Abstract
The initiation of cellular programs is orchestrated by key transcription factors and chromatin regulators that activate or inhibit target gene expression. To generate a compendium of chromatin factors that establish the epigenetic code during developmental hematopoiesis, a large-scale reverse genetic screen was conducted targeting orthologs of 425 human chromatin factors in zebrafish. A set of chromatin regulators was identified that target different stages of primitive and definitive blood formation, including factors not previously implicated in hematopoiesis. We identified 15 factors that regulate development of primitive erythroid progenitors and 29 factors that regulate development of definitive stem and progenitor cells. These chromatin factors are associated with SWI/SNF and ISWI chromatin remodeling, SET1 methyltransferase, CBP/P300/HBO1/NuA4 acetyltransferase, HDAC/NuRD deacetylase, and Polycomb repressive complexes. Our work provides a comprehensive view of how specific chromatin factors and their associated complexes play a major role in the establishment of hematopoietic cells in vivo.
The programs of gene expression required for maintenance and differentiation of cell types are tightly regulated by a network of transcription factors and associated chromatin modifying factors to facilitate or suppress gene expression. Epigenetic information consists of chemical modifications to both cytosine bases in DNA and histone proteins that fold the DNA into nucleosomes, as well as the repositioning, dissociation, and/or reconstitution of entire nucleosomes1. Mouse knockout models and zebrafish mutants have been used to investigate the role of chromatin factors in vertebrate development, but the majority of chromatin factors have yet to be characterized2-5.
Hematopoiesis is guided by cell-specific transcriptional regulators and associated chromatin factors that function to establish all mature blood cells6. This process is hallmarked by the establishment of hematopoietic stem cells (HSCs) and depends on the function of transcriptional regulators, Runx1, Scl, Lmo2, and chromatin factors, Mll and Bmi1, for stem cell production, self-renewal, and survival. Additional factors coordinate the specialization of HSCs into multilineage progenitors that generate differentiated cells of the peripheral blood lineages6. Members of the Polycomb (PcG) family have previously been identified as regulators of hematopoiesis. Bmi1 functions to positively regulate HSC proliferation by limiting cell cycle regulator expression, and HSCs with Bmi1 deficiency show impaired self-renewal capacity7. The Mi-2/NuRD complex regulates a set of HSC specific genes that maintain the HSC pool in the bone marrow. De-repression of these genes in Mi-2beta deficient HSCs exhausts the HSC pool8. Several chromatin factors have been identified as leukemic translocation partners, underscoring the importance they have in normal development. MLL is rearranged in the majority of infant leukemias with patients generally having poor clinical outcomes9. Similarly, translocations of PRDM16, another SET family member, is associated with a poor prognosis10. Both promote the development of normal HSCs and leukemic stem cells 9,11.
To identify chromatin factors that function during developmental hematopoiesis, we have undertaken a large-scale in vivo reverse genetic morpholino-based screen targeting zebrafish orthologs of 425 human chromatin factors. The zebrafish provides a suitable platform for rapid screening to assay the function of chromatin factors in hematopoiesis due to their high fecundity, rapid development, evolutionary conservation, and ease in generating genetic knockdowns. We have identified 44 factors that affect the development of primitive and definitive blood, including 28 factors that have yet to be associated with hematopoiesis. We have also characterized different developmental stages during which these factors function, from the induction of stem and progenitor cells to differentiation into erythroid cells. By incorporating protein interaction data, we predict the BAF/PBAF, ISWI, SET1, CBP/P300/HBO1/NuA4, HDAC/NuRD, and PRC1/PRC2 complexes as required for blood development. Taken together, our screen provides a valuable resource for elucidating the in vivo network of chromatin regulators of hematopoietic development.
Results
A screen for chromatin regulators of developmental hematopoiesis
To identify the chromatin remodeling factors that function in developmental hematopoiesis, we conducted a large-scale in vivo reverse genetic screen targeting chromatin factors (Fig. 1a). We designed antisense oligonucleotide morpholinos to knock down expression of 488 zebrafish orthologs of 425 human chromatin factors (Supplementary Table 1). Our gene list included most of the known human factors containing chromatin binding, modifying, or remodeling domains curated from several public databases: CREMOFAC, SMART domain by NRDB, CDD at NCBI, Pfam, and ChromDB12-16. 488 orthologous genes in zebrafish were identified by a reciprocal BLAST search of the unique human protein sequences into the zebrafish genome. Only 26 human proteins lacked a zebrafish ortholog.
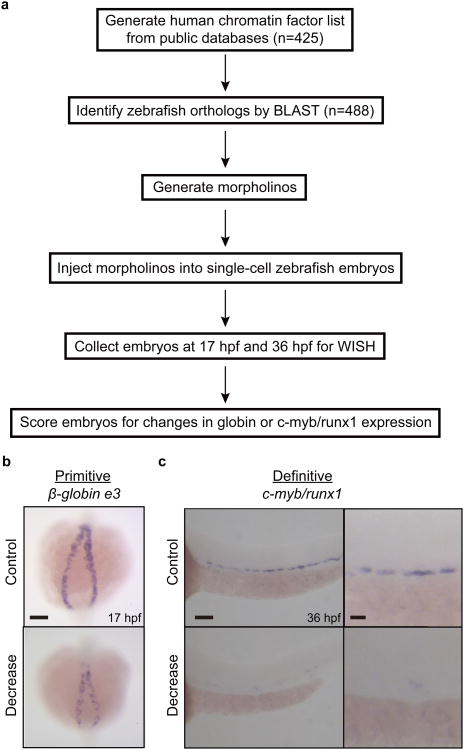
Figure 1.
Screen design for chromatin regulators of developmental hematopoiesis. (a) Schematic illustration of screening procedure. Knockdown of 488 zebrafish orthologs of 425 human chromatin factors was achieved using morpholinos. Embryos were subsequently collected at 17 hpf and 36 hpf to analyze changes in β-globin e3 expression in primitive erythroid cells and c-myb and runx1 expression in definitive HSPCs, respectively, by WISH. (b) Example of primitive screen phenotype observed for reduced β-globin e3 expression at 17 hpf. (c) Example of definitive screen phenotype observed for reduced c-myb and runx1 expression at 36 hpf. Scale bars: 100μm for low magnification and 25 μm for high magnification.
