Abstract
Individual diastereomeric phosphoramidites and mixtures of diastereomeric phosphoramidites were evaluated in the iridium-catalyzed amination of allylic carbonates. The original process was conducted with a phosphoramidite ligand containing a resolved 2,2-dihydroxy-1,1-binaphthyl (BINOL) group and a diastereomerically and enantiomerically pure bis(phenethyl)amino group. Evaluation of the structure of the active catalyst and relative rates for reactions in the presence of catalysts containing diastereomeric ligands led to the identification of a phosphoramidite that provided the amination product with enantiomeric excess similar to the original, more structurally and stereochemically complex ligand and that contains a racemic BINOLate and an N-benzylphenethylamino group on phosphorus.
Biologically active molecules with stereocenters alpha to nitrogen range from single enantiomer pharmaceuticals to alkaloid natural products (1, 2). The classic routes to such chiral amines include resolution with chiral acids or initiation of the synthesis with a member of the existing optically active chiral pool that includes naturally occurring amino acids. However, the resolution typically requires disposal of at least half of the chiral amine, and many targets require a multistep synthesis from materials in the chiral pool. Thus, many synthetic approaches have been investigated for the preparation of α-chiral amines in an enantioselective manner.
Two common approaches involve hydrogenation of an imine (3–5) and additions of nucleophiles to imines (6–12). The hydrogenation of imines is not yet a general process and can be used to form only primary or secondary amines. The addition of carbon nucleophiles to imines with high enantioselectivity has been limited also, and the highest selectivities typically require a chiral substituent at nitrogen that would ultimately be disposed or would require recycling (8). An alternative approach involves enantioselective formation of the carbon-nitrogen bond in the amine (6, 7, 13, 14).
Transition metal-catalyzed reactions of nitrogen nucleophiles with allylic electrophiles (15) provide the opportunity to form the carbon-nitrogen bond in an amine with stereoselectivity that is based on a catalytic amount of a chiral ligand bound to the transition metal. In most cases, a palladium catalyst has been used for this transformation (16, 17). Although exceptions have been found (18), most palladium catalysts generate a terminal achiral allylic amine product from a linear allylic electrophile or a racemic branched allylic electrophile. Thus, a catalyst that would convert either a racemic branched allylic electrophile or an achiral terminal electrophile enantioselectively to a branched chiral amine product would provide a valuable synthetic tool (19–21). We recently reported an iridium complex containing a phosphoramidite ligand that catalyzes the reaction of amines with achiral linear allylic carbonates to form the branched allylic amine products with high enantioselectivity and regioselectivity (Eq. 1 and ref. 22).
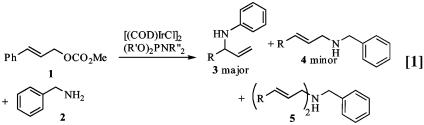 |
The phosphoramidite ligand (23–25) contained in the catalyst for this reaction possesses three stereochemical elements. It contains a resolved 2,2-dihydroxy-1,1-binaphthyl (BINOL) substituent and a diastereomerically and enantiomerically pure amino substituent containing two phenethyl groups. Because of the stereochemical complexity of these ligands, the cost of the ligand exceeds the cost of the iridium “precious metal.” We envisioned that structural information on the reaction could lead to catalysts that would possess simpler chiral ligands. Most recently, we showed that the iridium complex formed initially from [Ir(COD)Cl]2 (COD = 1,5-cyclooctadiene) and the phosphoramidite ligand undergoes an intramolecular C—H bond cleavage process to convert the monodentate phosphoramidite into a bidentate ligand bound to the metal through a new methylene unit and the phosphoramidite phosphorus (26). The complex formed by this cyclometallation process catalyzes the allylic amination with high rates and selectivities.
