Abstract
Background
Isolated caudate lobe resection remains a technical challenge even in the best hands. This is due to the difficult approach and its location between major vessels. This retrospective study aims to analyze our experience with isolated caudate lobe resections.
Methods
Of the 402 patients who underwent liver resections between January 2002 and December 2011, we identified 13 caudate lobectomies. We analyzed the operative parameters, hospital stay, morbidity and follow up of these patients.
Results
There were nine males and four females, age ranging between 30 and 72 years. The indications were hepatocellular carcinoma in nine patients, hilar cholangiocarcinoma in two, solitary fibrous tumor in one, and a regenerative nodule in one patient. Left-sided approach was employed in seven cases, right-sided approach in three cases and a combined approach in three cases. Operating time ranged between 125 and 225 min and blood loss ranged between 210 and 630 mL. There was no mortality in the post-operative period. No local recurrence was noted in the follow-up period ranging from 6 months to 7 years.
Conclusion
Caudate lobe resections, although technically challenging, can be successfully performed with minimal blood loss. Surgery offers potential cure in isolated caudate lobe tumors. The location and size of the tumor decides the approach.
Keywords: Caudate lobe tumors, isolated caudate lobe resection, hilar cholangiocarcinoma, hepatocellular carcinoma
Introduction
The caudate lobe of the liver is not an uncommon site of involvement by primary or metastatic liver neoplasms. Traditionally these lesions have been managed by resection of the caudate lobe along with the right or the left lobe. Isolated caudate lobe resection is an alternative to these major resections. Isolated caudate lobectomy was first described by Lerut et al in 1990 [1]. Caudate lobe resection is one of the most demanding procedures among hepatic resection, owing to its deep and complex location and its proximity to major vessels. The caudate lobe is located beneath the major hepatic veins, between the portal vein and ligamentum venosum, and anterior and partially circumferential to the inferior vena cava (IVC) [2].
Earlier studies claimed that the tumors arising from the caudate lobe had an early chance of intra or extrahepatic tumor spread due to infiltration of the portal vein or hepatic veins [3]. Survival of patients with hepatocellular carcinoma (HCC) in the caudate lobe was earlier reported to be poorer when compared to patients with HCC in other locations [4]. But recent studies have demonstrated the survival rates to be similar [5,6].
Materials and methods
All patients who underwent liver resections between January 2002 and December 2011 were entered into a prospective database. Out of 402 hepatic resections performed during that period, we identified 13 patients who underwent isolated caudate lobe resection. The records of these 13 patients were reviewed retrospectively. Eleven patients had a solitary tumor in the caudate lobe in the preoperative imaging and two patients had type II hilar cholangiocarcinoma. Patient records were scrutinized for radiological investigations done, surgical details, intra-operative events and patient outcomes. Post operative complications and morbidity were recorded. The histopathology reports were reviewed. Follow-up details were collected from review records and telephonic interviews. Data were reported as the median with range and percentage.
Anatomic considerations
The caudate lobe generally includes three parts, namely; the Spiegel’s lobe on the left, the paracaval portion on the right and the caudate process. The Spiegel’s lobe corresponds to Couinaud’s segment I and the paracaval portion is termed as Couinaud’s segment IX [7], and both are separated by the canal of Arantius which is embryonically the ligamentum venosum [8]. The arterial supply to the caudate lobe is predominantly from the left hepatic artery, but there may be contributions from the right hepatic artery as well. There may be several small branches from the portal vein to the caudate lobe. There is consistently one large branch about 1 mm thick, the ligation of which is a key step in caudate lobe resection [8,9]. The portal vein branches to the caudate lobe also come predominantly from the left portal vein, but it may also arise from the right portal vein or the portal vein bifurcation [9]. Venous drainage is along its posterior aspect directly into the IVC through multiple small branches of variable size, number and location. Biliary drainage includes small tributaries to both sides, but mainly to the left hepatic duct. The posterior edge of the caudate lobe on the left side normally has a fibrous attachment encircling the IVC to join the segment VII. In up to 50% of patients, this fibrous band is replaced by hepatic parenchyma that can actually embrace the IVC completely [10]. This may add to the difficulty in resecting the caudate lobe.
Surgical technique
The incision preferred is a bilateral subcostal with a midline extension if required. Laparotomy is done to confirm absence of peritoneal deposits. Either left- or right-sided approach was undertaken depending on the location and size of the tumor.