Morpholinos targeting each chromatin factor were injected into single cell embryos at three concentrations. These doses typically give a range of phenotypes from a hypomorph to a near complete knockdown for most mRNA products, similar to an allelic series. In some cases, complete knockdown could not be achieved because of lower targeting efficiency or embryonic lethality. Post-injection, embryos were collected at specific timepoints, using both standard morphological features of the whole embryo and hours post-fertilization (hpf) to stage to minimize differences in embryonic development caused by the morpholino injection17. The embryos were then assayed for hematopoietic defects by whole-mount in situ hybridization (WISH). We conducted two screens simultaneously for primitive and definitive blood formation. For the primitive screen, developing erythrocytes in the posterior mesoderm of the embryo were assayed by β-globin e3 expression at the 16 somite stage (ss), or 17 hpf (Fig. 1b)18. For the definitive screen, the establishment of hematopoietic stem and progenitor cells (HSPCs) in the aorta, gonad, mesonephros region (AGM) was detected with c-myb and runx1 expression at 36 hpf (Fig. 1c)19.
To establish the level of morpholino efficacy, the 21 splice blocking morpholinos targeting the chromodomain (CHD) gene family were assayed for splicing activity by reverse-transcription polymerase chain reaction (RT-PCR). Of these 21 morpholinos, 10 did not result in any hematopoietic defect. For the 10, one gene could not be evaluated because no PCR product was detected. Only one of the nine remaining morpholinos did not show altered splicing activity, resulting in an estimated false negative rate (FNR) of 11% for the screen (Supplementary Fig. 1a-b). To expand on this limited approach, we verified the splicing activity of an additional 48 splice blocking morpholinos that scored negative in both primitive and definitive screens. In total, 51 of 57 morpholinos caused altered splicing, resulting in the same estimated FNR of 11%. Furthermore, the knock down efficiencies were comparable to those that gave a hematopoietic defect (Supplementary Fig. 5).
Classification of screen results
Gene expression phenotypes observed in morpholino injected embryos, or morphants, were classified into one of three major categories: no change, decrease, or increase. Due to the range of decreased staining from subtle to complete absence of staining, we subdivided the decrease category into mild, intermediate, and strong (Fig. 2a-b, Supplementary Table 2). Any morphant showing changes in β-globin e3 or c-myb/runx1 expression was considered a screen hit. Morphants with developmental abnormalities were listed separately (Supplementary Fig. 2a-b, Supplementary Table 2). As morphologically normal morphants with the strongest decrease or increase in blood formation represented genes that were likely to be specific to blood development, these 26 primitive and 47 definitive factors were selected for further characterization.
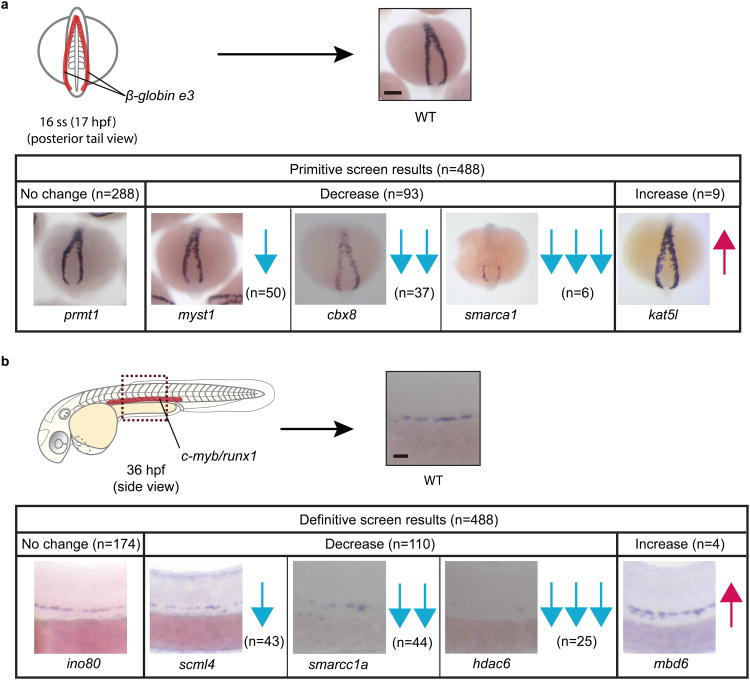
Figure 2.
Classification of screen results. (a) Summary of primitive screen WISH results. Developing β-globin e3+ erythroid cells are found as two bilateral stripes in the posterior of the embryo, as shown by the red highlights in the schematic of a 16 ss embryo. Knockdown of the different chromatin factors resulted in no change, decrease, or increase of β-globin e3 expression. (b) Summary of definitive screen WISH results. Induction of c-myb+ and runx1+ HSPCs occurs in the AGM region highlighted in red in the schematic of a 36 hpf embryo. Knockdown of the different chromatin factors screened resulted in no change, decrease, or increase of c-myb and runx1 expression. Representative WISH results are shown for each phenotypic category with additional categories continued in Supplementary Fig. 2. “n” is the number of chromatin factors with the indicated phenotype. Blue downward arrows represent reduced marker expression and magenta upward arrows represent increased marker expression. One arrow indicates a mild change, two arrows an intermediate change, and three arrows a strong change. Scale bars: 100μm
Chromatin factors regulate primitive blood development from the mesoderm
Of the 26 morphants with altered primitive erythropoiesis, knockdown of 13 chromatin factors reduced β-globin e3 expression and 13 factors increased β-globin e3 expression at 16 ss. To confirm these phenotypes, we rescreened for β-globin e3 expression. 16 of the 26 genes were verified, 6 from the reduced group and 10 from the increased group (Supplementary Table 3). Given that morpholinos can fail to inhibit their intended targets, splicing activity was confirmed by RT-PCR for the ten splice blocking morpholinos used, and a second, nonoverlapping morpholino was tested to verify the initial screen result for all factors. 15 of the 16 genes were validated in this manner and characterized further (Fig. 3a-b, Supplementary Fig. 5). To show that the morpholinos did not just affect globin expression, one gene from each decrease category was reevaluated with a second erythroid marker, band3. The decrease in band3 expression was consistent with the decrease in β-globin e3 expression and was not rescued by p53 loss (Fig. 3c), suggesting minimal, if any, morpholino toxicity. Overall, these additional tests provide further support for the validity of the screen results.
