Abstract
INTRODUCTION:
In 2007, the Canadian Paediatric Society (CPS) published guidelines aimed at preventing severe hyperbilirubinemia.
OBJECTIVES:
To determine whether hospitals had implemented these guidelines; to investigate how guideline-recommended care is organized; and to understand the factors influencing guideline implementation.
METHODS:
The present study was an online survey conducted from December 2011 to May 2012 of all Ontario hospitals offering maternal-newborn services.
RESULTS:
A total of 97 of 100 eligible hospitals responded. Seventy-seven of the 97 (79%) respondents reported having implemented universal neonatal bilirubin screening. Among these hospitals, hospital-based postdischarge follow-up was reported more frequently than follow-up at community-based locations: hospital laboratory (n=40 [52%]), mother-baby care unit (n=32 [42%]), outpatient clinic (n=25 [33%]), primary care provider in community (n=19 [25%]) and community laboratory (n=8 [10%]). The CPS guidelines were the most frequently reported factor influencing implementation (n=74 [96%]).
DISCUSSION:
The survey provides valuable insight into the impact of a complex guideline in Canada’s largest province. There was heterogeneity in how hospitals organized services, but there was a notable trend toward hospital-based postdischarge care. The shift to hospital-based care runs counter to current health policy directions and highlights the lack of integration among health care sectors.
CONCLUSION:
The majority of Ontario hospitals implemented universal bilirubin screening following the release of the CPS guidelines. Further analysis is needed to determine the impact that the guidelines and the differences in implementation have had on clinical outcomes and the utilization of health services.
Keywords: Guideline adherence, Hyperbilirubinemia, Jaundice, Practice guideline
Abstract
INTRODUCTION :
En 2007, la Société canadienne de pédiatrie (SCP) a publié des lignes directrices afin de prévenir l’hyperbilirubinémie grave.
OBJECTIFS :
Déterminer si les hôpitaux ont adopté ces lignes directrices, examiner l’organisation des soins recommandée dans les lignes directrices et comprendre les facteurs qui influent sur la mise en œuvre des lignes directrices.
MÉTHODOLOGIE :
La présente étude rend compte d’une enquête virtuelle menée de décembre 2011 à mai 2012 auprès de tous les hôpitaux ontariens offrant des services mère-enfant.
RÉSULTATS :
Au total, 97 des 100 hôpitaux admissibles ont répondu à l’enquête. Soixante-dix-sept des 97 répondants (79 %) ont déclaré avoir adopté le dépistage universel de la bilirubine néonatale. Dans ces hôpitaux, le suivi en milieu hospitalier après le congé était plus fréquent que le suivi en milieu communautaire : laboratoire de l’hôpital (n=40 [52 %]), unité de soins mère-enfant (n=32 [42 %]), consultations externes (n=25 [33 %]), dispensateur de soins de première ligne en milieu communautaire (n=19 [25 %]) et laboratoire en milieu communautaire (n=8 [10 %]). Les lignes directrices de la SCP étaient le plus souvent invoquées comme le facteur ayant suscité cette mise en œuvre (n=74 [96 %]).
EXPOSÉ :
L’enquête donne un aperçu précieux des répercussions de lignes directrices complexes dans la plus grande province canadienne. On a remarqué une organisation hétérogène des services dans les hôpitaux, mais une tendance nette vers des soins en milieu hospitalier après le congé. Le passage à des soins en milieu hospitalier va à l’encontre des directives de santé actuelles et fait ressortir l’absence d’intégration entre les secteurs de soins.
CONCLUSION :
La majorité des hôpitaux ontariens a adopté le dépistage universel de la bilirubine après la publication des lignes directrices de la SCP. Il faudra effectuer une analyse plus approfondie pour déterminer les répercussions de ces lignes directrices et des divers modes de mise en œuvre sur les résultats cliniques et l’utilisation des services de santé.
Severe hyperbilirubinemia is the leading cause of neonatal readmissions in Canada (1). Although chronic bilirubin encephalopathy is rare (affecting approximately one in 43,000 births), the consequences are devastating (2). Under-recognition of severe hyperbilirubinemia places otherwise healthy infants at risk for preventable harm (3,4). Efforts to counter this problem target system-based causes such as the limitations of visual assessment of jaundice, failure to recognize the severity of hyperbilirubinemia based on age in hours, lack of appropriate follow-up after early discharge and delays in treatment (5). In 2007, the Canadian Paediatric Society (CPS) published guidelines on the detection, management and prevention of hyperbilirubinemia aimed at addressing this problem (6). The impact of these guidelines has not been evaluated.