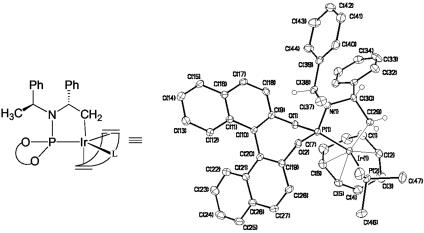
A crystal structure of a derivative of this complex formed by displacement of a κ′-bound phosphoramidite by PMe3 (26) is shown in Fig. 1. The structure of this complex suggests that the substituent of the original phosphoramidite located distal to the metal in the activated catalyst need not bear a stereocenter for the catalyst to react with high enantioselectivity. Most likely, the proximal stereocenter of the phenethyl substituent and the BINOL unit induce asymmetry. Thus we sought to test whether one achiral and one phenethyl substituent at nitrogen would generate a catalyst that would react with comparable enantioselectivity to the original catalyst we discovered. Second, early data (22) on the two diastereomers of the phosphoramidite showed that the (Ra,Rc,Rc) or (Sa,Sc,Sc) and (Sa,Rc,Rc) or (Ra,Sc,Sc) diastereomers of the phosphoramidite generated a catalyst that reacted with very different rates. The reactions that were catalyzed by complexes of the (Ra,Rc,Rc) diastereomer of the phosphoramidite occurred much faster than did reactions catalyzed by the (Sa,Rc,Rc) diastereomer. Thus, one could envision that one of the iridium complexes contained in a mixture of complexes generated from the two diastereomeric phosphoramidites would react with different rates. From the data discussed above, one would expect the more reactive complex to be the one generated from the (Ra,Rc,Rc) phosphoramidite. If the rates for reaction were different enough, a catalyst mixture generated from a combination of the phosphoramidites could form product with high enantioselectivity.
Fig. 1.
Line drawing and ortep drawing of the structure of the cyclometallated form of the iridium catalyst for allylic amination.
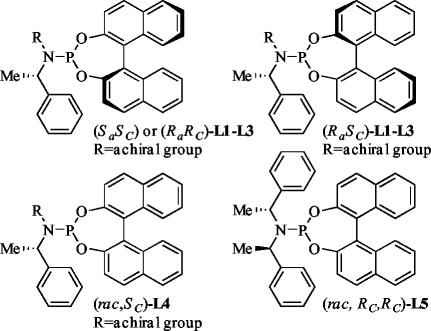
With these hypotheses in mind, we prepared four types of ligands (Fig. 2). We prepared phosphoramidites (Sa,SC)-L1–L3 containing the S enantiomer of BINOL and an amino group that contained one achiral and one (S)-phenethyl substituent. Second, we prepared phosphoramidite L4 containing (R)-BINOL and an amino group that contained an achiral benzyl substituent and an (S)-phenethyl substituent. Third, we prepared a mixture of diastereomeric phosphoramidites (rac,SC)-L3 from racemic BINOL and an amino group containing one achiral benzyl and one (S)-phenethyl substituent. Fourth, we prepared the version of the (Ra,RC,RC)-L5 with racemic BINOL (rac,RC,RC)-L5. For ligands L1–L3 containing one achiral and one (S)-phenethyl substituent at nitrogen (Fig. 3), we prepared phosphoramidites containing a methyl, diphenylmethyl, and benzyl group at nitrogen. The enantiopure amines for preparation for these phosphoramidites are all easily accessible by methylation, reductive amination, or nucleophilic substitution of the appropriate electrophile with an enantioenriched phenethylamine, which is among the least expensive enantioenriched amines. These three N-methyl or N-benzylamines then were used to prepare three phosphoramidites, each containing the (R)-BINOL group.
Fig. 2.
Structures of the phosphoramidites with reduced numbers of enantiopure stereochemical elements that were applied to the iridium-catalyzed amination of methyl cinnamylcarbonate.
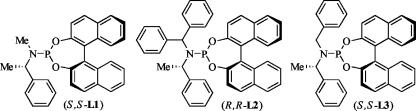
Fig. 3.
Structures of the phosphoramidites with one achiral and one (S)-phenethyl group on the nitrogen.
Reactions of benzylamine with methylcinnamylcarbonate in the presence of an iridium catalyst generated from [Ir-(COD)Cl]2 and the three phosphoramidites L1–L3 (Eq. 1) showed that the rates and enantioselectivities were highly dependent on the steric properties of the achiral substituent at nitrogen. Reactions in the presence of the catalyst generated from the phosphoramidite L1 containing the N-methyl, N-phenethylamino group occurred slowly and gave little of the allylic amine product. Reactions catalyzed by complexes generated from the phosphoramidite L2 containing the N-diphenylmethyl, N-phenethylamino group occurred in only 70% yield after 24 h at room temperature and in only 85% enantiomeric excess. However, reactions in the presence of the catalyst generated from the phosphoramidite L3 containing an N-benzyl, N-phenethylamino group occurred with yields similar to those of reactions catalyzed by the phosphoramidite containing a bis(phenethyl)amino group, and the allylic amine was formed with an enantiomeric excess of 91%.
