Abstract
There is no standardized definition of difficult polyps. However, polyps become difficult and challenging to remove endoscopically when they are large in size, flat in nature, situated in a high-risk location and when access to them is very awkward. Recently, an SMSA (Size, Morphology, Site, Access) classification has been proposed that helps to qualify the degree of difficulty by scoring on the above parameters. This article reviews the features that make polyps difficult to remove and provides some practical tips in managing these difficult polyps. We believe that ‘difficult polyp’ is a relative term and each endoscopist should define their own level of difficulty and what they would be able to handle safely. However, in expert trained hands, most difficult polyps can be safely removed by an endoscopic approach.
Keywords: Colonic polyp, polypectomy, complications, colonoscopy, EMR
Introduction
There has been a substantial increase in the number of colonoscopies being performed with the advent of national bowel cancer screening program. This has resulted in increased number of polyps detected. Hospital Episode Statistics (HES) database [1] from England reports that 334,753 polypectomy procedures were performed on 286,204 patients in English National Health Service (NHS) Trusts from 1997 to 2007 with a 55% increase in the total number of polypectomy procedures from 20,289 in 1997 to 31,503 in 2007. Most endoscopists performing colonoscopies are trained to remove the majority of polyps found. However, with the overall increased polyp detection, we are increasingly finding polyps which are technically more challenging, classified as difficult polyps. This review aims to provide some insight into some difficulties encountered when dealing with these polyps and aims to provide some tips and techniques to address those difficulties.
Difficult polyp
There are no well-defined criteria to define a difficult polyp. A number of factors can make removal of a polyp difficult. These factors can be summarized into those related to difficult morphology and those related to difficult location. Factors related to difficult morphology include size [2,3] (greater than 2 cm for sessile polyps and greater than 3 cm for pedunculated polyps), polyps occupying more than one third of the luminal circumference and polyps crossing 2 haustral folds [4,5]. Factors relating to location include peridiverticular polyps, rectal polyps close to the dentate line, polyps over the ileo-cecal valve or appendicear orifice, and clamshell polyps (polyps wrapped around a fold) [4-6].
Size, Morphology, Site, Access (SMSA) is a scoring system to grade the difficulty encountered during polypectomy [7]. The four factors for determining the complexity of a polypectomy are size (S), morphology (M), site (S) and access (A). After analyzing the expected complexity for each polyp type as proposed by experts in polypectomy, the SMSA scoring system was established, based on size (1-9 points), morphology (1-3 points), site (1-2 points) and access (1-3 points). Four polyp levels (with increasing level of complexity) were identified based on the range of expert scores obtained. Level 1 (4-5), Level 2 (6-9), Level 3 (10-12) and Level 4 (>12). This system provides an objective assessment of the complexity of a polyp and suggests that as the score increases, the complexity increases. It also proposes the level of expertise needed for the endoscopist to deal with these difficult polyps and might become a future guiding force (Table 1). Applying SMSA scoring system to our tertiary referrals for difficult polyps, we found that 63% were SMSA level 4, 32% were SMSA level 3 and 5% were SMSA level 2.
Table 1.
SMSA scoring system
We performed a literature review of series reporting endoscopic removal of large polyps, restricting our search to series with at least 100 polyps (Table 2). These show variable success, recurrence and complication rates.
Table 2.
Clinical outcome on selected series relating to difficult polyps
Difficult morphology
Size
Size is the most commonly encountered factor in most series [2,3]. With increasing size, the risk of complications, recurrence and malignancy also increase. Most endoscopists are trained to resect polyps less than 20 mm. Polyps greater than 20 mm are infrequent so endoscopists have less experience of dealing with them. Increase in size leads to a number of other problems which include: 1) increased complexity of resection; 2) increased duration of resection; 3) difficulty in seeing the far edge; 4) increased bleeding risk due to increased vascularity of larger pedunculated polyps; 5) increased risk of perforation due to diathermy effect delivered to the large flat polyps; 6) increased recurrence rates; and 7) increased risk of malignancy. Due to these challenges and concerns, many patients with large polyps are referred for surgery or to an expert endoscopist.
More than one-third of the circumference
The greater the circumferential spread of a polyp, the more difficult it is to remove it [8,9]. As a general rule, a polyp which involves more than one-third of the circumference of the colon wall is difficult to remove endoscopically. It is possible for polyps of this size to be removed by an expert endoscopist using multipiece endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) techniques but these procedures are technically challenging.
