in differences in connectivity.
In healthy neonates, the left cerebral hemisphere is larger than the right (Gilmore et al., 2007). However, the normal pattern of fronto-occipital asymmetry described in older children and adults, notably larger right than left frontal lobe and larger left than right occipital lobe (Chapple et al., 2004; LeMay, 1976; Sharma et al., 1999; Weinberger et al., 1982) is not present in neonates (Gilmore et al., 2007). Beyond the whole brain level, Witelson et al. (Witelson and Pallie, 1973) revealed a neonatal leftward asymmetry of the planum, a region known to be of significance for language function. Additional support is derived from a diffusion tensor imaging (DTI) technique that characterizes axonal organization of the brain white matter. Structural asymmetries in language- and motor-related fibers (e.g., the parieto-temporal part of the superior longitudinal fasciculus, the corticospinal tract, the superior thalamic radiations) are present in healthy (Dubois et al., 2009) as well as preterm infants (Liu et al., 2010). These findings are similar to the pattern seen in adults, suggesting that the observed neonatal anatomical asymmetry provides a structural basis for the adult pattern of the lateralization of language functions.
Our understanding of the structural asymmetry of the neonatal brain is still largely limited to the level of individual structures. Nevertheless, widespread brain areas are wired in a compact and economic manner and hence can easily transfer information in short and long distances to adapt to cognitive demands (Bullmore and Sporns, 2012). Recent advanced modern neuroimaging techniques allow for non-invasive investigation of the brain connectivity. Using DTI tractography and structural network techniques, the cortical network in adults was identified with small-worldness topology, suggesting that the cortex is highly interconnected and thus has a highly efficient topological organization (Gong et al., 2009b; Yan et al., 2011). Only recently, Iturria-Medina et al. (Iturria-Medina et al., 2011) employed the same techniques and showed that in adults the left cerebral hemisphere is significantly less efficient and interconnected than the right. Furthermore, the left hemisphere presents more central or indispensable regions for the whole-brain structural network than the right hemisphere. However, it is unclear whether this asymmetric pattern can be traced to the perinatal period. To examine this, we utilized DTI deterministic tractography and structural network analysis based on graph theory to determine the pattern of structural connectivity asymmetry in 124 normal neonates (born at 36 to 42 gestation weeks). We aimed to tract white matter axonal pathways characterizing interregional connections among cortical and subcortical regions and to infer left and right anatomical network properties. In parallel to the adults’ study (Iturria-Medina et al., 2011), we specially focused on graph measures of small-worldness, efficiency, and centrality of brain regions to 1) examine whether the information flow in both hemispheres of the neonatal brain is similar to that in adults; and 2) identify brain regions that play crucial roles in efficient communication in each cerebral hemisphere. To our knowledge, this is the first report on a normative reference on asymmetry of the neonatal brain structural connectivity. As the early life after birth may not only be a period of developmental vulnerability, but also a period in which therapeutic interventions have the greatest positive effects, our study provides potential insights on brain-based disorders that may originate from preexisting disruptions of anatomical connections at birth.
Materials and Methods
Subjects
Neonates scanned for this study were part of a larger ongoing birth cohort study of Growing Up in Singapore towards Healthy Outcomes (GUSTO). This birth cohort consists of pregnant Asian women aged 18 years and above attending the first trimester antenatal ultrasound scan clinic at the National University Hospital (NUH) and KK Women’s and Children’s Hospital (KKH) in Singapore. Both parents were Singapore citizens or Permanent Residents of Chinese, Malay or Indian ethnic background. Mothers on chemotherapy, psychotropic drugs, including antidepressant or anxiolytic medications, or with Type I Diabetes Mellitus were excluded. The study was approved by Centralized Institutional Review Boards of the Singapore Health Services and Domain Specific Review Board (DSRB) of National Health Care Group. One hundred eighty-nine eligible mothers agreed to participate in the brain imaging study and provided written consent.
Among 189 subjects, we excluded 65 subjects with incomplete demographic information, or birth weight less than 2000 g, or APGAR score less than 9, or absence of large motion artefacts with DTI. Hence, the present study included 124 subjects (58 females, gestational age: 40.2±1.3 weeks; range: 36.9 - 42.7 weeks; and 66 males, gestational age: 39.9±1.3 weeks; range: 36.6- 42.1 weeks). All brain scans were reviewed by a radiologist (M.V.F.).
MRI Data Acquisition
At 5 to 17 days of life, neonates underwent single-shot echo-planar diffusion weighted (DW) MRI scans (TR=7000 ms; TE=56 ms; flip angle = 90°, FOV=200 mm × 200 mm; matrix size= 256 × 256; 40 to 50 axial slices with 3.0 mm thickness; 19 diffusion directions with b=600 sec/mm2; 1 baseline image with b=0 sec/mm2) using a 1.5-Tesla GE scanner at the Department of Diagnostic and Interventional Imaging of the KKH. The scans were acquired when subjects were sleeping in the scanner. No sedation was used and precautions were taken to reduce exposure to the MRI scanner noise. A neonatologist was present during each scan. A pulse oximeter was used to monitor heart rate and oxygen saturation throughout the entire scans.