Although we have not yet extensively explored the scope of the reactions catalyzed by iridium complexes of this phosphoramidite, the observation of high enantiomeric excess extends beyond the reaction of benzylamine. For example, the reaction of allyl amine occurs with an equally high 92% enantiomeric excess. The product of this reaction is suitable for ring-closing metathesis (27–29) to form optically active 3-pyrrolines (19) or, after reduction of the C C bond, pyrrolidines.
C bond, pyrrolidines.
To further pare down the resolved stereochemical elements of the catalyst, we investigated whether the observation of distinct rates for reactions in the presence of catalysts bearing diastereomeric versions of the bis(phenethyl)amino ligand would extend to reactions catalyzed by complexes of the two diastereomeric phosphoramidites containing the N-benzyl, N-phenethylamino group. If so, then reactions catalyzed by complexes containing a diastereomeric mixture of phosphoramidites generated from racemic BINOL could occur with high enantioselectivities.
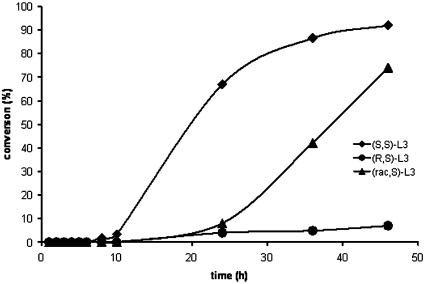
To test the feasibility of this concept, we measured qualitatively the rates for reactions of benzylamine with methyl cinnamylcarbonate in the presence of complexes of the R,S diastereomer and the S,S diastereomer of the N-benzyl ligands in Fig. 3. Indeed, as shown in Fig. 4, the reaction of benzylamine with methyl cinnamylcarbonate in the presence of the catalyst generated from the S,S diastereomer of the N-benzyl ligand occurred with convenient rates at room temperature. In contrast, the same reaction catalyzed by a complex generated from the R,S diastereomer occurred much more slowly. Because two ligands may be present at the metal center of the active catalyst or a reservoir of the active catalyst, the presence of two diastereomeric ligands in the same system can complicate translation of these kinetic data to a system in which two ligands are present in the same reaction mixture.
Fig. 4.
Profile of the reaction of benzylamine with cinnamylcarbonate in the presence of 1 mol % of [(COD)IrCl]2 and 2 mol % of the two diastereomeric phosphoramidites from N-benzylphenethylamines or the mixture of the two diastereomers together.
Nevertheless, reactions of allyl amine with methyl cinnamyl carbonate conducted in the presence of 2 mol % of the diastereomeric mixture of ligands and [Ir(COD)Cl]2 generated the allylic amination product in an isolated yield of 75% after 72 h of reaction time and 92% enantiomeric excess. This selectivity is identical to that obtained from reactions catalyzed by complexes of the diastereomerically pure phosphoramidite. Although we have not yet conducted detailed mechanistic studies to exclude cooperative effects of the two diastereomeric ligands, the observation of identical enantiomeric excess from reaction of the diastereomeric mixture of ligands and the enantiopure ligand suggests that the product is formed exclusively from the catalyst generated from the (S,S) diastereomer.
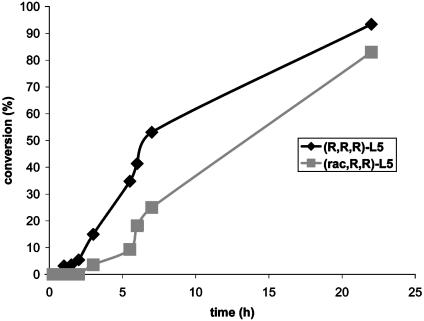
The same general observation was made when comparing reactions in the presence of the catalysts containing the (R,R,R) diastereomer of the ligand L5 we originally used in the allylic amination to reactions in the presence of a catalyst bearing the phosphoramidite (rac,Rc,Rc)-L5 generated from racemic BINOL and homochiral bis(phenethyl)amine. As shown in Fig. 5, the reaction catalyzed by the pure diastereomer was somewhat faster than that catalyzed by the mixture of diastereomers, but the enantiomeric excess was an identical 95% in both cases.