In the left-sided approach, the left lobe was mobilized and rotated to the right. The gastrohepatic ligament was exposed and divided. The retrocaval ligament was divided. The short hepatic veins from the caudate lobe to the IVC were progressively divided, proceeding in a caudal to cranial direction. Once the retrohepatic veins were divided, the caudate lobe could be lifted off the IVC and became more mobile. The caudate lobe was retracted to the left and the branch from the left portal vein to the caudate lobe was divided. Then parenchymal dissection was undertaken to separate the caudate lobe from the right lobe of the liver. This part of the procedure was the most difficult as there is no definite demarcation between the two. However, no counterstaining techniques were undertaken to identify the right margin of resection. A combination of harmonic scalpel, bipolar and monopolar cautery were commonly used for parenchymal transaction (Fig. 1-8).
Figure 1.
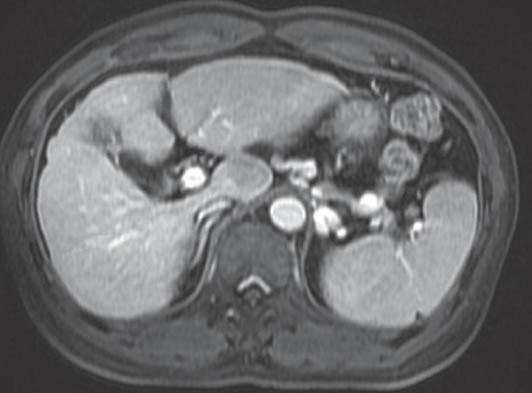
MRI picture showing a cirrhotic liver with a hypointense lesion in the caudate lobe of liver
Figure 8.
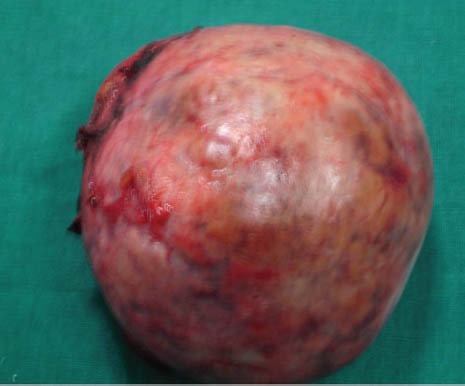
Resected specimen
Figure 2.
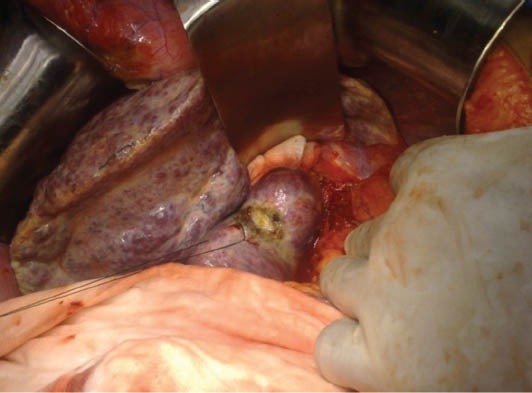
Operative picture showing a cirrhotic liver with a lesion in the caudate lobe
Figure 3.
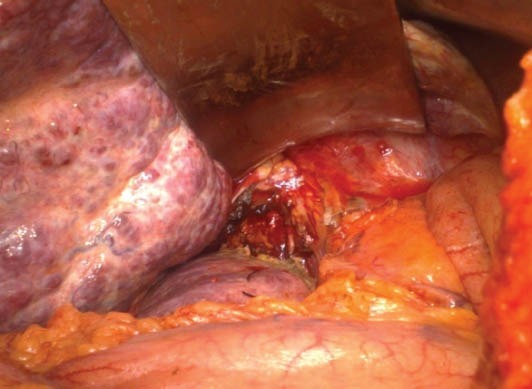
Picture of the region after resection of caudate lobe
Figure 4.
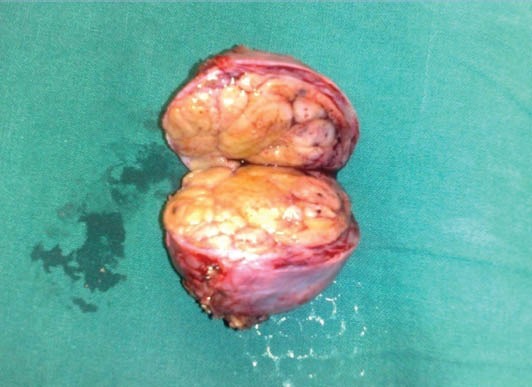
Cut surface of the well-encapsulated tumor
Figure 5.