The Construction of the Neonatal Brain Networks
Figure 1 illustrates the data analysis for constructing the anatomical networks of the two cerebral hemispheres. For each subject, DWIs were first corrected for motion and eddy current distortions using affine transformation to the image without diffusion weighting. Using multivariate least-square fitting, six elements of the diffusion tensor were determined from which fractional anisotropy (FA) was calculated. The FA image and the image without diffusion weighting were then simultaneously aligned via affine and nonlinear large deformation diffeomorphic metric mapping (LDDMM) transformations (Du et al., 2011) to those of the neonatal brain DTI atlas that was proposed by (Oishi et al., 2011) with manually labeled 32 cortical and sub-cortical structures per hemisphere (Table 1). We then employed the reorientation scheme of diffusion tensor using the preservation of principal direction (PPD) method, in which the reoriented tensor keeps its eigenvalues, yet its principal vector is transformed (Cao et al., 2005), to transform subjects’ DTI to the atlas.
Figure 1.
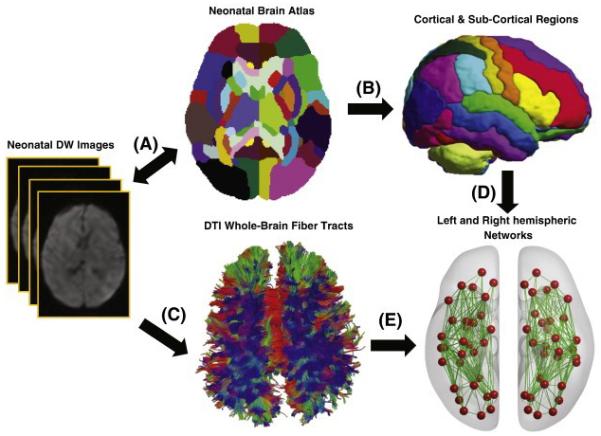
A schematic diagram illustrates the major processes involved in generating the neonatal brain networks. A. DW images of each subject are aligned to those of the neonatal atlas; B. the parcellation of cortical and subcortical regions using the neonatal brain atlas; C. the whole brain tractography using DTI deterministic tractography; D. the nodes (red balls) represent cortical and subcortical regions; E. weighted edges (green lines) obtained using the tract information.
Table 1.
The names and the corresponding abbreviations of the neonatal brain regions used in this study.
| Anatomical Structure | Abbreviation | Anatomical Structure | Abbreviation |
|---|---|---|---|
| Superior Frontal Gyrus | SFG | Fusiform | Fu |
| Middle Frontal Gyrus | MFG | Parahippocampus | PHG |
| Inferior Frontal Gyrus | IFG | Entorhinal cortex | ENT |
| medial Fronto-Orbital Gyrus | mFOG | Superior Occipital Gyrus | SOG |
| lateral Fronto-Orbital Gyrus | lFOG | Middle Occipital Gyrus | MOG |
| Gyrus Rectus | RG | Inferior Occipital Gyrus | IOG |
| PreCentral Gyrus | PrCG | Cuneus | Cu |
| PostCentral Gyrus | PoCG | Lingual gyrus | LG |
| Superior Parietal Lobule | SPL | Amygdala | Amyg |
| PreCuneus | PrCu | Hippocampus | Hippo |
| Cingulate Gyrus | CingG | Cerebellar hemisphere | Cereb |
| SupraMarginal Gyrus | SMG | Insular cortex | Ins |
| Angular Gyrus | AG | Thalamus | Thal |
| Superior Temporal Gyrus | STG | Putamen | Put |
| Middle Temporal Gyrus | MTG | Globus Pallidus | GP |
| Inferior Temporal Gyrus | ITG | Caudate nucleus | Caud |
Whole-brain fiber tractography was subsequently performed in the neonatal DTI atlas space using the fiber assignment by continuous tracking (FACT) (Mori et al., 1999) algorithm. The algorithm computes fiber trajectories starting from the deep white matter regions and ending at a voxel with FA value less than 0.1 or the turning angle between adjacent voxels was greater than 70°.
Two matrices with 32 × 32 elements were constructed based on the whole brain tractography to represent the anatomical networks among each intra-hemispheric regions. The (i, j)th element in the matrix was computed as the number of fiber tracts connecting regions i and j normalized by the mean volume of the two regions and thus represents the connectivity strength between regions i and j. To eliminate brain connections due to possible noise effects on the whole brain tractography, non-parametric one-tailed sign test was performed on each element of the matrices among all the subjects to determine the existence of the connection between any two regions, and thus determine whether the number of fiber tracts going through regions i and j is equal to zero at a significance level of 0.05. We now consider each of the matrices representing one network with the anatomical regions (listed in Table 1) as its nodes and the (i, j)th element of the matrix as its edge with weight information. The path length between any two nodes is the sum of the inverse weights of edges that must be traversed to go from one node to another.
Network Analysis
Small-worldness, global efficiency and local efficiency were computed to characterize the potential ease with which information can be transferred concurrently across a network and locally communicated within neighborhood. At a regional level, betweenness centrality was calculated to identify nodes of a network that play crucial roles in efficient communication.