Fig. 5.
Profile of the reaction of allylamine with cinnamylcarbonate in the presence of 0.5 mol % of [(COD)IrCl]2 and 1 mol % of the two diastereomeric phosphoramidites from homochiral bis(phenethyl)amine.
Thus, an effective catalyst is generated from a mixture of the diastereomeric ligands, which was generated from racemic BINOL and N-benzylphenethylamine. This ligand was prepared by a simple two-step sequence consisting of reductive amination of benzaldehyde with R phenethylamine and a one-pot combination of BINOL, the N-benzylphenethylamine, and phosphorus trichloride. The components of this ligand can be purchased commercially for less than $1 per gram.
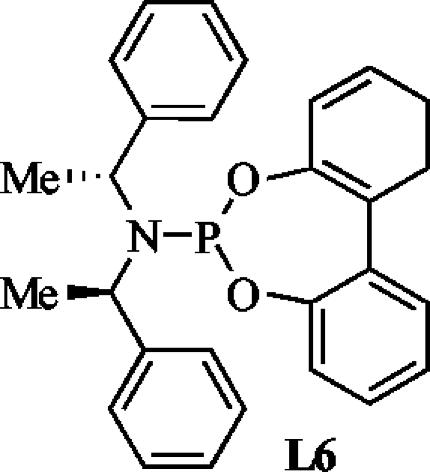
These results provide an explanation for a previous observation and several directions for future ligand design. In previous published work, we showed that a phosphoramidite L6 (Scheme 1) that was generated from (R,R)-bis(phenethyl)amine and achiral biphenol generated a catalyst for the reaction of benzylamine with methyl cinnamylcarbonate that formed product in 87% enantiomeric excess (22). The BINOL group in this ligand adopts a chiral conformation (24); our results suggest that the product is most likely generated from one confirmation of this ligand. This result, in combination with the work reported here, suggests that further derivatives of the N-benzyl ligand could lead to further improved enantiomeric excess.
Scheme 1.
Many studies have been conducted recently with phosphoramidites as ligands because they are prepared through a modular synthesis that allows for rapid variation of the structure. The ability to readily vary the achiral substituent at the nitrogen of the phosphoramidite ligand that induces high asymmetry in the allylic amination further increase the modularity of this framework. Many biphenol derivatives are readily available, as are racemic derivatives of BINOL. Moreover, many 1-arylethylamines are available in enantiomerically pure form by either classical or enzymatic resolution, and a wide variety of aromatic aldehydes are available as reagents for the reductive amination. Derivatives of the phosphoramidites reported here with altered steric and electronic properties are simple to prepare and may provide catalysts that react with broad scope and synthetically useful enantiomeric excesses with an asymmetry that is generated from a commodity chemical in enantiomerically pure form.
Acknowledgments
We thank the Merck Research Laboratories for support of this work. A.L. thanks the Fonds zur Förderung der wissenschaftlichen Forschung Austria for an Erwin Schrödinger fellowship.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BINOL, 2,2-dihydroxy-1,1-binaphthyl; COD, 1,5-cyclooctadiene.
References
- 1.Mealy, N. & Castaner, J. (2002) Drugs Future 24, 871. [Google Scholar]
- 2.Miguel-Hidalgo, J. J. (2000) Curr. Opin. Central Peripher. Nerv. Syst. Invest. Drugs 2, 438–453. [Google Scholar]
- 3.Ohkuma, T., Kitamura, M. & Noyori, R. (2000) in Catalytic Asymmetric Synthesis, ed. Ojima, I. (Wiley–VCH, New York), 2nd Ed., pp. 1–110.