The CPS hyperbilirubinemia guidelines are based on universal predischarge bilirubin screening, and use of a nomogram to guide follow-up and treatment. Evidence from three American cohort studies suggests that these measures reduce the incidence of severe hyperbilirubinemia (7–9). The guidelines also recommend timely follow-up for all infants after hospital discharge. This element of the guidelines adds complexity to their implementation, given the multiple sectors of the health care system (eg, hospitals, community-based care providers and public health) involved. Although clinical practice guidelines can effectively promote evidence-based practice, they are not consistently implemented in an effective and timely manner (10–12). Systematic reviews on successful guideline implementation suggests that more complex guidelines may be less likely to be adopted and followed (13).
As part of a larger project investigating the impact of the 2007 CPS hyperbilirubinemia guidelines in Ontario, we conducted a hospital survey to investigate hospital response to the guidelines. The survey objectives were: to determine whether and when hospitals had implemented universal bilirubin screening; to investigate how hospitals organize services to provide related follow-up and treatment; and to understand the processes used to implement screening and factors influencing these processes.
METHODS
Data were collected using an online questionnaire administered using Survey Monkey. The target population was all Ontario hospitals offering maternal-newborn services as of March 2011 (excluding children’s hospitals providing newborn services only) (14,15). The initial questionnaire was developed by the primary investigator (ED) using research-based principles of survey design (16–18) with input from the research team, which included expertise in nursing, paediatrics, midwifery and obstetrics. Questionnaire content was based on a review of the literature on the implementation of clinical guidelines, the research objectives and the reported experiences of a multidisciplinary work group that developed regional hyperbilirubinemia guidelines for the Champlain Local Health Integration Network (one of 14 health regions in Ontario). Questions regarding the implementation process were based primarily on theoretical constructs that focus on environmental context and resources (ie, organizational or system-level factors) (19–22). Closed-ended questions were used to gather information about whether and when hospitals had implemented screening, and about the organization of follow-up and treatment. Multiple-choice questions with the option of an open-ended response were used for questions about facilitators and challenges, and open-ended questions were used to gather information about new processes, strategies to address challenges and perceived successes.
Face validity was assessed by a four-member expert reference group with expertise in neonatology, nursing, hospital administration and research. Based on their input, the wording of four questions was revised, additional responses were added to seven multiple choice questions and one question was deleted. The revised survey was then pilot tested at five hospitals representing small- and large-volume centres. Respondents to the pilot survey provided feedback regarding question wording and acceptability. Based on their feedback, no further revisions were deemed necessary. Responses from hospitals participating in the pilot were retained for inclusion in the final analysis. Each version of the questionnaire was reviewed and approved by The Ottawa Hospital Research Ethics Board (Ottawa, Ontario) before its use.
Invitations to participate in the study were administered with assistance from the Better Outcomes Registry & Network (BORN) Ontario, which maintains contact with all Ontario hospitals providing maternal-child services. BORN distributed an e-mail to hospital contacts (primarily maternal-newborn program administrators) with a letter of information containing the link to the online survey and a copy of the survey questions. Participation was voluntary and participants retained the right to withdraw from the study at any time. No incentives were provided. Respondents from hospitals with multiple sites had the option of responding once for all sites or separately for each site. The detailed consent information from the letter of information was provided to participants again on the first screen of the online survey. Nonresponders were sent up to three reminder notices via e-mail. Following the final reminder notice, administrators who still had not responded were contacted by telephone and offered the opportunity to conduct the questionnaire verbally over the telephone.
Survey results were described by average birth volume of eligible hospitals, calculated using data from the Discharge Abstract Database accessed at the Institute for Clinical Evaluative Sciences (Toronto, Ontario).
RESULTS
The survey was piloted between December 2011 and January 2012. Remaining responses were collected between January and May 2012. Ninety-seven of 100 (97%) hospital sites responded (92 separate responses, with five respondents providing a single response for two sites) (Figure 1). Table 1 summarizes the characteristics of the responding hospitals. The three nonresponding hospitals were all from the North Local Health Integration Network region, provided level 1 newborn services and had average birth volumes of <500/year (15).
Figure 1).