Polyps crossing two haustral folds
Polyps that cross over two haustral folds present two distinct problems [10,11]. Firstly, the portion that lies in the valley between two interhaustral septae can be very challenging and difficult to access. Secondly, there is a risk of catching the full thickness of colonic wall in the snare, especially over the haustral fold, inadvertently leading to perforation. Submucosal injection-assisted EMR or ESD and operating in retroflexed position can help remove these polyps safely via an endoscopic approach.
Difficult location
Polyps behind folds
When part of the polyp is either hidden from view behind a fold or wrapped around a fold in clamshell fashion, endoscopists find it difficult to resect [10,11]. The solution to this problem when the proximal edge of the polyp is hidden is to inject the far side of the polyp. This is accomplished by passing the scope beyond the far edge of the polyp. While deflecting the tip toward the polyp, the injection should be made into the normal mucosa just at or near the edge of the polyp (cecal edge of the polyp). Injection into the wall on the far side of the polyp will raise that portion up on the fluid mound, making snare application easier and reducing the risk of perforation. Depending on the polyp size, several injections may be required to elevate the polyp so that snare placement is more readily accomplished. After the back portion of the polyp has been lifted, saline may be injected into the distal edge. Retroflexed endoscopes can also be effective in dealing with these types of polyps but require a lot more training and expertise.
Peridiverticular polyps
Diverticular disease leads to muscular hypertrophy and a fixed colon which makes any endoscopic manipulation a challenge [11]. Diverticular segments of bowel are generally very narrow and long with very little space for endoscope maneuring. Maintaining a stable position of the endoscope in a sigmoid colon with multiple diverticula can be difficult. If the polyp is large, it can become difficult for the endoscopist to maintain an adequate distance from diverticula so as to avoid perforation. These difficulties can often be overcome by using a gastroscope (short nose and thin diameter) and small snares.
When injecting submucosally, it is helpful to inject into the edge between the polyp and the diverticulum to create a cushion of fluid between them which allows adequate separation between the two and enables better polyp delineation by pushing the polyp distally. It is vital to start the injection at the edge of the polyp and move the polyp away from the diverticulum. For polyps inside the diverticulum, perforation risk is high.
Polyps involving the appendicular orifice
Polyps that involve the appendiceal orifice may extend into the appendix, and, although this phenomenon is rare, total removal of this type of polyp is problematic [11,12] and is associated with high risk of recurrence or perforation hence surgery is generally the preferred approach. However, for those polyps at the edge of the appendicular orifice, a similar principle to that used in peridiverticular polyps should be applied.
Rectal polyps touching the dentate line
Flat or sessile polyps in the distal rectum can spread downwards to the dentate line. These pose several challenges. It can be very difficult to exactly identify the distal edge of these polyps [13,14]. Limited space on the anal side of these polyps (to deploy the snare without endoscope falling out), and high risk of the snare touching the squamous epithelium (rich sensory nervous supply) risk causing discomfort to the patient. The other challenge in this area is a very rich vascular network which can result in intra-procedural and delayed bleeding. These challenges can be minimized by a series of measures. Injection of lignocaine locally into the dentate line will reduce the pain and discomfort for the patient. Using a gastroscope instead of a colonoscope in this situation, results in easier access to the polyp due to greater bending angle of the scope. Working in retroflexed position is often required to clear the anal side of the polyp and aid complete clearance. However, this is technically challenging and requires training not just for the endoscopist but also for the endoscopy nurse/assistant as snares can be difficult to open and shut in retroflexed position. Expertise in bleed control is essential and the use of ‘Coagrasper’ (Olympus) is very useful. ESD knives can also be very useful and effective in this situation.
Endoscopic resection: practical tips
Cap-assisted polypectomy
Cap & snare technique is an effective technique for performing EMR in the upper gastrointestinal (GI) tract. It involves submucosal injection and application of a suction cap with a preloaded snare. Recently this technique has been trialed in the colon. Flat adenomas can be difficult to catch in a snare despite submucosal injection and elevation of these lesions. Cap and suck technique can overcome this problem by sucking the flat adenoma into the cap before capturing it into a preloaded snare. Caution is urged when using this technique above the peritoneal reflection. This is a popular technique in upper GI tract and can be effectively used in the rectum [13] with subtle changes in technique such as using saline rather than colloid injection, avoiding full red out, moving the snare and releasing partially before completing polypectomy. However, this technique is still experimental in colon and should only be performed by experts.