Small-Worldness
A “Small-world” network model was originally proposed by (Watts and Strogatz, 1998), relating network clustering coefficient and characteristic path length. The clustering coefficient (C ) was defined as the ratio of the number of existing edges between neighbors to the number of all possible connections between neighbors (Onnela et al., 2005). A network with a high value of the clustering coefficient has tightly connected local clusters and hence the loss of an individual node has an impact on the structure of the network. The characteristic path length (L) was defined by the average shortest path length in a network (Watts and Strogatz, 1998), suggesting how far apart any two nodes are linked in the network. A real network is considered small-world if it meets the following criteria: and , or , where Crand and Lrand are the mean clustering coefficient and characteristic path length of random networks that preserve the same number of nodes, edges, and degree distribution as the real brain network (Watts and Strogatz, 1998), while Cbrain and Lbrain are the mean clustering coefficient and characteristic path length of the real brain network. is defined as small-worldness metric.
Global and Local Efficiencies
The global efficiency was defined as the mean of the inverse shortest path length in a network (Latora and Marchiori, 2001) and quantifies how efficiently information can be exchanged over the network, considering a parallel system in which each node sends information concurrently along the network. A high global efficiency value may indicate highly parallel information transfer in a network in which each node could efficiently send information concurrently.
In contrast, the local efficiency of a node was calculated as the global efficiency of the neighborhood sub-network of this node, indicating how well the information can be communicated within the neighbors of a given node when this node was removed. The local efficiency across all nodes within a network were further averaged to estimate the local efficiency of the network (Eloc) (Rubinov and Sporns, 2010). The local efficiency of a network reflects its potential tendency to present clusters of nodes that deal with common neural information. A high value of the local efficiency of a network may indicate efficiently information transfer in the immediate neighborhood of each node.
Betweenness Centrality
Betweenness centrality has been a widely-used measure to identify the most central nodes in a network, which act as bridges between the other nodes (Freeman, 1978). The betweenness centrality of a node was defined as the fraction of all shortest paths in the network that pass through the node i (Freeman, 1978). A node with high betweenness centrality is thus crucial to efficient communication.
Statistical Analysis
Brain network asymmetry was examined according to the aforementioned global network properties and betweenness centrality of each node. For this, the lateralization index of each parameter (X) was computed as
where X(right) and X(left) are the network metric values of the right and left hemispheric networks, respectively. The positive value of LI(X) suggests the rightward asymmetry in terms of X. The non-parametric sign test was used to examine whether all obtained LI values come from a distribution with a median value of zero. After Bonferroni correction for multiple comparisons, the significance level for individual statistical tests on the 3 global network metrics was chosen as 0.0167 (0.05/3), while the significance level for individual tests on the betweenness centrality was 0.0016 (0.05/32). We further conformed our findings using permutation analysis. In each trial of permutation analysis, LIs of the network metrics, including small-worldness, global efficiency, local efficiency, and betweenness centrality, were recomputed while left and right hemispheric labels were randomly assigned to each subject. The sign test was then performed to generate the test statistic. The empirical distribution of this sign test statistic was estimated by repeating the above random permutation for 10,000 trails. Finally, the p-value was computed as the percentage of the trials having the sign test statistic greater than that obtained from the original labeled data.
Multiple regression analyses were used to effects of age, gender and ethnicity on the asymmetry of the brain network metrics. Separate models evaluated each asymmetry measure of the brain network metrics as dependent variables. Age, gender, and ethnicity were entered as main independent variables.
Results
Asymmetry in Small-Worldness and Global Network Metrics
Compared to random networks with the same number of nodes, edges and degree distribution, the anatomical networks of both cerebral hemispheres showed the small-worldness metric greater than 1 (left: p<0.0001; right: p<0.0001), suggesting that the two cerebral hemispheres in neonates showed prominent small-world properties. However, there was no difference in the small-worldness metric between the two cerebral hemispheres (Table 2).
Table 2.
Efficiency measures and small-worldness of the left and right hemispheric networks.
| Left Hemisphere (mean ± SD) |
Right Hemisphere (mean ± SD) |
p-value | |
|---|---|---|---|
| Small-worldness | 2.049±1.455 | 2.023±1.293 | ns |
|
| |||
| Global Efficiency | 0.045±0.016 | 0.041±0.015 | 0.009 |
| Local Efficiency | 0.027±0.009 | 0.025±0.009 | <0.001 |
SD – standard deviation; ‘ns’ – non-significance.
Figure 2 shows the LIs of the global and local efficiencies of individual 124 neonates. The sign tests revealed that the median values of the LIs of these two global network metrics were significantly less than zero (Table 2), suggesting the left cerebral hemisphere with a better balance between local necessities and wide-scope interactions than the right cerebral hemisphere in the neonatal brain. Permutation analysis further confirmed the findings in Table 2.
Figure 2.
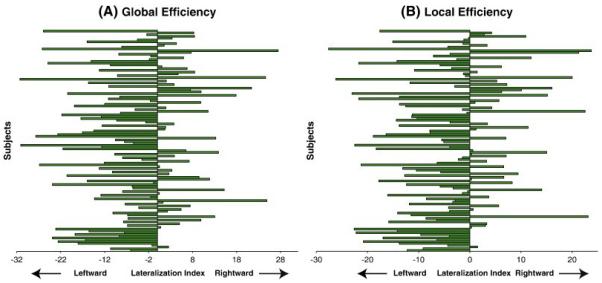
Lateralization indices of global efficiency (A) and local efficiency (B) for all the 124 subjects.