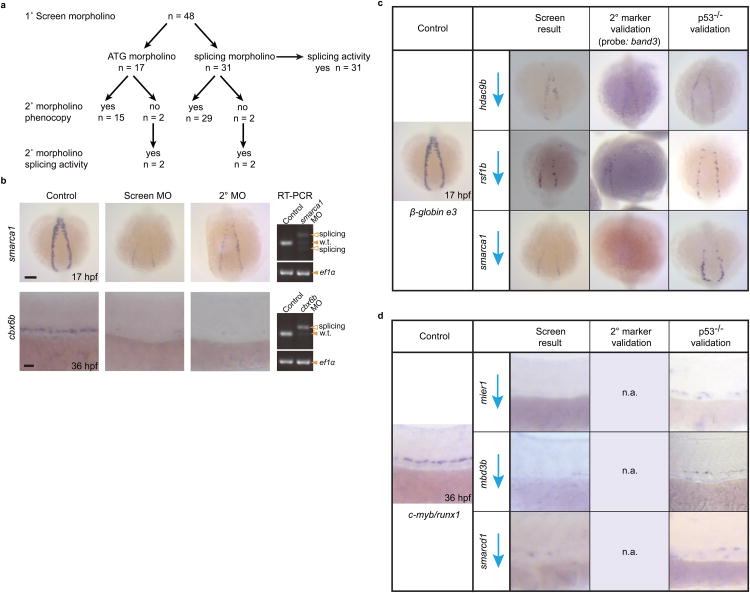
Figure 3.
Morpholino efficacy and secondary verification of screen phenotypes. (a) Flow chart of screening morpholino validation procedure. 44 of the 48 genes identified from the screen were verified with a second, nonoverlapping morpholino. The remaining 4 were recategorized. Splicing activity for splice blocking morpholinos were assayed and confirmed by RT-PCR for the 31 primary screen morpholinos and the 4 secondary morpholinos that did not phenocopy the primary screen results. n = is the number of morpholinos under each category. (b) Example of primitive and definitive screen hits that were verified with a secondary morpholino and show splicing activity by RT-PCR. ef1α was used as the control gene. Arrowheads mark the presence of a PCR band. Filled in arrowheads indicate wild-type bands and empty arrowheads indicate spliced bands. w.t stands for wild-type. (c) A subset of 3 primitive genes that resulted in decreased globin expression when knocked down were selected, one from each decrease category, to test for expression of a second erythroid marker, band3.(d) A subset of 3 definitive genes that resulted in decreased c-myb and runx1 expression when knocked down were selected, one from each category shown in Fig. 5. Given that two probes were used during the screen and expression of both genes were nearly abolished in the morphants, only the p53-/- rescue experiment was performed. n.a. stands for not applicable. Scale bars: 100μm for low magnification and 25 μm for high magnification.
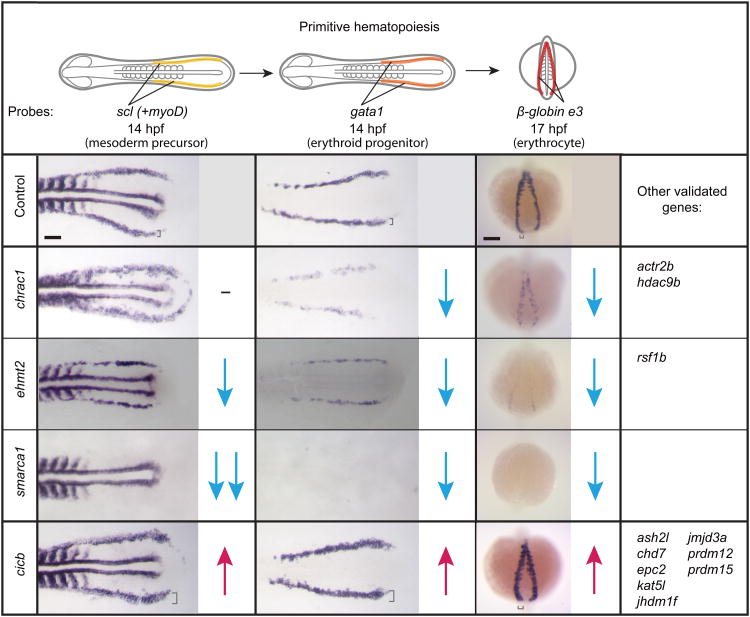
To examine early hematopoietic defects during the formation of mesodermal precursors and erythroid progenitors, we evaluated scl and gata1 expression at 10-12ss (14hpf), respectively (Fig. 4)20. Of the 15 morphants tested, 12 showed changes in scl and gata1 expression consistent with the changes in β-globin e3, suggesting that most of the factors categorized in the strong decrease or increase categories play a role in the formation of mesodermal precursors competent to make blood. Knockdown of one factor in particular, smarca1 (or snf2l), strikingly abrogated expression of all three markers, implicating a requirement for this gene early in hematopoietic development (Supplementary Fig. 4a). The remaining three, chrac1, actr2b, and hdac9b, had normal scl expression but reduced gata1 and β-globin e3 expression, indicating that these factors function to regulate the formation of erythroid progenitors from the mesoderm (Fig. 4). The majority of these factors represent previously unidentified regulators of erythroid development.
Figure 4.
