Abstract
An IR study of terminal acetylenes and Zn(OTf)2 in the presence of amine bases provides evidence that is consistent with the generation of Zn-acetylides.
The addition reactions of nucleophilic acetylides to ketones, aldehydes, and C N electrophiles are important processes in organic synthesis (1, 2). Such reaction processes can be intrinsically efficient in the production of useful building blocks of great synthetic versatility, because a new stereogenic center and a new C—C bond are established in a single operation. The acetylides typically used in such reactions are commonly prepared by deprotonation of terminal alkynes with strong bases (BuLi, RMgX, and R2NLi) (3, 4), or dialkyl zinc reagents, such as Et2Zn or Me2Zn (5–11). When it is of interest to effect additions to electrophiles such as aldehydes or ketones, the alkyne metallation must necessarily be carried out as a separate preparative step because of the incompatibility of reagents used in the metallation reaction with electrophilic substrates. We have reported a series of reactions in which terminal acetylenes undergo additions to aldehydes and nitrones in the presence of Zn(II) and amine bases (12–21). The propargylic alcohols or N-hydroxyl amines can serve as versatile useful building blocks (22, 23). In the presence of a chiral amino alcohol such as (–)-N-methyl ephedrine, optically active propargylic alcohols can be formed. As part of our working model, we hypothesize that under these conditions a Zn-acetylide is generated that subsequently participates in nucleophilic addition reactions (8). To provide insight on the mechanistic aspects, and in particular whether a Zn-acetylide is an intermediate in these processes, we decided to undertake a series of IR spectroscopic investigations involving ReactIR that we report herein. The outcome of these studies provides spectroscopic evidence that is consistent with the formation of a Zn-acetylide when a terminal acetylene is mixed with Zn(OTf)2 and amine base.
N electrophiles are important processes in organic synthesis (1, 2). Such reaction processes can be intrinsically efficient in the production of useful building blocks of great synthetic versatility, because a new stereogenic center and a new C—C bond are established in a single operation. The acetylides typically used in such reactions are commonly prepared by deprotonation of terminal alkynes with strong bases (BuLi, RMgX, and R2NLi) (3, 4), or dialkyl zinc reagents, such as Et2Zn or Me2Zn (5–11). When it is of interest to effect additions to electrophiles such as aldehydes or ketones, the alkyne metallation must necessarily be carried out as a separate preparative step because of the incompatibility of reagents used in the metallation reaction with electrophilic substrates. We have reported a series of reactions in which terminal acetylenes undergo additions to aldehydes and nitrones in the presence of Zn(II) and amine bases (12–21). The propargylic alcohols or N-hydroxyl amines can serve as versatile useful building blocks (22, 23). In the presence of a chiral amino alcohol such as (–)-N-methyl ephedrine, optically active propargylic alcohols can be formed. As part of our working model, we hypothesize that under these conditions a Zn-acetylide is generated that subsequently participates in nucleophilic addition reactions (8). To provide insight on the mechanistic aspects, and in particular whether a Zn-acetylide is an intermediate in these processes, we decided to undertake a series of IR spectroscopic investigations involving ReactIR that we report herein. The outcome of these studies provides spectroscopic evidence that is consistent with the formation of a Zn-acetylide when a terminal acetylene is mixed with Zn(OTf)2 and amine base.
The complexation of terminal alkynes with metals, such as Cu(I) or Ag(I), yields π complexes that labilize the terminal C–H bond such that weak amine bases can effect deprotonation and concomitant formation of the corresponding metal acetylide (24). Unfortunately, acetylides generated under these conditions, in general, fail to participate in nucleophilic C O additions. By contrast, it has been known for some time that with heating, Cu-acetylides will participate in imminiun additions (25, 26).
O additions. By contrast, it has been known for some time that with heating, Cu-acetylides will participate in imminiun additions (25, 26).