Asymmetry in Betweenness Centrality
Figure 3 illustrates the LI of the nodal betweenness centrality averaged across all the neonates. The sign tests revealed that the precentral gyrus, precuneus, fusiform, entorhinal cortex, and insular cortex showed leftward asymmetry, while the gyrus rectus, cingulate gyrus, hippocampus, and putamen exhibited rightward asymmetry in terms of betweenness centrality (Table 3). Figure 4 showed the anatomical locations of these regions in the cortical surface representation visualized using the BrainNet Viewer (http://www.nitrc.org/projects/bnv/). Permutation analysis further confirmed the findings in Table 3.
Figure 3.
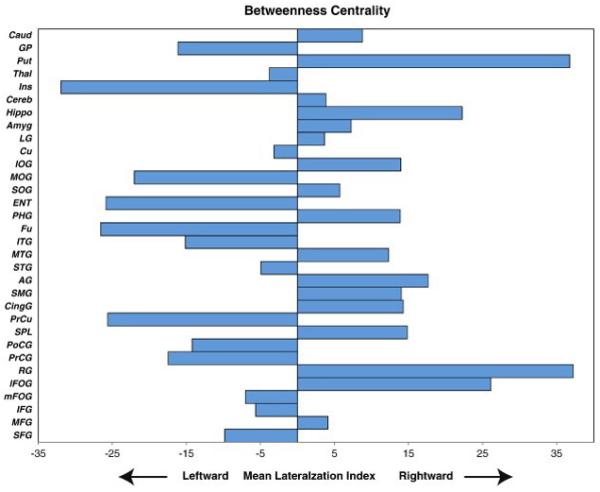
The mean lateralization index of betweenness centrality for the 32 defined cortical and subcortical regions. The abbreviations of the structures are given in Table 1.
Table 3.
Asymmetry in term of betweenness centrality.
| Region | Classification | p-value |
|---|---|---|
| left > right | ||
|
| ||
| Precentral gyrus | Primary | <0.001 |
| Precuneus | Association | <0.001 |
| Fusiform | Association | <0.001 |
| Entorhinal cortex | Association | <0.001 |
| Insular cortex | Association | <0.001 |
|
| ||
| right > left | ||
|
| ||
| Gyrus rectus | Association | <0.001 |
| Cingulate gyrus | Association | 0.001 |
| Hippocampus | Association | <0.001 |
| Putamen | Subcortical | <0.001 |
Figure 4.
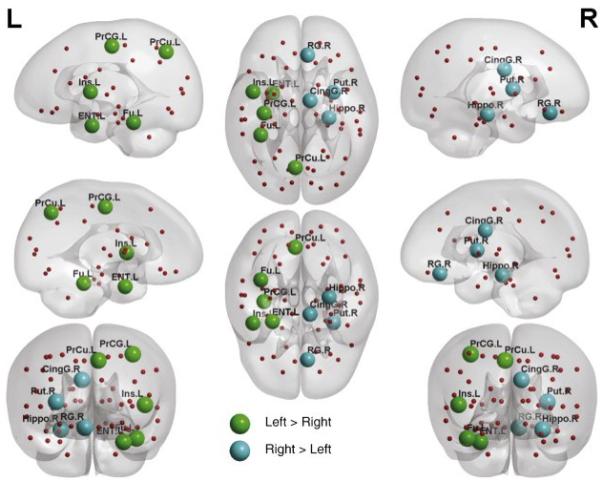
Neural substrates with structural asymmetry in the betweenness centrality. The neural substrates with leftward asymmetry are colored in green, while the neural substrates with right asymmetry are colored in blue. The abbreviations of these substrates are given in Table 1.
Effects of Age, Gender and Ethnicity on Structural Connectivity Asymmetry
Regression analysis did not reveal effects of age, gender, and ethnicity on the asymmetry of small-worldness (p>0.4), global efficiency (p>0.10), and local efficiency (p>0.15). Moreover, regression analysis also did not reveal effects of age and gender on the asymmetry in betweenness centralities of the structures listed in Table 3, including the precentral gyrus (p>0.40), precuneus (p>0.10), fusiform (p>0.25), entorhinal cortex (p>0.50), insular cortex (p>0.45), gyrus rectus (p>0.20), cingulate gyrus (p.0.45), hippocampus (p>0.10), and putamen (p>0.50). Even though most of the structures listed in Table 3 did not show differences in asymmetry of betweenness centrality among different ethnic groups (p>0.50), ethnic effect was discovered on the betweenness centrality asymmetry of the putamen (p=0.0180) but it was not survived after the correction of multiple comparisons.
Discussion
Our study utilized DTI deterministic tractography and structural network analysis and reported the first evidence of asymmetry of structural connectivity in the neonatal brain. In neonates, small-world characteristics were exhibited, but did not differ between the two cerebral hemispheres, suggesting that neighboring brain regions tightly connect to each other and one region is only a few paths away from any other region within each hemisphere. Moreover, the neonatal brain showed greater structural efficiency in the left hemisphere than that in the right, suggesting that the brain regions in the left hemisphere interconnect in better integration and segregation compared to the right hemisphere. Furthermore, in neonates, brain regions that at later ages are known to be involved in motor, language, and memory functions play crucial roles in efficient communications in the left hemisphere, while brain regions involved in emotional processes play crucial roles in efficient communications in the right hemisphere. Together these findings suggest that even at birth, the topology of each cerebral hemisphere is organized in an efficient and compact manner. Such organization may also appear lateralized to support specific lateralized brain functions at birth.