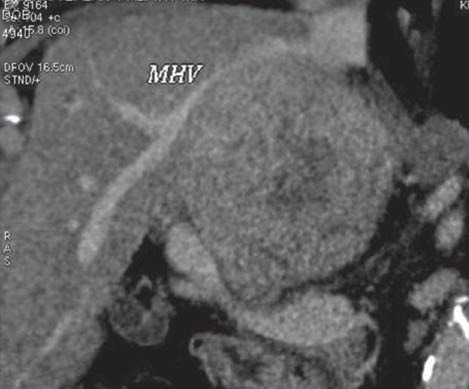
Contrast-enhanced computed tomography picture showing a large heterodense lesion in the caudate lobe of liver
MHV, middle hepatic vein
Figure 6.
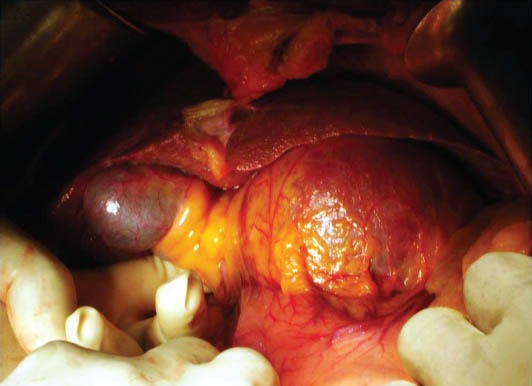
Operative picture showing a large lesion in the caudate lobe
Figure 7.
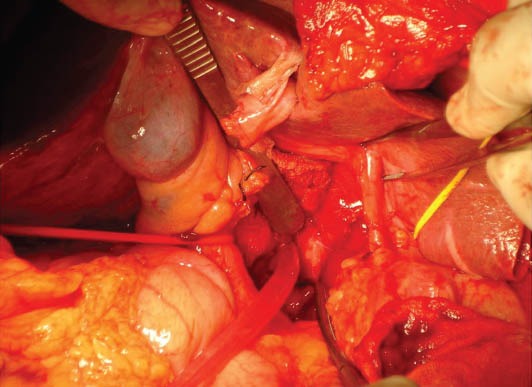
Post-resection picture showing bare vessels
Mobilization from the left was difficult in tumors located in the paracaval portion of the caudate lobe (n=2) and in case of bulky tumors (n=1). In such situations a right-sided approach was appropriate. The right liver was mobilized from the diaphragm until the lateral surface of the IVC was reached. Dissection was continued in a plane between the anterior surface of the IVC and the posterior surface of the caudate lobe. The retro hepatic veins from the caudate lobe were divided starting inferiorly behind the paracaval portion and extending superiorly until the major hepatic vein trunks were reached. Transection was carried along the right border of the caudate process to the root of the right hepatic vein in the cranial direction.
On three occasions when the tumor was very large (9 cm, 10 cm and 13 cm) it was difficult to complete the dissection from one side and a combined approach from both sides was necessary.
The third approach described is the anterior transhepatic approach where the caudate lobe is reached by splitting the liver parenchyma anteriorly along median fissure. It carries the advantage of providing a good view and access to perform a complete caudate lobe resection [11], but it is associated with a longer duration of surgery and an increased blood loss when compared to the conventional approaches. We have not used the anterior approach in any of our cases.
Results
Among the 13 patients who underwent isolated caudate lobe resection, nine were male. The median patient age was 48 years, with a range of 30 to 72 years. The most common indication was HCC (n=9). In two patients caudate lobe resection was done to achieve clearance along with resection of bile duct for type II hilar cholangiocarcinoma. Other diagnoses included regenerative nodule (n=1) and solitary fibrous tumor (n=1). Four among the 13 patients had a cirrhotic liver.
The procedure was performed through a left-sided approach in seven patients and a right-sided approach in three patients. In three patients a combined approach from both the sides was needed. The median operating time was 180 min (range, 125 to 225). Median blood loss was 450 mL (range, 210-630 mL). Only three patients (23%) required blood transfusion. Pringle’s maneuver was not used in any of our patients. There was no perioperative mortality. Two patients (15%) developed significant post-operative complications; bile leak which settled spontaneously in one patient and basal atelectasis in another patient. Median post-operative hospital stay was 14 days (range, 10-19 days). The resection margins were negative for tumor in all the patients (minimum distance of tumor from margin was 8 mm).