Piecemeal polypectomy
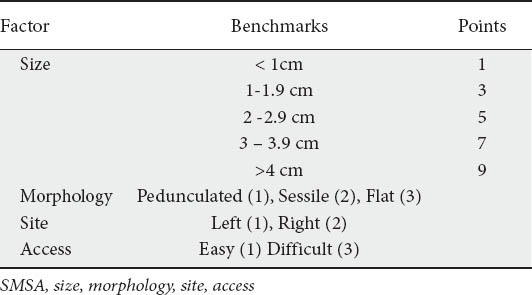
After careful inspection, if a decision to treat is made at the outset, the endoscopist should develop a very clear strategy for resecting the lesion. Following this, strategic submucosal injection is performed only to lift the area about to be resected. The first bite with the snare should include a cuff of normal mucosa. This is followed by further injections and resections in a step wise fashion without leaving any mucosal islands in between two bites (Fig. 1). During piecemeal polypectomy, the next placement of the snare may be immediately adjacent to the first, with the edge of the wire positioned into the denuded area just created by removal of the previous piece. In this fashion, multiple portions can be sequentially resected in an orderly fashion, with removal of each succeeding piece being facilitated by its predecessor. Careful attention should be paid to avoid catching the muscle in the denuded area. Several applications may be required to remove multiple fragments until satisfactory polypectomy is achieved [13,14]. Polyp fragments may be removed by suction into a trap if they are small, or retrieved with a Roth net or with a tripod grasper. The fulcrum technique may be used in difficult situations when space is tight or the polyp is very flat. The tip of the opened snare is impacted against the colonic wall behind the polyp. By keeping the tip fixed, slightly advancing the snare, and bending the endoscope tip to left or right the snare is pivoted to either side. If the tip of the opened snare is placed in front of the polyp, it can be flexed backwards along its long axis by advancing the snare and the tip of the endoscope. To prevent perforation, the wire loop should be pressed flat against the bowel wall to avoid entrapment of the muscle layer within the snare. This is a high-risk strategy and requires a fair amount of experience and skills to achieve safe and effective results.
Figure 1.

(A) Colonic polyp over a scarred base (indicated by the converging mucosal folds at the base. (B) Initial resection at the base performed using an ESD (Endoscopic submucosal dissection) knife. (C) Further resection using piece meal approach. (D) Polyp base after complete removal. This view highlights the difficulty in distinguishing circular muscle layer from the scar tissue
Laterally spreading tumors (LSTs)
LSTs are lesions greater than 10 mm with a low vertical axis that extend laterally along the luminal wall. Two distinct clinicopathological and phenotypic types are described [14-16] including LSTs granular-type (LST-G), which endoscopically consist of numerous nodules having a homogenous color in comparison with the surrounding colonic mucosa and LSTs non-granular-type (LST-NG) which consist of a smooth surface. Uraoka et al [15] showed that LST-NG had a higher frequency of submucosal invasion than LST-G (14% vs. 7%, P=0.01). Presence of a large nodule in LST-G was associated with higher submucosal invasion while pit pattern (invasive pattern), sclerous wall change, and larger tumor size were associated with higher submucosal invasion in LST-NG. The recommended therapeutic strategy for LST-G is piecemeal resection with the area including the large nodule resected first, whereas LST-NG should be removed en bloc because of the higher potential for malignancy, often multifocal and greater difficulty in diagnosing depth of submcucosal invasion (sm depth) and extent of invasion [14].
Pedunculated polyps
Large pedunculated polyps have a big vessel in the stalk which increases the risk of immediate or delayed bleeding [15-17]. In general, polyps with the longest pedicles tend to be located within the left colon. Large pedunculated polyps in the sigmoid within a narrow space pose difficulties in passing the snare over the head of the polyp. To decrease the occurrence of bleeding episodes different approaches have been utilized, including the use of endoloops, endoclips [17] and injection of adrenaline into the head or the stalk of the polyp. All these strategies are effective but the evidence comes from small, poorly designed studies. Each strategy poses a unique challenge as well. Endoloops are difficult to deploy and have a long tail which can get caught into the snare during polypectomy, impairing the conduction of current and may become stuck to the snare. Clips, if used, should be deployed at the base of the pedicle allowing enough space between the clip and the snare to avoid conduction of current down the clip. We believe that small amount of adrenaline and indigo carmine into the base followed by a very slow closure is an effective strategy. Immediate post-polypectomy inspection at the base is the key to reduce delayed bleeding. If any tiny vessel is visible, further hemostatic action (clip, endoloop, and diathermy) should be taken. Blue indigo carmine dye in the base highlights a pedicle vessel very well.
Other risks involved include transection through polyp whilst attempting polypectomy, and the risk of collateral burns. The role of changing the patient position cannot be over-emphasized in dealing with the pedunculated polyps. It is important to keep the polyp head towards the cecal side of the stalk base and keep the stalk base very close to the scope. The snare should be positioned halfway down the stalk. These measures aid careful assessment for any malignancy. In addition, if bleeding occurs, effective therapy can be applied easily.