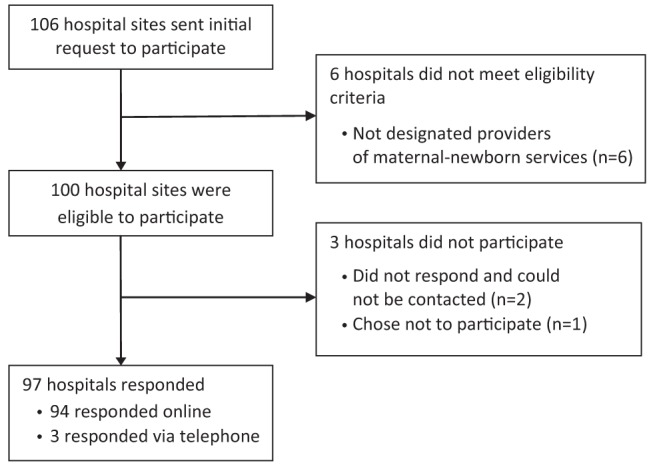
Study flow through invitation to participate, assessment of eligibility and survey response
TABLE 1.
Characteristics of responding hospitals
| Characteristic | Hospitals that implemented screening | Hospitals that did not implement screening |
|---|---|---|
| Neonatal level of care* | ||
| Level 1 | 31 (67) | 15 (33) |
| Level 2 | 39 (89) | 5 (11) |
| Level 3 | 7 (100) | 0 (0) |
| Local Health Integration Network (LHIN) | ||
| South West (LHINs 1 and 2) | 15 (100) | 0 (0) |
| Central West (LHINS 3 and 4) | 13 (87) | 2 (13) |
| Greater Toronto area (LHINS 5–9) | 24 (83) | 5 (17) |
| South East (LHINs 10–11) | 10 (71) | 4 (29) |
| North (LHINS 12–14) | 15 (63) | 9 (37) |
| Annual birth volume, number of newborns per year† | ||
| <240 | 16 (64) | 9 (36) |
| 240–500 | 9 (64) | 5 (36) |
| 501–1000 | 14 (93) | 1 (7) |
| >1000 | 38 (88) | 5 (12) |
Data presented as n (%).
Standardized criteria are used to define the level of neonatal services provided by Ontario hospitals, with level 1 being the most basic level of service and level 3 being the most specialized level of service (14);
Annual birth volumes were calculated using data from the Discharge Abstract Database housed at the Institute for Clinical Evaluative Sciences (Toronto, Ontario)
Universal screening
Seventy-seven of the 97 responding hospitals (79%) reported having implemented universal neonatal bilirubin screening. Nine of these hospitals (9%) reported having implemented universal screening before the release of the CPS guidelines. It took until May 2010 (three years following the release of the guidelines) for the next 45 hospitals to implement universal bilirubin screening. Table 2 shows the number of hospitals that implemented screening according to year. Sixty-three hospitals (82% of those conducting universal screening) reported that their approach was based on the CPS guidelines, and 12 hospitals (16%) reported that their approach was based on the guidelines with modifications.
TABLE 2.
Year of implementation of universal bilirubin screening
| Year |
Hospitals that implemented universal bilirubin screening
|
|
|---|---|---|
| n (%)* | Cumulative % | |
| 1993 | 1 (1) | 1 |
| 2003 | 2 (2) | 3 |
| 2005 | 3 (3) | 6 |
| 2006 | 1 (1) | 7 |
| 2007 | 12 (12) | 20 |
| 2008 | 13 (13) | 33 |
| 2009 | 18 (19) | 53 |
| 2010 | 12 (12) | 65 |
| 2011 | 9 (9) | 74 |
| 2012 | 2 (2) | 76 |
| Missing | 4 (4) | 80 |
Of 97 hospitals who responded to the survey
With respect to screening, 54 of the 77 hospitals (70%) that had implemented universal screening reported using total serum bilirubin measurements only for screening, 17 (22%) used both total serum bilirubin and transcutaneous bilirubin measurements, and five (6%) reported using only transcutaneous bilirubin for screening. Almost all hospitals (n=74 [96%]) indicated that if a baby required phototherapy before initial discharge from hospital, phototherapy treatment would normally be provided at that institution versus at another institution.