The small-world characteristics (high clustering and short paths) have made a great impact on understanding the topological organization of the brain network. The small-worldness topology was observed in the whole brain structural network of adults (Gong et al., 2009a) and functional networks of children and adults (Supekar et al., 2009). Recently, Yap et al. (Yap et al., 2011) also showed that the whole brain structural network exhibited the small-worldness property in the first two years of life. Our study provides additional support such that each cerebral hemisphere also exhibited the small-worldness properties in the neonatal period. These findings suggest that by even in very early postnatal life the brain favors locally dense communication and minimizes the number of long distance connections within and across two hemispheres. This topological organization of the brain at a global level persists through life, supporting an idea that the human brain has efficient neural architecture for maximizing the power of information processing from birth.
Our study demonstrated leftward asymmetric efficiency at both global and local levels, suggesting more information flow in the left hemisphere than that in the right as shorter paths in the left hemisphere allow speedy information transfer in both integrated and segregated manners among brain regions. With a large sample of 439 healthy subjects aged from 12 to 30 years, Dennisa et al. (Dennisa et al., 2012) found that the global efficiency was roughly the same between the two hemispheres in adolescence, but was greater in the left hemisphere in early adulthood. However, Iturria-Medina et al. (Iturria-Medina et al., 2011) concluded the rightward asymmetry in both global and local efficiencies in early adulthood from a relatively small sample of 11 subjects aged from 23 to 38 years. In spite of discrepancy, these results together with our findings suggested that leftward asymmetry in efficiency follows either a U-shape or a decreasing line during the development from birth to adulthood.
The leftward asymmetry in structural efficiency at birth is in line with previous structural findings. Gilmore et al. (Gilmore et al., 2007) found that the left cerebral hemisphere was larger than the right in healthy neonates. This is the opposite of the right-larger-than-left asymmetry observed in older children and adults (Caviness et al., 1996; Giedd et al., 1996; Good et al., 2001). Additionally, the left-greater-than-right asymmetry in cortical white matter was also found in neonates (Gilmore et al., 2007), whereas white matter volumes were symmetric in adults (Gur et al., 1999). A further provocative finding came in 1985 when Scheibel et al. (Scheibel et al., 1985) reported that the extent of high-order dendritic branching was greater in the left-hemisphere speech area than in their homologs on the right. Lower order dendrites were, however, longer in the right hemisphere in early life. The greater volume and high-order dendritic branching in parallel with more efficient communication in the left hemisphere could be a result of the adaption to lateralized functional needs (e.g., perceptual, language, and motor functions) at birth, which was also supported from our regional findings discussed below.
Our study found that the left precentral gyrus and insular cortex had more efficient communications than the right homologues. This idea is consistent with various structural findings in children and adults (Iturria-Medina et al., 2011; Luders et al., 2006; Tian et al., 2011) as well as leftward asymmetry in motor and language functions observed in most of right-handed people (Balsamo et al., 2006). Witelson and Pallie (Witelson and Pallie, 1973) showed that the neonate was born with an ability to process speech sounds. It has been suggested that the posture of the fetus may lead to motor and perceptual asymmetry at birth and persist through life (Previc, 1991). Two-thirds of fetuses have their right side face outwards. Lateralization of language perception may result from asymmetries in auditory experience. In an elaborate model of motor asymmetry, Previs (Previc, 1991) argued that asymmetrical vestibular stimulation in utero may produce behavioral asymmetries in later life.
Beyond key neural substrates for motor and language functions, our study also observed structural leftward asymmetries in the entorhinal cortex, fusiform, and precuneus in neonates in terms of betweenness centrality. Even though exact functions of these three neural substrates in the left hemisphere at the neonatal stage are unclear, we suspect that their functions in infants are likely similar to those in adults. This has been partially illustrated by a facial expression study in infants using a near infrared spectroscopy technique (Nakato et al., 2011). In adults, the entorhinal cortex plays an important role in episodic memories and is responsible for the familiarity of input signals. Functional MRI studies reveal increased activity in the left entorhinal cortex in tasks of smelling sense (Kjelvik et al., 2012) and of perceived pain (Ploghaus et al., 2001). The precuneus is involved with episodic memory, reflections upon self, and aspects of consciousness. The precuneus is involved with the left prefrontal cortex in the recall of episodic memories including past episodes related to the self (Lou et al., 2004). In the recollection of memories, the precuneus gates subsequent processing of perceptual features based on familiarity (Lundstrom et al., 2005). The fusiform is critical for face perception. Pascalis et al. (Pascalis et al., 2002) tested discrimination of human and monkey faces by 6-month-olds, 9-month-olds, and adults, using the visual paired-comparison procedure. Only the youngest group showed discrimination between individuals of both species; older infants and adults only showed evidence of discrimination of their own species. These results suggested that experience with faces is crucial to driving the development and specialization of the system that allows normal face recognition skill. Thus we would expect that in the neonatal period the entorhinal cortex, fusiform, and precuneus function as hubs in a widespread network in the left hemisphere for conscious perception and episodic memory and therefore play a crucial role in integrating experience of sensory information with memory systems. Hence, the left hemisphere could be more able to relate stimuli to past experience, either short or long-term in early life (Wada & Davis 1977).