Endoscopic submucosal dissection
ESD is a technique that allows en bloc resection of larger sessile and flat (usually more than 20 mm) mucosal as well as subepithelial lesions with the use of cutting devices (Fig. 2). ‘‘En bloc resection” is defined as resection in a single piece of tissue, and “piecemeal resection” is defined as resection in multiple pieces [15-17]. ESD for colorectal tumors is technically more challenging for various reasons: 1) the colonic wall is thinner than the gastric wall; 2) endoscopic control can be difficult because of paradoxical movement; and 3) tumors can be located on or behind a fold. Further, colonic perforation has a higher risk of peritonitis necessitating surgical repair. A multicenter study in Japan [14] of 1,111 ESDs showed en bloc and curative resection rates were 88% and 89%, respectively. Perforations occurred in 54 cases (4.9%) with 4 cases of delayed perforation (0.4%) and 17 cases of postoperative bleeding (1.5%). Two immediate perforations with ineffective endoscopic clipping and 3 delayed perforations required emergency surgery.
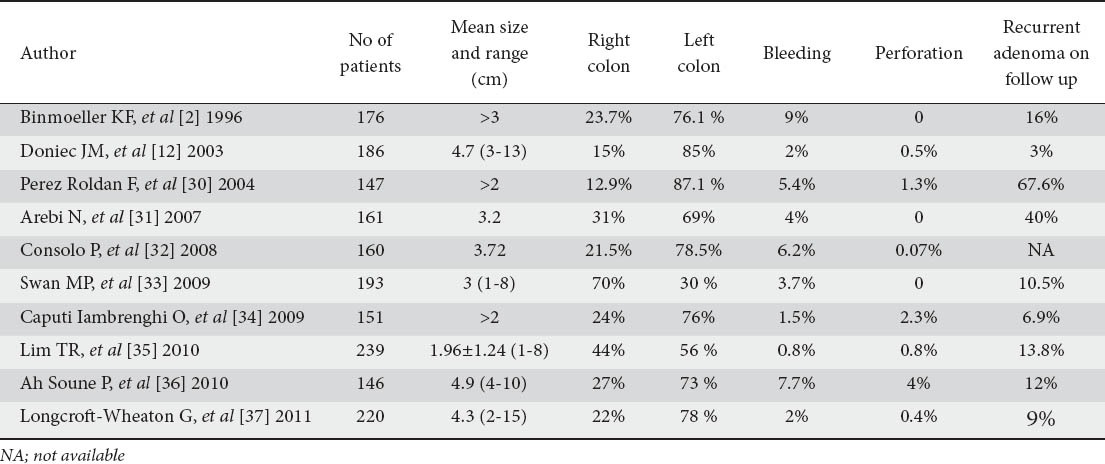
Figure 2.
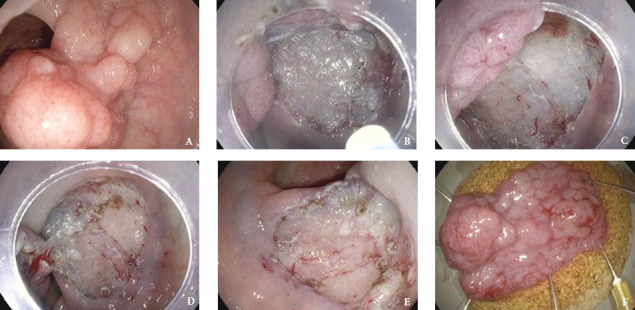
Endoscopic removal of a large rectal LST-G (Laterally spreading tumor – Granular). (A) Large rectal LST-G with a broad base. (B) Cap assisted ESD technique. The cap ensures the cutting field acting as a mean of support. (C) View of the circular muscle layer underneath the submucosa. (D, E) Complete en-bloc removal of the polyp using ESD technique. (F) Polyp specimen after removal
ESD is still developing in western countries. Experience is limited and training opportunities are few. A recent study in France by Farhat et al [16] showed that the short-term morbidity following ESD was 29% including 18% perforation and 11% bleeding during the first 24 h, although most of these were managed conservatively or endoscopically. En bloc resection was achieved in 77% of cases, with complete R0 resection in 73%. Outcomes and complication rates were clearly far inferior to Japanese data. Although ESD is a far superior technique to EMR for resection of large flat lesions, especially LST-NG, the safety of this technique in western settings remains unproven. It should only be performed by trained experts at high-volume centers.