We surmised that the ability to carry out nucleophilic additions to C O with metal acetylides generated under the mild conditions that parallel those used in the formation of Cu(I) or Ag(I) akynylides would offer new opportunities for catalytic C—C bond formation. We have observed that Zn(OTf)2, in combination with a tertiary amine base and a terminal alkyne, leads to the generation of a nucleophilic species that participates in aldehyde and nitrone addition reactions. The addition reactions to aldehydes were first reported employing stoichiometric quantities of Zn(II) and amine base (Eq. 1),
O with metal acetylides generated under the mild conditions that parallel those used in the formation of Cu(I) or Ag(I) akynylides would offer new opportunities for catalytic C—C bond formation. We have observed that Zn(OTf)2, in combination with a tertiary amine base and a terminal alkyne, leads to the generation of a nucleophilic species that participates in aldehyde and nitrone addition reactions. The addition reactions to aldehydes were first reported employing stoichiometric quantities of Zn(II) and amine base (Eq. 1),
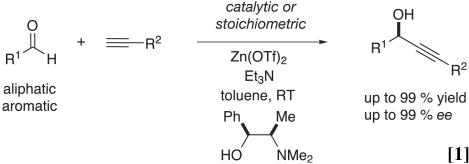 |
a protocol that offers great opportunity for reaction optimization, because the system can respond favorably to variation in the relative stoichiometry of the various components. Indeed, this procedure has been used in our own laboratories in the context of complex natural products synthesis (27–31) as well as by others (32). Subsequently, we have developed a catalytic variant of the addition reaction (Eq. 2).
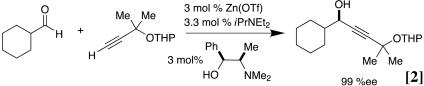 |
Key to this advance was the realization that catalytic turnover can be effected when the reaction is conducted at elevated temperatures, i.e. 60°C (Eq. 2) (12). The Zn(II) process is unique in that it can also be conducted neat, thus minimizing the generation of the usual waste in the form of solvent. In unpublished work, we have noted that for certain classes of substrates, this solvent-free protocol permits the use of catalyst loadings that are substantially lower than those we documented in our initial reports (Eq. 2). Subsequent to our work, other groups have established the use of transiently generated metal acetylides in catalytic enantioselective imine and carbonyl additions, including metal catalysts such as Cu, Ag, and Au (13, 33–37).
It has been known for some time that metal salts such as Cu(I), Ag(I), and Au(I) metallate terminal acetylenes in the presence of mild bases. This forms the basis of a number of useful C–C coupling reactions including Eglinton and Sonogoshira coupling reactions (2). The metallation reaction of terminal acetylenes by Cu(I) and other salts has been suggested to proceed by deprotonation of the corresponding metal-alkyne π complex (Eq. 3) (24). We have used a similar mechanistic model in the analysis of the Zn-mediated reactions: in its simplest rendition, following formation of a π complex, deprotonation of the terminal acetylene ensues with concomitant generation of a Zn-acetylide.
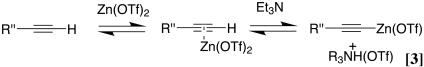 |
The ready availability of a convenient bench-top IR instrumentation, such as ReactIR, that permits real-time, continuous in situ observation of chemical reactions by using attenuated total reflection methods has provided a powerful tool for the investigation of reaction processes (38, 39). In prior work in our group, we have used this technique in studies aimed at understanding a new class of Mukaiyama aldol additions that proceed by enolate activation and thus complement the more common approach by using Lewis acid activation of aldehydes (40). In such investigations, the use of IR spectroscopic studies substantiated a process wherein the silyl enolate undergoes in situ activation by CuF·(p-tolBINAP) and conversion to the corresponding metalloenolate. In a similar fashion, we embarked on a study aimed at applying this same technique to the reaction of terminal acetylenes with Zn(OTf)2 and amines.