Two patients were lost to follow up. The remaining 11 patients were on follow up for a period ranging from 6 months to 7 years. Three patients with HCC had tumor recurrence, but none adjacent to the resection margin. The interval between caudate lobectomy and recurrence were 8, 11 and 16 months. All three patients were managed with radiofrequency ablation (RFA). One of the three patients with recurrence was a cirrhotic and the patient went in for decompensation. The patient succumbed to hepatorenal syndrome 19 months after undergoing RFA. Another patient was disease-free for 3 years and 5 months, before developing metastasis. The third patient is disease-free 16 months after RFA.
Discussion
Tumors originating in the caudate lobe have been managed by combined resection of a hemiliver with the caudate lobe to simplify the procedure. However, the frequent association of HCC with cirrhosis restricts the extent of major hepatic resections. Further, the additional resection of the overlying liver parenchyma would contribute little towards achieving a margin-free resection. In such a scenario, isolated caudate lobectomy would be an appropriate option if the tumor is confined to the caudate lobe without involving the major hepatic vascular structures [12]. But isolated caudate lobe resection has been considered a technically challenging procedure. Its location behind the major lobes and its close proximity to the portal triad, hepatic veins, and IVC are the reasons for the complexity of the procedure.
Even in major centers where high volume of liver resections are performed, caudate lobe excision comprises only 0.5% to 4% of the total number of hepatic resections and isolated resection of the caudate lobe is even rare [13,14]. The infrequency of the procedure is probably related to the concern of achieving a wide tumor-free margin for malignant tumors. The earlier belief was that malignant tumors in the caudate lobe carry a worse prognosis. Theoretically the location of a malignant tumor in the caudate lobe could potentiate easy spread to both intra- and extra-hepatic sites, when compared to tumors located in other regions [8], the reason being the close proximity to the major afferent vascular structures and the direct drainage into the IVC. However, recent data has demonstrated that the survival rates of patients with HCC in the caudate lobe were comparable to that of patients with HCC in other sites, following surgery [6]. These data support caudate lobe excision as an oncologically appropriate procedure for treating selected patients with tumors located in the caudate lobe [15]. In addition, it carries the advantage of preserving more functioning liver parenchyma in cirrhotics and reduced the chance of bleeding associated with the larger transection plane in major hepatectomies [2].
Analysis following caudate lobe resections has demonstrated subsegmental location of the tumor, liver cirrhosis and surgical margin to be the independent prognostic factors for overall survival [16]. Prognosis was better in patients with tumor located in the Spiegel’s lobe when compared to tumors located in the paracaval portion [16]. A surgical margin <5mm was found to be associated with poor survival. Although the significance of surgical margin in relation to survival is controversial, it is usually believed that the chance of recurrence is lower when the surgical margin is larger [17,18].
In case of hilar cholangiocarcinomas, the tumor frequently infiltrates the parenchyma of the caudate lobe and/ or invades its bile ducts. So, caudate lobe resection becomes frequently indispensable to achieve adequate tumor clearance [19]. Nimura et al in 1990 demonstrated microscopic tumor involvement in the caudate branches in nearly all patients in whom resection of the caudate lobe was performed, therefore advocating routine complete caudate lobe resection for hilar cholangiocarcinoma [20]. But isolated resection of caudate lobe is advisable only in hilar cholangiocarcinomas of small size, whereas a more extensive hepatectomy may be necessary in larger tumors to achieve radical tumor clearance [21].
Technically, we found the left-sided approach to be useful on most occasions, when the tumor was located in the Spiegel’s lobe. Rarely, we needed to approach the caudate lobe from both sides, when there was difficulty reaching from one side. We have never used the anterior transhepatic approach. Pringle’s maneuver was not needed in any of the patients. Moreover, we did not encounter problematic hemorrhage in our series. But there are centers which propose Pringle’s maneuver for caudate lobectomy [22].
Though three of our patients presented with recurrence, none were close to the resection margin. Six of nine patients with HCC are still on follow up with no recurrence. With these observations it may be possible to infer two facts. One is that isolated caudate lobe resections are possible with a negative resection margin. The other is that isolated caudate lobectomies are as effective as resections involving other parts of the liver for HCC.
We prefer a complete removal of the caudate lobe in patients with HCC, as recommended by studies [23]. But we do not practice the counterstaining technique to accurately delineate the right lateral margin of resection, routinely done in some centers in Japan [7].