Tattooing
Polyps which are large or are suspicious for invasive cancer should be tattooed for easier localization either by a surgeon during colectomy or by an endoscopist during future surveillance colonoscopy [18,19]. India ink is the preferred dye for tattooing polyps. Other dyes like indigo carmine and methylene blue are too rapidly resorbed to be useful. India ink is injected through an injection needle and targeted to the submucosal layer of the inter-haustral folds. Injecting at an oblique angle tangential to the colon wall can avoid penetration of the colon wall. We advocate dual injection technique [13,18] where 1 mL of saline is first injected creating a submucosal bleb. Once the saline bleb is made, the needle is left in place, the saline syringe is changed to an India ink syringe and about 2 mL of tattoo ink is then injected into the bleb space. We advocate tattooing all polyps >10 mm in size in view of the higher risk of cancer. Each unit should have a standard tattooing protocol so that the endoscopist and the surgeon always know where to expect the polyp/polypectomy site in relation to the tattoo. We always place a minimum of 2 tattoos about 5 cm distal (on the anal side).
Complications
The major risks of these techniques are perforation and bleeding. The Munich Polypectomy Study (MUPS) [20] analyzed the complications and risk factors in nearly 4,000 snare polypectomies in 2,257 patients with a mean polyp size of 1.1 cm and with 72% of the polyps being sessile. Complications occurred in 9.7% of patients (6.1% of polyps). 75% of the complications were minor; and the mortality rate was zero. Multivariate regression analysis revealed polyp size as the main risk factor for complications. Right-sided polyp location was shown to be a significant risk factor for major complications. It was shown that polyps larger than 1 cm in the right colon or 2 cm in the left colon, and multiple polyps carried an increased risk. The study also concluded that a cut-off value of 3% as an acceptable rate for major complications. We believe that the complications of polypectomies depend on the polyp characteristics as well as on the experience and skills of the endoscopist. We advocate that high-risk polypectomy should be performed by experts at a high-volume center.
Hemorrhage
EMR-related hemorrhage can be classified as “procedural bleeding” if it occurs during the procedure and can be Managing difficult polyps controlled endoscopically without further complications [21]. Bleeding is considered “immediate” when presenting during the first 24 h after the procedure and “delayed” when occurring more than 24 h after the procedure. There is a greater risk of immediate hemorrhage associated with cutting current and a greater risk of delayed hemorrhage with coagulation current. Risk of post-polypectomy hemorrhage ranges from 0.3% to 6% but can be as high as 24% in large polyps [14,22-24]. Preventive measures include prophylactic clips, endoloops [25-27] and injection of the adrenaline to the base [26]. In immediate bleeding, either adrenaline injection into the base of the polypectomy site or endoclip placement [24-27] can be used (Fig. 3). Endoloop placement can also be considered and applied to a stalk after removal of pedunculated polyps. Endoclips can be placed prophylactically at the polypectomy site after removal of the polyp. Most clips such as Quick clips (Olympus) and Resolution clips (Boston scientific) are through the-scope clips. Recently another clipping option is available in the form of OTSC-Over-The-Scope Clip (Ovesco). This clip is mounted over the scope and the endoscope has to be withdrawn in order to mount the clip. Once mounted, it can be very effective in clipping large vessels or tissue particularly in the rectum. We recommend using slow coagulation for pedunculated polyps, careful post-polypectomy inspection assisted by water jet and using coaggraspers. ‘Coagrasper’ (Olympus) can be extremely useful in treatment of bleeding during polypectomy. Achieving good views is vital to locate the source of bleeding and apply therapy to the exact site.
Figure 3.
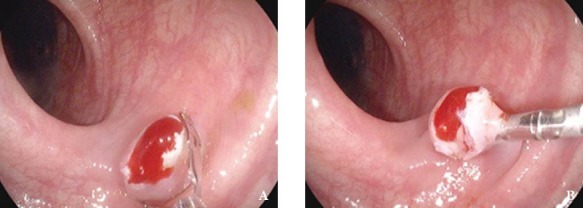
(A) Use of an endoclip in achieving hemostasis in post-polypectomy ooze - Endoclip positioned over the polyp stalk remnant at the bleeding vessel. (B) Use of an endoclip in achieving hemostasis in post-polypectomy ooze - Endoclip after closure
Endoloops
The endoloop is a detachable oval-shaped nylon snare. It is deployed in the same way as a standard snare, tightened and released around the stalk or base of the polyp prior to polypectomy (Fig. 4). Studies [25-28] have shown that the combination of epinephrine injection with endoloop placement is associated with much reduced rates of delayed bleeding compared to using epinephrine alone. However many problems with endoloops such as slipping off the polyp stalk, inadequate tightening, and persistence of bleeding despite endoloop placement were described in a retrospective study by Matsushita et al [29]. It is a good device for prophylactic use in high-risk pedunculated polyps but is cumbersome and requires expertise and skills.