Follow-up testing and readmission
Follow-up for babies requiring repeat bilirubin testing after hospital discharge was organized in a variety of ways. Table 3 summarizes where parents were directed to take their baby if retesting was required and where babies were admitted for inpatient phototherapy following hospital discharge. Free-text comments elaborating on other follow-up included: going to the emergency department; referral to an outpatient newborn assessment clinic at a neighbouring hospital; and direction to see the delivering family physician if the baby did not have a physician. Another response indicated that babies under midwifery care would be provided follow-up in the community by their midwife.
TABLE 3.
Location of follow-up for hyperbilirubinemia
| Location | Hospitals |
|---|---|
| Location of follow-up testing | |
| Hospital laboratory | 40 (52) |
| Mother-baby care unit | 32 (42) |
| Outpatient clinic | 25 (33) |
| Primary care provider in community | 19 (25) |
| Community laboratory | 8 (10) |
| Neonatal nursery | 6 (8) |
| Community-based family medicine clinic | 3 (4) |
| Community-based paediatric clinic | 1 (1) |
| Community health centre | 1 (1) |
| Another hospital in the region | 1 (1) |
| Location of readmission for treatment | |
| Paediatric unit | 48 (62) |
| Normal newborn/postpartum unit | 23 (30) |
| Neonatal nursery | 17 (22) |
| Nearest children’s hospital | 7 (9) |
| Another hospital in the region | 5 (6) |
Data presented as n (%)
Ensuring follow-up
Community-based care providers for newborns in Ontario include paediatricians, family physicians, midwives and nurse practitioners. Fifty hospitals (65%) reported that bilirubin screening results are routinely communicated to the baby’s primary care provider in the community, 18 (23%) only if the results were elevated and nine (12%) did not communicate results to community-based providers. Communication methods included: written document given to parent (n=38 [49%]), direct verbal communication (n=37 [48%]), fax (n=21 [27%]) and e-mail (n=1 [1%]). ‘Other’ methods described in open-ended text included discharge summary sent by mail (n=4 [5%]), laboratory report sent by mail, and electronic access via the hospital chart or laboratory result reporting system (n=2 [3%]). Several respondents noted that elevated results would always be communicated directly to the provider most responsible for the baby while in hospital.
Thirty-seven hospitals (48%) reported implementing a process to verify that babies return at the appropriate time for follow-up testing. Twenty-eight hospitals (36%) reported booking follow-up outpatient appointments or keeping a list of babies who required follow-up and contacting ‘no shows’. Other approaches to verification of follow-up reported include physicians ensuring follow-up for their patients (n=4 [5%]), hospitals contacting the community-based care provider if parents could not be contacted (n=2 [3%]), and verification of follow-up at health unit post-partum home visits (n=1 [1%]).
Thirty-four hospitals (44%) reported using specific strategies to help ensure appropriate follow-up regarding hyperbilirubinemia for babies at high risk for experiencing barriers in access to care. Open-ended responses included referrals to public health (n=15 [19%]), community-based physicians or midwives (n=7 [9%]), and hospital social workers (n=4 [5%]), and several other strategies.
Challenges, solutions and successes
Table 4 summarizes challenges encountered in implementing universal bilirubin screening. Table 5 summarizes the more common new processes or services that respondents described in open-ended text. Forty-two hospitals (55%) reported that they developed new processes or services to implement the CPS guidelines; in many cases, these changes were introduced to address challenges identified in Table 4. Forty-one respondents (53%) described in open-ended text the successes they believed had been achieved in implementing universal bilirubin screening. Perceived successes included: improved early identification of infants at risk or in need of treatment (n=11 [14%]), better follow-up (n=8 [10%]), decreased length of stay (n=5 [6%]), high satisfaction with approach (n=3 [4%]), streamlining of care (n=3 [4%]), consistency in care (n=2 [3%]), reassurance for care providers that cases were less likely to be missed (n=2 [3%]) and reduction in painful procedures (blood draws) for newborns (n=2 [3%]).
TABLE 4.
Challenges experienced with implemention of universal bilirubin screening
| Challenges of implementation | Hospitals |
|---|---|
| Arranging postdischarge follow-up on weekends and holidays | 34 (44) |
| Delays in newborn discharge from hospital | 33 (43) |
| Arranging postdischarge follow-up for babies who live far from hospital | 30 (39) |
| Ensuring appropriate communication of results | 22 (29) |
| Arranging access to total serum bilirubin or transcutaneous bilirubin testing in the community | 20 (26) |
| Cost | 13 (17) |
| Overtesting | 11 (14) |
| Resistance from care providers to screen all babies | 7 (9) |
Data presented as n (%)
TABLE 5.