- 4.Blaser, H. U., Buser, H. P., Hausel, R., Jalett, H. P. & Spindler, F. (2001) J. Organomet. Chem. 621, 34–38. [Google Scholar]
- 5.Xiao, D. & Zhang, X. (2001) Angew. Chem. Int. Ed. Engl. 40, 3425–3428. [DOI] [PubMed] [Google Scholar]
- 6.Boezio, A. A., Pytkowicz, J., Cote, A. & Charette, A. B. (2003) J. Am. Chem. Soc. 125, 14260–14261. [DOI] [PubMed] [Google Scholar]
- 7.Boezio, A. A. & Charette, A. B. (2003) J. Am. Chem. Soc. 125, 1692–1693. [DOI] [PubMed] [Google Scholar]
- 8.Ellman, J. A., Owens, T. D. & Tang, T. P. (2002) Acc. Chem. Res. 35, 984–995. [DOI] [PubMed] [Google Scholar]
- 9.Fujihara, H., Nagai, K. & Tomoika, K. (2000) J. Am. Chem. Soc. 122, 12055–12056. [Google Scholar]
- 10.Denmark, S. E. & Stiff, C. M. (2000) J. Org. Chem. 65, 5875–5878. [DOI] [PubMed] [Google Scholar]
- 11.Alvaro, G., Pacioni, P. & Savoia, D. (1997) Chem. Eur. J. 3, 726–731. [Google Scholar]
- 12.Denmark, S. E., Nakajima, N. & Nicaise, O. J. C. (1994) J. Am. Chem. Soc. 116, 8797–8798. [Google Scholar]
- 13.Dahmen, S. & Brase, S. (2002) J. Am. Chem. Soc. 124, 5940–5941. [DOI] [PubMed] [Google Scholar]
- 14.Hermanns, N., Dahmen, S., Bolm, C. & Brase, S. (2002) Angew. Chem. Int. Ed. Engl. 41, 3692–3694. [DOI] [PubMed] [Google Scholar]
- 15.Johannsen, M. & Jorgensen, K. A. (1998) Chem. Rev. (Washington, D.C.) 98, 1689–1708. [DOI] [PubMed] [Google Scholar]
- 16.Acemoglu, L. & Williams, J. M. J. (2002) in Handbook of Organopalladium Chemistry for Organic Synthesis, ed. Negishi, E.-i (Wiley Interscience, New York), Vol. 2, p. 1945. [Google Scholar]
- 17.Trost, B. M. (2002) Chem. Pharm. Bull. 50, 1–14. [DOI] [PubMed] [Google Scholar]
- 18.You, S.-L., Zhu, X.-Z., Luo, Y.-M., Hou, X.-L. & Dai, L.-X. (2001) J. Am. Chem. Soc. 123, 7471–7472. [DOI] [PubMed] [Google Scholar]
- 19.Evans, P. A., Robinson, J. E. & Nelson, J. D. (1999) J. Am. Chem. Soc. 121, 6761–6762. [Google Scholar]
- 20.Evans, P. A., Robinson, J. E. & Moffett, K. K. (2001) Org. Lett. 3, 3269–3271. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi, R., Ue, N., Tanabe, K., Yamashita, K. & Shiga, N. (2001) J. Am. Chem. Soc. 123, 9525–9534. [DOI] [PubMed] [Google Scholar]
- 22.Ohmura, T. & Hartwig, J. F. (2002) J. Am. Chem. Soc. 124, 15164–15165. [DOI] [PubMed] [Google Scholar]
- 23.Feringa, B. L. (2000) Acc. Chem. Res. 33, 346–353. [DOI] [PubMed] [Google Scholar]
- 24.Alexakis, A., Rosset, S., Allamand, J., March, S., Guillen, F. & Benhaim, C. (2001) Synlett, 1375–1378.
- 25.Feringa, B. L., Pineschi, M., Arnold, L. A., Imbos, R. & De Vries, A. H. M. (1997) Angew. Chem. Int. Ed. Engl. 36, 2620–2623. [Google Scholar]
- 26.Kiener, C. A., Shu, C., Incarvito, C. & Hartwig, J. F. (2003) J. Am. Chem. Soc. 125, 14272–14273. [DOI] [PubMed] [Google Scholar]
- 27.Furstner, A. (2000) Angew. Chem. Int. Ed. Engl. 39, 3012–3043. [PubMed] [Google Scholar]
- 28.Trnka, T. M. & Grubbs, R. H. (2001) Acc. Chem. Res. 34, 18–29. [DOI] [PubMed] [Google Scholar]
- 29.Schrock, R. R. & Hoveyda, A. H. (2003) Angew. Chem. Int. Ed. Engl. 42, 4592–4633. [DOI] [PubMed] [Google Scholar]