Alternate modalities of therapy like transarterial chemoembolization (TACE), percutaneous RFA and percutaneous ethanol injection are difficult and inaccurate for tumors located in the caudate lobe [2,23]. The use of percutaneous ethanol injection and RFA are limited in the treatment of caudate lobe tumors due to the risk of injury to the adjacent blood vessels and other important structures, during penetration and injection [24]. Recent studies have established the use of RFA for caudate lobe tumors. But the long distance from the skin to the caudate lobe makes the correct placement of the needle difficult [25]. Detection of the tumor by ultrasonography is also difficult because of the depth. The use of these treatment modalities is also limited by the size of the tumor. Ultimately, surgery may be the only valid option left behind apart from transplantation. In cirrhotic patients, where major liver resections are not possible, isolated caudate lobe resection may be the sole chance of cure.
In summary, major hepatic resections can be avoided for tumors confined to the caudate lobe. Careful technique and detailed knowledge of the caudate lobe anatomy are essential to perform isolated caudate lobectomy. However, it has the advantage of maximally sparing hepatic parenchyma in patients with cirrhosis or hepatic dysfunction. Alternate modalities of therapy like TACE, RFA and percutaneous ethanol injection are largely inaccurate and difficult for caudate lobe tumors. Surgical resection may offer the only chance of cure. Isolated caudate lobe resection can be safely performed with minimal blood loss, low morbidity and mortality, in carefully selected patients.
Summary Box
What is ready known:
Hepatocellular carcinomas located in the caudate lobe were considered to have a poor prognosis when compared to tumors located in other parts of the liver
Traditionally major hepatectomies (right or left hepatectomy) including the caudate lobe was the treatment for caudate lobe tumors
For hepatocellular carcinomas located in the caudate lobe in a cirrhotic background, major resections were ruled out and transplantation was the only option
What the new findings are:
This study demonstrates that although isolated caudate lobe resection is technically demanding, it is possible for such tumors with minimal blood loss, morbidity and mortality
Isolated caudate lobe resection maximally preserves functioning liver parenchyma, reducing the chance of post-operative complications related to major liver resections, particularly in cirrhotic patients
It provides a valid option for cure in these patients in centers where liver transplantation is not possible
Other modalities of therapy like transarterial chemoembolization, radiofrequency ablation and percutaneous ethanol injection may not be possible for these tumors as they are technically difficult for caudate lobe tumors
Biography
Government Stanley Medical College, Chennai, India
Footnotes
Conflict of Interest: None
References
- 1.Lerut J, Gruwez JA, Blumgart LH. Resection of the caudate lobe of the liver. Surg Gynecol Obstet. 1990;171:160–162. [PubMed] [Google Scholar]
- 2.Elias D, Lasser PH, Desruennes E, Mankarios H, Jiang Y. Surgical approach to segment I for malignant tumors of the liver. Surg Gynecol Obstet. 1992;175:17–24. [PubMed] [Google Scholar]
- 3.Takayasu K, Muramatsu Y, Shima Y, et al. Clinical and radiologic features of hepatocellular carcinoma originating in the caudate lobe. Cancer. 1986;58:1557–1562. doi: 10.1002/1097-0142(19861001)58:7<1557::aid-cncr2820580729>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka S, Shimada M, Shirabe K, et al. Surgical outcome of patients with hepatocellular carcinoma originating in the caudate lobe. Am J Surg. 2005;190:451–455. doi: 10.1016/j.amjsurg.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Ikegami T, Ezaki T, Ishida T, Aimitsu S, Fujihara M, Mori M. Limited hepatic resection for hepatocellular carcinoma in the caudate lobe. World J Surg. 2004;28:697–670. doi: 10.1007/s00268-004-7341-4. [DOI] [PubMed] [Google Scholar]