Chromatin factors regulate distinct steps of primitive erythroid development. 15 primitive genes were screened for changes in scl expression marking hematopoietic mesodermal precursors (yellow highlight) and gata1 expression marking erythroid progenitor formation (orange highlight) illustrated in the top panel (flatmount view). β-globin e3 staining was repeated to verify primary screen results (posterior view). myoD probe for labeling somites was included as a staging marker. Blue downward arrow represents reduced expression. Magenta upward arrow represents increased expression. A hyphen indicates no change in gene expression. Square brackets indicate the thickness of the stripes. Scale bar: 100μm
Chromatin factors regulate the induction of HSPCs
As in the primitive screen, we rescreened the 47 definitive genes (41 with a strong decrease and 6 with increase runx1/c-myb expression) and confirmed 31 genes: 26 morphants recapitulated the initial strong decrease in c-myb and runx1 expression in the AGM at 36 hpf, and 5 were verified for increased c-myb and runx1 expression (Supplementary Table 3). For morphants in the decrease category, expression of both markers was nearly abolished in the AGM. Morpholinos injected in a p53-/- background did not rescue the loss of expression of AGM markers, indicating these phenotypes were not the result of morpholino toxicity (Fig. 3d). To verify the efficacy of the morpholinos, splicing activity was assessed and confirmed for all 21 splice blocking morpholinos used. 29 of the 31 genes were validated with a second, nonoverlapping morpholino and selected for further characterization (Fig. 3a-b, Supplementary Fig. 5). Collectively, these data provide additional verification of our definitive screen data.
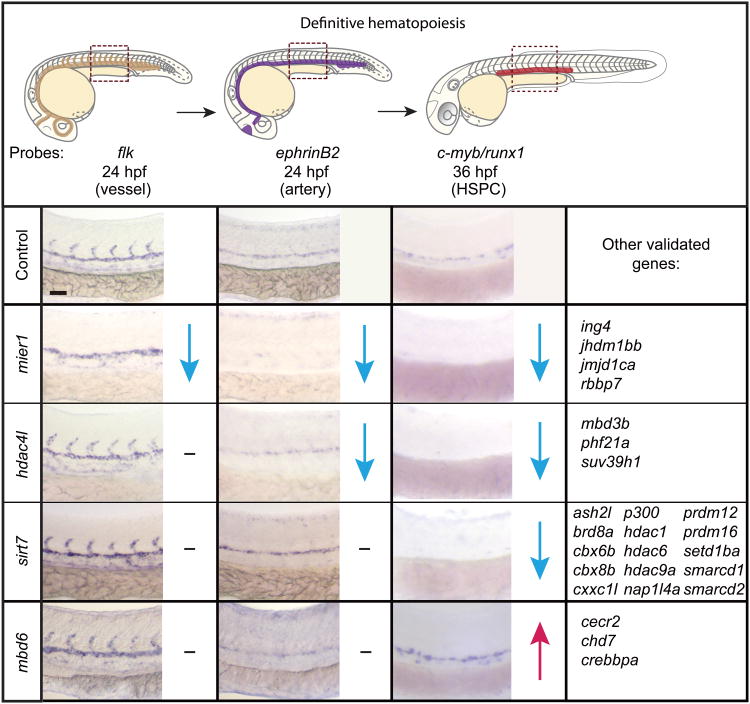
Previous work has shown that HSPCs emerge from the hemogenic endothelium of the dorsal aorta and that proper vessel development and establishment of artery identity is necessary for AGM stem cell induction23,24. The expression of vascular marker flk1 and arterial identity marker ephrinB2 were analyzed for the 29 verified genes. The majority (20 of 29) showed normal flk1 and ephrinB2 expression. While these results do not directly demonstrate a cell autonomous mechanism, they do suggest that the chromatin factors identified in the strong decrease and increase categories function in the specification or maintenance of HSPCs from the hemogenic endothelium, as the tissues that arise most proximal to HSC specification were intact (Fig. 5). Both known stem cell regulators, such as hdac1 and prdm16, and unknown factors, including brd8a, cbx6b, jmjd1c, and nap1l4a, were identified11,24.
Figure 5.
Chromatin factors regulate distinct steps of definitive HSPC development. 29 definitive genes were screened for changes in flk1 expression marking the vessels (tan highlight) and ephrinB2 expression marking the artery endothelium (purple highlight) illustrated in the top panel. c-myb and runx1 staining was repeated to verify primary screen results. Blue downward arrow represents reduced expression. Magenta upward arrow represents increased expression. A hyphen indicates no change in gene expression. Scale bars: 100μm for low magnification and 25 μm for high magnification.
The remaining ten chromatin factors were found to function at earlier stages of vessel specification based on the presence of vascular defects. Four of the factors, hdac4l, mbd3b, phf21a, and suv39h1, showed normal flk1 levels but reduced ephrinB2 expression, suggesting they function in the establishment of aorta identity upstream of HSPC formation . Finally, 5 morphants, mier1, jhdm1bb, jmjd1ca, ing4, and rbbp7, lost both intersomitic flk1 and arterial ephrinB2 expression, hence the loss of HSPCs in these morphants is likely due to the absence of hemogenic endothelium. Overall, our definitive screen uncovered chromatin regulators involved in the development of HSPCs from the AGM and during vascular development.
Genes associated with mild to moderate knockdown phenotypes are important regulators of hematopoietic development
In addition to chromatin factors with strong decrease or increase phenotypes, other factors with moderate to mild phenotypes also function in hematopoietic development. (Supplementary Table 3). We characterized seven genes from the primitive screen that showed only an intermediate reduction in β-globin e3 expression upon reinjection. Of these seven morphants, six had normal scl but reduced gata1 expression, suggesting that they likely function at the erythroid specification stage. Two of these factors, CHD4 and CBX8, associate with the FOG1/GATA1 transcriptional complex and the TIF1-γ elongation complex, respectively; both complexes are key regulators of erythroid development25,26. Finally, kat5 showed a decrease in β-globin e3 without loss of scl and gata1 expression, indicating it plays a role in erythroid cell differentiation. In comparison to the 15 genes with the strongest phenotypes, those with more mild phenotypes likely function at later stages of erythropoiesis after induction of the scl+ mesoderm.
Similarly, for genes with moderate phenotypes from the definitive screen, we did not observe any defects in flk1 and ephrinB2 expression for 10 definitive morphants that were reconfirmed in the intermediate decrease category, suggesting these genes also regulate HSPC development. In summary, our screen has identified a large number of chromatin factors that contribute to different stages of primitive and definitive hematopoiesis.