Figure 4.
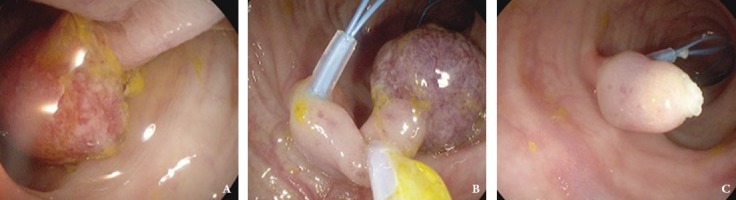
(A) Prophylactic use of an endoloop for hemostasis in a pedunculated polyp. (B) The endoloop applied near the stalk base to create enough space for polyp snaring. (C) Polyp stalk remnant with endoloop applied
Perforation
Perforation remains the most serious complication of polypectomy. Mechanical stress from the scope, barotrauma, electrocautery, and the depth of the polyp resection can all contribute to the risk of perforation. Risk of perforation increases with flat or sessile polyps, longer electrocautery time, larger polyps, and location in the cecum [30]. Risk of perforation is higher in right-sided polyps and blended cut is recommended over coagulation current. Hot biopsy is not recommended in the right colon to avoid the risk of delayed perforation. Post-EMR inspection of the site and prophylactic clipping [30-32] are recommended to reduce the risk of delayed perforation. If a perforation is seen during endoscopy, an attempt at closure with endoclips can be made. OTSC clips can be particularly useful for grabbing the large areas of mucosa and also come with clever grasping forceps which allows bringing the two edges of the perforation together before firing the clip. Delayed perforation can often be managed conservatively but occasionally requires surgery.
Post-polypectomy syndrome
Post-polypectomy syndrome is similar to perforation but less serious and occurs when there is a transmural burn not resulting in perforation. Post-polypectomy syndrome presents with leukocytosis, fever and abdominal pain in the absence of free air on imaging. This occurs due to deeper penetration of current and necrosis of muscle fibers causing some local peritonitis and inflammatory response. Treatment is usually conservative involving antibiotics, fluids, and bowel rest with very close observation in a hospital setting.
Diathermy
It is essential for the endoscopist to be familiar with the diathermy machine available in their unit and the appropriate settings before attempting polypectomy. Settings vary between machines and units and a detailed discussion of different types of diathermy machines available is beyond the scope of this article. In general, use of blue paddle is for coagulation current and yellow for cutting current. As a general rule, pedunculated polyps have a higher risk of bleeding and coagulation current is preferred whereas sessile and flat polyps have a higher risk of perforation and blended cutting current is preferred. Gentle movement is the key in order to avoid collateral burns and there must not be any vigorous shaking.
Polypectomy learning curve
Various factors influence the endoscopist’s learning curve. The learning curve should include developing skills, knowledge and attitude. The skills and knowledge come with a lot of training and experience. We believe that it takes around 1,000 colonoscopies to acquire the necessary scope handling skills. Endoscopist should make a stepwise progress in the type of lesion they can deal with. Generally pedunculated polyps and sessile polyps are less challenging than the flat polyps. It is vital to be proficient in hemostatic techniques before taking on the challenging lesions. Scarred polyps are also very challenging and may need ESD in order for the safe and effective removal. Animal models can be a very useful adjunct particularly in ESD training. All endoscopists should be aware of their own limitations and should operate within their own limits and undertake further training before taking on the more challenging lesions.
Conclusion
This review has highlighted the features which make a polyp difficult to resect. We have also discussed some practical tips to help overcome these challenges. We believe that difficulty is relative and each endoscopist should identify features that make polypectomy difficult for them. These difficulties can then be addressed by adequate training. Difficult polyps can be dealt with endoscopic approach if the endoscopist has the experience, expertise and skills. However, it is a team effort and requires expert nurses, pathologist and surgeon to achieve good outcomes.