In contrast, in early life the right cerebral hemisphere could be better able to process stimuli that are not easily identifiable or referable, such as emotion (Schore, 2000; Wada and Davis, 1977). This idea appears consistent with our findings of the rightward asymmetry in the betweenness centrality of the limbic structures (cingulate and hippocampus) and the striatum (putamen). These neural substrates function as hubs in the right hemisphere for emotion processes and mother and child interaction. Among adults individual differences in activity of the right hippocampus predicts the magnitude of the neuroendocrine response to stress (Pruessner et al., 2008). Small hippocampal volumes at term associated with school-age social-emotional development in preterm children (Rogers et al., 2012). The cingulate gyrus, particularly the anterior segment, maintains rich interconnections with hippocampus and striatum as well as other neural substrates (e.g., amygdala, thalamus, and frontal cortex) and hence appears to be a supra-modal area that is associated with processing and modulating emotion. There is evidence for the differential activation of the right anterior cingulate cortex in emotional processing, especially that of self-referential emotional stimuli (Pujol et al., 2002; Sturm et al., 2012; Yoshimura et al., 2009), emotional regulation (Beauregard et al., 2001; Casey et al., 1997) and trait differences in emotional function (Pujol et al., 2002). The anterior cingulate gyrus, in conjunction with the right frontal lobe, appears to provide the foundation for mother-infant communication and the generation of separation anxiety (Davidson and Fox, 1989).
We notice that gender specific asymmetries are often found in the human brain. For instance, females have advantages in the left-lateralized language processing (Toga and Thompson, 2003). Resting-state functional MRI studies showed that male adults have higher clustering coefficient in right hemisphere and lower clustering coefficient in left hemisphere comparing with females (Tian et al., 2011). However, our study did not reveal gender differences in structural connectivity asymmetry in neonates. Hence, gender-hemisphere interaction was not considered in our study. Moreover, our study did not reveal any age effects on structural connectivity asymmetry even though the brain in terms of size and synaptic proliferation grows rapidly during the first two weeks of life (Qiu et al., 2013) (Gilmore et al., 2007). This is not surprising as synaptic pruning and fine-tuning that could influence structural connectivity occur later than this early stage of life (Petanjek et al., 2008). Finally, consistent with previous findings on individual brain structural differences among Asian ethnic groups (Bai et al., 2012), our study showed ethnic effects on the asymmetry of betweenness centrality in the putamen, one of the earliest developing brain structures. It undergoes rapid morphological growth to adapt to the needs of sensorimotor functions in early life.
There are several concerns on structural network analysis of the neonatal brain. In the neonatal brain, the white matter is largely unmyelinated and hence has low diffusion anisotropy. This could cause potential termination of fiber tracking in deterministic tractography, especially in the periphery white matter region (region close to the cortex). To overcome this issue, our study employed the neonatal brain atlas (Oishi et al., 2011) in which the cortical parcellation incorporates its corresponding periphery white matter region (see illustration in Figure 1). Moreover, the deterministic tractographic technique (FACT) rather than probabilistic tractographic technique was used in our structural network analysis. Although the FACT algorithm may not be robust against noise, it was used in this present study mainly because it has been validated in the infant brain (Dubois et al., 2006).
In conclusion, this study provided the first evidence of hemispheric differences in structural connectivity of the neonatal brain. The neonatal brain is well equipped to process cognitive functions needed at birth. This is particularly achieved by two topologically well-organized hemispheres with distinct and lateralized functions.
Acknowledgements
We acknowledged Jordan Bai (Department Bioengineering, National University of Singapore) for his contribution to DTI data preprocessing. This study is supported by National Medical Research Council (NMRC; NMRC/TCR/004-NUS/2008), Agency for Science, Technology and Research (A*STAR; SICS-09/1/1/001), the Young Investigator Award at the National University of Singapore (NUSYIA FY10 P07), the National University of Singapore MOE AcRF Tier 1, and Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2012-T2-2-130).