New processes and services accompanying implementation
| New process/services | Hospitals |
|---|---|
| Creation of new paediatric or neonatal outpatient clinic | 11 (14) |
| Changes in staff roles | 11 (14) |
| Arrangements for outpatient laboratory access | 11 (14) |
| Implementation of transcutaneous bilirubin testing | 8 (10) |
| Arrangements for outpatient follow-up via existing locations (paediatric department, mother-baby unit, breastfeeding clinics) | 6 (8) |
| New readmission procedures or location | 4 (5) |
Data presented as n (%)
Factors influencing implementation
Factors that influenced the decision to implement universal bilirubin screening are summarized in Table 6. Seventeen (22%) hospitals reported that there had been regional coordination of guideline implementation, and 38 (49%) hospitals reported regional engagement about a broader set of issues around the optimal provision of newborn health services following hospital discharge. Hospitals also reported engagement with local community-based primary care providers (n=32 [42%]), regional perinatal partnerships (n=21 [27%]) and local public health (n=19 [25%]).
TABLE 6.
Factors contributing to implementation of universal bilirubin screening
| Factor contributing to implementation | Hospitals |
|---|---|
| Release of Canadian Paediatric Society guidelines | 74 (96) |
| Leadership from physicians providing paediatric care | 55 (71) |
| Leadership from maternal-newborn program leaders | 55 (71) |
| Standard practices of other hospitals in region | 41 (53) |
| Risk-management considerations | 40 (52) |
| Leadership from nurse educator/clinical specialist | 37 (48) |
| Activities of regional perinatal program | 29 (38) |
| Leadership from an interdisciplinary committee | 28 (36) |
| Medicolegal considerations | 25 (32) |
| Case(s) of severe hyperbilirubinemia in hospital/community | 17 (22) |
Data presented as n (%)
Hospitals that reported not having implemented universal bilirubin screening (n=20) were asked to identify barriers to implementation. These responses are summarized in Table 7. ‘Other’ barriers described in open-ended text were: universal screening perceived as unnecessary because other processes were in place (eg, risk-based screening) (15%); physician preference (5%); and lack of knowledge (5%).
TABLE 7.
Barriers to implementation in hospitals without universal screening (n=20)
| Barrier to implementation | Hospitals |
|---|---|
| Lack of human resources to coordinate implementation of clinical protocols | 7 (35) |
| Resistance from care providers to screen all babies | 6 (30) |
| Cost | 5 (25) |
| Difficulties arranging postdischarge follow-up on weekends and holidays | 3 (15) |
| Difficulties arranging access to total serum bilirubin or transcutaneous bilirubin testing in the community | 3 (15) |
| Patient population living at a significant distance from hospital | 3 (15) |
Data presented as n (%)
DISCUSSION
The findings provide valuable insight into the impact of a complex paediatric guideline in the largest Canadian province. Uptake of the CPS hyperbilirubinemia guidelines was gradual over the first five years since their release; however, as of May 2012, approximately 88% of births in Ontario occurred at a hospital that had implemented universal bilirubin screening. There is substantial heterogeneity in how hospitals have organized services to implement the guidelines. This likely reflects differences in availability of services (including laboratory services), the mix of providers, the degree of involvement of community-based physicians in hospital care, work flow and administrative processes, birth volumes, patient demographics and the size of the geographical area served. One notable trend is that the majority of hospitals in the province have extended their responsibility for babies at higher risk of severe hyperbilirubinemia to include care during the first few days following discharge from hospital. The shift to hospital-based neonatal follow-up has been influenced by limitations in the availability of timely bilirubin testing in the community as well as limitations in access to community-based physicians within the recommended time frames.
Hospitals experienced a variety of challenges in implementing the guidelines, and the solutions they developed to address these challenges often involved creating new processes or reorganizing existing services. Several of the more frequently identified challenges that hospitals encountered have notable implications for health care resources. While a small number of respondents indicated that they had been able to reduce lengths of stay with universal bilirubin screening, a greater number of respondents perceived that screening had led to delays in hospital discharge. These delays were attributed to both the time required to screen babies and interpret the results, and to decisions to keep babies in hospital for longer when they require follow-up testing but not treatment. Some respondents also noted that challenges in interpreting the guidelines sometimes contributed to overtesting and overtreatment. Also, hospitals have not been allocated extra resources to fund the provision of postdischarge follow-up services. Another common theme in reported challenges was difficulty arising from a lack of integration among different sectors of the health care system (eg, hospitals and community-based physicians).