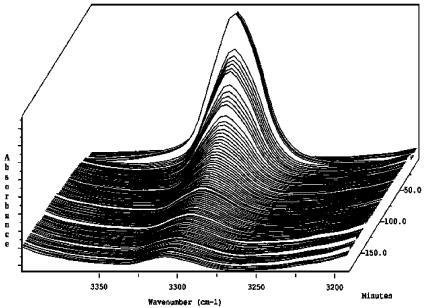
In the initial investigations, a control experiment was carried out in which phenylacetylene was deprotonated under standard conditions; namely, by treatment with an organometallic base. Thus, at t = 0 a solution of phenylacetylene in CH2Cl2 displays a prominent C—H stretch resonance at 3,296 cm–1 in the IR spectrum. As expected, treatment of this solution with 1 eq EtMgBr resulted in the quantitative disappearance of this band within 150 min at ambient temperature, signifying that the terminal alkyne had undergone deprotonation (Fig. 1). Although additional changes in the spectrum can be observed in the fingerprint region, interpretation of these shifts are rather difficult; thus, the spectral window spanning 3,250–3,360 cm–1 proved most effective for analysis.
Fig. 1.
ReactIR spectra showing the disappearance of the acetylenic C–H stretch resonance at 3,296 cm–1 due to deprotonation of phenylacetylene by EtMgBr (t = 0 at rear back right corner).
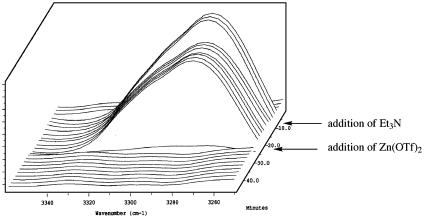
We next monitored by IR spectroscopy the stepwise reaction of phenyl-acetylene, Zn(OTf)2, and triethylamine, sequentially in acetonitrile at 23°C. Although the Zn-mediated acetylide addition reactions can be successfully conducted in a number of different solvents and even solvent-free, acetonitrile was chosen as the media for the spectroscopic investigations, because the reactions conducted in this solvent are routinely homogeneous, thus facilitating the spectroscopic studies. As shown in Fig. 2, the C—H stretch signal of a solution of phenylacetylene in acetonitrile is observed at 3,277 cm–1. Treatment of this solution with excess Et3N leads to a small diminution in the absorbance intensity due to dilution effects. In a separate control experiment, we could show that a solution of various aldehydes along with phenylacetylene and Et3N did not lead to product formation; thus, background deprotonation by Et3N can be reasonably excluded. Importantly, however, subsequent addition of 1 eq Zn(OTf)2 led to rapid disappearance of the signal at 3,277 cm–1 within 4 min. Similar observations were made when the IR experiment was carried out in the presence of added amino alcohol ligand and otherwise identical conditions to those described above. Thus, analysis by ReactIR of a reaction mixture consisting of phenylacetylene, Zn(OTf)2, NEt3, and (–)-N-methyl ephedrine in acetonitrile reveals a rapid disappearance of the terminal C—H stretch, in an analogous manner to that observed in Fig. 2.
Fig. 2.
ReactIR spectra of the C–H stretch resonance signal of phenylacetylene in CH3CN. The IR band at 3,277 cm–1 completely disappears within 4 min after the addition of Zn(OTf)2 to a mixture of phenylacetylene and NEt3.
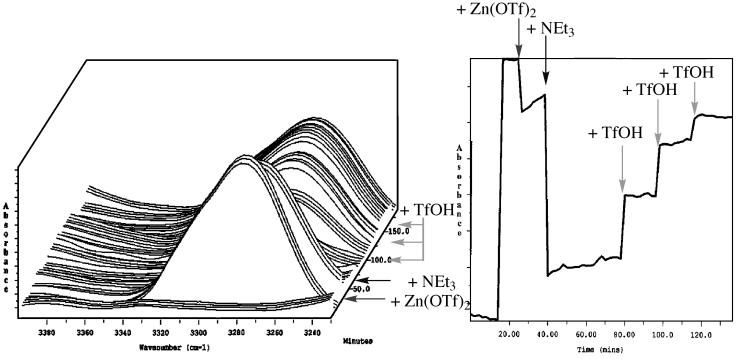
Further evidence for the metallation reaction of terminal acetylenes on exposure to Zn(OTf)2 and base was obtained by examining whether the metallation reaction was reversible. Indeed, this could be demonstrated by the addition of Brønsted acid to the reaction mixture produced as described above from mixing phenylacetylene, Zn(OTf)2, and Et3N. Thus, when trifluoromethane sulfonic acid was added in a piecemeal fashion in proportion to the amount of base used, the signal corresponding to the terminal C—H stretch for phenylacetylene was observed to resurface (Fig. 3). Subsequent to reappearance of the C—H stretch, addition of base to the solution in amounts exceeding the stoichiometry of acid used led again to disappearance of the C—H signal. The fact that we have never observed any side products derived exclusively from the acetylene fragment in these reactions, even when conducted on large scale, in conjunction with the IR studies delineated above, is consistent with a simple metallation process for Zn(II), in analogy with that accepted for Cu(I) and Ag(I).