A chromatin factor interaction network for hematopoietic cell development
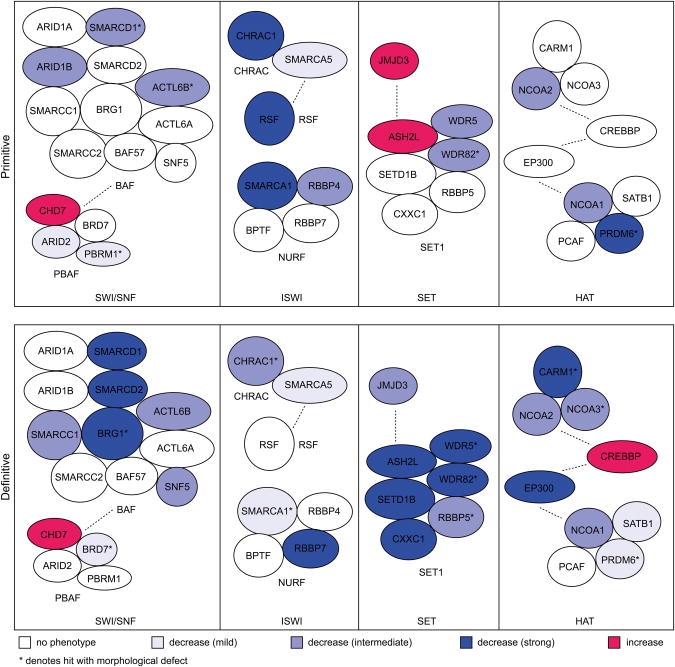
Given that chromatin factors often function in multisubunit complexes, we sought to identify complexes important for hematopoietic development by mapping known protein interactions for the genes identified in our screen. We started by annotating the protein complexes for the 44 validated genes from the primitive and definitive screens and included additional genes from the screen that were also present in these complexes27. Our results implicate multiple chromatin factor complexes required for developmental hematopoiesis: BAF/PBAF and ISWI (chromatin remodeling), SET1 (histone methylation - activation), HDAC/NuRD (histone deacetylation), NuA4/P300/CBP/HBO1 (histone acetylation), and PRC1/2 (histone methylation - repression) (Fig. 6, Supplementary Fig. 3).
Figure 6.
Identification of chromatin modifying complexes using protein interaction data for the 44 validated primitive and definitive genes. Protein complexes associated with the 44 chromatin factors from the decrease (strong) and increase categories were identified, and additional genes from the screen present in these complexes were included (Uniprot). Results for SWI/SNF, ISWI, SET1, and CBP/P300 are illustrated with additional complexes shown in Supplementary Figure 3. Filled in circles represent chromatin factors that were identified as screen hits with their respective phenotypic classification denoted by different colors. Dotted lines indicate alternative complex associations.
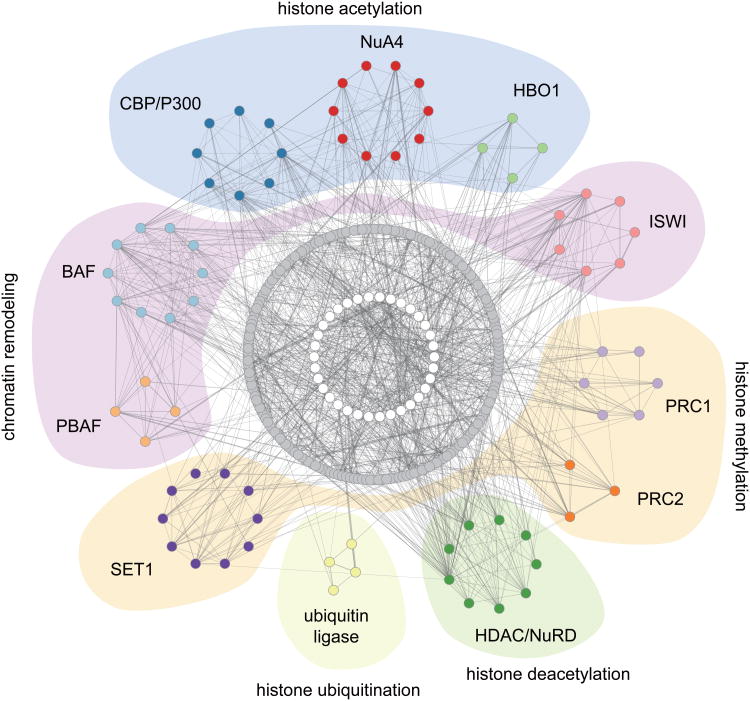
To identify all possible chromatin complexes represented by our screen datasets, we generated a human protein-protein interaction map of the 425 chromatin factors screened and mapped the factors found in our screen (102 primitive and 116 definitive factors) onto the network. We identified the same complexes as we did in the previous analysis with the addition of a ubiquitination complex (Fig. 7, Supplementary Fig. 3).
Figure 7.
A protein-protein interaction network for the 425 human chromatin factors screened. Screen results for 102 primitive and 116 definitive genes were mapped onto the network, and highly interconnected modules containing factors identified from the screens were isolated, representing the chromatin complexes important for hematopoietic development39. Detailed maps of the modules are in Supplementary Figure 5. Each module is assigned a unique color, and those that share the same chromatin function are highlighted in the same background color. Grey circles represent chromatin factors not associated with the isolated complexes, and white circles mark additional factors that were added to increase the connectivity of the network.
As chromatin factors associated with the same complex likely share target binding sites, we analyzed 34 published ChIP-seq (chromatin immunoprecipitation followed by sequencing) datasets in K562 erythroleukemia cells of chromatin factors in our screen28. Two major complexes were found. The first group includes SIN3A, CHD4, HDAC1, TAF1, and JARID1C associated with the HDAC/NuRD complex, and the second group includes RNF2, SUZ12, CBX2, and CBX8 from the Polycomb complexes. We ranked triplet combinations of these factors together with all other groups of three factors based on the percent overlap of target genes. The HDAC/NuRD and PRC1/2 complex combinations predicted from our screen, including those which have been shown to interact biochemically, fell within the top 20% of all possible combinations of three factors (Supplementary Fig. 4a,c). After excluding a subset often factors that have large target gene lists (>8,000 target genes), which could skew the distribution, this filtered analysis resulted in the predicted interactions falling within the top 5% of all combinations (Supplementary Fig. 4b,c). Both predicted interactions are significantly enriched in the upper tail of the distribution in the two analyses, therefore suggesting that our screen has identified chromatin factors that function in distinct complexes to regulate hematopoietic development.