The results of the survey suggest that efficiency may be gained from provincial coordination of guideline implementation. While some diversity in service delivery models is to be expected given the contextual variations across the province, a provincially coordinated approach to guideline implementation may better support hospitals to benefit from the experiences of others by sharing solutions to challenges, and may also facilitate timelier implementation in low-volume hospitals with limited human resources for guideline implementation. Provincially led implementation may also ensure that changes arising from the implementation of a guideline develop in directions that are consistent with current health policy. For example, increased use of hospital-based neonatal follow-up care runs counter to current Ontario health policy directions, which aim to deliver services in the community when that option is safe, effective and less expensive (23). Current Ontario initiatives, such as the Health Links program (24), which facilitates coordination of care between different health care sectors and providers, may provide a model for the development of a coordinated system of community-based postdischarge maternal-newborn care in the province, which could reduce the need for hospital-based follow-up for neonatal hyperbilirubinemia.
A major strength of our study was that we achieved a 97% response rate to the survey. One limitation to the survey was that responses may have been subject to errors of recall or limited by the extent of experience of the respondent with respect to the questions being asked. We attempted to minimize this problem by providing an electronic copy of the survey in advance to allow time to gather responses and encouraging respondents to gather input from others as needed. Our findings are not universally generalizable outside of Ontario, but the Ontario experience may be of relevance in settings where universal bilirubin screening has been adopted in an ad hoc manner by hospitals or in settings where a coordinated system of universal community-based postdischarge maternal-newborn care is absent.
Additional research is needed to determine the impact that the guidelines have had on clinical outcomes and the utilization of health services. Recent surveillance data collected by the Canadian Paediatric Surveillance Program should provide basic information related to the impact of the guidelines on the incidence of severe neonatal hyperbilirubinemia across Canada. Data collected in our study on the month and year of screening implementation at each hospital will enable us to examine the impact of the guidelines while taking into account the lag between guideline release and implementation. Further analyses are planned, which will determine the impact on health service utilization and recommended newborn follow-up.
Acknowledgments
The authors acknowledge the assistance of BORN Ontario in distributing invitations to participate in the survey, and the efforts of all the hospital administrators who took the time to complete the questionnaire. The authors are also grateful for input provided by Kevin Coughlin, Sandra Dunn, Melissa Dougherty and Jennifer Medves during the development of the survey questionnaire. Elizabeth Darling was funded by a CIHR Vanier Canada Graduate Scholarship. Astrid Guttmann is funded by a CIHR Applied Chair in Child Health Services and Policy Research.
Footnotes
INSTITUTION WHERE THE WORK ORIGINATED: University of Ottawa.
REFERENCES
- 1.Liu S, Wen SW, McMillan D, Trouton K, Fowler D, McCourt C. Increased neonatal readmission rate associated with decreased length of hospital stay at birth in Canada. Can J Public Health. 2000;91:46–50. doi: 10.1007/BF03404253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canadian Paediatric Surveillance System. 2008 Results. 2009 < www.cpsp.cps.ca/publications/annual-cpsp-results/Results-2008.pdf> (Accessed October 10, 2013) [Google Scholar]
- 3.Bhutani VK, Johnson L. Kernicterus in the 21st century: Frequently asked questions. J Perinatol. 2009;29(Suppl 1):S20–4. doi: 10.1038/jp.2008.212. [DOI] [PubMed] [Google Scholar]
- 4.Sgro M, Campbell D, Shah V. Incidence and causes of severe neonatal hyperbilirubinemia in Canada. CMAJ. 2006;175:587–90. doi: 10.1503/cmaj.060328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhutani VK, Johnson LH, Maisels MJ, et al. Kernicterus: Epidemiological strategies for its prevention through systems-based approaches. J Perinatol. 2004;24:650–62. doi: 10.1038/sj.jp.7211152. [DOI] [PubMed] [Google Scholar]