- 6.Sakamoto Y, Nara S, Hata S, et al. Prognosis of patients undergoing hepatectomy for solitary hepatocellular carcinoma originating in the caudate lobe. Surgery. 2011;150:959–967. doi: 10.1016/j.surg.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Kosuge T, Yamamoto J, Takayama T, et al. An isolated, complete resection of the caudate lobe, including the paracaval portion, for hepatocellular carcinoma. Arch Surg. 1994;129:280–284. doi: 10.1001/archsurg.1994.01420270056013. [DOI] [PubMed] [Google Scholar]
- 8.Sarmiento JM, Que FG, Nagorney DM. Surgical outcomes of isolated caudate lobe resection: A single series of 19 patients. Surgery. 2002;132:697–709. doi: 10.1067/msy.2002.127691. [DOI] [PubMed] [Google Scholar]
- 9.López-Andújar R, Montalvá E, Pareja E, Mir J. Step-by-step isolated resection of segment 1 of the liver using the hanging maneuver. Am J Surg. 2009;198:e42–e48. doi: 10.1016/j.amjsurg.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Chaib E, Ribeiro MAF, Jr, Collet Silva F, Saad WA, Cecconello I. Caudate lobectomy: tumor location, topographic classification, and technique using right- and left-sided approaches to the liver. Am J Surg. 2008;196:245–251. doi: 10.1016/j.amjsurg.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 11.Sasada A, Ataka K, Tsuchiya K, Yamagishi H, i Maeda H, Okada M. Complete Caudate Lobectomy: Its Definition, Indications, and Surgical Approaches. HPB Surgery. 1998;11:87–95. doi: 10.1155/1998/92312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SH, Park SJ, Lee S, Lee WJ, Park JW, Kim CM. Isolated caudate lobectomy using the hanging maneuver. Surgery. 2006;139:847–850. doi: 10.1016/j.surg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Nagasue N, Kohno H, Yamanoi A. Resection of the caudate lobe of the liver for primary and recurrent hepatocellular carcinomas. J Am Coll Surg. 1997;184:1–8. [PubMed] [Google Scholar]
- 14.Takayama T, Makuuchi M, Watanabe K, et al. A new method for marking hepatic subsegment: counterstaining identification technique. Surgery. 1991;109:226–229. [PubMed] [Google Scholar]
- 15.Bartlett D, Fong Y, Blumgart LH. Complete resection of the caudate lobe of the liver. Br J Surg. 1996;83:1076–1081. doi: 10.1002/bjs.1800830812. [DOI] [PubMed] [Google Scholar]
- 16.Liu P, Yang J-M, Niu W-Y, et al. Prognostic factors in the surgical treatment of caudate lobe hepatocellular carcinoma. World J Gastroenterol. 2010;16:1123–1128. doi: 10.3748/wjg.v16.i9.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SG, Hwang S, Jung JP, Lee YJ, Kim KH, Ahn CS. Outcome of patients with huge hepatocellular carcinoma after primary resection and treatment of recurrent lesions. Br J Surg. 2007;94:320–326. doi: 10.1002/bjs.5622. [DOI] [PubMed] [Google Scholar]
- 18.Laurent C, Blanc JF, Nobili S, et al. Prognostic factors and longterm survival after hepatic resection for hepatocellular carcinoma originating from noncirrhotic liver. J Am Coll Surg. 2005;201:656–662. doi: 10.1016/j.jamcollsurg.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 19.Malago M, Frilling A, Li J, Lang H, Broelsch CE. Cholangiocellular carcinoma - the role of caudate lobe resection and mesohepatectomy. HPB. 2008;10:179–182. doi: 10.1080/13651820801992500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nimura Y, Hayakawa N, Kamiya J, Kondo S, Shionoya S. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg. 1990;14:535–543. doi: 10.1007/BF01658686. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins WG, DeMatteo RP, Cohen MS, et al. Caudate hepatectomy for cancer: a single institution experience with 150 patients. J Am Coll Surg. 2005;200:345–352. doi: 10.1016/j.jamcollsurg.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 22.Yanaga K, Matsumata T, Hayashi H, Shimada M, Urata K, Sugimachi K. Isolated hepatic caudate lobectomy. Surgery. 1994;115:757–761. [PubMed] [Google Scholar]
- 23.Yamamoto J, Kosuge T, Shimada K, Yamazaki S, Takayama T, Makuuchi M. Anterior transhepatic approach for isolated resection of the caudate lobe of the liver. World J Surg. 1999;23:97–101. doi: 10.1007/s002689900572. [DOI] [PubMed] [Google Scholar]
- 24.Wen ZQ, Yan YQ, Yang JM, Wu MC. Precautions in caudate lobe resection: report of 11 cases. World J Gastroenterol. 2008;14:2767–2770. doi: 10.3748/wjg.14.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kariyama K, Nouso K, Wakuta A, et al. Percutaneous radiofrequency ablation for treatment of hepatocellular carcinoma in the caudate lobe. AJR. 2011;197:W571–W575. doi: 10.2214/AJR.11.6893. [DOI] [PubMed] [Google Scholar]