Genetic interaction of predicted chromatin complex subunits
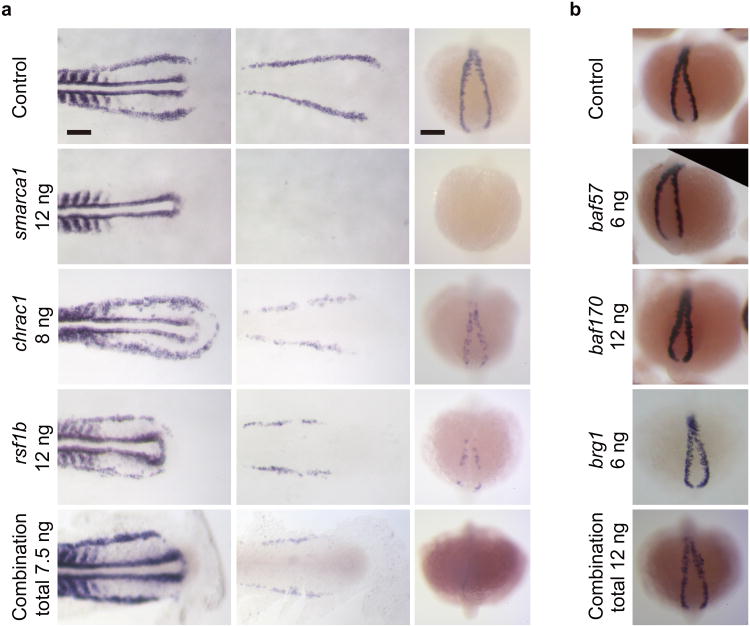
Based on complexes identified, we expected that chromatin factor subunits that scored positive in our screen would interact genetically. We tested the interaction by combinatorial knockdown of ISWI subunits required for primitive erythropoiesis, smarca1, chrac1, and rsf1b (Fig. 6)29,30. As described in the previous section, loss of these factors individually resulted in decreased gata1 and β-globin e3 expression. Knockdown of smarca1 and rsf1b also reduced scl expression. In knocking down all three factors at suboptimal doses, scl expression is retained while gata1 and β-globin e3 expression is highly reduced as in the single morphants (Fig. 8a). In contrast, knockdown of SWI/SNF components brg1, baf57, and baf170, which were not identified as primitive screen hits, did not result in primitive hematopoietic defects individually or in combination (Fig. 8b)31,32. These data suggest that the chromatin factors we identified from our screen interact genetically to regulate hematopoietic development.
Figure 8.
Genetic interaction of ISWI chromatin factors by combinatorial knockdown. (a) gata1 and β-globin e3 expression levels were examined by injecting single and combined suboptimal doses of morpholinos against smarca1, chrac1, and rsf1b. Combined dose of 7.5 ng includes 4 ng of smarca1, 0.8 ng of chrac1, and 2.7 ng of rsf1b.(b) Knockdown of SWI/SNF chromatin factors baf57, baf170, and brg1, which were not identified as hits from the screen, were tested for β-globin e3 expression either individually or in combination. Combined dose of 12 ng includes 4 ng of each of the three morpholinos. Morpholino doses are indicated as nanograms (ng). Scale bars: 100μm
Discussion
Hematopoietic stem cells undergo proliferation and differentiation under the control of cell-specific transcription factors whose function is facilitated by chromatin factors. These factors establish an epigenetic landscape that controls self-renewal and provides lineage priming, driving differentiation. To better understand the epigenetic regulation of the hematopoiesis, we undertook the first reverse genetic approach to define the function of chromatin factors in the zebrafish. A library of zebrafish genes orthologous to 425 human chromatin factors were identified, containing canonical ‘readers’, ‘writers’, and ‘erasers’ of chromatin and other, less characterized, families.
In this study, we characterized a cohort of 15 chromatin factors that regulate primitive hematopoiesis and 29 that regulate definitive hematopoiesis, including both known and previously unidentified factors. Based on our validation work, the data suggest that these factors function at the level of erythroid and HSPC specification. Our analysis of several blood-specific markers at several distinct timepoints has been used to describe differentiation defects in many zebrafish blood mutants33,34. However, a delay in blood development would also readout as a decrease phenotype. As well, it is possible that an accelerated emergence of blood cells could confound our analysis except when it results in an overall increase in blood production. Regardless of whether blood development is selectively delayed or accelerated, our results ultimately show expression of the blood markers is altered, whether directly or indirectly affecting any number of pathways, such as metabolism, transcriptional elongation, and cell cycle regulation. Additional work will be required to determine the mechanism of action on hematopoiesis and whether these phenotypes are cell autonomous.
Disruption of both positive and negative regulators of chromatin frequently resulted in the same phenotype in our screen. For example, knockdown of p300, which functions to acetylate histones, and hdac6, which deacetylates histones, each resulted in loss of c-myb+ and runx1+ cells in the AGM. While they likely serve opposing roles in regulation of their respective target genes, their functions are both required for proper HSC specification. These data are in concordance with proteomics data showing transcription factors, such as GATA1, recruiting both positive and negative regulators to activate and repress target genes, respectively35.
While members of the same chromatin family could compensate for each other, individual knockdown of many of these factors still resulted in a hematopoietic phenotype, suggesting nonredundant functions among related chromatin factors. Two factors that were identified from our screen, p300 and crebbp, share similar functions but showed opposing phenotypes. p300 and CBP are homologous proteins that share a bromodomain and histone acetyltransferase domain36. Mouse knockouts of p300 and Cbp exhibit similar phenotypes37. Despite their overlapping roles, evidence of differential regulation has been accumulating. In HSCs, Cbp plays an important role in HSC self-renewal whereas p300 regulates HSC differentiation. Recent ChIP-seq results identified distinct binding sites between the two factors38,39. Consequently, the effect chromatin factors have in vivo cannot be predicted based solely on their domain function. Future in vivo studies will be important for our understanding of chromatin regulation and gene expression.