References
- Bai J, Abdul-Rahman MF, Rifkin-Graboi A, Chong YS, Kwek K, Saw SM, Godfrey KM, Gluckman PD, Fortier MV, Meaney MJ, Qiu A. Population Differences in Brain Morphology and Microstructure among Chinese, Malay, and Indian Neonates. PLoS One. 2012;7:e47816. doi: 10.1371/journal.pone.0047816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsamo LM, Xu B, Gaillard WD. Language lateralization and the role of the fusiform gyrus in semantic processing in young children. Neuroimage. 2006;31:1306–1314. doi: 10.1016/j.neuroimage.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. The economy of brain network organization. Nature reviews. Neuroscience. 2012;13:336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- Cao Y, Miller MI, Winslow RL, Younes L. Large deformation diffeomorphic metric mapping of vector fields. IEEE Trans Med Imaging. 2005;24:1216–1230. doi: 10.1109/tmi.2005.853923. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor R, Giedd J, Vauss Y, Vaituzis CK, Hamburger S, Kozuch P, Rapoport JL. The role of the anterior cingulate in automatic and controlled processes: a developmental neuroanatomical study. Developmental psychobiology. 1997;30:61–69. [PubMed] [Google Scholar]
- Caviness JVS, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The Human Brain Age 7-11 Years: A Volumetric Analysis Based on Magnetic Resonance Images. Cerebral Cortex. 1996;6:726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- Chapple B, Grech A, Sham P, Toulopoulou T, Walshe M, Schulze K, Morgan K, Murray RM, McDonald C. Normal cerebral asymmetry in familial and non-familial schizophrenic probands and their unaffected relatives. Schizophr Res. 2004;67:33–40. doi: 10.1016/s0920-9964(03)00095-1. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Fox NA. Frontal brain asymmetry predicts infants’ response to maternal separation. Journal of abnormal psychology. 1989;98:127–131. doi: 10.1037//0021-843x.98.2.127. [DOI] [PubMed] [Google Scholar]
- Dennisa E, Jahanshada N, McMahonb K, de Zubicarayc G, Martind N, Hickiee I, Toga A, Wrightc W, Thompsona P. Development of brain structural connectivity between ages 12 and 30: A 4-Tesla diffusion imaging study in 439 adolescents and adults. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Younes L, Qiu A. Whole brain diffeomorphic metric mapping via integration of sulcal and gyral curves, cortical surfaces, and images. Neuroimage. 2011;56:162–173. doi: 10.1016/j.neuroimage.2011.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Hertz-Pannier L, Cachia A, Mangin JF, Le Bihan D, Dehaene-Lambertz G. Structural asymmetries in the infant language and sensori-motor networks. Cerebral Cortex. 2009;19:414–423. doi: 10.1093/cercor/bhn097. [DOI] [PubMed] [Google Scholar]
- Dubois J, Hertz-Pannier L, Dehaene-Lambertz G, Cointepas Y, Le Bihan D. Assessment of the early organization and maturation of infants’ cerebral white matter fiber bundles: a feasibility study using quantitative diffusion tensor imaging and tractography. Neuroimage. 2006;30:1121–1132. doi: 10.1016/j.neuroimage.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Freeman LC. Centrality in social networks: conceptual clarification. Soc. Netw. 1978;1:215–239. [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative Magnetic Resonance Imaging of Human Brain Development: Ages 4-18. Cerebral Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YS, Knickmeyer RC, Evans DD, Smith JK, Hamer RM, Lieberman JA, Gerig G. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci. 2007;27:1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, He Y, Concha L, Lebel C, Gross DW, Evans AC, Beaulieu C. Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex. 2009a;19:524–536. doi: 10.1093/cercor/bhn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, Rosa-Neto P, Carbonell F, Chen ZJ, He Y, Evans AC. Age- and gender-related differences in the cortical anatomical network. J Neurosci. 2009b;29:15684–15693. doi: 10.1523/JNEUROSCI.2308-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1999;19:4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturria-Medina Y, Perez Fernandez A, Morris DM, Canales-Rodriguez EJ, Haroon HA, Garcia Penton L, Augath M, Galan Garcia L, Logothetis N, Parker GJ, Melie-Garcia L. Brain hemispheric structural efficiency and interconnectivity rightward asymmetry in human and nonhuman primates. Cerebral Cortex. 2011;21:56–67. doi: 10.1093/cercor/bhq058. [DOI] [PubMed] [Google Scholar]
- Kjelvik G, Evensmoen HR, Brezova V, Haberg AK. The human brain representation of odor identification. J Neurophysiol. 2012;108:645–657. doi: 10.1152/jn.01036.2010. [DOI] [PubMed] [Google Scholar]
- Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett. 2001;87:198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- LeMay M. Morphological cerebral asymmetries of modern man, fossil man, and nonhuman primate. Ann N Y Acad Sci. 1976;280:349–366. doi: 10.1111/j.1749-6632.1976.tb25499.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Baleriaux D, Kavec M, Metens T, Absil J, Denolin V, Pardou A, Avni F, Van Bogaert P, Aeby A. Structural asymmetries in motor and language networks in a population of healthy preterm neonates at term equivalent age: a diffusion tensor imaging and probabilistic tractography study. Neuroimage. 2010;51:783–788. doi: 10.1016/j.neuroimage.2010.02.066. [DOI] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, Keenan JP, Nowak M, Kjaer TW, Sackeim HA, Lisanby SH. Parietal cortex and representation of the mental Self. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6827–6832. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Rex DE, Jancke L, Toga AW. Hemispheric asymmetries in cortical thickness. Cerebral Cortex. 2006;16:1232–1238. doi: 10.1093/cercor/bhj064. [DOI] [PubMed] [Google Scholar]