Fig. 3.
ReactIR data obtained during deprotonation and reprotonation of phenylacetylene in acetonitrile in the presence of Zn(OTf)2 and triethylamine. (Left) Accumulated spectra, time-resolved. (Right) Profile taken at the absorbance maximum at 3,277 cm–1.
In summary, we carried out ReactIR spectroscopic investigations of the nucleophilic acetylide species generated from terminal alkynes in the presence of Zn(OTf)2 and NEt3. The spectroscopic observations strongly support the working hypothesis in these reactions wherein the combination of an amine base and Zn(OTf)2 leads to the generation of a Znacetylide. The discovery and study of new reactive intermediates is of critical importance for the continuing evolution of asymmetric catalysis. The results reported herein permit the rationalization of the results that have been observed and encourage additional developments and experimentation in the use of zinc alkynylides as reactive intermediates in asymmetric catalysis.
Acknowledgments
We thank the Eidgenössische Technische Hochschule (TH Gesuch) and the Swiss National Science Foundation for funding this work. R.F. acknowledges the generous support of a student fellowship from Hoffman–La Roche.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Stang, P. J. & Diederich, F., eds. (1995) Modern Acetylene Chemistry (VCH, Weinheim, Germany).
- 2.Brandsma, L. (1988) Preparative Acetylene Chemistry (Elsevier, Amsterdam), 2nd Ed.
- 3.Wakefield, B. J. (1988) Organolithium Methods (Academic, London), pp. 32–44.
- 4.Wakefield, B. J. (1995) Organomagnesium Methods in Organic Synthesis (Academic, London), pp. 46–48.
- 5.Yamamoto, Y., Nishii, S. & Maruyama, K. (1986) J. Chem. Soc. Chem. Commun., 102–103.
- 6.Corey, E. J. & Cimprich, K. A. (1994) J. Am. Chem. Soc. 116, 3151–3152. [Google Scholar]
- 7.Ahn, J. H., Joung, M. J. & Yoon, N. M. (1995) J. Org. Chem. 60, 6173–6175. [Google Scholar]
- 8.Evans, D. A., Halstead, D. P. & Allison, B. D. (1999) Tetrahedron Lett. 40, 4461–4462. [Google Scholar]
- 9.Santelli, M. & Pons, J.-M. (1996) Lewis Acids and Selectivity in Organic Synthesis (CRC, Boca Raton, FL), Chaps. 3 and 5.