In a recent study investigating histone modifications on differentiating erythroid cells in mouse fetal liver, five histone marks were induced during this transition, H3K4me2, H3K4me3, H3K9Ac, H4K16Ac, and H3K79me240. Consistent with these findings, our strongest primitive hits are composed of chromatin factors involved in methylation and acetylation of histones, including H3K4 methylation. Although similar work characterizing changes in histone marks during various stages of definitive HSC formation has not been done, we predict that they will include histone modifications such as methylation of H3K4, H3K9, and H3K36 based on the chromatin factors identified in our screen.
By examining our screen results, we identified relevant chromatin modifying complexes for blood development including BAF/PBAF, ISWI, HDAC/NuRD, NuA4/P300/CBP/HBO1, SET1, and PRC1/2 complexes. Hypotheses regarding the subunit composition of the chromatin factor complexes can be generated using our data set. One of the most striking results from our primitive screen was the knockdown of smarca1, which abrogates scl, gata1, and β-globin e3 expression in the embryo. chrac1 and rsf1b, other components that form the ISWI complex, were also identified in the screen. In mammalian data, these factors form a complex with another family member, smarca5. Our data suggests that ISWI chromatin remodeling is important for primitive and definitive hematopoiesis and that the complex contains smarca1 (not smarca5), chrac1, and rsf1b. As well, by comparing chromatin occupancy of complex members, we observed higher proportions of bound genes among factors predicted to be in the same complex. Taken together, these data suggest that our screen has identified chromatin factors that function in distinct complexes to regulate hematopoiesis.
Overall, we have identified a set of genes involved in the regulation of developmental hematopoiesis, including primitive erythropoiesis and definitive HSPC specification, and provide a resource for the identification and characterization of previously unidentified regulators. Studies focusing on the interactions between hematopoietic transcription factors and our chromatin factors will provide a more complete transcriptional network of gene regulation in blood development. In combination with other genetic and biochemical studies, our screen helps to unravel the epigenetic code that establishes the programs of gene expression for self-renewal and differentiation in hematopoietic cells.
Supplementary Material
Acknowledgments
We thank O. Tamplin, T.V. Bowman, P. Cahan, and C.K. Kaufman for helpful discussions. The work was supported by NIH NIDDK 5R01DK053298-15, NIH NHLBI 5R01HL048801-21, NIH NIDDK 5P30 DK49216-19, NIH NIDDK DK53298-15, NIH NIDDK R24 DK092760-02, HHMI (to L.I.Z.)), NIH NHLBI T32 HL066987-09 and NIH NIDDK 1F32DK089876-01 (to K.L.K)). L.I.Z. is a founder and stock holder of Fate, Inc. and Scholar Rock, and a scientific advisor for Stemgent.
Footnotes
Author Contributions: H.T.H. and K.L.K. performed all experiments and data analysis. A.B. and Z.G. assisted with morpholino microinjection, WISH, and data collection. Y-H.H. assisted with morpholino microinjection. T.P.W., Y.Z., A.S., and A.D. developed the screen database. Y.Z. initiated and assisted with bioinformatic analysis of chromatin factors. O.H. and W.H. generated the protein interaction network. S.T. performed the distribution analysis for the ChIP-seq data. H.T.H. and L.I.Z. conceived the study.
References
- 1.Li B, Carey M, Workman J. The role of chromatin during transcription. Cell. 2007;128:707–726. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Ho L, Crabtree G. Chromatin remodelling during development. Nature. 2010;463:474–484. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunliffe V. Histone deacetylase 1 is required to repress Notch target gene expression during zebrafish neurogenesis and to maintain the production of motoneurones in response to hedgehog signalling. Development (Cambridge, England) 2004;131:2983–2995. doi: 10.1242/dev.01166. [DOI] [PubMed] [Google Scholar]
- 4.Gregg R, Willer G, Fadool J, Dowling J, Link B. Positional cloning of the young mutation identifies an essential role for the Brahma chromatin remodeling complex in mediating retinal cell differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6535–6540. doi: 10.1073/pnas.0631813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller C, Maves L, Kimmel C. moz regulates Hox expression and pharyngeal segmental identity in zebrafish. Development (Cambridge, England) 2004;131:2443–2461. doi: 10.1242/dev.01134. [DOI] [PubMed] [Google Scholar]
- 6.Orkin S, Zon L. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takihara Y. Role of Polycomb-group genes in sustaining activities of normal and malignant stem cells. International journal of hematology. 2008;87:25–34. doi: 10.1007/s12185-007-0006-y. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida T, et al. The role of the chromatin remodeler Mi-2beta in hematopoietic stem cell self-renewal and multilineage differentiation. Genes Dev. 2008;22:1174–1189. doi: 10.1101/gad.1642808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krivtsov A, Armstrong S. MLL translocations, histone modifications and leukaemia stem-cell development. Nature reviews Cancer. 2007;7:823–833. doi: 10.1038/nrc2253. [DOI] [PubMed] [Google Scholar]
- 10.Duhoux FP, et al. PRDM16 (1p36) translocations define a distinct entity of myeloid malignancies with poor prognosis but may also occur in lymphoid malignancies. Br J Haematol. 2012;156:76–88. doi: 10.1111/j.1365-2141.2011.08918.x. [DOI] [PubMed] [Google Scholar]
- 11.Chuikov S, Levi B, Smith M, Morrison S. Prdm16 promotes stem cell maintenance in multiple tissues, partly by regulating oxidative stress. Nature cell biology. 2010;12 doi: 10.1038/ncb2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shipra A, Chetan K, Rao MR. CREMOFAC--a database of chromatin remodeling factors. Bioinformatics. 2006;22:2940–2944. doi: 10.1093/bioinformatics/btl509. [DOI] [PubMed] [Google Scholar]