Biography
Queen Alexandra Hospital, Portsmouth Hospitals NHS Trust, Portsmouth, UK
Footnotes
Conflict of Interest: None
References
- 1.Almoudaris AM, Gupta S, Bottle A, et al. Polypectomy at colonoscopy and sigmoidoscopy in England: a review of national data between 1997 and 2007. Gut. 2011;60:A40–A41. [Google Scholar]
- 2.Binmoeller KF, Bohnacker S, Seifert H, Thonke F, Valdeyar H, Soehendra N. Endoscopic snare excision of ‘giant’ colorectal polyps. Gastrointest Endosc. 1996;43:183–188. doi: 10.1016/s0016-5107(96)70313-9. [DOI] [PubMed] [Google Scholar]
- 3.Tamegai Y, Saito Y, Masaki N, et al. Endoscopic submucosal dissection: a safe technique for colorectal tumors. Endoscopy. 2007;39:418–422. doi: 10.1055/s-2007-966427. [DOI] [PubMed] [Google Scholar]
- 4.Hintze RE, Adler A, Veltzke W. Endoscopic resection of large colorectal adenomas: a combination of snare and laser ablation. Endoscopy. 1995;27:665–670. doi: 10.1055/s-2007-1005784. [DOI] [PubMed] [Google Scholar]
- 5.Iishi H, Tatsuta M, Iseki K, et al. Endoscopic piecemeal resection with submucosal saline injection of large sessile colorectal polyps. Gastrointest Endosc. 2000;51:697–700. doi: 10.1067/mge.2000.104652. [DOI] [PubMed] [Google Scholar]
- 6.Kanamori T, Itoh M, Yokoyama Y, Tsuchida K. Injection-incision– assisted snare resection of large sessile colorectal polyps. Gastrointest Endosc. 1996;43:189–195. doi: 10.1016/s0016-5107(96)70314-0. [DOI] [PubMed] [Google Scholar]
- 7.Gupta S, Miskovic D, Bhandari P, et al. The “SMSA” scoring system for determining the complexity of a polyp. Gut. 2011;60:A129. [Google Scholar]
- 8.Repici A, Tricerri R. Endoscopic polypectomy: techniques, complications and follow-up. Tech Coloproctol. 2004;8(Suppl 2):S283. doi: 10.1007/s10151-004-0178-x. [DOI] [PubMed] [Google Scholar]
- 9.Bedogn G, Bertoni G, Ricci E, et al. Colonoscopic excision of large and giant colorectal polyps. Technical implications and results over eight years. Dis Colon Rectum. 1986;29:831–835. doi: 10.1007/BF02555357. [DOI] [PubMed] [Google Scholar]
- 10.Higaki S, Hashimoto S, Harada K, et al. Long-term follow-up of large flat colorectal tumors resected endoscopically. Endoscopy. 2003;35:845–849. doi: 10.1055/s-2003-42622. [DOI] [PubMed] [Google Scholar]
- 11.Kudo SE, Kashida H. Flat and depressed lesions of the colorectum. Clin Gastroenterol Hepatol. 2005;3(7 Suppl 1):S33–S36. doi: 10.1016/s1542-3565(05)00283-1. [DOI] [PubMed] [Google Scholar]
- 12.Doniec JM, Lohnert MS, Schniewind B, et al. Endoscopic removal of large colorectal polyps: prevention of unnecessary surgery? Dis Colon Rectum. 2003;46:340. doi: 10.1007/s10350-004-6553-x. [DOI] [PubMed] [Google Scholar]
- 13.Tada M, Inoue H, Yabata E, Okabe S, Endo M. Feasibility of the transparent cap-fitted colonoscope for screening and mucosal resection. Dis Colon Rectum. 1997;40:618–621. doi: 10.1007/BF02055390. [DOI] [PubMed] [Google Scholar]
- 14.Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopy submucosal dissections. Gastrointest Endosc. 2010;72:1217–1225. doi: 10.1016/j.gie.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Uraoka T, Saito Y, Matsuda T, et al. Endoscopic indications for endoscopic mucosal resection of laterally spreading tumours in the colorectum. Gut. 2006;55:1592–1597. doi: 10.1136/gut.2005.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farhat S, Chaussade S, Ponchon T, et al. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy. 2011;43:664–670. doi: 10.1055/s-0030-1256413. [DOI] [PubMed] [Google Scholar]
- 17.Beilstein MC, Ginsberg GG. EMR for colonic pathology. Gastrointest Endosc. 2003;5:166–171. [Google Scholar]
- 18.Brandimarte G, Tursi A. Endoscopic snare excision of large pedunculated colorectal polyps: a new, safe, and effective technique. Endoscopy. 2001;33:854–857. doi: 10.1055/s-2001-17329. [DOI] [PubMed] [Google Scholar]
- 19.Heldwein W, Dollhopf M, Rösch T, et al. The Munich Polypectomy Study (MUPS): prospective analysis of complications and risk factors in 4000 colonic snare polypectomies. Endoscopy. 2005;37:1116–1122. doi: 10.1055/s-2005-870512. [DOI] [PubMed] [Google Scholar]