- Lundstrom BN, Ingvar M, Petersson KM. The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. Neuroimage. 2005;27:824–834. doi: 10.1016/j.neuroimage.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Nakato E, Otsuka Y, Kanazawa S, Yamaguchi MK, Kakigi R. Distinct differences in the pattern of hemodynamic response to happy and angry facial expressions in infants--a near-infrared spectroscopic study. Neuroimage. 2011;54:1600–1606. doi: 10.1016/j.neuroimage.2010.09.021. [DOI] [PubMed] [Google Scholar]
- Oishi K, Mori S, Donohue PK, Ernst T, Anderson L, Buchthal S, Faria A, Jiang H, Li X, Miller MI, van Zijl PC, Chang L. Multi-contrast human neonatal brain atlas: application to normal neonate development analysis. Neuroimage. 2011;56:8–20. doi: 10.1016/j.neuroimage.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onnela JP, Saramaki J, Kertesz J, Kaski K. Intensity and coherence of motifs in weighted complex networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;71:065103. doi: 10.1103/PhysRevE.71.065103. [DOI] [PubMed] [Google Scholar]
- Pascalis O, de Haan M, Nelson CA. Is face processing species-specific during the first year of life? Science. 2002;296:1321–1323. doi: 10.1126/science.1070223. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Kostovic I, Uylings HB. Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: a layer-specific pattern. Cerebral Cortex. 2008;18:915–929. doi: 10.1093/cercor/bhm124. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21:9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previc FH. A general theory concerning the prenatal origins of cerebral lateralization in humans. Psychological review. 1991;98:299–334. doi: 10.1037/0033-295x.98.3.299. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, Lupien S. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry. 2008;63:234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Pujol J, Lopez A, Deus J, Cardoner N, Vallejo J, Capdevila A, Paus T. Anatomical variability of the anterior cingulate gyrus and basic dimensions of human personality. Neuroimage. 2002;15:847–855. doi: 10.1006/nimg.2001.1004. [DOI] [PubMed] [Google Scholar]
- Qiu A, Fortier MV, Bai J, Zhang X, Chong YS, Kwek K, Saw SM, Godfrey KM, Gluckman PD, Meaney MJ. Morphology and microstructure of subcortical structures at birth: a large-scale Asian neonatal neuroimaging study. Neuroimage. 2013;65:315–323. doi: 10.1016/j.neuroimage.2012.09.032. [DOI] [PubMed] [Google Scholar]
- Rogers CE, Anderson PJ, Thompson DK, Kidokoro H, Wallendorf M, Treyvaud K, Roberts G, Doyle LW, Neil JJ, Inder TE. Regional cerebral development at term relates to school-age social-emotional development in very preterm children. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:181–191. doi: 10.1016/j.jaac.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Scheibel AB, Paul LA, Fried I, Forsythe AB, Tomiyasu U, Wechsler A, Kao A, Slotnick J. Dendritic organization of the anterior speech area. Experimental neurology. 1985;87:109–117. doi: 10.1016/0014-4886(85)90137-2. [DOI] [PubMed] [Google Scholar]
- Schore AN. Attachment and the regulation of the right brain. Attachment & human development. 2000;2:23–47. doi: 10.1080/146167300361309. [DOI] [PubMed] [Google Scholar]
- Sharma T, Lancaster E, Sigmundsson T, Lewis S, Takei N, Gurling H, Barta P, Pearlson G, Murray R. Lack of normal pattern of cerebral asymmetry in familial schizophrenic patients and their relatives--The Maudsley Family Study. Schizophr Res. 1999;40:111–120. doi: 10.1016/s0920-9964(99)00143-7. [DOI] [PubMed] [Google Scholar]
- Sturm VE, Sollberger M, Seeley WW, Rankin KP, Ascher EA, Rosen HJ, Miller BL, Levenson RW. Role of right pregenual anterior cingulate cortex in self-conscious emotional reactivity. Social cognitive and affective neuroscience. 2012 doi: 10.1093/scan/nss023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS biology. 2009;7:e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Wang J, Yan C, He Y. Hemisphere- and gender-related differences in small-world brain networks: a resting-state functional MRI study. Neuroimage. 2011;54:191–202. doi: 10.1016/j.neuroimage.2010.07.066. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- Wada JA, Davis AE. Fundamental nature of human infant’s brain asymmetry. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 1977;4:203–207. doi: 10.1017/s0317167100025233. [DOI] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Luchins DJ, Morihisa J, Wyatt RJ. Asymmetrical volumes of the right and left frontal and occipital regions of the human brain. Ann Neurol. 1982;11:97–100. doi: 10.1002/ana.410110118. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Pallie W. Left hemisphere specialization for language in the newborn. Neuroanatomical evidence of asymmetry. Brain. 1973;96:641–646. doi: 10.1093/brain/96.3.641. [DOI] [PubMed] [Google Scholar]
- Yan C, Gong G, Wang J, Wang D, Liu D, Zhu C, Chen ZJ, Evans A, Zang Y, He Y. Sex- and brain size-related small-world structural cortical networks in young adults: a DTI tractography study. Cerebral Cortex. 2011;21:449–458. doi: 10.1093/cercor/bhq111. [DOI] [PubMed] [Google Scholar]
- Yap PT, Fan Y, Chen Y, Gilmore JH, Lin W, Shen D. Development trends of white matter connectivity in the first years of life. PLoS One. 2011;6:e24678. doi: 10.1371/journal.pone.0024678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S, Ueda K, Suzuki S, Onoda K, Okamoto Y, Yamawaki S. Self-referential processing of negative stimuli within the ventral anterior cingulate gyrus and right amygdala. Brain and Cognition. 2009;69:218–225. doi: 10.1016/j.bandc.2008.07.010. [DOI] [PubMed] [Google Scholar]