- 13.Schultz J, Milpetz F, Bork P, Ponting CP. SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci U S A. 1998;95:5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchler-Bauer A, et al. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 2011;39:D225–229. doi: 10.1093/nar/gkq1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Punta M, et al. The Pfam protein families database. Nucleic Acids Res. 2012;40:D290–301. doi: 10.1093/nar/gkr1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gendler K, Paulsen T, Napoli C. ChromDB: the chromatin database. Nucleic Acids Res. 2008;36:D298–302. doi: 10.1093/nar/gkm768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimmel C, Ballard W, Kimmel S, Ullmann B, Schilling T. Stages of embryonic development of the zebrafish. Developmental dynamics: an official publication of the American Association of Anatomists. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 18.Brownlie A, et al. Characterization of embryonic globin genes of the zebrafish. Developmental biology. 2003;255:48–61. doi: 10.1016/s0012-1606(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 19.North T, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao E, et al. SCL/Tal-1 transcription factor acts downstream of cloche to specify hematopoietic and vascular progenitors in zebrafish. Genes & development. 1998;12:621–626. doi: 10.1101/gad.12.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muralidhar SA, Ramakrishnan V, Kalra IS, Li W, Pace BS. Histone deacetylase 9 activates gamma-globin gene expression in primary erythroid cells. J Biol Chem. 2011;286:2343–2353. doi: 10.1074/jbc.M110.115725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, et al. ASH2L: alternative splicing and downregulation during induced megakaryo cytic differentiation of multipotential leukemia cell lines. Journal of molecular medicine (Berlin, Germany) 2001;79:399–405. doi: 10.1007/s001090100222. [DOI] [PubMed] [Google Scholar]
- 23.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 24.Burns C, et al. A genetic screen in zebrafish defines a hierarchical network of pathways required for hematopoietic stem cell emergence. Blood. 2009;113:5776–5782. doi: 10.1182/blood-2008-12-193607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong W, et al. FOG-1 recruits the NuRD repressor complex to mediate transcriptional repression by GATA-1. The EMBO journal. 2005;24:2367–2445. doi: 10.1038/sj.emboj.7600703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bai X, et al. TIF1gamma controls erythroid cell fate by regulating transcription elongation. Cell. 2010;142:133–143. doi: 10.1016/j.cell.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Apweiler R JMM, et al. Reorganizing the protein space at the Universal Protein Resource (UniProt) Nucleic Acids Res. 2012;40:D71–75. doi: 10.1093/nar/gkr981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peter BB. A User' Guide to the Encyclopedia of DNA Elements (ENCODE) PLoS biology. 2011;9 doi: 10.1371/journal.pbio.1001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LeRoy G, Orphanides G, Lane WS, Reinberg D. Requirement of RSF and FACT for transcription of chromatin templates in vitro. Science. 1998;282:1900–1904. doi: 10.1126/science.282.5395.1900. [DOI] [PubMed] [Google Scholar]
- 30.Varga-Weisz P, Becker P. Chromatin-remodeling factors: machines that regulate? Current opinion in cell biology. 1998;10:346–399. doi: 10.1016/s0955-0674(98)80010-0. [DOI] [PubMed] [Google Scholar]
- 31.Wang W, et al. Architectural DNA binding by a high-mobility-group/kinesin-like subunit in mammalian SWI/SNF-related complexes. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:492–498. doi: 10.1073/pnas.95.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang W, et al. Diversity and specialization of mammalian SWI/SNF complexes. Genes & development. 1996;10:2117–2130. doi: 10.1101/gad.10.17.2117. [DOI] [PubMed] [Google Scholar]
- 33.Ransom D, et al. Characterization of zebrafish mutants with defects in embryonic hematopoiesis. Development (Cambridge, England) 1996;123:311–319. doi: 10.1242/dev.123.1.311. [DOI] [PubMed] [Google Scholar]
- 34.Thompson M, et al. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Developmental biology. 1998;197:248–269. doi: 10.1006/dbio.1998.8887. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez P, et al. GATA-1 forms distinct activating and repressive complexes in erythroid cells. The EMBO journal. 2005;24:2354–2420. doi: 10.1038/sj.emboj.7600702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogryzko V, Schiltz R, Russanova V, Howard B, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–962. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 37.Yao T, et al. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell. 1998;93:361–433. doi: 10.1016/s0092-8674(00)81165-4. [DOI] [PubMed] [Google Scholar]
- 38.Rebel V, et al. Distinct roles for CREB-binding protein and p300 in hematopoietic stem cell self-renewal. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14789–14883. doi: 10.1073/pnas.232568499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramos Y, et al. Genome-wide assessment of differential roles for p300 and CBP in transcription regulation. Nucleic acids research. 2010;38:5396–5804. doi: 10.1093/nar/gkq184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong P, et al. Gene induction and repression during terminal erythropoiesis are mediated by distinct epigenetic changes. Blood. 2011;118:38. doi: 10.1182/blood-2011-03-341404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawrence C, Adatto I, Best J, James A, Maloney K. Generation time of zebrafish (Danio rerio) and medakas (Oryzias latipes) housed in the same aquaculture facility. Lab Anim (NY) 2012;41:158–165. doi: 10.1038/laban0612-158. [DOI] [PubMed] [Google Scholar]
- 42.Berghmans Sp, et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:407–412. doi: 10.1073/pnas.0406252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 44.Shimoda N, Yamakoshi K, Miyake A, Takeda H. Identification of a gene required for de novo DNA methylation of the zebrafish no tail gene. Developmental dynamics: an official publication of the American Association of Anatomists. 2005;233:1509–1525. doi: 10.1002/dvdy.20455. [DOI] [PubMed] [Google Scholar]
- 45.Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nature protocols. 2008;3:59–128. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- 46.Turner B, et al. iRefWeb: interactive analysis of consolidated protein interaction data and their supporting evidence. Database (Oxford) 2010;2010:baq023. doi: 10.1093/database/baq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopes CT, et al. Cytoscape Web: an interactive web-based network browser. Bioinformatics. 2010;26:2347–2348. doi: 10.1093/bioinformatics/btq430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McLean C, et al. GREAT improves functional interpretation of cis-regulatory regions. Nature biotechnology. 2010;28:495–501. doi: 10.1038/nbt.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.