- 20.Puli SR, Kakugawa Y, Gotoda T, Antillon D, Saito Y, Antillon MR. Meta-analysis and systematic review of colorectal endoscopic mucosal resection. World J Gastroenterol. 2009;15:4273–4277. doi: 10.3748/wjg.15.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad NA, Ginsberg GG. Safety and complications of endoscopic mucosal resection. Tech Gastrointest Endosc. 2002;4:10–14. doi: 10.1067/mge.2002.121881. [DOI] [PubMed] [Google Scholar]
- 22.Parra-Blanco A, Kaminaga N, Kojima T, et al. Hemoclipping for postpolypectomy and postbiopsy colonic bleeding. Gastrointest Endosc. 2000;51:37–41. doi: 10.1016/s0016-5107(00)70384-1. [DOI] [PubMed] [Google Scholar]
- 23.Waye JD, Lewis BS, Yessayan S. Colonoscopy. A prospective report of complications. J Clin Gastroenterol. 1992;15:347–351. [PubMed] [Google Scholar]
- 24.Norton ID, Wang L, Levine SA, et al. Efficacy of colonic submucosal saline solution injection for the reduction of iatrogenic thermal injury. Gastrointest Endosc. 2002;56:95–99. doi: 10.1067/mge.2002.125362. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka S, Oka S, Kaneko I, et al. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc. 2007;66:100–107. doi: 10.1016/j.gie.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 26.Matsushita M, Hajiro K, Takakuwa H, et al. Ineffective use of detachable snare for colonoscopic polypectomy of large polyps. Gastrointest Endosc. 1998;47:496–499. doi: 10.1016/s0016-5107(98)70251-2. [DOI] [PubMed] [Google Scholar]
- 27.Conio M, Repici A, Demarquay JF, Blanchi S, Dumas R, Filiberti R. EMR of large sessile colorectal polyps. Gastrointest Endosc. 2004;60:234–241. doi: 10.1016/s0016-5107(04)01567-6. [DOI] [PubMed] [Google Scholar]
- 28.Monkemuller K, Neumann H, Fry LC, Ivekovic H, Malfertheiner P. Polypectomy techniques for difficult colon polyps. Dig Dis. 2008;26:342–346. doi: 10.1159/000177020. [DOI] [PubMed] [Google Scholar]
- 29.Doniec J.M. Endoscopy removal of large colorectalk polyps. Dis Colon Rectum. 2003;46:340–348. doi: 10.1007/s10350-004-6553-x. 169. [DOI] [PubMed] [Google Scholar]
- 30.Pérez Roldán F, González Carro P, Legaz Huidobro ML, et al. Endoscopic resection of large colorectal polyps. Rev Esp Enferm Dig. 2004;96:36–47. doi: 10.4321/s1130-01082004000100006. [DOI] [PubMed] [Google Scholar]
- 31.Arebi N, Swain D, Suzuki N, Fraser C, Price A, Saunders BP. Endoscopic mucosal resection of 161 cases of large sessile or flat colorectal polyps. Scand J Gastroenterol. 2007;42:859–866. doi: 10.1080/00365520601137280. [DOI] [PubMed] [Google Scholar]
- 32.Consolo P, Luigiano C, Strangio G, et al. Efficacy, risk factors and complications of endoscopic polypectomy: Ten year experience at a single center. World J Gastroenterol. 2008;14:2364–2369. doi: 10.3748/wjg.14.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swan MP, Bourke MJ, Alexander S, Moss A, Williams SJ. Large refractory colonic polyps: is it time to change our practice? A prospective study of the clinical and economic impact of a tertiary referral colonic mucosal resection and polypectomy service. Gastrointest Endosc. 2009;70:1128–1136. doi: 10.1016/j.gie.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 34.Caputi Iambrenghi O, Ugenti I, Martines G, Marino F, Francesco Altomare D, Memeo V. Endoscopic management of large colorectal polyps. Int J Colorectal Dis. 2009;24:749–753. doi: 10.1007/s00384-009-0684-4. [DOI] [PubMed] [Google Scholar]
- 35.Lim TR, Mahesh V, Singh S, et al. Endoscopic mucosal resection of colorectal polyps in typical UK hospitals. World J Gastroenterol. 2010;16:5324–5328. doi: 10.3748/wjg.v16.i42.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ah Soune P, Ménard C, Salah E, Desjeux A, Grimaud J-C, Barthet M. Large endoscopic mucosal resection for colorectal tumors exceeding 4 cm. World J Gastroenterol. 2010;16:588–595. doi: 10.3748/wjg.v16.i5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Longcroft-Wheaton G, Mead R, Duku M, Bhandari P. Endoscopic mucosal resection of colonic polyps: a large prospective single centre series. Gut. 2011;60:A14–A15. [Google Scholar]