- 10.Marshall, J. A. (1996) Chem. Rev. 96, 31–48. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto, Y. & Asao, N. (1993) Chem. Rev. 93, 2207–2293. [Google Scholar]
- 12.Frantz, D. E., Fässler, R. & Carreira, E. M. (1999) J. Am. Chem. Soc. 121, 11245–11246. [Google Scholar]
- 13.Fässler, R., Frantz, D. E., Oetiker, J. & Carreira, E. M. (2002) Angew. Chem. Int. Ed. Engl. 41, 3054–3056. [DOI] [PubMed] [Google Scholar]
- 14.Frantz, D. E., Fässler, R. & Carreira, E. M. (2000) J. Am. Chem. Soc. 122, 1806–1807. [Google Scholar]
- 15.Boyall, D., Lopez, F., Sasaki, H., Frantz, D. & Carreira, E. M. (2000) Org. Lett. 2, 4233–4236. [DOI] [PubMed] [Google Scholar]
- 16.Anand, N. K. & Carreira, E. M. (2001) J. Am. Chem. Soc. 123, 9687–9688. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki, H., Boyall, D. & Carreira, E. M. (2001) Helv. Chim. Acta 84, 964–971. [Google Scholar]
- 18.Boyall, D., Frantz, D. E. & Carreira, E. M. (2002) Org. Lett. 4, 2605–2606. [DOI] [PubMed] [Google Scholar]
- 19.Diez, R. S., Adger, B. & Carreira, E. M. (2002) Tetrahedron 58, 8341–8344. [Google Scholar]
- 20.Frantz, D. E., Fässler, R., Tomooka, C. S. & Carreira, E. M. (2000) Acc. Chem. Res. 33, 373–381. [DOI] [PubMed] [Google Scholar]
- 21.Pinet, S., Pandya, S. U., Chavant, P. Y., Ayling, A. & Vallee, Y. (2002) Org. Lett. 4, 1463–1466. [DOI] [PubMed] [Google Scholar]
- 22.El-Sayed, E., Anand, N. K. & Carreira, E. M. (2001) Org. Lett. 3, 3017–3020. [DOI] [PubMed] [Google Scholar]
- 23.Aschwanden, P., Frantz, D. E. & Carreira, E. M. (2000) Org. Lett. 2, 2331–2333. [DOI] [PubMed] [Google Scholar]
- 24.Cotton, F. A., Wilkinson, G., Murillo, C. A. & Bochmann, M. (1999) Advanced Inorganic Chemistry (Wiley, New York), 6th Ed., pp. 681–683.
- 25.Brannock, K. C., Buhpitt, R. D. & Thweatt, J. G. (1963) J. Org. Chem. 28, 1462–1464. [Google Scholar]
- 26.Knöpfel, T. F. & Carreira, E. M. (2003) J. Am. Chem. Soc. 125, 6054–6055. [DOI] [PubMed] [Google Scholar]
- 27.Fettes, A. & Carreira, E. M. (2003) J. Org. Chem. 68, 9274–9283. [DOI] [PubMed] [Google Scholar]
- 28.Reber, S., Knöpfel, T. F. & Carreira, E. M. (2003) Tetrahedron 59, 6813–6817. [Google Scholar]
- 29.Fettes, A. & Carreira, E. M. (2002) Angew. Chem. Int. Ed. Engl. 41, 4098–4101. [DOI] [PubMed] [Google Scholar]
- 30.Bode, J. W. & Carreira, E. M. (2001) J. Org. Chem. 66, 6410–6424. [DOI] [PubMed] [Google Scholar]
- 31.Bode, J. W. & Carreira, E. M. (2001) J. Am. Chem. Soc. 123, 3611–3612. [DOI] [PubMed] [Google Scholar]
- 32.Maezaki, N., Kojima, M., Tominaga, H. & Tanaka, T. (2002) Org. Lett. 4, 2977–2980. [DOI] [PubMed] [Google Scholar]
- 33.Wei, C. & Li, C.-J. (2003) J. Am. Chem. Soc. 125, 9584–9585. [DOI] [PubMed] [Google Scholar]
- 34.Wei, C. & Li, C.-J. (2002) J. Am. Chem. Soc. 124, 5638–5639. [DOI] [PubMed] [Google Scholar]
- 35.Moore, D. & Pu, L. (2002) Org. Lett. 4, 1855–1857. [DOI] [PubMed] [Google Scholar]
- 36.Gommermann, N., Koradin, C., Polborn, K. & Knochel, P. (2003) Angew. Chem. Int. Ed. Engl. 42, 5763–5766. [DOI] [PubMed] [Google Scholar]
- 37.Fischer, C. & Carreira, E. M. (2001) Org. Lett. 3, 4319–4321. [DOI] [PubMed] [Google Scholar]
- 38.Jenkins, F. A. & White, H. E. (1976) Fundamentals of Optics (McGraw–Hill, New York), 4th Ed.
- 39.Urban, M. W. (1996) Attenuated Total Reflectance Spectroscopy of Polymers: Theory and Practice, Polymer Surfaces and Interfaces Series (Am. Chem. Soc., Washington, DC).
- 40.Pagenkopf, B. L., Krüger, J., Stojanovic, A. & Carreira, E. M. (1998) Angew. Chem. Int. Ed. Engl. 37, 3124–3126. [DOI] [PubMed] [Google Scholar]