- 6.Barrington KJ, Sankaran K, Canadian Paediatric Society, Fetus and Newborn Committee Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks’ gestation) Abridged version: Paediatr Child Health. 2007;12(Suppl B):1B–12B. < www.cps.ca/en/documents/position/hyperbilirubinemia-newborn> (Accessed October 10, 2013) [Google Scholar]
- 7.Eggert LD, Wiedmeier SE, Wilson J, Christensen RD. The effect of instituting a prehospital-discharge newborn bilirubin screening program in an 18-hospital health system. Pediatrics. 2006;117:e855–62. doi: 10.1542/peds.2005-1338. [DOI] [PubMed] [Google Scholar]
- 8.Kuzniewicz MW, Escobar GJ, Newman TB. Impact of universal bilirubin screening on severe hyperbilirubinemia and phototherapy use. Pediatrics. 2009;124:1031–9. doi: 10.1542/peds.2008-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mah MP, Clark SL, Akhigbe E, et al. Reduction of severe hyperbilirubinemia after institution of predischarge bilirubin screening. Pediatrics. 2010;125:e1143–e8. doi: 10.1542/peds.2009-1412. [DOI] [PubMed] [Google Scholar]
- 10.Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39(8 Suppl 2):II46–54. doi: 10.1097/00005650-200108002-00003. [DOI] [PubMed] [Google Scholar]
- 11.Ploeg J, Davies B, Edwards N, Gifford W, Miller PE. Factors influencing best-practice guideline implementation: Lessons learned from administrators, nursing staff, and project leaders. Worldviews Evid Based Nurs. 2007;4:210–9. doi: 10.1111/j.1741-6787.2007.00106.x. [DOI] [PubMed] [Google Scholar]
- 12.Davies B, Edwards N, Ploeg J, Virani T. Insights about the process and impact of implementing nursing guidelines on delivery of care in hospitals and community settings. BMC Health Serv Res. 2008;8:29. doi: 10.1186/1472-6963-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francke A, Smit M, de Veer A, Mistiaen P. Factors influencing the implementation of clinical guidelines for health care professionals: A systematic meta-review. BMC Med Inform Decis Mak. 2008;8:38. doi: 10.1186/1472-6947-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Provincial Council for Maternal and Child Health. Standardized Maternal and Newborn Levels of Care Definitions. 2011
- 15.Provincial Council for Maternal and Child Health. Maternal and Newborn Level of Care Designations. 2011
- 16.Burns KEA, Duffett M, Kho ME, et al. A guide for the design and conduct of self-administered surveys of clinicians. CMAJ. 2008;179:245–52. doi: 10.1503/cmaj.080372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boynton PM, Greenhalgh T. Selecting, designing, and developing your questionnaire. BMJ. 2004;328:1312–5. doi: 10.1136/bmj.328.7451.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eysenbach G. Improving the quality of web surveys: The Checklist for Reporting Results of Internet E-Surveys (CHERRIES) J Med Internet Res. 2004;6:e34. doi: 10.2196/jmir.6.3.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michie S, Johnston M, Abraham C, Lawton R, Parker D, Walker A, ‘Psychological Theory’ Group Making psychological theory useful for implementing evidence based practice: A consensus approach. Qual Saf Health Care. 2005;14:26–33. doi: 10.1136/qshc.2004.011155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramanian U, Sutherland J, McCoy K, Welke K, Vaughn T, Doebbeling B. Facility-level factors influencing chronic heart failure care process performance in a national integrated health delivery system. Med Care. 2007;45:28–45. doi: 10.1097/01.mlr.0000244531.69528.ee. [DOI] [PubMed] [Google Scholar]
- 21.Barry A, Loewen P, De Lemos J, Lee K. Reasons for non-use of proven pharmacotherapeutic interventions: Systematic review and framework development. J Eval Clin Pract. 2012;18:49–55. doi: 10.1111/j.1365-2753.2010.01524.x. [DOI] [PubMed] [Google Scholar]
- 22.Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci. 2012;7:37. doi: 10.1186/1748-5908-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ontario Government. Ontario’s Action Plan For Health Care: Better patient care through better value from our health care dollars. Queen’s Printer for Ontario; 2012. < www.health.gov.on.ca/en/ms/ecfa/healthy_change/docs/rep_healthychange.pdf> (Accessed October 10, 2013) [Google Scholar]
- 24.Ontario Government. Transforming Ontario’s Health Care System: Community Health Links provide coordinated, efficient and effective care to patients with complex needs. 2013. < www.health.gov.on.ca/en/pro/programs/transformation/community.aspx> (Accessed October 10, 2013)


